Abstract
The effects of early heat conditioning on the acute heat stress response in broilers were investigated via the growth performance, dopamine, serotonin, and corticosterone and the expression of heat shock proteins (HSP) and heat shock factors. One-day-old chicks (n = 144) were divided into 3 groups in a 35-d experiment (48 chicks per each group). Group 1 (C) was treated with an optimum temperature, group 2 (CH) was treated with 40°C ± 1°C on day 35 (5 h), and group 3 (HH) was treated with 40°C ± 1°C on day 5 (24 h) and day 35 (5 h). On day 7, the body weight gain was lower (P < 0.05) in HH than in C and CH. On day 35, the heat-treated groups (CH and HH) had lower weight gains than the C group (P < 0.05), whereas the feed conversion ratio was lower in HH (P < 0.05). Serum corticosterone was higher in CH than in C, but HH and C did not differ (P < 0.05). Liver HSP70 protein expression was higher in CH than HH and C (P < 0.05), which did not differ, and HSP40 protein expression was higher in CH than C (P < 0.05). These results suggest that early heat conditioning may reduce acute heat stress on broiler.
Key words: heat stress, acute, heat shock protein, broiler
Introduction
In the poultry industry, acute heat exposure before marketing has negative effects. Broilers are thermophilic, with a body temperature of approximately 41°C. Being different from other animals, birds do not have sweat glands for releasing body heat; therefore, they release heat through breathing (i.e., panting) (Yousaf et al., 2019). Thus, birds are susceptible to high temperatures. Heat exposure can cause physiological changes, including blood parameter, hormone concentrations, immunity, water and feed conversion ratios (FCR), and feed intake and marketing weights, all of which decrease productivity and lead to economic losses in the poultry industry (Lara and Rostagno, 2013). Therefore, safeguards against high temperatures in poultry are important. Studies of broiler heat stress are divided into short-term, long-term, and cyclic heat conditioning. To reduce stress and increase poultry productivity, studies have sought to alleviate high-temperature stress by adding acid–base controls, taurine, vitamin C, betaine, and selenium to the feed and/or water (Mahmoud et al., 2004; Saeed et al., 2017). Alternative methods involve exposing broilers to initial heat stress and restricting feed (Zulkifli et al., 2003).
Early heat conditioning is a promising management method to reduce heat stress. This method is inexpensive, making it suitable for immediate implementation on an array of farms, and positive effects have been reported. Arjona et al. (1988) reported significantly reduced weight gain in the first week of early heat exposure (3-, 4-, or 6-d-old), but the growth accelerated to achieve higher marketing weights than in the control. The mortality rate also decreased, and the feed efficiency improved (Arjona et al., 1988). Yahav and McMurtry (2001) reported that early heat exposure improves the feed efficiency and body weight (BW) before marketing, with reduced triiodothyronine concentrations in the blood and less heat production during heat exposure.
Heat shock proteins (HSP) bind to other cellular proteins to aid intracellular transport, act as chaperones to facilitate protein structures and fold formation, and prevent protein aggregation during stress (Wynn et al., 1994). Therefore, HSP expression is used for heat stress studies. Yeğenoğlu and Lgen (2009) analyzed the expression levels of HSP70 during chronic heat stress (34°C for 7 h/d, 21–49 d) and reported decreased HSP70 levels in the early heat exposed groups compared with the control. In broilers, early heat exposure (40.5°C for 3 h/d, 15–17 d of the incubation period) lead to significant increase in HSP90a, HSP90b, HSP70, HSP60, and HSP27 protein expression in the late heat stress group compared with the early + late heat-treated and control groups (Vinoth et al., 2015). There were lower HSP expressions in the early heat-exposed groups because the high ambient temperatures were not recognized as stressors at the cellular level.
Previous studies have mainly focused on improvements to the productivity and physiological parameters such as oxidative damage and hormonal changes in broilers (Arjona et al., 1988; Günal, 2013). Information on the molecular level heat stress responses after heat conditioning is limited but would be beneficial for poultry production. Therefore, the purpose of this study was to investigate the effects of early heat conditioning on the resistance to acute heat stress in broilers by analyzing the growth performance, stress hormones in the blood, and the expression of HSP (protein and mRNA) and heat shock factors (HSF) in the liver.
