Abstract
Fibrosis has also been recorded as a prominent pathological feature within wooden breast (WB) myopathy of broiler chickens. This study was conducted to evaluate the accumulation of fibril collagen, deposition of the extracellular matrix (ECM) components, and the underlying mechanism mediating the pathogenic fibrotic process in the pectoralis major (PM) muscle of WB-affected birds. Broiler chickens were categorized into the control and WB groups based on the evaluation of myopathic lesions. Results indicated that the total content and area of collagen in cross-sections of the PM muscle, as well as the augmented expression of collagen-I and fibronectin in the ECM, were greatly increased in birds with WB. Wooden breast myopathy upregulated expressions of transforming growth factor-beta (TGF-β) and the phosphorylation of Smad 2 and 3, thereby activating TGF-β-mediated Smad signaling pathway, which further enhanced the transcription of profibrotic mediators. In addition, regulators involved in collagen biosynthesis and cross-linking including prolyl 4-hydroxylase, lysyl oxidase, lysyl hydroxylase, and decorin were increased in the WB muscle. Finally, the expressions of both matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinases (TIMP) were increased in the WB muscle, which might be related with reduced ECM remodeling. Overall, WB myopathy induces severe fibrosis by enhancing ECM deposition and collagen cross-linking in the PM muscle of broiler chickens, possibly via the activation of TGF-β signaling and the dysregulation of the MMP and TIMP system.
Key words: wooden breast, fibrosis, transforming growth factor-beta, collagen remodeling, broiler chicken
Introduction
Wooden breast (WB) myopathy emerges as a serious muscle disorder affecting modern broiler chickens that causes tremendous economic losses to the global poultry industry (Petracci et al., 2019). The WB shows pale and outbulging areas of hardened consistency in the pectoralis major (PM) muscle and is also occasionally covered with clear viscous fluid and hemorrhages. The affected areas show histological changes characterized by polyphasic myodegeneration and necrosis, infiltration of macrophages and heterophils, as well as interstitial connective-tissue accumulation (Sihvo et al., 2014). Wooden breast disorder impairs the appearance and technological and nutritional properties of breast meat and negatively affects consumer purchases (Soglia et al., 2015; Xing et al., 2020). Considerable studies have been conducted to investigate the possible etiologies leading to the development of WB myopathy, suggesting that localized hypoxia, oxidative stress, inflammatory responses, fiber-type switching, and metabolic shift are multifactorial components of this myopathy (Petracci et al., 2019). However, the molecular mechanisms mediating these underlying pathogenic processes remain poorly understood.
Fibrosis is a process that typically results from chronic tissue injury and inflammation and involves excessive deposition of the extracellular matrix (ECM) that compromises tissue function (Wynn, 2008). The fibrotic process in the skeletal muscle can be observed in various inherited and acquired myopathies including Duchenne muscular dystrophy (DMD), amyotrophic lateral sclerosis, and muscle atrophy as well as in aging muscle (Rayavarapu et al., 2013). Generally, fibrosis reduces motile and contractile function of skeletal muscle and limits the amount of muscle available for therapy and repair (Ismaeel et al., 2019). Interstitial connective-tissue accumulation or fibrosis has also been recorded as a prominent pathological feature within WB myopathy of broiler chickens. Velleman et al. (2017) proposed that the palpable hardness of WB-affected PM muscle might be due to the fibrosis resulting from the deposition of highly cross-linked collagen fibrils. In addition, WB-affected broilers usually exhibit physiological behavior changes of increased locomotor difficulties, decreased wing mobility, and higher occurrence of dorsal recumbency, which seriously affect their welfare (Gall et al., 2019; Norring et al., 2019). Therefore, investigating the ECM deposition and defining the mechanisms mediating the fibrotic process within WB myopathy can promote an understanding of its etiology and help develop effective therapies for this myopathy.
Transforming growth factor-beta (TGF-β) functions as a central profibrotic cytokine contributing to the pathogenesis of fibrotic disorders through promoting ECM synthesis, enhancing expression of profibrotic genes and suppressing the activity of ECM degradation proteins (Ismaeel et al., 2019). The TGF-β promotes pathological fibrosis via the activation of canonical Smad-dependent pathway to initiate signal transduction (Leask and Abraham, 2004). Elevated expression levels of TGF-β have been observed in the serum and skeletal muscle of patients with DMD and in the skeletal muscle during chronic inflammation (Burks and Cohn, 2011). Recent transcriptomic data revealed that gene expression of TGFB3 is upregulated in the PM muscle of WB-affected broilers (Mutryn et al., 2015), and this result was also observed by Velleman and Clark (2015). The TGF-β plays important roles in the pathogenesis of myopathic disorders. For example, TGF-β1 promotes the transformation of myoblasts into fibrotic cells in regenerating skeletal muscle after injury (Serrano and Muñoz-Cánoves, 2010). Furthermore, Allen and Boxhorn (1987) demonstrated that TGF-β inhibits satellite cell proliferation and myofiber fusion, thereby negatively affecting skeletal muscle regeneration.
The present study was designed to evaluate the deposition of collagen fibril and other ECM proteins and to assess the activation of canonical TGF-β-mediated Smad signaling to elucidate the pathogenic mechanisms regulating fibrosis in PM muscle of WB myopathic broilers. Furthermore, we also examined expression patterns of genes involved in collagen biosynthesis, cross-linking, and remodeling to further comprehend this fibrotic process.
