Abstract
Slow-growing broilers offer differentiation in the chicken meat market for consumers who have distinct preferences based on perceived higher welfare indices and willingness to pay a higher price for the product. Although breeding for slow-growing broilers is relatively advanced in Europe and the United States, it is limited in Australia. Crossbreeding is one of the approaches taken to developing slow-growing broiler strains. Thus, the aim of this study was to compare performance, immune response, leg health, carcass characteristics, and meat quality of a novel crossbred slow-growing broiler breed (SGB) with the conventional, fast-growing Cobb 500 broiler (CB) to assess their suitability as an alternative for chicken meat production in Australia. A total of 236 one-day-old broiler chicks (116 SGB and 120 fast-growing CB) were reared on standard commercial diet in an intensive production system. Birds and feed were weighed on a weekly basis and feed intake and feed conversion ratio calculated. At 21 d of age, a 2% suspension of sheep red blood cells was injected subcutaneously into 8 broilers of each breed to compare their antibody response. Birds from both breeds were grown to a final live weight of 2.0–2.2 kg, before a latency-to-lie (LTL) test, carcass analysis and apparent metabolizable energy (AME) assay were performed. The SGB reached the target weight at 55 d of age compared with 32 d in CB. However, SGB stood for longer during LTL, had higher thigh, drumstick, and wing yields (as a percentage of carcass weight) as well as darker and redder meat in comparison with the CB. The CB had better feed conversion efficiency, higher antibody (IgM) production, higher AME, heavier breast yield, and lower meat drip loss than the SGB. Although fast-growing CB outperformed the SGB for traditional performance parameters, the crossbred in this study was comparable with other slow-growing broiler breeds and strains across different countries and is thus a suitable candidate for a slow-growing alternative in Australia.
Key words: performance, carcass composition, crossbred, slow-growing broiler, chicken meat
Introduction
Selective breeding, efficient production systems, improved diets, and veterinary care have all resulted in the exponential growth rates of meat chickens contributing to their high levels of production (Fanatico et al., 2007). Although many consumers are driven by lower prices of fast-growing meat chickens, some seek additional attributes that add apparent value when making choices about their purchases. The expansion of slow-growing broiler breeds can be attributed to characteristics that indicate better welfare such as improved leg health, adaptability to different rearing conditions, and lower mortality rates (Fanatico et al., 2008; Sirri et al., 2011; Comert et al., 2016; Wilhelmsson et al., 2019). Producers of slower-growing meat chickens can capitalize on these attributes by marketing their chickens as a perceived higher welfare product.
Slower-growing breeds make up nearly 40% of Dutch broiler production, 24% of French broiler production, and 11% of production in the United Kingdom (Davies, 2019). One of the most successful specialty poultry production systems in Europe is the “French Label Rouge” program which uses broilers with a growing period of at least 81 d. In Poland, certified chickens are available for pasture-based poultry production, and a good example of a free-range rearing program is “kurczak zagrodowy z Podlasia” (“organically raised chickens of Podlasie”) (Mikulski et al., 2011). Global Animal Partnership (GAP), creator of North America's most comprehensive farm animal welfare standards, has announced their intention to replace 100 percent of fast-growing chicken breeds with slower-growing breeds for all levels of its 5-Step Rating Program by the year 2024.
As there has been a 10-fold increase in chicken meat consumption per capita from 5.9 kg (in 1965) to 47.4 kg (in 2019) and, it has been forecast, to reach approximately 52 kg per capita by 2022/2023 (Mullumby, 2018), the Australian chicken meat industry needs to continue to increase its production. Contrary to most developed countries that have shown some diversification in the chicken meat market with slow-growing broilers, the predominant chicken meat available in Australia is from the fast-growing strains including those grown in free-range production systems. Research suggests slow-growing broilers may be better suited to free-range and pasture poultry systems than their fast-growing counterparts (Rack et al., 2009). Some studies found slow-growing broilers to have better meat quality, and lower nutrient requirements and cost of rearing (Attia et al., 2007, 2009, 2011, 2017). To date, Australia has not conducted research into developing any specific slow-growing broiler breed. Adjusting the amount or nutritional quality of the feed delivered to the fast-growing broiler strains is not an alternative solution to slow their growth rates as it inflicts stress on the birds (Mench, 2002). Thus, there is need to develop a slow-growing broiler breed that would provide an option for producing a welfare-oriented and differentiated chicken meat market. Furthermore, increased awareness among Australian consumers about the welfare of production animals, as reflected by the increased demand for free-range poultry products (Scott et al., 2017), presents an opportunity for the slow-growing broiler breeds to be introduced into the market. The consumers who are interested in other premium labels such as organic, free-range, pasture poultry, etc., are more likely than the average customer to pay the premium price for slow-growing broilers (Fisher, 2017).
Slow-growing broilers can either be imported as existing genetics from overseas or developed locally through domestic breeding programs. Coles Supermarkets Australia Pty Ltd., recently launched “Slow Hills Chicken”, a new special breed of chicken from the poultry genetics company Hubbard based in France. However, importation of poultry genetic stock into Australia is not only costly but also requires strict quarantine measures. Moreover, customers in Australia may have different requirements regarding performance and characteristics of the chickens, for example, some may prefer breast meat while others may prefer the bone portions. Furthermore, there are large differences in the environments (housing facilities, ambient temperature, altitude, available nutritional sources, quality of the water, etc.) and it might be that chickens of one strain or crossbred producing well in one environment will perform poorly in another (EFSA, 2010). Slow-growing broilers in Australia need to be suitable for free-range conditions and Australia's often extreme climate. They also need to develop at a rate that allows for all body parts to grow proportionately to perform natural behaviors and movement and be grown as per RSPCA approved farming scheme standards (RSPCA, 2020). Thus, it is increasingly important for SGB breeds to be locally developed and their performance and suitability determined for the Australian market.
The objective of this study was to compare the performance, serological response, leg health, carcass characteristics, and meat quality of a locally developed novel crossbred slow-growing broiler (SGB) with a fast-growing commercial Cobb 500 broiler (CB), both grown in identical housing conditions and fed the same diet. The results of this study provide a better understanding of the productivity, feed conversion ratio, and meat quality of a candidate slow-growing broiler breed in an Australian context.
Materials and methods
Birds, Housing, and Experimental Conditions
All procedures within this study were approved by the University of Sydney Animal Ethics Committee (2018/1389) and the Birling Animal Ethics Committee (BAEC Ref No: 1069).
One hundred sixteen one-day-old novel crossbred SGB chicks and 120 fast-growing Cobb 500 chicks (CB), were vaccinated for Marek's disease via subcutaneous injection and coarse-sprayed against infectious bronchitis and Newcastle disease. The day-old chicks were randomly allocated into 8 mixed-sex pens consisting of either 30 CB or 29 SGB. Each pen was provided with 3.5 m2 floor space, 2 tube feeders, a line of nipple drinkers, a double-level perch and wood shavings as litter. Water was available via fonts in the first 4 d and then nipple drinkers for the rest of the trial. A thermostatically controlled gas-fired space heater was used during brooding, with the shed temperature at 32°C on day 1 and decreasing by 1°C every second day until 21°C was reached at 21 d. Artificial lighting for both groups were provided at 20 Lux with a duration of 23 h light for the first 2 d, which was then reduced to 16 h from day 4, 12 h from day 9, 11 h from day 16, and finally 11.5 h of light from day 22 until day 55. Environmental enrichment in the form of perches ((30 cm (l) × 45 cm (w) × 15 cm (h)) was provided in all pens for the birds from 7 d old.