Materials and methods
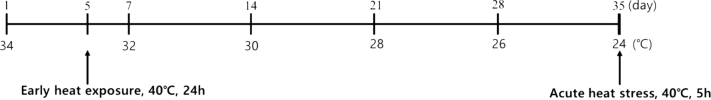
Animal Experiments
The animal experiments conducted in this study were approved by the Animal Ethics Committee of Jeonbuk National University, Republic of Korea (approval number: CBNU2018-097). A total of one hundred forty-four 1-day-old Ross broiler chickens were obtained from the Dongwoo hatchery (Iksan, Korea). The chicks were weighed and randomly divided into 3 groups (48 chicks per group). The cage floating 50 cm from the bottom was used for reared all birds (130 × 100 × 60, W × D × H) (12 birds per each cages). The heat conditions for the treatment groups were as follows: (1) C, the control without any heat treatment, (2) CH, heat exposure on day 35 for 5 h, and (3) HH, heat exposure both at day 5 for 24 h and on day 35 for 5 h (Figure 1). The heat-treated and control groups were raised in different rooms for each exposure temperature. The chicks were housed at 34°C, which was reduced by 2°C every week until 24°C. On day 5, the HH group was exposed to 40°C for 24 h (the C and CH groups were kept in the normal temperature). On day 35, the CH and HH groups were exposed to 40°C for 5 h (the C group was kept at 24°C). Feed and water were offered ad libitum, and the humidity was maintained at 57 ± 3%. The individual BW of the birds and the feed weight of each cage was measured on day 1, 7, 28, and 35. The BW of the birds was measured after heat exposure on day 35. Birds body weight was measured for calculate daily body weight gain and FCR, and feed weight for feed intake, average daily feed intake (ADFI), and FCR. Daily body weight gain and ADFI was calculated from the weekly data (1–7 and 28–35 d). After the 5 h heat treatment on day 35, chicks were randomly selected from each cage (10 birds from each treatment for analysis replication) and immediately weighed and sampled for blood. Blood samples was taken from the wing vein (volume: 2 mL) using 3 mL syringes (23 G needles) and collected to Eppendorf tube. Serum samples were obtained after centrifuging the blood samples at 2,000 × g for 15 min. The serum was stored at −20°C until analysis. Then, the birds were then sacrificed for liver tissues. The liver tissues were immediately frozen in liquid nitrogen and stored at –80°C.
Figure 1.
Experimental schedule of the heat exposure method. Abbreviations: C, raised at optimum temperature without any heat exposure; CH, exposed acute heat stress on 35 d; HH, exposed early heat exposure on 5 d and acute heat stress on 35 d.
Dopamine, Serotonin, and Corticosterone Analysis
Dopamine (PHR1090, Supelco, St. Louis, MO), serotonin (14,927, Supelco), and corticosterone (27,840, Sigma-Aldrich, St. Louis, MO) were used as reference materials. For pretreatment of the samples, 100 μL of serum was added to 900 μL of methanol and vortexed. The mixture was incubated at −20°C for 1 h and centrifuged, and the supernatant was collected. Each supernatant (5 μL) was assayed using liquid chromatography with tandem mass spectrometry (LC-MS/MS; Waters Xevo TQ-S, Waters Corporation, Milford, MA) and separated via a Synergi Hydro-RP column (4 μm, 150 × 2 mm; 00F-4375-B0, Waters Corporation) at 35°C and a flow rate of 0.2 mL/min. The mobile phase consisted of 0.1% formic acid in distilled water (A) and 0.1% formic acid in methanol (B). The gradient program was as follows: initiation to 1 min, 0% B; 1 to 4 min, linear increase to 100% B and hold at 100% B for 30 s; 4.5 to 5 min, linear decrease to 0% B and hold at 0% B for 5 min. For data transformation, the standard curve was made using the area value of each standard materials, and the concentration was calculated by applying the sample value to each functional formula.
