Abstract
Diets enriched with phytogenic feed additives (PFA) such as AV/HGP/16 premix (AVHGP), Superliv concentrate premix (SCP), and bacteriostatic herbal growth promotor (BHGP) with essential oils have been shown to improve feed efficiency (FE) in broilers. This FE improvement was achieved via modulation of hypothalamic neuropeptides, which results despite feed intake reduction, in increased breast yield without changes in body weight compared to the control group. To gain further insights into the mode of action of these PFA, the present study aimed to determine the potential involvement of signaling pathways associated with lipid and protein metabolism. One day-old male Cobb 500 chicks were randomly assigned into 1 of 4 treatments, comprising 8 replicates per treatment in a completely randomized design. The dietary treatments included a basal diet (control) or 0.55 g/kg diet of AVHGP, SCP, or BHGP. The birds had ad libitum access to water and feed. On day 35, after blood sampling, the liver, abdominal adipose tissue (AT), and breast muscle samples were collected. The levels of phosphorylated mechanistic target of rapamycin (mTOR)Ser2481 as well as its levels of mRNA and those of its downstream mediator RPS6B1 were significantly upregulated in the muscle of the PFA-fed groups compared with the control group. In the liver, the phosphorylated levels of acetyl-CoA carboxylase alpha at Ser79, the rate-limiting enzyme in fat synthesis, was significantly induced in the PFA-fed groups compared with the control group, indicating a lower hepatic lipogenesis. The hepatic expression of hepatic triglyceride lipase (LIPC) and adipose triglyceride lipase (ATGL) was significantly upregulated in the AVHGP-fed group compared with the control group. These hepatic changes were accompanied by a significant downregulation of hepatic sterol regulatory element-binding protein cleavage-activating protein in all the PFA groups and an upregulation of peroxisome proliferator–activated receptor alpha and gamma in the SCP-fed compared with the control group. In the AT, the mRNA abundances of ATGL and LIPC were significantly increased in both SCP- and BHGP-fed birds compared with the control group. Together these data indicate that PFA improve FE via modulation of muscle mTOR pathway and hepatic lipolytic/lipogenic programs, thus, favoring muscle protein synthesis and lowering hepatic lipogenesis.
Key words: phytogenic feed additives, hepatic lipogenesis, lipolysis, muscle protein synthesis, mTOR, ACCα, broilers
Introduction
The main goal of the poultry industry is to produce affordable and high protein quality to meet the high nutritional demand worldwide. The demand for poultry meat and products is rising globally and estimated to be higher in the next century (Mulder, 1997; Reynnells, 1999; Van Boeckel et al., 2015; Mekonnen et al., 2019). Although the spectacular progress in nutrition, genetics, and management strategies have improved broiler chicken growth performance and breast meat yield (Havenstein et al., 2003a; Havenstein et al., 2003b), the expected increase in the human population by 2050 (Bongaarts, 2009) will require the poultry industry to implement innovative and effective strategies to cope with various challenges and increase meat production by 73% to feed the future (Bruce, 2016).
The global heightened concerns on emerging drug-resistant superbugs and the critical need for antibiotic alternatives in livestock generally and poultry, particularly, are the most significant challenges that the poultry industry is facing. Since their discovery in the 1920s, in-feed antibiotics have played a crucial role in improving growth performance and feed conversion efficiency in poultry production (Castanon, 2007). Owing to antimicrobial (cross)-resistance that threats human health and increased public awareness (Marshall and Levy, 2011; Tang et al., 2017), the European Union and the United States banned the use of antibiotics in animal production in 2006 and 2017, respectively (Castanon, 2007; Tang et al., 2017).
The quest and search for antibiotics alternatives have remarkably intensified and became hot research spots in the recent years (Gadde et al., 2017). On being empowered by consumer demand for poultry products from “No Antibiotics Ever, NAE” flocks, several classes of alternatives including probiotics, organic acids, prebiotics, synbiotics, enzymes, antimicrobial peptides, hyperimmune egg antibodies, bacteriophages, clay, and metals are available (Gadde et al., 2017). Of particular interest, consumer's' changing tastes, values, and preferences for natural products have triggered the popularity of feed phytogenics as favorable alternatives to antibiotic growth promoters. The phytogenic market worldwide is expected to increase between 2018 and 2023, from about 631.4 million to over 962.5 million US dollars (Stevanovic et al., 2018).