The same standard broiler diet (Table1) was allocated to both CB and SGB based on the number and weight of birds in a pen. The ration consisted of a starter diet (crumble, 500 gm/bird to CB and SGB over first 2 wk), a grower diet (pelleted, 1.2 kg/bird over 1 wk to CB and over 3 wk to SGB) and a finisher diet (pelleted, 2 kg/bird over 2 wk to the CB and 2.4 kg/bird over 4 wk to SGB). All diets contained Maxiban (Elanco Animal Health, Australia) at 500 g/tonne for coccidiosis control. Birds were grown to a final live weight of 2.0–2.2 kg for both breeds. Mortality was recorded daily, and postmortem analysis was conducted to determine the cause of death.
Table 1.
Ingredient and calculated composition of starter, grower, and finisher diets fed to both Cobb (CB) and slow-growing broiler (SGB).
| Ingredient | Starter |
Grower |
Finisher |
|---|---|---|---|
| % | |||
| Wheat | 57.9 | 61.0 | 65.4 |
| Soya meal | 31.0 | 26.4 | 20.3 |
| Canola meal | 5.0 | 6.0 | 7.5 |
| Canola oil | 1.82 | 3.32 | 4.00 |
| L-Lysine HCL | 0.274 | 0.230 | 0.234 |
| DL-Methionine | 0.276 | 0.219 | 0.163 |
| L-Threonine | 0.119 | 0.0964 | 0.0826 |
| L-Valine 98% | 0.0479 | 0.0183 | 0.01 |
| Salt | 0.136 | 0.147 | 0.133 |
| Sodium bicarbonate | 0.161 | 0.153 | 0.182 |
| Limestone flour | 1.26 | 1.02 | 0.86 |
| Dicalcium phosphate | 1.60 | 1.03 | 0.83 |
| Xylanase | 0.005 | 0.005 | 0.005 |
| Phytase | 0.01 | 0.01 | 0.01 |
| Choline chloride | 0.08 | 0.07 | 0.06 |
| Vitamin-mineral premix1 | 0.2 | 0.2 | 0.2 |
| MaxibanTM (500 g/t)2 | 0.05 | 0.05 | 0.05 |
| BACECO 150TM (267 g/t)3 | 0.027 | 0.027 | 0.027 |
| Calculated composition | |||
| DM% | 90.4 | 90.5 | 90.6 |
| ME, kcal/kg | 2,892 | 3,040 | 3,126 |
| Digestible CP, % | 20.2 | 18.7 | 16.9 |
| Digestible Lys, % | 1.28 | 1.15 | 1.03 |
| Digestible Met, % | 0.58 | 0.51 | 0.44 |
| Digestible Met + Cys, % | 0.93 | 0.85 | 0.76 |
| Digestible Thr, % | 0.84 | 0.77 | 0.69 |
| DEB4, Meq/Kg | 254 | 233 | 207 |
| Digestible Ca, % | 0.65 | 0.54 | 0.48 |
| Available P | 0.52 | 0.43 | 0.40 |
The vitamin-mineral premix supplied per tonne of feed: [MIU] retinol 12, cholecalciferol 5, [g] tocopherol 50, menadione 3, thiamine 3, riboflavin 9, pyridoxine 5, cobalamin 0.025, niacin 50, pantothenate 18, folate 2, biotin 0.2, copper 20, iron 40, manganese 110, cobalt 0.25, iodine 1, molybdenum 2, zinc 90, selenium 0.3.
Maxiban (Elanco Australasia Pty Ltd., Australia), provided 40 g of active ingredients narasin and nicarbazin per tonne of complete feed.
BACECO 150 (IAH Sales Pty Ltd., Australia), provided 40 g bacitracin activity per tonne of complete feed.
Dietary electrolyte balance.
Weight, Feed Intake, and Performance
The birds and feed were weighed on a weekly basis. Feed intake, body weight gain, and feed conversion ratio (FCR) were calculated weekly for the duration of the study.
Antibody Response Test
At 21 d of age, 2 mL of blood was drawn from a total of 16 birds (8 each of CB and SGB), randomly selected, wing tagged, and injected subcutaneously in the neck with 0.25 mL of a 2% suspension of sheep red blood cells (SRBC) to evaluate their antibody response (Kreukniet et al., 1992; Parmentier et al., 1993; Haghighi et al., 2005). Hemagglutination was used to evaluate the total antibody response after 7 d. A minimum of 50% SRBC agglutination was required for a positive result to be recorded. Subsequently, 0.2 M of 2-mercaptoethanol (2-ME; source: Sigma-Aldrich) were administered to the serum samples to inactivate IgM and determine the IgG antibodies. The IgM titer was determined by subtracting the IgG titers from the total antibody as per protocols described by Haghighi et al. (2005).
Latency-to-Lie Test
Severe leg weakness is a significant issue in commercial broiler production and the latency-to-lie (LTL) technique, which is based on the chicken's natural aversion to water (Berg and Sanotra, 2003), is an objective measure used to assess broiler leg strength (Weeks et al., 2002). Latency-to-lie test was conducted at the final mean weight of 2.0–2.2 kg, where a total of 60 male birds (30 each of CB and SGB) were randomly selected and individually placed into a tub with 3 cm of tepid water (30°C–33°C) and timed until the bird sat down or to a maximum of 5 min. The results (in seconds) collected from the LTL were compared across the 2 breeds using Kaplan-Meier survival analysis, with data for birds that remained standing at 300 s being censored. Comparisons between the 2 broiler breeds in LTL were performed using the Gehan's Wilcoxon test (Groves and Muir, 2017). Only the male birds were compared in the LTL test as the distinction between their LTL time was considered to be more apparent (Groves and Muir, 2017).
Carcass Analysis
A total of 80 broilers (forty (20 female and 20 male) each of CB and SGB) were randomly selected at their final weights of 2.0–2.2 kg, for carcass analysis. After 11 h of fasting the final body weight was determined and the broilers were slaughtered using cervical dislocation followed by exsanguination. Plucked weight (PW) (weight of whole bird after removal of feathers) was determined. Length of the duodenum, jejunum, ileum, and caeca, as well as empty weight of the crop, proventriculus, gizzard, pancreas, liver, heart, duodenum, jejunum, ileum, and caeca, were determined as a relative percentage to plucked weight (%PW). The carcasses were dissected into breast meat (without skin and adherent fat), thighs, drumsticks, whole wings. Carcass weight (CW) was determined by adding the weights of the breast, thighs, drumsticks, wings, and rest of carcass (back + rib cage). Length and width of the breast, length of the thigh, drumstick and wing, weight of the abdominal fat pad, breast, 2 thighs, 2 drumsticks and 2 wings were calculated as relative percentage to carcass weight (%CW). The color of the skin on top of the right pectoralis major was determined at 3 places with a Konica Minolta Chromameter 400 (Konica Minolta Sensing Singapore Pte Ltd.) applying the CIE (1978) system color profile of lightness (L∗), redness (a∗), and yellowness (b∗). Ultimate pH24 of the right pectoralis major was measured using a professional portable meat pH meter (Instrument Choice, Australia) 24 h after slaughter. Samples from left pectoralis major muscle were subjected to drip loss measurement by weighing and then storing them on a suspended net in a plastic container at 4°C on day 1, 3, 6, and 10 after slaughter. Drip loss was expressed as a percentage of the initial muscle weight (Fanatico et al., 2005a).