Western Blotting
Total proteins were extracted from the liver samples. Briefly, 100 mg of liver tissue was mixed with RIPA buffer, homogenized, and centrifuged to collect the supernatant. Protein concentrations were determined using the DC Protein Assay kit (5000111, BioRad Laboratories, Richmond, CA). Equal amounts of protein extract (20 μg) were separated by 12% SDS-PAGE. The separated proteins were transferred to a polyvinylidene fluoride membrane. The membrane was incubated with 5% skim milk in TBST (20 mM Tris, 137 mM sodium chloride (NaCl), 5 mM potassium chloride (KCl), and 0.05% Tween 20) at room temperature for 1.5 h, followed by incubation at 4°C overnight with the primary antibodies glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5,000, monoclonal; MA5-15738, Invitrogen, Rockford, IL), HSP70 (1:2,500, monoclonal, ADI-SPA-820, Enzo Life Sciences, Farmingdale, NY), HSP60 (1:2,500; monoclonal, ADI-SPA-806, Enzo Life Sciences), and HSP40 (1:1,250, polyclonal, ADI-SPA-400, Enzo Life Sciences). All the primary and secondary antibodies were diluted with 5% skim milk in TBST. After washing with TBST, the membrane was incubated with the secondary antibodies at room temperature for 1.5 h. That secondary antibodies were used goat anti-mouse (ADI-SAB-100, Enzo Life Sciences) for GAPDH (1:5,000), HSP70 (1:5,000), and HSP60 (1:5,000), and goat anti-rabbit (ADI-SAB-300, Enzo Life Sciences) for HSP40 (1:2,500). Protein expression was detected using an ECL kit (SuperSignal WestPico Plus, 32,106, Thermo Fisher Scientific, Rockford, IL) and imaged on an iBright CL100 Imaging System (Thermo Fisher Scientific, San Jose, CA). The data were normalized by GAPDH and expressed as relative value.
Real-Time qPCR
Gene-specific primers for chicken HSP70, HSP60, HSP40, HSF1, HSF2, HSF3, and GAPDH were designed using Gallus genes by Primer 3 software (Primer3, v.0.4.0, http://primer3.ut.ee). The primers are listed in Table 1. Total RNA was extracted from the liver samples using the AccuZol Total RNA extraction kit (Bioneer, Daejeon, Korea) according to the manufacturer's instructions. Total RNA quality and concentration was determined using μDrop plate (NanoDrop, Thermo Fisher Scientific, Wilmington, DE). Complementary DNA was synthesized with AccuPower Cycle Script RT Premix (dt20) (Bioneer, Korea) from 1 μg of total RNA. Real-time quantitative PCR was conducted using SsoFastTM EvaGreen Supermix (BioRad Laboratories, Hercules, CA) on a CFX96 real-time PCR detection system (BioRad Laboratories, Hercules, CA). The thermal cycling conditions were 95°C for 5 min, followed by 40 cycles of 95°C for 5 s (denaturation) and 60°C for 30 s (annealing and extension). Annealing temperatures were set with reference to the Tm value (Table 1). The specific gene expression levels were normalized against GAPDH, calculated via the 2−ΔΔCt method (Livak and Schmittgen, 2001) and expressed as fold-change.
Table 1.
Primers for real time qPCR.
| Gene | Sequence (5′-3′) | Accession number | Tm (°C)1 |
|---|---|---|---|
| HSP70 | F: GGTAAGCACAAGCGTGACAATGCT | NM_001006685.1 | 55°C |
| R: TCAATCTCAATGCTGGCTTGCGTG | |||
| HSP60 | F: TGTGTGGAGCAGCAAGACAGAGA | NM_001012916.1 | 55°C |
| R: TTCATGAGCTCCCAATCCCAGACA | |||
| HSP40 | F: GGGCATTCAACAGCATAGA | NM_001199325.1 | 60°C |
| R: TTCACATCCCCAAGTTTAGG | |||
| HSF1 | F: AAGTCACCAGCGTGTCCAG | NM_001305256.1 | 60°C |
| R: GCCTCGTTCTCATGCTTCA | |||
| HSF2 | F: TACTGCATTTCCGCTGCTC | NM_001167764.2 | 60°C |
| R: AGGGGTTTGTCCACAGAGG | |||
| HSF3 | F: ACGACGTCATCTGCTGGAG | NM_001305041.1 | 60°C |
| R: TTGAGCTGTCGGATGAAGC | |||
| GAPDH | F: AGAACATCATCCCAGCGT | NM_204305.1 | 60°C |
| R: AGCCTTCACTACCCTCTTG |
Abbreviations: HSP, heat shock proteins; HSF, heat shock factor; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
The Tm value was referred to the annealing temperatures of each genes for RT-qPCR analyzed.
Statistical Analysis
The experimental data were analyzed in SAS 9.4 (SAS Institute) and expressed as the mean ± SE. Analysis of variance was used to analyze the treatment effects, and Duncan's multiple range test was used to assess the statistical differences between the groups. Differences were considered statistically significant at P < 0.05.