Materials and methods
Selection of Experimental Broiler Chickens
All experimental procedures and bird managements were approved by Nanjing Agricultural University Institutional Animal Care and Use Committee under protocol number SYXK 2017-0007. Broiler chickens used in this study were all Arbor Acres males raised in layered cages and received commercial diets and husbandry routines. At 6 wk of age, a total of 300 broilers were examined independently for WB myopathy by 3 experienced individuals following the methods described by Papah et al. (2017) and Velleman et al. (2017). Then, 63 suspected WB-affected and 20 WB-unaffected broilers were selected for further management. These broilers were electrically stunned (50 V, alternating current, 400 Hz for 5 s each) and slaughtered via exsanguination (Zhang et al., 2017). Immediately after slaughter, broilers were necropsied, and samples from the superficial layer of the cranial part of the right PM muscle were collected either snap-frozen in liquid nitrogen for molecular analysis or fixed in 4% paraformaldehyde for histological evaluation or immunostaining.
Histological Analysis, Scoring of Lesions, and Sample Collection
For histological analysis, paraffin-embedded muscle tissue cross-sections were cut into 8-μm thickness and subjected to hematoxylin and eosin staining. Images were acquired using a light microscope (Axio Scope.A1, Carl Zeiss, Oberkochen, Germany). Four fields per section and 2 sections from each sample were randomly selected for scoring of lesions according to an ordinal scoring system (Gibson-Corley et al., 2013). The ordinal grades were assigned based on the severity of myopathic lesions showing vascular damage, lipid infiltration, myodegeneration, myositis, interstitial edema, occasional myoregeneration, and fibrosis recorded by Papah et al. (2017). After scoring for each PM muscle slide, 10 unaffected (with no presence of myopathic lesion, fibrosis, or lipidosis, control [CON]) and 10 affected (with moderate to severe lesions, WB) muscle samples were randomly chosen for further biochemical analysis.
Masson Trichrome Staining and Collagen Area Analysis
Paraffin-embedded tissues were sectioned at 8 μm, rehydrated by a series of incubations in xylene and ethanol solutions and then used for Masson Trichrome staining according to the procedures described by Huang et al. (2010), which stains muscle fibers red, nuclei black, and collagen blue. Images were acquired under identical conditions at the same magnification using a light microscope. Eight fields per sample were randomly selected for collagen area quantification using the Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc., Rockville, MD). The area of collagen was expressed as the percentage of the captured image areas.
Collagen Concentration Measurement
Collagen concentration was calculated from the hydroxyproline concentration determined using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), in accordance with the manufacturer's instructions. The total collagen concentration was calculated assuming collagen weighs 7.25 times hydroxyproline (Zimmerman et al., 2001), and the results were expressed as mg/g dry muscle mass.
RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
Total RNA extraction was performed using Trizol reagent (Takara Biotechnology Co., Ltd., Dalian, China) following the manufacturer's instructions. Total RNA was quantified by measuring the absorbance at 260 nm with a NanoDrop ND-100 spectrophotometer (NanoDrop Technologies, Rockland, DE), and the purity was assessed by determining the ratio of the absorbance at 260 and 280 nm. cDNA was reverse-transcribed using a commercial cDNA Synthesis Kit (PrimeScript RT Master Mix, Takara). Quantitative real-time PCR was performed on an Applied Biosystems 7500 instrument (Foster City, CA) using SYBR Premix EX Taq (Takara). Primer sets used for quantitative RT-PCR analysis are listed in Supplementary Table 1. All gene expressions are calculated as the relative fold changes compared with CON, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal reference to normalize the expression of targets genes. Relative mRNA expression was calculated by the 2−ΔΔCT method.
Total Protein Extraction and Western Blot Analysis
Frozen muscle samples were homogenized in RIPA lysis buffer (Beyotime Biotechnology, Jiangsu, China) containing protease and phosphatase inhibitors (Roche Applied Science, Indianapolis, IN). The homogenate was centrifuged at 12,000 g for 20 min at 4°C, and the supernatant (avoiding the fat layer) was collected. The bicinchoninic acid assay was used to determine the protein concentration (Pierce Chemical Co., Rockford, IL). For electrophoresis, an appropriate volume of each sample and an equal amount of loading buffer (10-mM Tris-HCl, 2.5% SDS, 1% β-mercaptoethanol, 10% glycerol, and 0.01% bromophenol blue, pH 6.8) were combined together and heated for 5 min at 95°C. Equal amounts of total protein were resolved on 10% SDS-PAGE using a BioRad Electrophoresis System (Richmond, CA) before being transferred to a nitrocellulose membrane (Millipore, Merck, Germany). After transfer, the membranes were incubated in a blocking buffer consisting of 5% nonfat dry milk powder dissolved in Tris-buffered saline (50 mM Tris-HCl, 150 mM NaCl, pH 7.6) with 0.1% Tween-20 for 1 h at room temperature. Membranes were then incubated in primary antibodies against TGF-β, collagen-I, α-smooth muscle actin (α-SMA), GAPDH (Servicebio Biological Technology, Wuhan, China), phosphor-Smad 2 and 3 (Ser423/425) (Cell Signaling Technology, Danvers, MA), and Smad 2 and 3 (Boster Biological Technology, Wuhan, China) diluted in Tris-buffered saline (50 mM Tris-HCl, 150 mM NaCl, pH 7.6) with 0.1% Tween-20 overnight at 4°C. The membranes were washed and incubated with the corresponding horseradish peroxidase–conjugated secondary antibodies (Bioworld, Nanjing, China) for 1 h. Finally, the membranes were visualized using ECL reagents (Pierce, IL) and scanned using ImageQuant LAS4000 (GE, CT). The density of the bands was quantified by using Quantity One software (Bio-rad). GAPDH was used as the house-keeping protein for normalization.