Apparent Metabolizable Energy Assay
At the respective final weights of 2.0–2.2 kg for each breed, a total of 48 birds (24 each of CB and SGB) were housed in 8 bioassay cages. The total collection of excreta and feed intake on a per cage basis over 48 h was measured. Excreta were pooled within a cage, mixed well using a blender, and representative samples per pen were taken. The samples were oven dried and ground to pass through a 0·5 mm sieve. The gross energy of diets and excreta were determined by bomb calorimetry using an adiabatic calorimeter (Parr 1281 bomb calorimeter; Parr Instruments Co. Moline, IL). The apparent metabolizable energy (AME) of the diet was calculated as outlined by Ravindran et al. (2000).
Statistical Analyses
Data were analyzed with a one-way analysis of variance using Genstat 18th Edition (VSN International Ltd., UK, 2017) with breed as the main effect and pen as an experimental unit. The least mean squares were compared using the Tukey–Kramer option and considered to be significantly different at P < 0.05.
Results and discussion
Performance (Body Weight, Feed Consumption, Feed Conversion Ratio)
Day-old body weight of CB (47.3 g) was higher (P < 0.001) than that of SGB (39.8 g) (Figure 1). Final market weight of 2.0–2.5 kg was achieved on day 32 for CB (2.16 kg) and on day 55 for SGB (2.02 kg) (Figure 1). Body weight gain on a weekly basis was found to be significantly higher (P < 0.001) in CB than in SGB, until day 32, as would be expected from several previous studies (Fanatico et al., 2005b, 2008; Mikulski et al., 2011; Sarica et al., 2014a; Canoğulları Doğan et al., 2019), where weight gain of fast-growing broilers exceeded that of the slow-growing genotype. The lower body weight of SGB in this study on day 32 could be attributed to the genetic effect of the layer strain as one of its parents. However, the number of days needed for SGB to achieve market weight of 2–2.5 kg at 55 d was found to be either comparable or better to the commercially available slow-growing broiler breeds such as Rowan Ranger (Aviagen, 2018) and CobbSasso (Cobb-Vantress, 2007), and many other slow-growing strains and breeds investigated in several other studies (Fanatico et al., 2005b; Sarica et al., 2014b; McCrea et al., 2014; Cruz et al., 2018; Mueller et al., 2018) that estimated time to reach market weight at 50 to 105 d.
Figure 1.
Average body weight (kg/bird) on a weekly basis, for Cobb (CB) until day 32 and slow-growing broiler (SGB) until day 55, grown to a market weight of 2.0–2.2 kg.
There was a significant difference in the cumulative weekly feed consumption between genotypes with that of the SGB (1.41 kg) being lower than that of CB (3.04 kg) on day 32 (P < 0.01). While CB consumed nearly 76% more feed than SGB on day 32, the cumulative feed intake to achieve target market weight was significantly lower for CB (3.04 kg per bird) than for SGB (4.03 kg per bird) (P < 0.05) (Figure 2), confirming results from previous studies (Fanatico et al., 2005b; McCrea et al., 2014; Canoğulları Dogan et al., 2019). However, in a study by Sarica et al. (2019), total feed consumption was found to be higher for the fast-growing broiler genotype than for the slow-growing broiler strains, although they were still more efficient.
Figure 2.
The weekly cumulative feed consumption (kg/bird) of Cobb (CB) until day 33 and slow-growing broiler (SGB) until day 55, grown to a market weight of 2.0–2.2 kg.
The cumulative FCR was significantly lower for SGB on day 7 (0.681 (CB) vs. 0.207 (SGB); (P < 0.001)) and day 21 (1.22 (CB) vs. 1.16 (SGB); P < 0.05)). However, SGB had a higher FCR of 1.98 on processing at target weight, which was 0.54 points higher than the CB at 1.44 (Figure 3), and in agreement with earlier findings (Fanatico et al., 2005b, 2008; Mikulski et al., 2011; McCrea et al., 2014; Sarica et al., 2016). The slow-growing broilers are believed to have higher maintenance requirements due to increased mobility (Gordon and Charles, 2002), thus affecting their feed efficiency (Fanatico et al., 2008). The crossbred SGB in this study performed better with an FCR of 1.98 at day 55 when compared with the established commercial slow-growing CobbSasso, where FCR is expected to be 2.14 at day 56 (Cobb-Vantress, 2007).
Figure 3.
Weekly cumulative FCR of Cobb (CB) until day 33 and slow-growing broiler (SGB) until day 55, grown to a market weight of 2.0–2.2 kg.
Apparent Metabolizable Energy
Difference in AME values of 13.62 MJ/kg for CB as compared with 13.36 MJ/kg for SGB (P < 0.05) indicated a 1.91% higher AME obtained from the same diet in CB. Apparent metabolizable energy is the energy used for growth, reproduction, and metabolic processes. The growth rates of birds are likely to affect the rates of gastrointestinal development and enzyme production, consequently, influencing nutrient utilization by the birds (Santos et al., 2015).
The higher body weight gain of CB could be attributed to their higher feed intake and thus a higher AME, resulting in more energy being available for growth. Moreover, it is likely that the SGB utilized the metabolizable energy to perform increased physical activities thus contributing to their slower and lower weight gain on a weekly basis. The energy utilization of SGB (13.36 MJ/kg) was similar to that of the slow-growing Cobb Sasso (13.31 MJ/kg) reported in the production manual (Cobb-Vantress, 2007), implying that the results of this study are comparable with slow-growing broiler industry standards.
Antibody Response Test
The antibody response test was designed to expose the birds to an antigen (SRBC) to which they were naïve allowing a robust evaluation of their antibody (IgM and IgG) response, as assessed by a direct hemagglutinin reactions (Haghighi et al., 2005). The CB had a significantly higher mean total anti-SRBC antibody titer (IgM and IgG) of 13 in comparison with the SGB titer of 6 (P < 0.05) 7 d after exposure. The CB also had a significantly higher mean IgM titer of 11, compared with 4 for the SGB (P < 0.05). However, for the 2-mercaptoethanol–treated serum samples, the IgG titer reported for both broiler breeds was 2, indicating undetectable levels of anti-SRBC IgG.
A stronger immune response to SRBC antigen was observed in the fast-growing CB as compared with SGB. This is similar to observations in turkeys (Li et al., 2000), where the subline selected for increased 16-wk body weight had higher total anti-SRBC, IgM, and IgG titers than the slower-growing random bred control line, probably due to the higher proportion of helper T cells, which promote B cell proliferation and maturation in the faster-growing line in comparison with the slow-growing. However, it should be noted that the T-cell subpopulation in the birds of the present study was not measured. The variation in the SGB immune response could also be due to the genetic influences of the layer line in the crossbred, as Koenen et al. (2002) found higher IgM titers in response to SRBC in meat chickens indicating a strong short-term humoral response (good IgM response but a poor IgG response) in comparison with the layer-line chickens. However, contrary to our findings, some studies found that chicken lines which were selected for increased growth had compromised immune functions (Cheema et al., 2003; van der Most et al., 2011). Immunization followed by pathogen challenge would be a more conclusive comparison of immune response between SGB and fast-growing chickens, in future studies.