Results
Growth Performance of Whole Chickens
The chicks were placed in the cages without significant weight differences. At placement, the average body weight was not different between treatment groups (Table 2). Lower body weight, DBG, feed intake, and ADFI were observed in the HH group compared with CH and C groups at first week, which was after the day 5 heat exposure. However, the final body weight did not differ between the CH and HH groups. After 35 d, the CH and HH groups had lower body weight and DBG than the C group (P < 0.05), whereas feed intake, ADFI, and FCR was lower in the HH group than in the C or CH groups (P < 0.05). Thus, early exposure to heat stress may influence feed intake. The HH chicks (early heat stress) had decreased feed intake but similar body weight as CH, but the CH chicks did not decrease feed intake to the levels observed in the HH group.
Table 2.
Early and chronic heat effects on growth performance of broiler chickens.
| Age (d) | Measurements | C | CH | HH |
|---|---|---|---|---|
| 0 | Initial weight (g) | 36.04 ± 0.73 | 35.97 ± 0.81 | 35.05 ± 0.58 |
| 7 | Body weight (g) | 167.33 ± 3.14a | 165.54 ± 3.96a | 137.49 ± 3.67b |
| 1–7 | DBG (g) | 23.9 ± 0.45a | 23.65 ± 0.57a | 19.64 ± 0.52b |
| Feed intake (g) | 165.36 ± 1.67a | 167.25 ± 1.4a | 146.01 ± 1.03b | |
| ADFI (g) | 23.62 ± 0.24a | 23.89 ± 0.2a | 20.86 ± 0.15b | |
| FCR | 1.03 ± 0.01 | 1.04 ± 0.01 | 1.04 ± 0.01 | |
| 35 | Body weight (g) | 2327.06 ± 32.39a | 2106.33 ± 86.9b | 2078.05 ± 37.19b |
| 28–35 | DBG (g) | 332.44 ± 4.63a | 300.9 ± 12.41b | 296.86 ± 5.31b |
| Feed intake (g) | 3305.42 ± 53.72a | 3260.4 ± 49.51a | 3102.99 ± 16.78b | |
| ADFI (g) | 472.2 ± 7.67a | 465.77 ± 7.07a | 443.28 ± 2.4b | |
| FCR | 1.51 ± 0.03a | 1.5 ± 0.03a | 1.43 ± 0.01b |
Data are shown as mean ± SEM. a,b Significant differences (P < 0.05) are indicated by different letters.
Abbreviations: ADFI, average daily feed intake; C, control group that raised optimum temperature without any heat exposure; CH, acute heat exposure group that exposed on day 35 for 5 h; DBG, daily body weight gain; FCR, Feed conversion ratio; HH, early heat exposure group that exposed both on day 5 for 24 h and day 35 for 5 h.
Dopamine, Serotonin, and Corticosterone Concentrations in Blood
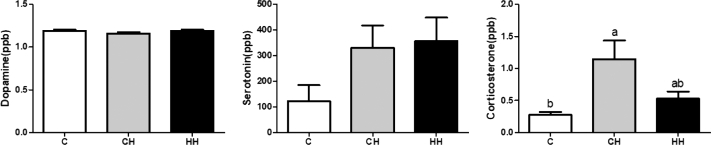
The serum levels of dopamine (ppb), serotonin (ppb), and corticosterone (ppb) after acute heat exposure on day 35 are shown in Figure 2. For hormone analysis, 10 serum samples from each group were used. The concentrations of dopamine and serotonin were similar, but the corticosterone levels were higher in the CH group compared with the C group (P < 0.05), whereas the C and HH groups did not differ.
Figure 2.
Dopamine, serotonin, and corticosterone of serum concentrations (ppb) after acute heat exposure at day 35 of broilers. Abbreviations: C, control group that raised optimum temperature without any heat exposure; CH, acute heat exposure group that exposed on day 35 for 5 h; HH, early heat exposure group that exposed both on day 5 for 24 h and day 35 for 5 h. Graph and error bar are shown as mean and SEM, respectively. a,bSignificant differences (P < 0.05) are indicated by different letters.