Immunofluorescence Microscopic Analysis
Immunofluorescence microscopic analysis was performed according to a previous study (Xing et al., 2016). Briefly, frozen muscle cross-sections were cut into 8-μm thickness and placed on 3-aminopropyl-triethoxysilane–coated slides. The slides were fixed in 4% paraformaldehyde and blocked using normal goat serum diluted in 0.01-M PBS (135 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, and 8 mM K2HPO4, pH 7.2) at 37°C for 1 h, and then incubated in primary antibodies against collagen-I and fibronectin 1 (Servicebio Biological Technology, Wuhan, China) diluted in PBS overnight at 4°C. The slides were washed and incubated with corresponding Alexa Fluor 488 or 647-conjugated secondary antibodies (Molecular Probes, Eugene, OR) at 37°C for 1 h. Subsequently, the sections were counterstained with 4′,6-diamidino-2-phenylindole. Finally, the images of fluorochrome-stained sections were acquired using a fluorescence microscope (Axio Scope.A1, Carl Zeiss, Oberkochen, Germany). Four fields per sample were randomly selected for fluorescence intensity quantification using Image-Pro Plus software (Media Cybernetics, Inc., Rockville, MD). Results are expressed as the fold difference between the CON and WB groups.
Statistical Analysis
Data were analyzed by ANOVA using SAS 9.12 (2003; SAS Inst. Inc., Cary, NC) and differences between individuals were compared using Student's t-tests. Data were reported as means ± SE. Significance was considered when P ≤ 0.05.
Results
Collagen Content in PM Muscle of WB Myopathic Birds
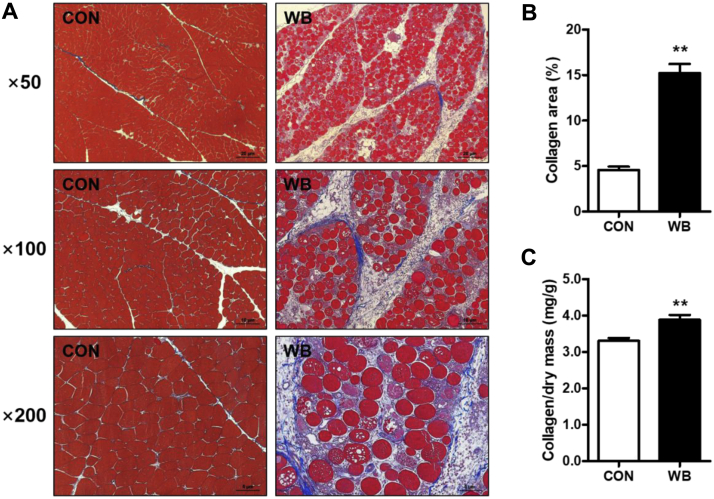
The Masson Trichrome staining showed more collagen deposition in the endomysial and perimysial spaces of PM muscles in WB myopathic broilers than those of the CON broilers (Figure 1A). The calculated collagen area was significantly higher in the WB muscle than in the CON (15.22 ± 1.00 vs. 4.56 ± 0.37%; P < 0.01; Figure 1B). In addition, collagen concentration, as measured by the hydroxyproline concentration, was significantly elevated in the PM muscle of WB in comparison with CON (3.31 ± 0.08 vs. 3.88 ± 0.14 mg/g; P < 0.01; Figure 1C).
Figure 1.
Collagen content for the pectoralis major (PM) muscle of wooden breast–unaffected (CON) and –affected (WB) broiler chickens. (A) Representative images of Masson Trichrome staining of PM muscle from CON and WB broiler chickens at different magnifications. (B) The percentage of the collagen area normalized to the captured image area. (C) Collagen concentration calculated based on the hydroxyproline concentration. Data are expressed as the mean ± SE (n = 10). ∗∗P < 0.01. Abbreviations: CON, control; WB, wooden breast.
Extracellular Matrix Deposition in the PM Muscle of WB Myopathic Birds
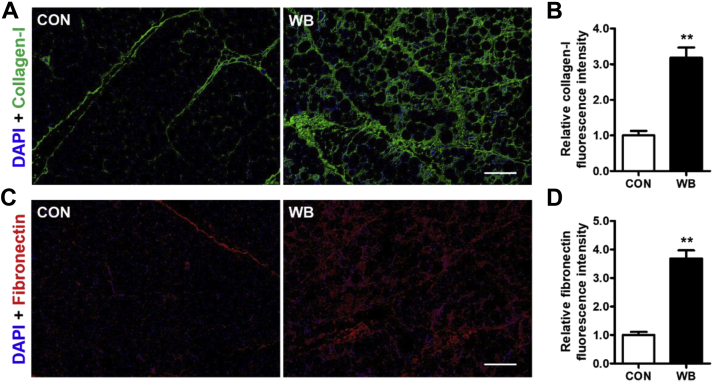
To confirm the increased fibrosis suggested by Masson Trichrome staining, we further detected the distribution of representative ECM proteins including collagen-I and fibronectin using immunofluorescence staining (Figures 2A and 2C). The fluorescence staining pattern of these 2 fibrotic proteins was obviously enhanced in WB myopathic birds when compared with the CON birds, showing increased staining distributing either in the perimysium or endomysium. Consistently, the calculated relative collagen-I and fibronectin fluorescence intensities were significantly higher in the PM muscle of the WB group than those in the CON group (P < 0.01; Figures 2B and 2D).
Figure 2.