Latency-to-Lie-Test
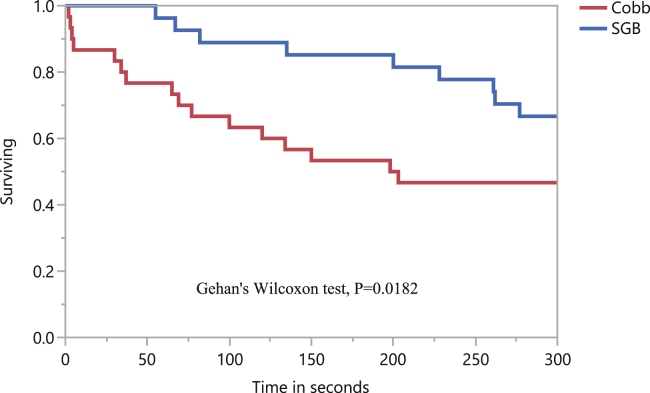
Latency-to-lie test conducted at the target market weight of 2.0–2.2 kg resulted in shorter median standing time by the CB (200.5 s) than by the SGB (300 s) (P = 0.0182) (Figure 4), which could be a consequence of their higher and faster growth rates resulting in the skeleton and joints still being immature when they are subjected to the exceptionally heavier weight loads (Webster, 2004; Shim et al., 2012; Alves et al., 2016). Increased sternal load largely increases metabolic and energetic costs associated with prolonged standing in birds (Tickle et al., 2012, 2013, 2018) and could be associated to the impact of a larger breast muscle mass on fast-growing broiler energetics, thus reducing their time to stand. Moreover, the potentially adverse thermoregulatory effects of rapid growth rate and body mass increases, contribute to the increased sedentary resting and decreased locomotor behavior observed in large broilers as compared with SGB (Dukic-Stojcic and Bessei, 2011; Tickle and Codd, 2019). Slow-growing broilers have been reported to have higher tibia ash, tibia density, and tibia breaking strength than fast-growing broilers which have more porous bones (Shim et al., 2012) and reduced mineralization (Williams, 2000). Tahamtani et al. (2018) found a high prevalence of impaired walking ability (measured by gait score) and severe lameness in conventional fast-growing as compared with organic slow-growing broilers.
Figure 4.
Kaplan-Meier survival analysis and Gehan's Wilcoxon test for latency-to-lie (LTL) of male Cobb (CB) and slow-growing broilers (SGB) timed for 300 s. Total number of birds censored were 14 of 30 for CB with a median standing time of 200.5 (SEM: 19.05) s, and 18 of 28 for SGB with a median standing time of 300 (SEM: 20.08) s.
Carcass Analysis (Carcass Characteristics, Gastrointestinal Segments, and Offal Weights)
Carcass weight of SGB (1.49 kg) was significantly lighter than CB (1.62 kg) (P < 0.05) (Table 2). The body and breast were longer and narrower (P < 0.01) and the length of shank, thigh, drumstick, and wing was higher (P < 0.001) in SGB as compared with CB. Apart from the weight of breast which was 1.53 times higher in CB (P < 0.001), the thighs, drumsticks, wings, head, and the rest of the carcass, were heavier (P < 0.01) in SGB (Table 2). Of the gastrointestinal segments and offal weights (% PW), crop, proventriculus, jejunum, ileum, liver, and abdominal fat pad were heavier (P < 0.05) in CB, whereas the gizzard, duodenum, and caeca were heavier (P < 0.001) in SGB (Table 3).
Table 2.
Carcass characteristics for Cobb (CB) on day 32 and slow-growing broiler (SGB) on day 55, grown to a market weight of 2–2.2 kg.
| Carcass characteristics | Genotype |
SEM | P-value | |
|---|---|---|---|---|
| Cobb | SGB | |||
| Weight of the bird (BW) | 2,143 | 2,049 | 42.130 | 0.117 |
| Plucked weight (PW)1 | 2,025 | 1,892 | 40.247 | 0.0219 |
| Carcass weight (CW)2 | 1,626a | 1,495b | 32.76 | <0.001 |
| Length of body (cm) | 23.53b | 30.31a | 0.345 | <0.0001 |
| Width of body (cm) | 18.84a | 17.63b | 0.213 | 0.0001 |
| Length of breast (cm) | 17.93b | 18.78a | 0.196 | 0.003 |
| Width of breast (cm) | 15.23a | 13.68b | 0.145 | <0.0001 |
| Length of shank (cm) | 5.79b | 7.23a | 0.118 | <0.0001 |
| Length of thigh (cm) | 9.84b | 12.29a | 0.152 | <0.0001 |
| Length of drumstick (cm) | 9.44b | 12.85a | 0.190 | <0.0001 |
| Length of wing (cm) | 23.10b | 29.01a | 0.334 | <0.0001 |
| Breast weight (% CW3) | 27.00a | 17.58b | 0.205 | <0.0001 |
| Two thighs (% CW) | 13.56b | 14.53a | 0.177 | <0.0001 |
| Two drumsticks (% CW) | 11.90b | 15.16a | 0.133 | <0.0001 |
| Two wings (% CW) | 10.38b | 12.26a | 0.107 | <0.0001 |
| Rest of carcass (% CW) | 37.15b | 40.44a | 0.256 | <0.0001 |
| Head (% CW) | 3.06b | 3.76a | 0.061 | <0.0001 |
a,bMeans within a column lacking a common superscript differ (P < 0.05).
Plucked weight (PW) is the weight of whole bird after removal of feathers.
Carcass weight (CW) was determined by adding the weights of breast (without skin and adherent fat), thighs, drumsticks, wings, and rest of carcass (back + rib cage).
Percentage of total carcass weight.
Table 3.
Gastrointestinal sections and offal weights for Cobb (CB) on day 32 and slow-growing broiler (SGB) on day 55, grown to a market weight of 2–2.2 kg.
| Organ (%PW) 1 | CB | SGB | SEM | P-value |
|---|---|---|---|---|
| Crop | 0.34a | 0.29b | 0.013 | 0.0084 |
| Proventriculus | 0.45a | 0.42b | 0.009 | 0.0196 |
| Gizzard | 1.57b | 2.62a | 0.059 | <0.0001 |
| Duodenum | 0.49b | 0.58a | 0.011 | <0.0001 |
| Jejunum | 1.14a | 1.07b | 0.019 | 0.0281 |
| Ileum | 0.93a | 0.75b | 0.015 | <0.0001 |
| Both caeca | 0.33b | 0.44a | 0.010 | <0.0001 |
| Rectum | 0.10 | 0.12 | 0.027 | 0.7701 |
| Liver | 2.54a | 2.06b | 0.035 | <0.0001 |
| Pancreas | 0.22 | 0.22 | 0.005 | 0.7293 |
| Heart | 0.61 | 0.62 | 0.014 | 0.9261 |
| Abdominal fat pad | 1.58a | 1.30b | 0.066 | 0.0047 |
a,bMeans within a column lacking a common superscript differ (P < 0.05).
Expressed as a percentage of total plucked weight.