A growing body of scientific papers has reported many health- and growth-promoting activities of phytogenics. Amad et al. have shown that phytogenics improve growth performance in poultry (Amad et al., 2011). Although the mode of phytogenics action is not fully defined, their beneficial effects are attributed to their antimicrobial, immunomodulatory, and antioxidant properties (Kim et al., 2010, 2013; Settle et al., 2014). Recently, we have shown that PFA modulate the expression of feeding-related hypothalamic neuropeptides and result in feed efficiency (FE) improvement and a slight increase in breast yield (Orlowski et al., 2018; Flees et al., 2020). As FE is also controlled by peripheral intermediary metabolisms, we sought to determine here the effects of PFA on lipid metabolism– and protein synthesis–associated signaling pathways.
Materials and methods
Ethical Statement
All the animal experiments were approved by the University of Arkansas Animal Care and Use Committee (protocol number 16084) and were in accordance with the recommendations in NIH's Guide for the Care and Use of Laboratory Animals.
Experimental Animal Husbandry and Diets
All animal husbandry, diet formulations, and experimental design were previously described (Flees et al., 2020). Briefly, a day-old male Cobb 500 broiler chicks (Gallus gallus domesticus, n = 384, Cobb-Vantress, Inc., Siloam Springs, AR) were individually tagged and randomly allotted (12 birds/pen) into 32 floor pens with fresh pine wood shavings equipped with separate feeders (Choretime feeders; Georgia Poultry, Newton Grove, NC) and waterlines (Ziggity water system, Georgia Poultry, Newton Grove, NC). Eight pens were randomly assigned into 1 of 4 treatments in a completely randomized design. The dietary treatments included a basal diet (control) or 0.55 g/kg diet of AV/HGP/16 premix (AVHGP), Superliv concentrate premix (SCP), or bacteriostatic herbal growth promotor (BHGP). The composition of the 3 phytogenic feed additives (PFA) are proprietary to Ayurvet Ltd. (Kaushambi, Ghaziabad, India) and are a polyherbal formulation of prestandardized and tested herbs. AV/HGP/16 is a phytoadditive intended for use across different species of livestock, consisting of many protein-rich ingredients, predominantly Cicer arietinum, Phaseolus mungo, and Mucuna pruriens, that are reputed for their antioxidant, immunomodulatory, and growth-promoting activities besides their ability to supplement commonly deficient amino acids. Superliv concentrate premix, containing several liver-stimulating, antioxidant, and growth-promoting ingredients, such as Achyranthes aspera, Andrographis paniculata, and Tinospora cordifolia, to name a few., is a polyherbal liver tonic and growth promoter for monogastric species of livestock. Bacteriostatic herbal growth promotor, commercially available as Nbiotic, is an herbal growth promoter with essential oils, intended for use in both poultry as well as in other species of livestock and comprises ingredients such as Allium sativum, Zingiber officinale, Cichorium intybus, eucalyptus oil, etc. that are reputed for their antimicrobial, antioxidant, immunomodulatory, and growth-promoting activities (Nadkarni, 2005).
All the birds were offered ad libitum access to feed and water and were reared under gradually decreasing ambient temperatures of 32°C for day 1 to 3, 31°C for day 4 to 6, 29°C for day 7 to 10, 27°C for day 11 to 14, and 25°C thereafter. A relative humidity of about 20 to 40%, and a 23 h light to 1 h dark photoperiod was maintained until the end of the experiment. The pen feed intake was measured daily, and the individual body weights were recorded weekly. The bird welfare was assessed daily. On day 35 at 8:00 am, one of the birds per pen was randomly euthanized by cervical dislocation in the necropsy area for the collection of blood for serum, liver tissue from the caudal region of the left lobe, abdominal adipose tissue (AT), and pectoralis major (breast) muscle tissue from the left breast. All the tissue samples were snap frozen in liquid nitrogen and stored at -80°C until further analysis. The sample size was based on previous experiments and power analysis.