Extracellular matrix (ECM) components deposition in the pectoralis major (PM) muscle of wooden breast–unaffected (CON) and –affected (WB) broiler chickens. (A) Representative images of immunofluorescence staining of collagen-I in PM muscle of CON and WB. (B) Relative fluorescence intensity of collagen-I. (C) Representative images of immunofluorescence staining of fibronectin in the PM muscle of CON and WB. (D) Relative fluorescence intensity of fibronectin. Data are expressed as the mean ± SE (n = 10). ∗∗P < 0.01. Abbreviations: CON, control; WB, wooden breast.
Activation of TGF-β-mediated Smad Signaling Pathway
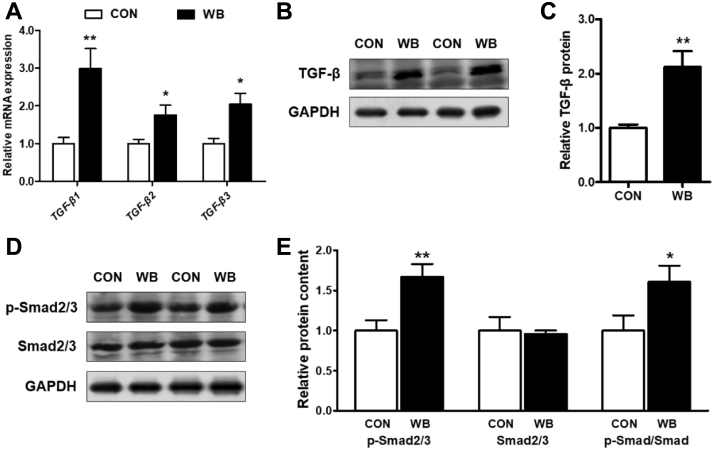
The TGF-β superfamily contains 3 prototypes namely TGF-β1, 2, and 3, and they are synthesized as latent precursors complexed with TGF-β–binding proteins (Leask and Abraham, 2004). As exhibited in Figure 3A, WB myopathy induced the enhancement of mRNA expression of all the 3 TGF-β prototypes in the PM muscle as compared with the CON group (P < 0.05). Consistently, the relative TGF-β protein content was significantly higher in the WB group than in the CON group (Figures 3B and 3C, P < 0.01). In addition, we observed that the phosphorylation of Smad 2 and 3 at Ser423/425 was increased in WB compared with CON (Figures 3D and 3E, P < 0.05, for original Western blot frames containing all 10 bands, see Supplementary Figure 1). Collectively, these results suggest that TGF-β-mediated Smad signaling pathway was activated in the PM muscle of WB myopathy–affected birds.
Figure 3.
Transforming growth factor-β (TGF-β-mediated) Smad signaling pathway in pectoralis major (PM) muscle of wooden breast–unaffected (CON) and –affected (WB) broiler chickens. (A) Relative mRNA expression of TGF-β1, TGF-β2, and TGF-β3 in the PM muscle of CON and WB. (B and C) Relative protein content of TGF-β in the PM muscle of CON and WB. (D and E) Relative protein content of phosphorylated Smad 2 and 3 and total Smad 2 and 3 in the PM muscle of CON and WB. Data are expressed as the mean ± SE (n = 10). ∗∗P < 0.01 and ∗ P < 0.05. Abbreviations: CON, control; WB, wooden breast.
Expression of Profibrotic Mediators
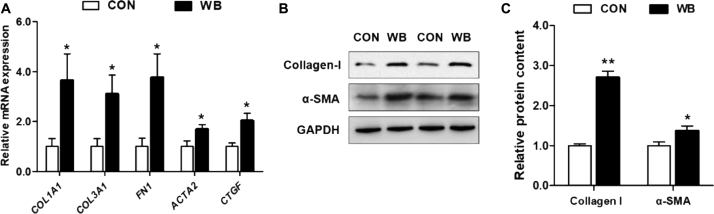
The mRNA expression of ECM components and profibrotic mediators including collagen type I alpha 1 chain, collagen type III alpha 1 chain, fibronectin 1, α-smooth muscle actin, and connective tissue growth factor (CTGF) were significantly increased (P < 0.05) in the PM muscle of the WB group compared with the CON group (Figure 4A). We further tested the protein contents of representative ECM components; results indicated that collagen-I (P < 0.01) and α-SMA (P < 0.05) were both significantly upregulated in WB compared with CON (Figures 4B and 4C).
Figure 4.
Relative expression of extracellular matrix components and profibrotic mediators in the pectoralis major (PM) muscle of wooden breast–unaffected (CON) and –affected (WB) broiler chickens. (A) Relative mRNA expression of profibrotic genes including collagen type I alpha 1 chain (COL1A1), collagen type III alpha 1 chain (COL3A1), fibronectin 1 (FN1), α-smooth muscle actin (ACTA2), and connective-tissue growth factor (CTGF) in the PM muscle of CON and WB. (B and C) Relative protein content of collagen-I and α-SMA in the PM muscle of CON and WB. Data are expressed as the mean ± SE (n = 10). ∗∗P < 0.01 and ∗ P < 0.05. Abbreviation: CON, control.