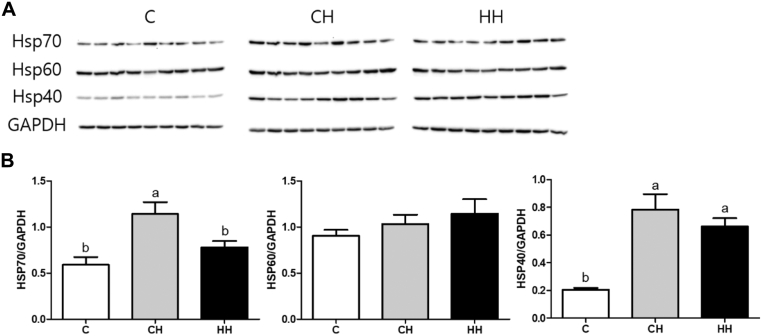
HSP Expression Analyzed by Western Blot
Figure 3 shows the Western blot results for the HSP (HSP70, HSP60, and HSP40) in the liver after acute heat exposure at day 35. The expression of HSP70 was higher only in the CH group (P < 0.05) and did not differ between the HH and C groups. Heat shock protein 40 expression was higher in the CH and HH groups compared with the C group. The expression of HSP60 did not differ among the groups.
Figure 3.
HSP protein expression by Western blot in the liver tissue after acute heat exposure at day 35 of early and acute heat exposed broilers. The Western blot (A) banding patterns of individual birds and (B) protein expression data that normalized by GAPDH (target protein expression/GAPDH expression) and calculated by fold change. Abbreviations: C, control group that raised optimum temperature without any heat exposure; CH, acute heat exposure group that exposed on day 35 for 5 h; HH, early heat exposure group that exposed both on day 5 for 24 h and day 35 for 5 h HSP, heat shock protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Graph and error bar are shown as mean and SEM, respectively. a,bSignificant differences (P < 0.05) are indicated by different letters.
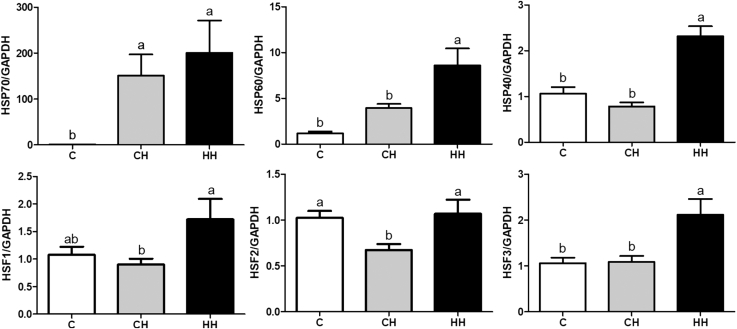
mRNA Expression of HSP and HSF Analyzed by Real-Time Quantitative PCR
We analyzed the mRNA levels of the HSP (HSP70, HSP60, and HSP40) and HSF (HSF1, HSF2, and HSF3) in the liver after acute heat exposure at day 35 (10 samples from each group) (Figure 4). Heat shock protein 70 mRNA expression was higher in the CH and HH groups compared with the C group (P < 0.05). The HSP60, HSP40, HSF1, and HSF3 mRNA expression was higher in HH than in C and CH groups (P < 0.05), but the mRNA expression did not differ between C and CH. The HSF2 expression was lower in the CH group compared with the HH and C groups (P < 0.05).
Figure 4.
HSP and HSF gene expression by RT-qPCR in the liver tissue after acute heat exposure at day 35 of early and acute heat exposed broilers (fold change). Data were normalized by GAPDH (target gene expression/GAPDH gene expression) and calculated by fold change. Abbreviations: C, control group that raised optimum temperature without any heat exposure; CH, acute heat exposure group that exposed on day 35 for 5 h; HH, early heat exposure group that exposed both on day 5 for 24 h and day 35 for 5 h; HSP, heat shock protein; HSF, heat shock factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Graph and error bar are shown as mean and SEM, respectively. a,bSignificant differences (P < 0.05) are indicated by different letters.