RNA Isolation, Reverse Transcription, and Real-Time Quantitative Polymerase Chain Reaction
The RNA from the liver, AT, and muscle samples was extracted using TRIzol reagent (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer's recommendations. The integrity and quality of the RNA was assessed by 1% agarose gel electrophoresis, whereas the concentrations and purity were determined for each sample by Take 3 Micro-Volume Plate using a Synergy HT multimode microplate reader. The RNA samples were RQ1 RNase-free DNase treated (Promega, Madison, WI), and RNA (1 μg) was reverse transcribed using qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD). The reverse transcribed products (cDNA) were amplified by real-time quantitative PCR (Applied Biosystems 7500 Real-Time PCR system) by using 5 μL of 10 × diluted cDNA with SYBR Green Master Mix (Thermo Fisher Scientific) combined with 0.5 μmol of each forward and reverse specific primer in a total of 20 μL reaction as previously described (Piekarski et al., 2018; Greene et al., 2019). Oligonucleotide primers specific for chicken ATP citrate lyase (ACLY), acetyl-CoA carboxylase alpha (ACCα), fatty acid synthase (FASN), malic enzyme (ME), sterol regulatory element–binding protein 1 and 2 (SREBP-1/2), SREBP cleavage–activating protein (SCAP), insulin-induced gene 2, lipoprotein lipase (LPL), hepatic triglyceride lipase (LIPC), adipose triglyceride lipase (ATGL), peroxisome proliferator–activated receptor alpha and gamma (PPARα/γ), adiponectin (AdipoQ), adiponectin receptor 1 and 2 (AdipoR1/2), visfatin (NAMPT), mechanistic target of rapamycin (mTOR), ribosomal protein S6 kinase B1 (RPS6KB1), AMP-activated protein kinase alpha 1 and 2 (AMPKα1/2), and 18S ribosomal subunit as a housekeeping gene, as described previously (Nguyen et al., 2015; Blankenship et al., 2016; Flees et al., 2017; Rajaei-Sharifabadi et al., 2017; Ferver et al., 2020), were used. Oligonucleotide primers specific for chicken AMPKβ1/2 and AMPKγ1-3 are presented in Table 1. The cycling conditions were 50°C for 2 min and 95°C for 10 min followed by 40 cycles of a 2-step amplification process (95°C for 15 s and 58°C for 1 min). After the PCR, melting curve analysis was applied using the dissociation protocol from the Sequence Detection system to exclude samples with nonspecific products. The PCR products were also confirmed for 1 specific size band by agarose gel electrophoresis. The relative expression of the target genes was normalized to the expression of 18S rRNA and calculated using the 2−ΔΔCt method (Schmittgen and Livak, 2008) with the control group as the calibrator.
Table 1.
Oligonucleotide primers for real-time quantitative PCR.
| Gene | Accession number1 | Primer sequence (5’ → 3′) | Orientation | Product size (bp) |
|---|---|---|---|---|
| AMPKβ1 | NM_001039912 | TTGGCAGCAGGATCTGGAA | Forward | 60 |
| AAGACTGTTGGTCGAGCTTGAGT | Reverse | |||
| AMPKβ2 | NM_001044662 | TGTGACCCGGCCCTACTG | Forward | 56 |
| GCGTAGAGGTGATTGAGCATGA | Reverse | |||
| AMPKγ1 | NM_001034827 | CAAGCCGTTGGTCTGCATCT | Forward | 56 |
| GGGAGGAGACGGCATCAA | Reverse | |||
| AMPKγ2 | NM_001278142 | TGCCATGCCATTCTTGGA | Forward | 62 |
| CCACCTTGCGAGAAGCATTT | Reverse | |||
| AMPKγ3 | NM_001031258 | CCCAAGCCACGCTTCCTA | Forward | 57 |
| ACGGAAGGTGCCGACACA | Reverse |
Abbreviation: AMPK, AMP-activated protein kinase.
Accession number refer to Genbank (NCBI).