Regulators Catalyzing Collagen Biosynthesis and Cross-linking
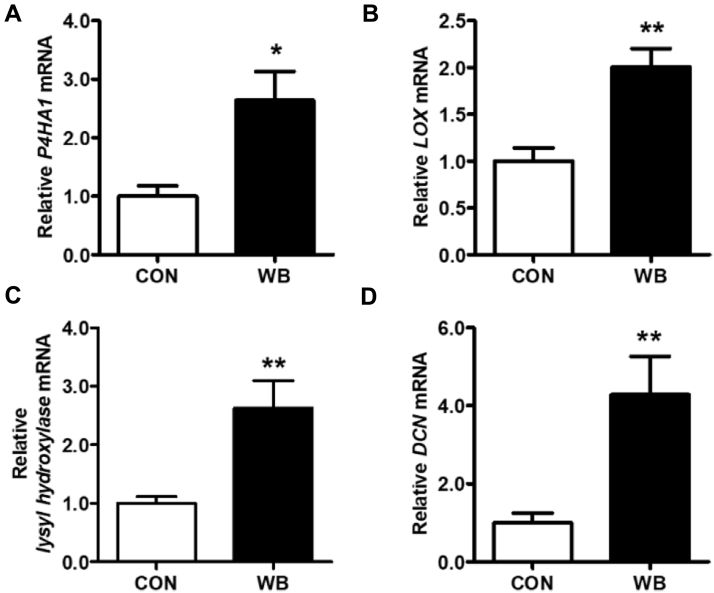
Collagen biosynthesis and intermolecular cross-linking are complicated biological processes mediated by a series of key regulators. As exhibited in Figure 5, mRNA expressions of prolyl 4-hydroxylase (P4HA1), lysyl oxidase (LOX), lysyl hydroxylase, and decorin were increased by 164.3 ± 49.0% (P < 0.05), 100.7 ± 19.6% (P < 0.01), 203.1 ± 67.8% (P < 0.05), and 328.3 ± 98.4% (P < 0.01), respectively, in the PM muscle from WB compared with CON broiler chickens.
Figure 5.
Relative mRNA expression of enzymes regulating collagen biosynthesis and cross-linking in PM muscle of wooden breast–unaffected (CON) and –affected (WB) broiler chickens. (A) Prolyl 4-hydroxylase (P4HA1). (B) Lysyl oxidase (LOX). (C) Lysyl hydroxylase. (D) Decorin (DCN). Data are expressed as the mean ± SE (n = 10). ∗∗P < 0.01 and ∗ P < 0.05. Abbreviation: CON, control.
Enzymes Mediating ECM Remodeling
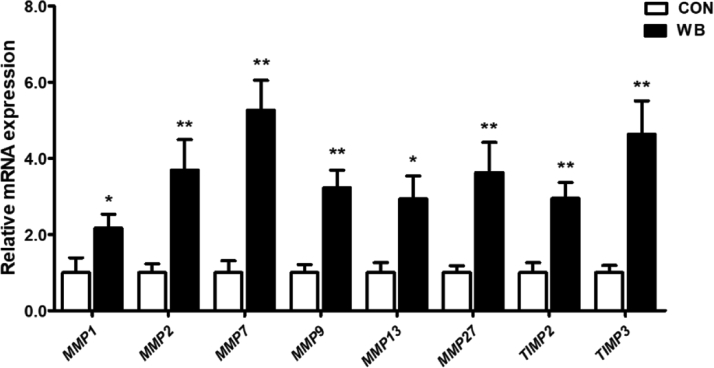
Matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinases (TIMP) are key enzymes regulating ECM remodeling. We observed that PM muscle of WB broiler chickens expressed significantly higher MMP1 (P < 0.05, by 117.4 ± 36.3%), MMP2 (P < 0.01, by 270.4 ± 79.2%), MMP7 (P < 0.01, 426.8 ± 78.4%), MMP9 (P < 0.01, 223.3 ± 45.6%), MMP13 (P < 0.05, by 194.4 ± 59.7%), and MMP27 (P < 0.01, by 262.9 ± 79.2%) mRNA levels than that of the CON group. In addition, mRNA expression of TIMP2 and TIMP3 were greatly increased in WB compared with CON (Figure 6).
Figure 6.
Relative mRNA expression of matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinases (TIMPs) in the PM muscle of wooden breast–unaffected (CON) and –affected (WB) broiler chickens. Data are expressed as the mean ± SE (n = 10). ∗∗P < 0.01 and ∗ P < 0.05. Abbreviation: CON, control.
Discussion
Genetic selection for accelerated growth rate and increased breast muscle yield has been associated with the development of WB myopathy in modern broiler chickens (Petracci et al., 2019). Despite the recently conducted research characterizing clinical and gross features, histological lesions as well as molecular and metabolic alterations of this muscle abnormality, its etiology still remains incompletely understood. The deposition of variable amounts of connective tissue in the epimysia and endomysia areas of the affected areas has been identified as a prominent histopathological feature of WB myopathy (Sihvo et al., 2014; Soglia et al., 2015). In addition, the defining phenotypic feature of palpation hardness of WB-affected tissues is strongly linked to the enhanced accumulation of highly cross-linked collagen fibrils (Velleman et al., 2017). Therefore, the purpose of this study was to investigate the fibrotic status and to define the underlying mechanisms mediating the fibrotic process so as to promote the understanding of its etiology and developing effective pharmacotherapy to attenuate muscle fibrosis in WB myopathic birds.
Fibrosis results from the dysregulation of tissue repair under chronic inflammation status induced by various stimuli including persistent infections, chemical insults, and tissue injury (Wynn, 2008). In severe muscular dystrophies such as DMD, amyotrophic lateral sclerosis, and spinal muscular atrophy as well as in the aging process, the aberrant fibrotic process characterized by excessive production, deposition, and contraction of ECM components has been the most recognized histopathological symptom (Serrano and Munoz-Canoves, 2010). Similarly, prominent fibrosis including variable amount of loose connective tissue, granulation tissue, and collagen-rich connective tissue accumulation has been described in chickens (Fujii et al., 1983; Kuttappan et al., 2013) and turkeys (Sosnicki et al., 1991) affected by various myopathies or dystrophies. In the present study, a higher collagen content was observed in WB myopathy–affected PM muscle of broiler chickens as directly evidenced by the Masson Trichrome staining and the determination of the hydroxyproline concentration. Furthermore, the PM muscle from birds with WB displayed markedly increased perimysial and endomysial deposition of collagen-I and fibronectin compared with a CON muscle biopsy. Overall, the replacement of normal muscle fiber by excessive ECM molecules in WB-affected muscle of broiler chickens indicated that the fibrotic process has been established, which might further lead to the aggravation of this myopathy (Lieber and Ward, 2013).