Discussion
Heat stress negatively impacts the production of animals. To increase heat resistance in broiler chickens, previous studies have used embryo heat treatment, feed restriction, and housing (Zulkifli et al., 2003; Rajkumar et al., 2015). Early heat exposure was applied to the embryo or postnatally, and a study of the 2 periods (prehatched vs. posthatched) (Tona et al., 2008) found that embryo heat treatment induced broiler growth at normal temperatures, but it did not affect heat resistance in heat-exposed 3-day-old chicks. The embryo is not the sensitive stage for developing heat resistance, but early heat exposure to specific areas (e.g., the muscles, hypothalamus, or brain) may affect growth, immunity, and biological responses. Early heat control is a promising management method for improved heat resistance in broilers (Lin et al., 2006). Yahav (1999) reported higher weight gain in early heat-exposed chicks (5-d-old, 36°C, 24 h) after late heat treatment (42-d-old, 35°C, 6 h) compared with the control group with only heat exposure on the 42nd d. Günal (2013) found that early heat exposure (5-d-old, 36°C, 24 h, with 28–42 d, 32–35°C for 7 h/d) significantly increased the body weight gain compared with the control (28–42 d, 32–35°C for 7 h/d). These results are consistent with our observations—the final body weight of both CH and HH group were lower than the C group but demonstrating that heat exposure at day 5 was an effective method to increase productivity and reduce feed costs (Table 2).
Blood cortisol or corticosterone levels are commonly analyzed to screen for responses to stress stimuli in animal welfare studies (Mahmoud et al., 2004). In this study, the serum corticosterone concentration increased after acute heat stress (P < 0.05). Hormonal changes in the blood are also sensitive to acute stimuli, and rapid changes in the hormone levels may be an indicator of stress. The level of corticosterone was higher in the CH group with acute heat stress compared with the C group (P < 0.05), but there were no differences between the HH and C groups. Previous studies have also analyzed corticosterone as a stress index in chickens and found that it increased in several tissues with stress (Mahmoud et al., 2004). Tona et al. (2008) conducted initial thermal conditioning of 3-day-old chicks and compared the corticosterone concentrations immediately before and after heat treatment on the 42nd d. The concentration of corticosterone immediately after heat treatment was higher in the CH group (P < 0.05), but there were no changes in the C group. These results indicate that initial heat treatment encourages the heat stress response in broilers and improves heat resistance.
Heat shock proteins are ubiquitous proteins for all living organisms. Heat shock proteins are expressed under normal conditions and induced (P < 0.05) when the tissues are damaged by environmental stimuli such as heat, transport, densification, or noise (Lara and Rostagno, 2013). Thus, HSP expression has been used as an indicator of environmental stress in living organisms. The upregulation and accumulation of HSP can improve the repair and replacement of cells damaged in stressful conditions (Li et al., 1995). In a prior study, the protein expression levels of HSP90, HSP60, and HSP70 were significantly upregulated in chronic heat stress conditions (15–42 d, 35°C) (Vinoth et al., 2015), and HSP70 was also upregulated in acute heat stress conditions (30 d, 36°C, 3 h) (Soleimani et al., 2011). This suggests that heat stress may increase HSP expression in broilers. However, other studies have reported inconsistent effects of early heat treatment on HSP expression. For example, Liew et al. (2003) did not find a significant effect of initial heat exposure on HSP70 expression at 1 to 21 d (36°C, 1 h/d). Yahav et al. (1997) also reported no effects of initial heat treatment (5-d-old, 36°C, 24 h) on the expression of HSP70 or HSP90 when heat-stressed just before marketing. Therefore, the authors concluded that early heat treatment does not play an important role in heat resistance. In the present study, we observed similar HSP70 expression in the HH (with early heat conditioning) and C groups, but both were lower than the CH group. These results suggest that heat exposure before marketing can upregulate the expression of HSP70 and HSP40 in the liver. Toplu et al. (2014) similarly reported lower HSP70 expression in the liver, brain, and kidneys of early heat-treated (5-d-old, 36°C, 24 h) chicks after chronic heat stress (22–42 d, 35°C, 6 h/d) than in the chronic heat-stressed group without early heat treatment. These variable results may be because of differences in the broiler breeds, sample collections, heat treatments, and environmental conditions. Therefore, the effects of heat resistance on early heat exposure may be breed- and environment-dependent.