Protein Isolation and Western Blot Analysis
The liver, AT, and muscle tissues were homogenized in lysis buffer, containing protease and phosphatase inhibitors, as previously described (Greene et al., 2019). The total protein concentrations were determined using a Bradford assay kit (BioRad, Hercules, CA), with bovine serum albumin used to establish a standard curve and read using a Synergy HT multimode microplate reader. The proteins (80 μg) were separated on 4 to 12% gradient Bis-Tris gels (Life Technologies, Waltham, MA) and transferred into polyvinylidene difluoride membranes in an XCell II blot system (Life Technologies). After transfer, the membranes were blocked in 5% nonfat dry milk and incubated with primary antibodies (1:1500 to 1:1000 dilution) overnight at 4°C. The polyclonal antibodies used were as follows rabbit anti-FASN, rabbit anti-ACCα, rabbit anti-phospho ACCαSer79, rabbit anti-ACLY, rabbit anti-phospho mTORSer 2481, rabbit anti-mTOR, rabbit anti-ME, rabbit anti-PPARγ, rabbit anti-ATGL, rabbit anti-phospho HSLSer 855/554, and rabbit anti-HSL. Rabbit anti-glyceraldehyde 3-phosphate dehydrogenase and rabbit ant-β-actin antibodies were used as the housekeeping proteins. All the antibodies were from Cell Signaling Technologies (Danvers, MA), except the anti-FASN antibody that was from Novus Biologicals (Littleton, CO) and the anti-ACLY antibody from LSBio (Seattle, WA). After several washes, the membranes were incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX), at 1:5000 dilution, for 60 min at room temperature. A prestained molecular weight marker (Precision Plus Protein Dual Color) was used as a standard (BioRad). The signal was visualized by enhanced chemiluminescence (ECL plus; GE Healthcare Bio-Sciences, Buckinghamshire, UK) and captured by using a FluorChem M MultiFluor System (ProteinSimple, Santa Clara, CA). The image acquisition and analysis were performed by AlphaView software (Version 3.4.0, 1993–2011).
Statistical Analysis
The gene and protein expression data were analyzed using one-way ANOVA or Student t-test when appropriate and Graph Pad Prism version 6.0 for Windows (Graph Pad Software, La Jolla, CA). The differences were considered significant at P < 0.05.
Results
Phytogenic Feed Additive–Enriched Diets Reduced Hepatic ACCα Activity
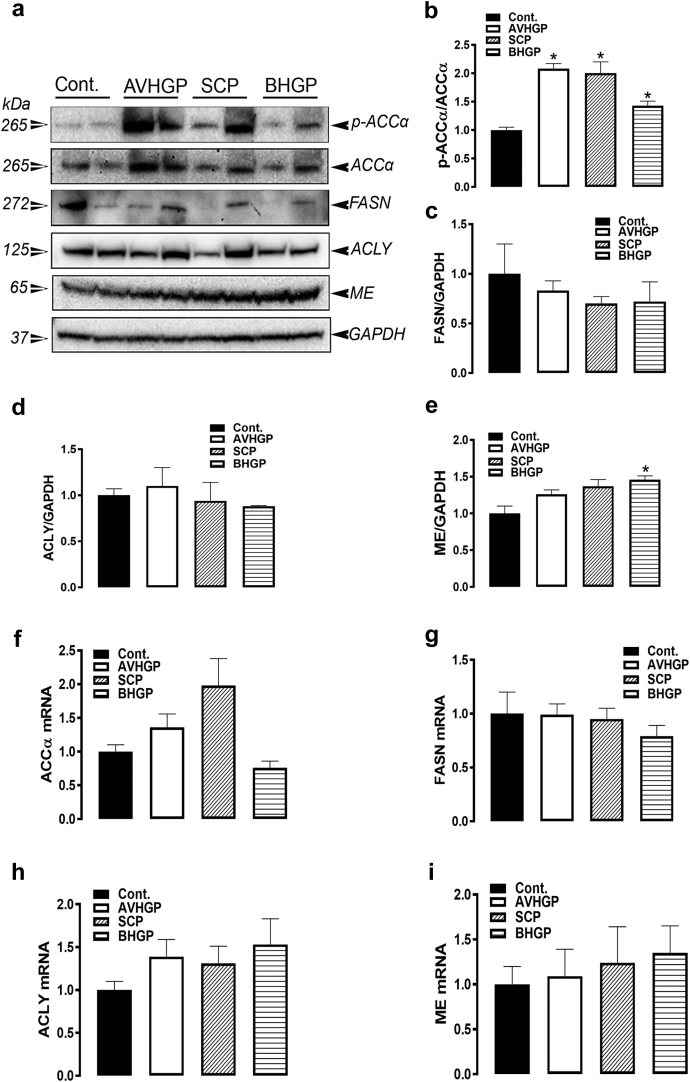
Phytogenic feed additive (AVHGP, SCP, and BHGP) supplementations significantly increased the levels of phosphorylated ACCα at Ser79 site, which indicates its inactivation compared with the control group (Figures 1A and 1B). The expression of FASN and ACLY proteins was not affected by the PFA administration (Figures 1A, 1C and 1D). Only BHGP supplementation upregulated ME protein levels compared with the control diet (P < 0.05, Figures 1A and 1E). The mRNA abundances of ACCα, FASN, ACLY, and ME remained unchanged among all the groups (Figures 1F–1I).