Mutryn et al. (2015) indicated that 2-oxoglutarate 5-dioxygenase 2 (PLOD2), MMP2, and TGFB3 genes related with ECM deposition and remodeling were upregulated in PM muscle of WB-affected birds as evidenced by RNA-seq analysis. However, the intrinsic mechanisms regulating this fibrotic response remain unknown. The ECM proteins are mainly produced by activated tissue mesenchymal fibroblasts, whereas the activation of fibroblasts is stimulated by fibrogenic cytokines. Of which, TGF-β is the most potent fibrogenic cytokine contributing to the pathogenesis of fibrotic disorders. The TGF-β signals through its transmembrane serine/threonine kinase receptors (type I and type II), which in turn phosphorylates receptor-regulated Smad2 and/or Smad3 and induces their translocation into the nucleus, where they bind to DNA and regulate transcription of fibrotic genes (Leask and Abraham, 2004). The elevated expression patterns of TGF-β and its receptors have been extensively observed in a variety of inherited and acquired myopathies (Burks and Cohn, 2011). Furthermore, evidence suggested that neutralization of the TGF-β using a specific antibody reduces fibrosis and restores muscle architecture in neuromuscular disease model of mdx mice (Cohn et al., 2007). This indicates the important role for this fibrogenic cytokine in pathogenic muscle fibrosis. Here, we observed that TGF-β was highly expressed in PM muscle of WB myopathic birds, which was in accordance with a previous finding (Velleman and Clark, 2015). In addition, the phosphorylation of Smad 2 and 3 at Ser423/425 was induced that correlates with promoted transcription of profibrotic genes including collagen type I alpha 1 chain, collagen type III alpha 1 chain, fibronectin 1, α-smooth muscle actin, and CTGF, as well as high levels of ECM proteins collagen-I and α-SMA. Among the examined profibrotic factors, CTGF exerts coregulatory effects with the TGF-β on fibroblast proliferation, adhesion, and ECM production through enhancing the binding ability of the TGF-β to its receptors (Abreu et al., 2002). Furthermore, TGF-β treatment of myoblasts and myotubes induces the synthesis of CTGF, which inhibits myogenesis and promotes dedifferentiation of myoblasts, thereby inducing fibrosis (Vial et al., 2008). Collectively, our results further confirmed that the activation of TGF-β-mediated Smad cascade initiated the downstream profibrotic signaling transduction and promoted ECM protein deposition in PM muscle of myopathic broiler chickens.
Fibrosis-induced tissue hardness is affected by both the extent and the structure of fibrillar collagen. Collagen biosynthesis involves the conversion of prolyl residues to hydroxyproline, and P4HA is the key enzyme catalyzing the synthesis of hydroxyprolyl residues (Han et al., 1999). We observed that mRNA expression of P4HA1 was higher in PM muscle of WB-affected birds. Fibril diameter and density, cross-linking, and glycosaminoglycan content are important factors contributing to the structural stability of collagen fibrils. LOX catalyzes the oxidative deamination of telopeptide and helical collagen lysine residues extracellularly and allows the initiation of the cross-linking process. Lysyl hydroxylase catalyzes the hydroxylation of lysyl residues intracellularly and promotes the formation of nonreversible trivalent hydroxylysylpyridinoline cross-link (Reiser et al., 1992), which increases the collagen fibril diameter and contributes to tissue hardness. Inflammation could induce the expression of LOX and lysyl hydroxylase via hypoxia-inducible factor 1α (Hofbauer et al., 2003), which reasonably explained the abundance of both LOX and lysyl hydroxylase in WB compared with CON muscle as evidenced by the inflammatory and hypoxia status observed in WB myopathic birds (Sihvo et al., 2014; Malila et al., 2019). Decorin is an extracellularly located small leucine-rich proteoglycan that binds to collagens and TGF-β, playing a major role in the formation of hydroxylysylpyridinoline cross-link. In a variety of muscular dystrophies, the enhanced decorin levels have been closely correlated with increased fibrosis (Zanotti et al., 2005). Here, the expression of decorin was higher in WB muscle than in the CON group, which was consistent with a previous observation (Velleman et al., 2017). Overall, the enhanced expression of these collagen synthesis and cross-linking regulators would help explain the deposition of collagen in WB muscle and the palpation hardness of the affected tissue of broiler chickens.