Heat shock proteins are associated with corticosterone levels and growth performance. The HSP70 increases in stressful conditions and is used as an indicator of heat stress. The HSP70 protects cells by preventing the degradation of protein function via the inhibition of other protein aggregation. However, HSP synthesis comes at a physiological cost, as the composition of other proteins is reduced, thereby negatively affecting growth (Mahmoud and Edens, 2005). The highest corticosterone concentration in the CH group may reflect an inflammatory state caused by heat stress. If HSP70 increases, it binds to the toll-like receptor (TLR), and TLR2 or TLR4 induces the expression of IL-6 (Vanhooren et al., 2008). The IL-6 expression decreases IGF-1 and reduces growth. Increased corticosterone levels induced by inflammation are also related to IL-6. Compared with the HH and CH groups, HSP70 expression, corticosterone levels, and FCR were lower in the HH group. Lower FCR is an indicator of improved growth. Thus, the heat stress–related results for HSP70 expression, corticosterone, and FCR suggest that broiler growth may be retarded by HSP70 and other related cytokines and corticosterone.
High-temperature exposure increases the concentration of HSP by increasing the activity of heat shock modulators and their mRNA levels. Tamzil et al. (2013) found that HSP70 expression increased in 2 breeds after exposure to acute heat stress (40°C for 0, 0.5, 1, and 1.5 h). Heat exposure causes stress by inducing higher expression of the HSP genes. Similar to Tamzil et al. (2013), the mRNA expression of HSP70 was higher in the CH group compared with the C group. Moreover, the HH group had higher expression of the HSP and HSF than the other groups. However, the effect of heat stress on protein synthesis showed different trend. Inconsistencies between the mRNA and protein expression levels have also been reported in other studies (Vogel and Marcotte, 2012) and probably reflects the heat stress–induced increase in mRNA expression (transcription) and suppression of HSP synthesis. Together, these results showed that the heat stress was more severe in the HH group than in the CH group.
In conclusion, we investigated the effects of early heat conditioning on the broiler response to acute heat exposure by analyzing the corticosterone levels and gene/protein expression of HSP. Early heat exposure group showed lower body weight gain and FCR was reduced (P < 0.05), indicating a positive effect on feed efficiency. Acute heat stress to 35-day-old broilers increased the blood corticosterone levels and HSP expression (protein and gene). The HSP70 expression in the early heat treatment group was similar to the control. The corticosterone concentrations in the HH group did not differ from CH, but this is a positive result because it decreased compared with CH and did not differ between the C and HH groups. Together, these results suggest that early age heat conditioning may induce acute heat stress resistance in broilers.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (No. 2020R1I1A3A04038058).
Disclosures
The authors declare no conflicts of interest.
References
- Arjona A., Denbow D., Weaver W., Jr. Effect of heat stress early in life on mortality of broilers exposed to high environmental temperatures just prior to marketing. Poult. Sci. 1988;67:226–231. doi: 10.3382/ps.0670226. [DOI] [PubMed] [Google Scholar]
- Günal M. The effects of early-age thermal manipulation and daily short-term fasting on performance and body temperatures in broiler exposed to heat stress. J. Anim. Physiol. Anim. Nutr. (Berl) 2013;97:854–860. doi: 10.1111/j.1439-0396.2012.01330.x. [DOI] [PubMed] [Google Scholar]
- Lara L., Rostagno M. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Mivechi N.F., Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int. J. Hyperthermia. 1995;11:459–488. doi: 10.3109/02656739509022483. [DOI] [PubMed] [Google Scholar]
- Liew P., Zulkifli I., Hair-Bejo M., Omar A., Israf D. Effects of early age feed restriction and heat conditioning on heat shock protein 70 expression, resistance to infectious bursal disease, and growth in male broiler chickens subjected to heat stress. Poult. Sci. 2003;82:1879–1885. doi: 10.1093/ps/82.12.1879. [DOI] [PubMed] [Google Scholar]