Figure 1.
The effects of PFA-enriched diets on hepatic expression of lipogenic markers in the broilers. The protein levels were measured by Western blot (A–E), and the mRNA abundances were determined by qPCR (F-I) using 2−ΔΔCt method (Schmittgen and Livak, 2008). The data are presented as mean ± SEM (n = 8/group). ∗denotes significant difference compared with the control group at P < 0.05. Abbreviations: ACC, acetyl-CoA carboxylase; ACLY, ATP citrate lyase; AVHGP, AV/HGP/16 premix; BHGP, bacteriostatic herbal growth promotor; FASN, fatty acid synthase; ME, malic enzyme; PFA, phytogenic feed additive; SCP; Superliv concentrate premix.
Phytogenic Feed Additives Modulated the Hepatic Expression of PPARγ and SCAP
The protein levels of PPARγ were significantly upregulated by SCP supplementation only but not by AVHGP or BHGP supplementation (Figures 2A and 2B). None of these treatments elicited any changes in PPARγ, PPARα, SREBP-1, SREBP-2, or insulin-induced gene 2 mRNA abundances; however, they all significantly downregulated SCAP mRNA expression compared with the control group (Figures 1C–1H).
Figure 2.
The effects of PFA-enriched diets on hepatic expression of key transcription factors involved in hepatic lipogenesis. The protein levels of PPARγ were measured by Western blot (A, B), and the mRNA abundances were determined by qPCR (C–H) using the 2−ΔΔCt method (Schmittgen and Livak, 2008). The data are presented as mean ± SEM (n = 8/group). ∗indicates significant difference compared with the control group at P < 0.05. Abbreviations: AVHGP, AV/HGP/16 premix; BHGP, bacteriostatic herbal growth promotor; INSIG2, insulin-induced gene 2; PPAR, peroxisome proliferator–activated receptor; PFA, phytogenic feed additive; SCAP, sterol regulatory element–binding protein cleavage–activating protein; SCP; Superliv concentrate premix; SREBP, sterol regulatory element–binding protein.
Phytogenic Feed Additives Upregulated the Hepatic Expression of AdipoQ
The AVHGP and BHGP supplementations upregulated the hepatic expression of AdipoQ gene expression but not that of its related receptors (AdipoR1 and AdipoR2) or visfatin (NAMPT) compared with the control group (Table 2).
Table 2.
The effects of PFA on hepatic adipokine expression in broilers1.
| Target genes3 | Cont. | Experimental groups2 |
SCP | BHGP |
|---|---|---|---|---|
| AVHGP | ||||
| AdipoQ | 1 ± 0.1 | 1.78 ± 0.34 | 1.03 ± 0.2 | 0.67 ± 0.14 |
| AdipoR1 | 1 ± 0.1 | 1.97 ± 0.7 | 1.16 ± 0.1 | 1.01 ± 0.08 |
| AdipoR2 | 1 ± 0.1 | 1.02 ± 0.1 | 1.10 ± 0.06 | 1.23 ± 0.1 |
| NAMPT | 1 ± 0.1 | 1.58 ± 0.3 | 1.24 ± 0.1 | 1.22 ± 0.1 |
Abbreviation: PFA, phytogenic feed additive.