To further explore mechanisms leading to the enhanced ECM deposition in PM muscle of WB myopathic birds, we examined major determinant enzymes catalyzing ECM remodeling. The MMP are extracellular zinc-dependent endopeptidases participating in the degradation and remodeling of the ECM by direct cleavage of collagen, activation of latent enzymes, and liberation of structural or signaling molecules. Tissue inhibitor of metalloproteinases functions exclusively as endogenous inhibitors of MMP, thereby modulating ECM remodeling (Alameddine and Morgan, 2016). The balance of MMP and TIMP regulates ECM homeostasis, perturbation of this balance occurs in various physiological and pathological remodeling situations including muscular dystrophies, neuromuscular diseases, and inflammatory myopathies (Alameddine, 2012). In the present study, mRNA expression of examined MMP including MMP1, MMP2, MMP7, MMP9, MMP13, and MMP21 was higher in the PM muscle of the bird with WB. Of which, MMP1 and MMP13 are collagenases, MMP2 and MMP9 are gelatinases, MMP7 is a matrilysin enzyme (Page-McCaw et al., 2007). Generally, these MMP coordinate and exhibit activities toward growth factors, cytokines and chemokines, acting on the degradation of a broad and diverse array of ECM substrates (Yamamoto et al., 2015). In addition to hydrolytic roles, MMP are multifunctional regulators involved in cell migration and fusion in skeletal muscles. Upregulation of MMP correlates with the inflammatory process in muscular dystrophies or inflammatory myopathies, which probably contributes to muscle regeneration (Kherif et al., 1999; Miyazaki et al., 2011). Herein, we observed that TIMP2 and TIMP3 were overexpressed in WB myopathic birds, which might inhibit the activities of MMP and suppress ECM degradation and remodeling. Meanwhile, TIMP have also been implicated in myoblasts fusion, myofibers maturation, and mediation of angiogenesis (Visse and Nagase, 2003). Overall, the elevation of both MMP and TIMP mRNA levels in PM muscle of WB myopathic bird implied the possible involvement of the MMP and TIMP system in the regulation of hydrolysis of ECM components and their synthesis. However, due to their equally important regulatory functions of the extracellular signaling network during muscle injury or disease, mechanisms responsible for the alterations of MMP and TIMP balance and the role of certain MMP and TIMP during the dynamic pathogenic fibrosis phases of WB myopathy require further investigation.
Conclusions
Our findings demonstrate that WB myopathy induces fibrosis and the deposition of ECM components including collagen-I, fibronectin, and α-SMA in PM muscle of broiler chickens, which might be associated with the activation of TGF-β-mediated Smad signaling. Wooden breast myopathy also upregulates expression of genes related with collagen synthesis and cross-linking, which might contribute to the palpation hardness of the affected tissues. In addition, the MMP and TIMP system might be involved in the pathological remodeling of ECM in birds with WB.
Acknowledgments
This study was supported by the Natural Science Foundation of Jiangsu Province in China (BK20190516), the China Postdoctoral Science Foundation Grant (2018M640492, 2019T120432), the Jiangsu Postdoctoral Science Foundation (2019K013), the National Natural Science Foundation of China (31872374), the National Key Research and Development Program of China (2018YFD0500405), and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2020]407).
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.10.058.
Supplementary data
References
- Abreu J.G., Ketpura N.I., Reversade B., De Robertis E.M. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat. Cell Biol. 2002;4:487–494. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alameddine H.S. Matrix metalloproteinases in skeletal muscles: friends or foes? Neurobiol. Dis. 2012;48:508–518. doi: 10.1016/j.nbd.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Alameddine H.S., Morgan J.E. Matrix metalloproteinases and tissue inhibitor of metalloproteinases in inflammation and fibrosis of skeletal muscles. J. Neuromuscul. Dis. 2016;3:455–473. doi: 10.3233/JND-160183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R.E., Boxhorn L.K. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J. Cell. Physiol. 1987;133:567–572. doi: 10.1002/jcp.1041330319. [DOI] [PubMed] [Google Scholar]
- Burks T.N., Cohn R.D. Role of TGF-β signaling in inherited and acquired myopathies. Skelet. Muscle. 2011;1:19. doi: 10.1186/2044-5040-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R.D., Van Erp C., Habashi J.P., Soleimani A.A., Klein E.C., Lisi M.T., Gamradt M., Ap Rhys C.M., Holm T.M., Loeys B.L. Angiotensin II type 1 receptor blockade attenuates TGF-β–induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Murota K., Tanzer M.L. Abnormal collagen synthesis in skeletal muscle of dystrophic chicken. Biochem. Bioph. Res. Co. 1983;111:933–938. doi: 10.1016/0006-291x(83)91389-x. [DOI] [PubMed] [Google Scholar]
- Gall S., Suyemoto M.M., Sather H.M., Sharpton A.R., Barnes H.J., Borst L.B. Wooden breast in commercial broilers associated with mortality, dorsal recumbency, and pulmonary disease. Avian Dis. 2019;63:514–519. doi: 10.1637/11995-111218-Case.1. [DOI] [PubMed] [Google Scholar]
- Gibson-Corley K.N., Olivier A.K., Meyerholz D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013;50:1007–1015. doi: 10.1177/0300985813485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Wang W., Myllyla R., Virtanen P., Karpakka J., Takala T.E.S. mRNA levels for α-subunit of prolyl 4-hydroxylase and fibrillar collagens in immobilized rat skeletal muscle. J. Appl. Physiol. 1999;87:90–96. doi: 10.1152/jappl.1999.87.1.90. [DOI] [PubMed] [Google Scholar]
- Hofbauer K., Gess B., Lohaus C., Meyer H.E., Katschinski D., Kurtz A. Oxygen tension regulates the expression of a group of procollagen hydroxylases. FEBS J. 2003;270:4515–4522. doi: 10.1046/j.1432-1033.2003.03846.x. [DOI] [PubMed] [Google Scholar]