This study found that genotype was a major factor impacting carcass weight of broilers with SGB being nearly 0.13 kg lighter at final market weight with carcass yields being 75.82 and 72.90% for CB and SGB, respectively. Similar effects of genotype on carcass yield have been observed in previous studies (Fanatico et al., 2008; Mikulski et al., 2011; Cruz et al., 2018; Mueller et al., 2018, 2020; Devatkal et al., 2019) that found carcass yield to decrease in slow-growing broiler strains. However, by contrast, Cruz et al. (2018) found carcass yields of slow-growing strains could be increased by delaying slaughter age to 105 d of age.
The breast weight (% PW) in the present study was significantly higher in CB (27%) as compared with SGB (17.58%) (P < 0.001), which is possibly a consequence of intensive selection for this trait in fast-growing broilers and leads to the reduction in the relative yield of other carcass components (Fanatico et al., 2005b, 2008). Greater breast muscle weight in fast-growing meat chickens has been attributed to the higher insulin-like growth factor concentrations in the serum and breast muscle as compared with the slow-growing genotype (Xiao et al., 2017). Higher relative breast weight in fast-growing broiler strains in comparison with slow-growing breeds were also observed previously (Fanatico et al., 2008; Mikulski et al., 2011; Cruz et al., 2018; Mueller et al., 2018; Sarica et al., 2019; Jaspal et al., 2020).
Weight (% PW) of the thighs, drumsticks, and wings were heavier for SGB in this study. A high leg weight percentage in the slow-growing genotype has also been observed by Canoğulları Doğan et al. (2019), Sarica et al. (2014a, 2016), Cömert et al. (2016), Sirri et al. (2011), and Fanatico et al. (2005b, 2008). The higher wing yield observed in SGB could be a result of higher activity levels which promote the bone mass and supporting muscle mass accumulation, compared with CB, (Gordon and Charles, 2002; Abdullah and Buchtova, 2016). Moreover, it has been found that layer-type males tend to have a higher percentage of leg yield and less value parts of the carcass than broilers (Gerken et al., 2003). This suggests that the higher thigh-drumstick yield in SGB in our study could be a result of the layer strain genotype in the parental cross.
A significantly heavy gizzard (P < 0.0001) in the SGB as compared with CB in this study is comparable with observations in studies by Alshamy et al. (2018) and Mohammadigheisar et al. (2020), where both the body weight and genetic line of bird had a significant influence on gizzard mass. Reduction in visceral organ weight relative to body weight has been associated with a higher growth rate in modern broiler breeds (Havenstein et al., 2003) due to a reduction in maintenance energy required and therefore increased overall energy utilization and efficiency (Tallentire et al., 2016).
The abdominal fat pad was significantly heavier in CB than in SGB (P < 0.001). Similarly, Smith et al. (2012) reported that a longer growing period resulted in a leaner bird. When selecting for lower residual feed intake, Wen et al. (2018) identified that the weight and percentage of abdominal fat pad was lower in slow-growing birds. This may explain the lower abdominal fat pad weight as a consequence of lower feed intake of SGB in the present study. By contrast, Quentin et al. (2003) and Mikulski et al. (2011) reported that slow-growing birds provided with dietary energy and protein in excess of their nutritional requirements resulted in more abdominal fat.
Meat Quality (Color, pH, Water-Holding Capacity)
The CIE (1978) system color profile of lightness (L∗), redness (a∗), and yellowness (b∗) was used to evaluate breast meat color. A lower L∗ results in redder meat color, which is perceived to be an indicator of good meat quality and is also often the first trait observed by consumers. Breast meat color of CB was lighter with a higher L∗ value (51.93) than that of the SGB (45.39) (P < 0.001), whereas SGB showed a higher (P < 0.05) redness (a∗: 2.64 vs. 2.08) and lower (P < 0.001) yellowness (b∗: 3.84 vs. 4.97) (Table 4). The redder pectoralis major of SGB found in this study was in agreement with earlier findings (Berri et al., 2001; Quentin et al., 2003; Smith et al., 2012; Sarica et al., 2014a) whereby the redness (a∗) was higher for the alternative broiler type than for the conventional broilers selected for rapid growth. The varying degree of redness could be a result of the difference in slaughter age given that the myoglobin content (heme pigment) of the breast meat of broilers increases with age (Berri et al., 2001; Gordon and Charles, 2002). The redder breast muscle of SGB can also be explained by the increased blood circulation as a consequence of prolonged wing flapping associated with slaughter (Berri et al., 2005a,b). By contrast, Fanatico et al. (2005a) and Canoğulları Doğan et al. (2019) found thinner fillets from the slow-growing broilers to have higher L∗ values (lighter) than thicker fillets of the commercial fast-growing genotype.
Table 4.
Meat quality (color, ultimate pH, and drip loss) of the pectoralis major of Cobb (CB) on day 32 and slow-growing broiler (SGB) on day 55, grown to a market weight of 2–2.2 kg.
| Meat Quality Attributes | Cobb | SGB | SEM | P-value |
|---|---|---|---|---|
| Breast color L∗ (lightness) | 51.93a | 45.39b | 0.413 | <0.0001 |
| Breast color a∗ (redness) | 2.08b | 2.64a | 0.166 | 0.0204 |
| Breast color b∗ (yellowness) | 4.97a | 3.84b | 0.145 | <0.0001 |
| pH241 | 5.88a | 5.74b | 0.013 | <0.0001 |
| Drip loss2@ 24 h (%) | 2.93b | 6.17a | 0.400 | <0.001 |
| Drip loss @ 3 d (%) | 5.78b | 9.13a | 0.462 | <0.001 |
| Drip loss @ 6 d (%) | 8.26b | 9.76a | 0.473 | 0.032 |
| Drip loss @ 10 d (%) | 8.58b | 11.21a | 0.468 | <0.001 |
a,bMeans within a column lacking a common superscript differ (P < 0.05).
pH at 24 h after processing.
Drip loss calculated as a percentage of initial muscle weight at processing.
The ultimate pH24 of breast meat was lower (P < 0.001) in SGB, and the drip loss in pectoralis major (calculated as a percentage of initial muscle weight) on day 10 after slaughter was higher at 11.21% for SGB in comparison with 8.58% for CB (P < 0.05) (Table 4). In the event of the conversion of muscle to meat, the extent of postmortem pH decline is crucial as it not only affects meat color, but also its water-holding capacity and texture (Aberle et al., 2001). The pH findings in this study were consistent with previous results (Quentin et al., 2003; Fanatico et al., 2007; Canoğulları Doğan et al., 2019), with the slow-growing genotype reporting a lower ultimate pH in comparison with the fast-growing genotype. Berri et al. (2001) concluded that the intense selection for increased growth rates and breast meat yield of broilers leads to diminished postmortem glycolysis, resulting in less pyruvic acid being released, leading to a higher ultimate pH. However, Henckel (1996) suggested that the muscles of birds subjected to a period of concentrated growth would likely be in a constant state of hypoxia. Consequently, the demand for energy by the muscle cells leads to anaerobic glycolysis which converts glycogen into lactate, lowering the pH. This has been observed in several studies (Sarica et al., 2014a; Hoan and Khoa, 2016; Devatkal et al., 2019) which contrasts to observations in this study. Nonetheless, in the present study, given that the 2 broiler breeds were processed at different ages, the impact of the age on muscle metabolism and composition must also be considered.