Data are means ± SEM.
AVHGP, AV/HGP/16; BHGP, bacteriostatic herbal growth promoter with essential oil; Cont., control; SCP, superliv concentrate premix.
AdipoQ, adiponectin; AdipoR, adiponectin receptor; NAMPT, visfatin.
Denotes significant difference compared with the control group at P < 0.05.
Phytogenic Feed Additives Increased the Expression of Hepatic and Adipose Tissue Lipases
ATGL and LIPC mRNA levels were significantly induced by AVHGP supplementation in the liver and by SCP- and BHGP-supplemented diets in the AT compared with the control group (Figures 3B and 3C and Figures 4D and 4F). The expression of LPL gene did not change by the treatments in both tissues (liver and AT) (Figure 3A and Figure 4E). The ratio of phosphorylated hormone-sensitive lipase (pHSL)/ HSL and ATGL/actin also remained unchanged in all the groups (Figures 4A–4C).
Figure 3.
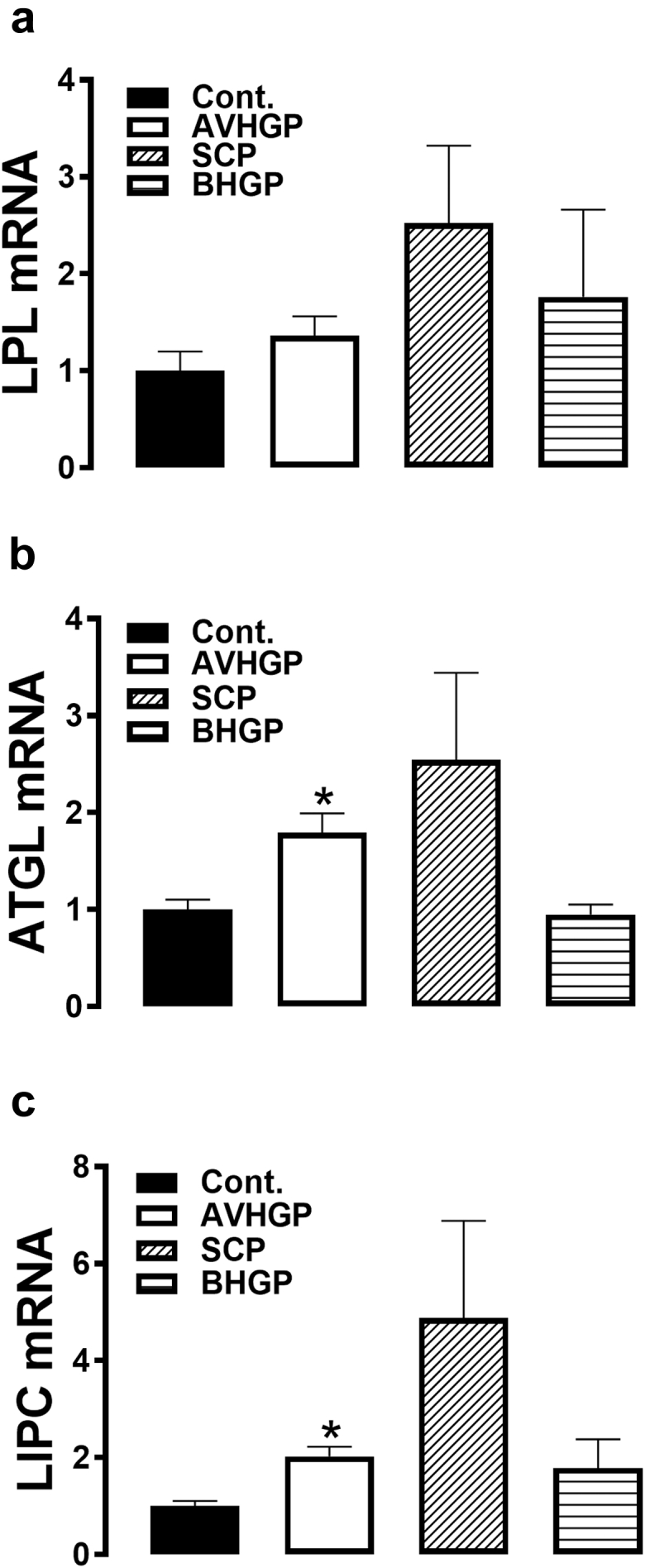
The effects of PFA-enriched diets on hepatic expression of lipolysis-related genes. The relative expression of LPL (A), ATGL (B), and LIPC (C) was measured by real-time RT-PCR using the 2−ΔΔCt method (Schmittgen and Livak, 2008). The data are presented as mean ± SEM (n = 8/group). ∗indicates significant difference compared to the control group at P < 0.05. Abbreviations: ATGL, adipose triglyceride lipase; AVHGP, AV/HGP/16 premix; BHGP, bacteriostatic herbal growth promotor; LIPC, hepatic triacylglycerol lipase; LPL, lipoprotein lipase; PFA, phytogenic feed additive; SCP; Superliv concentrate premix.