- Huang Y., Yan X., Zhu M.J., Mccormick R.J., Ford S.P., Nathanielsz P.W., Du M. Enhanced transforming growth factor-? signaling and fibrogenesis in ovine fetal skeletal muscle of obese dams at late gestation. Am. J. Physiol. Endocrinol. Metab. 2010;298:E1254–E1260. doi: 10.1152/ajpendo.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaeel A., Kim J., Kirk J.S., Smith R.S., Bohannon W.T., Koutakis P. Role of transforming growth factor-β in skeletal muscle fibrosis: a Review. Int. J. Mol. Sci. 2019;20:2446. doi: 10.3390/ijms20102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kherif S., Lafuma C., Dehaupas M., Lachkar S., Fournier J.G., Verdiere-Sahuque M., Fardeau M., Alameddine H.S. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev. Biol. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.L., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., Mckee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Leask A., Abraham D.J. TGF-β signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Lieber R.L., Ward S.R. Cellular Mechanisms of Tissue Fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am. J. Physiol. Cell. Physiol. 2013;305:C241–C252. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malila Y., Thanatsang K., Arayamethakorn S., Uengwetwanit T., Srimarut Y., Petracci M., Strasburg G.M., Rungrassamee W., Visessanguan W. Absolute expressions of hypoxia-inducible factor-1 alpha (HIF1A) transcript and the associated genes in chicken skeletal muscle with white striping and wooden breast myopathies. PLoS One. 2019;14:e0220904. doi: 10.1371/journal.pone.0220904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutryn M.F., Brannick E.M., Fu W., Lee W.R., Abasht B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genomics. 2015;16:399. doi: 10.1186/s12864-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki D., Nakamura A., Fukushima K., Yoshida K., Takeda S., Ikeda S.I. Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum. Mol. Genet. 2011;9:1787–1799. doi: 10.1093/hmg/ddr062. [DOI] [PubMed] [Google Scholar]
- Norring M., Valros A., Valaja J., Sihvo H.-K., Immonen K., Puolanne E. Wooden breast myopathy links with poorer gait in broiler chickens. Animal. 2019;13:1690–1695. doi: 10.1017/S1751731118003270. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Bio. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papah M.B., Brannick E.M., Schmidt C.J., Abasht B. Evidence and role of phlebitis and lipid infiltration in the onset and pathogenesis of Wooden Breast Disease in modern broiler chickens. Avian Pathol. 2017;46:623–643. doi: 10.1080/03079457.2017.1339346. [DOI] [PubMed] [Google Scholar]
- Petracci M., Soglia F., Madruga M., Carvalho L., Ida E., Estévez M. Wooden-breast, white striping, and Spaghetti meat: causes, consequences and consumer Perception of emerging broiler meat Abnormalities. Compr. Rev. Food Sci. F. 2019;18:565–583. doi: 10.1111/1541-4337.12431. [DOI] [PubMed] [Google Scholar]
- Rayavarapu S., Coley W., Kinder T.B., Nagaraju K. Idiopathic inflammatory myopathies: pathogenic mechanisms of muscle weakness. Skelet. Muscle. 2013;3:13. doi: 10.1186/2044-5040-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser K.M., Mccormick R.J., Rucker R.B. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 1992;6:2439–2449. doi: 10.1096/fasebj.6.7.1348714. [DOI] [PubMed] [Google Scholar]
- Serrano A.L., Muñoz-Cánoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp. Cell Res. 2010;316:3050–3058. doi: 10.1016/j.yexcr.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Sihvo H.-K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Soglia F., Mudalal S., Babini E., Di Nunzio M., Mazzoni M., Sirri F., Cavani C., Petracci M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2015;95:651–659. doi: 10.3382/ps/pev353. [DOI] [PubMed] [Google Scholar]
- Sosnicki A., Cassens R., Vimini R., Greaser M. Histopathological and ultrastructural alterations of Turkey skeletal muscle. Poult. Sci. 1991;70:349–357. doi: 10.3382/ps.0700349. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L. Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Dis. 2015;59:410–418. doi: 10.1637/11097-042015-Reg.1. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Clark D.L., Tonniges J.R. Fibrillar collagen organization associated with broiler wooden breast fibrotic myopathy. Avian Dis. 2017;61:481–490. doi: 10.1637/11738-080217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Vial C., Zuniga L.M., Cabelloverrugio C., Canon P., Fadic R., Brandan E. Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J. Cell. Physiol. 2008;215:410–421. doi: 10.1002/jcp.21324. [DOI] [PubMed] [Google Scholar]
- Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Wynn T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T., Wang P., Zhao L., Liu R., Zhao X., Xu X., Zhou G. A comparative study of heat shock protein 70 in normal and PSE (pale, soft, exudative)-like muscle from broiler chickens. Poult. Sci. 2016;95:2391–2396. doi: 10.3382/ps/pew181. [DOI] [PubMed] [Google Scholar]
- Xing T., Zhao X., Zhang L., Li J., Zhou G., Xu X., Gao F. Characteristics and incidence of broiler chicken wooden breast meat under commercial conditions in China. Poult. Sci. 2020;99:620–628. doi: 10.3382/ps/pez560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Murphy G., Troeberg L. Extracellular regulation of metalloproteinases. Matrix Biol. 2015;44:255–263. doi: 10.1016/j.matbio.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Zanotti S., Negri T., Cappelletti C., Bernasconi P., Canioni E., Blasi C.D., Pegoraro E., Angelini C., Ciscato P., Prelle A. Decorin and biglycan expression is differentially altered in several muscular dystrophies. Brain. 2005;128:2546–2555. doi: 10.1093/brain/awh635. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang X., Li J., Zhu X., Gao F., Zhou G. Creatine Monohydrate Enhances Energy status and reduces Glycolysis via inhibition of AMPK pathway in pectoralis major muscle of Transport-Stressed broilers. J. Agric. Food Chem. 2017;65:6991. doi: 10.1021/acs.jafc.7b02740. [DOI] [PubMed] [Google Scholar]
- Zimmerman S.D., Thomas D.P., Velleman S.G., Li X., Mccormick R.J. Time course of collagen and decorin changes in rat cardiac and skeletal muscle post-MI. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1816–H1822. doi: 10.1152/ajpheart.2001.281.4.H1816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.