Fanatico et al. (2007), Hoan and Khoa (2016), and Devatkal et al. (2019) found breast muscle of slow-growing broilers had a lower water-holding capacity than the fast-growing breed (P < 0.05), which is in agreement with the present study, where the breast weight (% CW) of SGB was lower in comparison with the CB, suggesting smaller and thinner breast fillets from SGB. Therefore, SGB are expected to have a larger surface area with regards to the muscle mass exposed to air, thus, being vulnerable to greater drip loss (Fanatico et al., 2005a, 2007). However, fillet thickness was not objectively measured in this study and was concluded based on visual observations only.
Berri et al. (2005a) suggested a strong negative correlation between breast muscle pH at 24 h postmortem and drip loss similar to that seen in the current study, where SGB were reported to have a higher drip loss corresponding to a lower ultimate pH. Because rigor mortis proceeds at a faster rate in the SGB as compared with the fast-growing broilers, the initial rate of pH drop is hastened, thus, leading to poorer water-holding capacity (Mikulski et al., 2011).
Conclusion
In this study the SGB were grown for 55 d to reach the target weight of 2–2.5 kg, compared with the 32 d for CB. However, SGB showed longer standing times during LTL test, higher thigh, drumstick and wing yields (as a percentage of carcass weight) as well as darker and redder meat in comparison with the CB. Although CB outperformed the SGB for traditional performance parameters such as feed conversion ratio, antibody (IgM) production, AME, breast yield, and meat with lower drip loss, the crossbred SGB showed comparable outcomes to other more established slow-growing broiler breeds. The main advantage of the slow-growing genotype is their improved welfare over the fast-growing broilers, as indicated by better leg strength. Moreover, the difference in body conformation between the genotypes helps to differentiate the slow-growing brand from the conventional broiler. However, production costs have been found to be 11–25% per pound higher for slower-growing breeds than for modern breeds, depending on the target end point, and consumer willingness to pay would need to increase by 10.8% to offset the producer losses. (Lusk et al., 2019). The advantages, over and above the higher cost of production arising from the longer growing period, would likely attract a niche market and be able to fetch premium pricing for the SGB, making it a suitable candidate for a slow-growing welfare-oriented alternative for the Australian market. However, a cost-benefit analysis would be useful to determine the feasibility of growing SGB in Australia. Further research is also needed to evaluate meat nutrient content and taste characteristics of SGB.
Acknowledgments
The authors are most grateful for the exemplary animal care provided throughout this experiment by Jadranka Velnic of Zootechny Pty Ltd. The research was financially supported by Poultry Research Foundation at the Sydney School of Veterinary Science, University of Sydney, Australia.
Disclosures
The authors declare that they have no conflict of interest and competing interests.
References
- Abdullah F., Buchtova H. Comparison of qualitative and quantitative properties of the wings, necks and offal of chicken broilers from organic and conventional production systems. Vet. Med. (Praha) 2016;61:643–651. [Google Scholar]
- Aberle E.D., Forrest J.C., Gerrad D.E., Mills E.W. Kendall/Hunt Publ. Co.; Dubuque, IA: 2001. Principles of Meat Science. [Google Scholar]
- Alshamy Z., Richardson K.C., Hünigen H., Hafez H.M., Plendl J., Al Masri S. Comparison of the gastrointestinal tract of a dual-purpose to a broiler chicken line: a qualitative and quantitative macroscopic and microscopic study. PLoS One. 2018;13:e0204921. doi: 10.1371/journal.pone.0204921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M.C.F., Paz I.C.d.L.A., Nääs I.d.A., Garcia R.G., Caldara F.R., Baldo G.A.d.A., Garcia E.A., Molino A.d.B. Locomotion of commercial broilers and indigenous chickens. Rev. Bras. de Zootec. 2016;45:372–379. [Google Scholar]
- Attia Y.A., Abd-Elhamidb A.-E.E., Mustafac M., M A A.-H., Muhammadb M. Response of slow-growing chickens to feed restriction and effects on growth performance, blood constituents and immune markers. Rev. Mex. Cienc. Pecu. 2017;8:175–184. [Google Scholar]
- Attia Y.A., Hassan R.A., Qota E.M.A. Recovery from adverse effects of heat stress on slow-growing chicks in the tropics 1: effect of ascorbic acid and different levels of betaine. Trop. Anim. Health Prod. 2009;41:807–818. doi: 10.1007/s11250-008-9256-9. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Hassan R.A., Tag El-Din A.E., Abou-Shehema B.M. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J. Anim. Physiol. Anim. Nutr. 2011;95:744–755. doi: 10.1111/j.1439-0396.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- Attia Y., Qota E.-S.A., Abd ElHamid A.E.-H., Sadaka T. The response of slow-growing chicks to the supplementations with different methionine levels and/or two types of enzymes. Emir J. Food Agric. 2007;19:48–63. [Google Scholar]
- Aviagen Rowan ranger broiler performance objectives. 2018. http://en.aviagen.com/assets/Tech_Center/Rowan_Range//RowanRanger-Broiler-PO-18-EN.pdf
- Berg C., Sanotra G. Can a modified latency-to-lie test be used to validate gait-scoring results in commercial broiler flocks? Anim. Welf. 2003;12:655–659. [Google Scholar]
- Berri C., Debut M., Santé-Lhoutellier V., Arnould C., Boutten B., Sellier N., Baéza E., Jehl N., Jego Y., Duclos M. Variations in chicken breast meat quality: implications of struggle and muscle glycogen content at death. Br. Poult. Sci. 2005;46:572–579. doi: 10.1080/00071660500303099. [DOI] [PubMed] [Google Scholar]
- Berri C., Le Bihan-Duval E., Baéza E., Chartrin P., Picgirard L., Jehl N., Quentin M., Picard M., Duclos M.J. Further processing characteristics of breast and leg meat from fast-, medium-and slow-growing commercial chickens. Anim. Res. 2005;54:123–134. [Google Scholar]
- Berri C., Wacrenier N., Millet N., Le Bihan-Duval E. Effect of selection for improved body composition on muscle and meat characteristics of broilers from experimental and commercial lines. Poult. Sci. 2001;80:833–838. doi: 10.1093/ps/80.7.833. [DOI] [PubMed] [Google Scholar]
- Canoğulları Doğan S., Baylan M., Bulancak A., Ayaşan T. Differences in performance, carcass characteristics and meat quality between fast- and slow-growing broiler genotypes. Prog. Nutr. 2019;21:558–565. [Google Scholar]
- Cheema M., Qureshi M., Havenstein G. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1519–1529. doi: 10.1093/ps/82.10.1519. [DOI] [PubMed] [Google Scholar]
- CIE (Commission Internationale de l'Eclairage). 1978. Recommendations on uniform color spaces-color difference equations, psychometric color terms, Supplement No. 2 to CIE Publication No. 15 (E-1.3.1) 1971/(TC-1·3), Paris, France.