- Lin H., Jiao H., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. World's Poult. Sci. J. 2006;62:71–86. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mahmoud K.Z., Edens F. Influence of organic selenium on hsp70 response of heat-stressed and enteropathogenic Escherichia coli-challenged broiler chickens (Gallus gallus) Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2005;141:69–75. doi: 10.1016/j.cca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Mahmoud K.Z., Edens F., Eisen E., Havenstein G. Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2004;137:35–42. doi: 10.1016/j.cbpc.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Rajkumar U., Vinoth A., Shanmugam M., Rajaravindra K., Rama Rao S. Effect of embryonic thermal exposure on heat shock proteins (HSPs) gene expression and serum T3 concentration in two broiler populations. Anim. Biotechnol. 2015;26:260–267. doi: 10.1080/10495398.2015.1022183. [DOI] [PubMed] [Google Scholar]
- Saeed M., Babazadeh D., Naveed M., Arain M.A., Hassan F.U., Chao S. Reconsidering betaine as a natural anti-heat stress agent in poultry industry: a review. Trop. Anim. Health Prod. 2017;49:1329–1338. doi: 10.1007/s11250-017-1355-z. [DOI] [PubMed] [Google Scholar]
- Soleimani A., Zulkifli I., Omar A., Raha A. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011;90:1435–1440. doi: 10.3382/ps.2011-01381. [DOI] [PubMed] [Google Scholar]
- Tamzil M., Noor R., Hardjosworo P., Manalu W., Sumantri C. Acute heat stress responses of three lines of chickens with different heat shock protein (HSP)-70 genotypes. Int. J. Poult. Sci. 2013;12:264–272. [Google Scholar]
- Tona K., Onagbesan O., Bruggeman V., Collin A., Berri C., Duclos M., Tesseraud S., Buyse J., Decuypere E., Yahav S. Effects of heat conditioning at day 16 to 18 of incubation or during early broiler rearing on embryo physiology, posthatch growth performance and heat tolerance. Arch. Geflügelk. 2008;72:75–83. [Google Scholar]
- Toplu H.D.O., Tunca R., Aypak S.U., Coven F., Epikmen E.T., Karaarslan S., Yagin O. Effects of heat conditioning and dietary ascorbic acid supplementation on heat shock protein 70 expression, blood parameters and fear-related behavior in broilers subjected to heat stress. Acta Sci. Vet. 2014;42:1–8. [Google Scholar]
- Vanhooren V., Liu X.-E., Desmyter L., Fan Y.-D., Vanwalleghem L., Van Molle W., Dewaele S., Praet M., Contreras R., Libert C. Over-expression of heat shock protein 70 in mice is associated with growth retardation, tumor formation, and early death. Rejuvenation Res. 2008;11:1013–1020. doi: 10.1089/rej.2008.0783. [DOI] [PubMed] [Google Scholar]
- Vinoth A., Thirunalasundari T., Tharian J.A., Shanmugam M., Rajkumar U. Effect of thermal manipulation during embryogenesis on liver heat shock protein expression in chronic heat stressed colored broiler chickens. J. Therm. Biol. 2015;53:162–171. doi: 10.1016/j.jtherbio.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Vogel C., Marcotte E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012;13:227. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn R.M., Davie J.R., Cox R.P., Chuang D.T. Molecular chaperones: heat-shock proteins, foldases, and matchmakers. J. Lab. Clin. Med. 1994;124:31–36. [PubMed] [Google Scholar]
- Yahav S. Effect of early-age thermal conditioning and food restriction on performance and thermotolerance of male broiler chickens. Br. Poult. Sci. 1999;40:120–126. doi: 10.1080/00071669987944. [DOI] [PubMed] [Google Scholar]
- Yahav S., McMurtry J. Thermotolerance acquisition in broiler chickens by temperature conditioning early in life—the effect of timing and ambient temperaturey. Poult. Sci. 2001;80:1662–1666. doi: 10.1093/ps/80.12.1662. [DOI] [PubMed] [Google Scholar]
- Yahav S., Shamai A., Haberfeld A., Horev G., Hurwitz S., Einat M. Induction of thermotolerance in chickens by temperature conditioning: heat shock protein expression. Ann. New York Acad. Sci. 1997;813:628–636. doi: 10.1111/j.1749-6632.1997.tb51757.x. [DOI] [PubMed] [Google Scholar]
- Yeğenoğlu E., Lgen G. World Poultry Science Association (WPSA), 2nd Mediterr. Summit WPSA; Antalya, Turkey: 2009. Effects of thermal conditioning treatments on brain HSP70 level in broilers under heat stress; pp. 139–142. October 4–7, 2009. [Google Scholar]
- Yousaf A., Jabbar A., Rajput N., Memon A., Shahnawaz R., Mukhtar N., Farooq F., Abbas M., Khalil R. Effect of environmental heat stress on performance and carcass yield of broiler chicks. World Vet. J. 2019;9:26–30. [Google Scholar]
- Zulkifli I., Liew P., Israf D., Omar A., Hair-Bejo M. Effects of early age feed restriction and heat conditioning on heterophil/lymphocyte ratios, heat shock protein 70 expression and body temperature of heat-stressed broiler chickens. J. Therm. Biol. 2003;28:217–222. [Google Scholar]