- Cobb-Vantress . 2007. CobbSasso breeder management Supplement.https://www.cobb-vantress.com/assets/Cobb-Files/product-guides/6c1436d72b/CobbSasso_Breeder_Management_Supplement_v1_EN.pdf [Google Scholar]
- Comert M., Sayan Y., Kirkpinar F., Bayraktar O.H., Mert S. Comparison of carcass characteristics, meat quality, and blood parameters of slow and fast grown female broiler chickens raised in organic or conventional production system. Asian-Australas. J. Anim. Sci. 2016;29:987–997. doi: 10.5713/ajas.15.0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F.L., Saraiva L.K.V., Silva G.E., Nogueira T.M., Silva A.P., Faria P.B. Growth and carcass characteristics of different crosses of broiler chickens reared under an alternative system. Semin.Ciênc. Agrár. 2018;39:317–328. [Google Scholar]
- Davies J. Slow-growing birds are fast becoming mainstream. 2019. https://www.poultryworld.net/Meat/Articles/2019/7/Slow-growing-birds-are-fast-becoming-mainstream-454287E/
- Devatkal S., Naveena B., Kotaiah T. Quality, composition, and consumer evaluation of meat from slow-growing broilers relative to commercial broilers. Poult. Sci. 2019;98:6177–6186. doi: 10.3382/ps/pez344. [DOI] [PubMed] [Google Scholar]
- Dukic-Stojcic M., Bessei W. The effect of weight load on the legs of broilers behaviour. Biotechnol. Anim. Husbandry. 2011;27:1667–1671. [Google Scholar]
- EFSA Scientific Opinion on the influence of genetic parameters on the welfare and the resistance to stress of commercial broilers. EFSA J. 2010;8 1667. [Google Scholar]
- Fanatico A., Pillai P.B., Emmert J., Owens C. Meat quality of slow-and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007;86:2245–2255. doi: 10.1093/ps/86.10.2245. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Cavitt L.C., Pillai P.B., Emmert J.L., Owens C.M. Evaluation of slower-growing broiler genotypes grown with and without outdoor access: meat quality. Poult. Sci. 2005;84:1785–1790. doi: 10.1093/ps/84.11.1785. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Cavitt L.C., Owens C.M., Emmert J.L. Evaluation of slower-growing broiler genotypes grown with and without outdoor access: growth performance and carcass yield. Poult. Sci. 2005;84:1321–1327. doi: 10.1093/ps/84.8.1321. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Hester P.Y., Falcone C., Mench J.A., Owens C.M., Emmert J.L. Performance, livability, and carcass yield of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2008;87:1012–1021. doi: 10.3382/ps.2006-00424. [DOI] [PubMed] [Google Scholar]
- Fisher T. Management of slow growing broilers for Profit. 2017. http://midwestpoultry.com/wp-content/uploads/Fisher-Tatijana.pdf Accessed Jan. 2020.
- Gerken M., Jaenecke D., Kreuzer M. Growth, behaviour and carcass characteristics of egg-type cockerels compared to male broilers. Worlds Poult. Sci. J. 2003;59:46–49. [Google Scholar]
- Gordon S., Charles D. Nottingham Univ. Press; Nottingham, UK: 2002. Niche and Organic Chicken Products. [Google Scholar]
- Groves P.J., Muir W. Earlier hatching time predisposes Cobb broiler chickens to tibial dyschondroplasia. Animal. 2017;11:112–120. doi: 10.1017/S1751731116001105. [DOI] [PubMed] [Google Scholar]
- Haghighi H.R., Gong J., Gyles C.L., Hayes M.A., Sanei B., Parvizi P., Gisavi H., Chambers J.R., Sharif S. Modulation of antibody-mediated immune response by probiotics in chickens. Clin. Diagn. Lab Immunol. 2005;12:1387–1392. doi: 10.1128/CDLI.12.12.1387-1392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenstein G., Ferket P., Qureshi M. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Henckel P. Foulum; Denmark: 1996. Pages 79–89 in Physiology and biochemistry of muscle fibres in poultry. Proceedings of the 2nd European Poultry Breeders Roundtable. [Google Scholar]
- Hoan N.D., Khoa M.A. Meat quality comparison between fast growing broiler Ross 308 and slow growing Sasso laying males reared in free range system. J. Sci. Dev. 2016;214:101–108. [Google Scholar]
- Jaspal M.H., Ali S., Rajput N., Naeem M., Talpur F.N., Rehman I. 7. Fatty acid profiling and comparative evaluation of carcass cut up yield, meat quality traits of Cobb Sasso, commercial broiler and native aseel chicken. Pure Appl. Biol. (Pab) 2020;9:56–65. [Google Scholar]
- Koenen M.E., Boonstra-Blom A.G., Jeurissen S.H. Immunological differences between layer-and broiler-type chickens. Vet. Immunol. Immunopathol. 2002;89:47–56. doi: 10.1016/s0165-2427(02)00169-1. [DOI] [PubMed] [Google Scholar]
- Kreukniet M., Van der Zijpp A., Nieuwland M. Effects of route of immunization, adjuvant and unrelated antigens on the humoral immune response in lines of chickens selected for antibody production against sheep erythrocytes. Vet. Immunol. Immunopathol. 1992;33:115–127. doi: 10.1016/0165-2427(92)90039-s. [DOI] [PubMed] [Google Scholar]
- Li Z., Nestor K.E., Saif Y.M., Anderson J. Antibody responses to sheep red blood cell and Brucella abortus antigens in a Turkey line selected for increased body weight and its randombred control. Poult. Sci. 2000;79:804–809. doi: 10.1093/ps/79.6.804. [DOI] [PubMed] [Google Scholar]
- Lusk J.L., Thompson N.M., Weimer S.L. The cost and market impacts of slow-growth broilers. J. Agr. Resour. Econ. 2019;44:536–550. [Google Scholar]
- McCrea B.A., Mills A.F., Matthews K., Hutson J. Performance and carcass characteristics of Delaware chickens in comparison with broilers. J. Appl. Poult. Res. 2014;23:586–592. [Google Scholar]
- Mench J.A. Broiler breeders: feed restriction and welfare. Worlds Poult. Sci. J. 2002;58:23–29. [Google Scholar]
- Mikulski D., Celej J., Jankowski J., Majewska T., Mikulska M. Growth performance, carcass traits and meat quality of slower-growing and fast-growing chickens raised with and without outdoor access. Asian-Australas. J. Anim. Sci. 2011;24:1407–1416. [Google Scholar]
- Mohammadigheisar M., Shouldice V.L., Torrey S., Widowski T., Kiarie E.G. Research Note: comparative gastrointestinal, tibia, and plasma attributes in 48-day-old fast-and slow-growing broiler chicken strains. Poult. Sci. 2020;99:3086–3091. doi: 10.1016/j.psj.2020.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Kreuzer M., Siegrist M., Mannale K., Messikommer R.E., Gangnat I.D.M. Carcass and meat quality of dual-purpose chickens (Lohmann Dual, Belgian Malines, Schweizerhuhn) in comparison to broiler and layer chicken types. Poult. Sci. 2018;97:3325–3336. doi: 10.3382/ps/pey172. [DOI] [PubMed] [Google Scholar]
- Mueller S., Taddei L., Albiker D., Kreuzer M., Siegrist M., Messikommer R., Gangnat I. Growth, carcass, and meat quality of 2 dual-purpose chickens and a layer hybrid grown for 67 or 84 D compared with slow-growing broilers. J. Appl. Poult. Res. 2020;29:185–196. [Google Scholar]
- Mullumby J. Chicken meat: Outlook to 2022-23. Agric. Commod. 2018;8:113. [Google Scholar]
- Parmentier H.K., Schrama J.W., Meijer F., Nieuwland M.G.B. Cutaneous Hypersensitivity responses in chickens Divergently selected for antibody responses to sheep red blood cells. Poult. Sci. 1993;72:1679–1692. doi: 10.3382/ps.0721679. [DOI] [PubMed] [Google Scholar]
- Quentin M., Bouvarel I., Berri C., Le Bihan-Duval E., Baéza E., Jégo Y., Picard M. Growth, carcass composition and meat quality response to dietary concentrations in fast-, medium-and slow-growing commercial broilers. Anim. Res. 2003;52:65–77. [Google Scholar]
- Rack A.L., Lilly K.G.S., Beaman K.R., Gehring C.K., Moritz J.S. The effect of genotype, choice feeding, and season on organically reared broilers fed diets devoid of synthetic methionine. J. Appl. Poult. Res. 2009;18:54–65. [Google Scholar]
- Ravindran V., Cabahug S., Ravindran G., Selle P., Bryden W. Response of broiler chickens to microbial phytase supplementation as influenced by dietary phytic acid and non-phytate phosphorous levels. II. Effects on apparent metabolisable energy, nutrient digestibility and nutrient retention. Br. Poult. Sci. 2000;41:193–200. doi: 10.1080/00071660050022263. [DOI] [PubMed] [Google Scholar]
- RSPCA . RSPCA Australia; Deakin, Australia: 2020. RSPCA Approved Farming Scheme Standard - Meat Chickens (v1.1) [Google Scholar]
- Santos F.R.d., Stringhini J.H., Freitas N.F.d., Minafra C.S., Oliveira P.R., Duarte E.F., Guimaraes G.S., dos Santos F.R., de Freitas N.F. Morphological and morphometric aspects of the digestive apparatus, serum biochemical measures and activity of pancreatic enzymes of slow- and fast-growing broilers Aspectos morfologicos e morfometricos do aparelho digestorio, perfil bioquimico serico e atividade de enzimas pancreaticas de frangos de crescimento lento e rapido. Revis. Bras. Cienc. Agrar. (Agraria) 2015;10:322–327. [Google Scholar]
- Sarica M., Ceyhan V., Yamak U.S., Ucar A., Boz M.A. Comparison of slow growing synthetic broiler genotypes with commercial broilers in terms of growth, carcass traits and some economic parameters. Tarim Bilim. Derg. 2016;22:20–31. [Google Scholar]
- Sarica M., Yamak U., Boz M., Erensoy K., Cilavdaroglu E., Noubandiguim M. Performance of fast, medium and slow growing broilers in indoor and free-range production systems. S. Afr. J. Anim. Sci. 2019;49:1127–1138. [Google Scholar]
- Sarica M., Yamak U.S., Boz M.A. Comparing growth and carcass traits of slow growing chicken parents with pure egg type parents and commercial broilers. J. Anim. Prod. 2014;55:1–8. [Google Scholar]
- Sarica M., Yamak U.S., Turhan S., Boz M.A., Saricaoglu F.T., Altop A. Comparing slow-growing chickens produced by two- and three-way crossings with commercial genotypes 2 Carcass quality and blood parameters. Eur. Poult. Sci. 2014;78:30. [Google Scholar]
- Scott A.B., Singh M., Toribio J.-A., Hernandez-Jover M., Barnes B., Glass K., Moloney B., Lee A., Groves P. Comparisons of management practices and farm design on Australian commercial layer and meat chicken farms: cage, barn and free range. PLoS One. 2017;12:e0188505. doi: 10.1371/journal.pone.0188505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim M.Y., Karnuah A.B., Mitchell A.D., Anthony N.B., Pesti G.M., Aggrey S.E. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult. Sci. 2012;91:1790–1795. doi: 10.3382/ps.2011-01968. [DOI] [PubMed] [Google Scholar]
- Sirri F., Castellini C., Bianchi M., Petracci M., Meluzzi A., Franchini A. Effect of fast-, medium-and slow-growing strains on meat quality of chickens reared under the organic farming method. Animal. 2011;5:312. doi: 10.1017/S175173111000176X. [DOI] [PubMed] [Google Scholar]
- Smith D., Northcutt J., Steinberg E. Meat quality and sensory attributes of a conventional and a Label Rouge-type broiler strain obtained at retail. Poult. Sci. 2012;91:1489–1495. doi: 10.3382/ps.2011-01891. [DOI] [PubMed] [Google Scholar]
- Tahamtani F.M., Hinrichsen L.K., Riber A.B. Welfare assessment of conventional and organic broilers in Denmark, with emphasis on leg health. Vet. Rec. 2018;183:192. doi: 10.1136/vr.104817. [DOI] [PubMed] [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Breeding for efficiency in the broiler chicken: a review. Agron. Sustain. Dev. 2016;36:66. [Google Scholar]
- Tickle P.G., Codd J.R. Thermoregulation in rapid growing broiler chickens is compromised by constraints on radiative and convective cooling performance. J. Therm. Biol. 2019;79:8–14. doi: 10.1016/j.jtherbio.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Tickle P.G., Hutchinson J.R., Codd J.R. Energy allocation and behaviour in the growing broiler chicken. Sci. Rep. 2018;8:4562. doi: 10.1038/s41598-018-22604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle P.G., Lean S.C., Rose K.A., Wadugodapitiya A.P., Codd J.R. The influence of load carrying on the energetics and kinematics of terrestrial locomotion in a diving bird. Biol. Open. 2013;2:1239–1244. doi: 10.1242/bio.20135538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle P.G., Nudds R.L., Codd J.R. Barnacle geese achieve significant energetic savings by changing posture. PLoS One. 2012;7:e46950. doi: 10.1371/journal.pone.0046950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most P.J., de Jong B., Parmentier H.K., Verhulst S. Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct. Ecol. 2011;25:74–80. [Google Scholar]
- Webster A. Welfare implications of avian osteoporosis. Poult. Sci. 2004;83:184–192. doi: 10.1093/ps/83.2.184. [DOI] [PubMed] [Google Scholar]
- Weeks C., Knowles T., Gordon R., Kerr A., Peyton S., Tilbrook N. New method for objectively assessing lameness in broiler chickens. Vet. Rec. 2002;151:762–764. [PubMed] [Google Scholar]
- Wen C., Yan W., Zheng J., Ji C., Zhang D., Sun C., Yang N., Wen C.L., Yan W., Zheng J.X., Ji C.L., Zhang D.X., Sun C.J., Yang N. Feed efficiency measures and their relationships with production and meat quality traits in slower growing broilers. Poult. Sci. 2018;97:2356–2364. doi: 10.3382/ps/pey062. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson S., Yngvesson J., Jonsson L., Gunnarsson S., Wallenbeck A. Welfare Quality assessment of a fast-growing and a slower-growing broiler hybrid, reared until 10 weeks and fed a low-protein, high-protein or mussel-meal diet. Livest Sci. 2019;219:71–79. [Google Scholar]
- Williams B.G. University of Glasgow, PhD Thesis; 2000. Aspects of Bone Quality in the Broiler Chicken. [Google Scholar]
- Xiao Y., Wu C., Li K., Gui G., Zhang G., Yang H., Xiao Y.P., Wu C.F., Li K.F., Gui G.H., Zhang G.L., Yang H. Association of growth rate with hormone levels and myogenic gene expression profile in broilers. J. Anim. Sci. Biotechnol. 2017;8:1–7. doi: 10.1186/s40104-017-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]