Figure 4.
The effect of PFA-enriched diets on the expression of lipolytic markers in the broiler adipose tissue. The protein levels were measured by Western blot (A–C), and the mRNA abundances were determined by qPCR (D–F) using the 2−ΔΔCt method (Schmittgen and Livak, 2008). Data are presented as mean ± SEM (n = 8/group). ∗indicates significant difference compared with the control group at P < 0.05. Abbreviations: ATGL, adipose triglyceride lipase; AVHGP, AV/HGP/16 premix; BHGP, bacteriostatic herbal growth promotor; HSL, hormone sensitive lipase; LIPC, hepatic triacylglycerol lipase; LPL, lipoprotein lipase; PFA, phytogenic feed additive; SCP; Superliv concentrate premix.
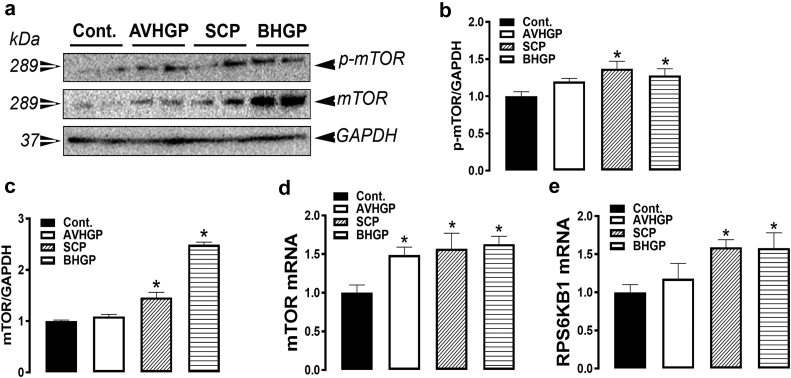
Phytogenic Feed Additive Modulated the Muscle Expression of mTOR and RPS6KB1
All the PFA treatment upregulated muscle mTOR gene expression, but only SCP- and BHGP-enriched diets increased RPS6KB1 mRNA levels compared with the control group (Figures 5D and 5E). Both SCP and BHGP supplementations induced the phosphorylated and total mTOR protein levels in the broiler muscles compared with the control group (Figures 5A–5C). The mRNA levels of AMPK regulatory subunits (β2 and γ1) were upregulated by all the tested PFA; however, AMPKβ1 was upregulated by SCP and BHGP, and AMPKγ3 was induced by AVHGP and BHGP compared with the control group (P < 0.05, Table 3). The expression of the AMPK catalytic subunit α2 was significantly upregulated by AVHGP and BHGP treatments compared with the control group (Table 3).
Figure 5.
The effect of PFA-enriched diets on the expression of protein synthesis-associated pathway in the broiler muscle. The phosphorylated and pan protein levels of mTOR were determined by Western blot (A–C). The relative expression of mTOR (D) and RPS6KB1 (E) was measured by qPCR using the 2−ΔΔCt method (Schmittgen and Livak, 2008). The data are presented as mean ± SEM (n = 8/group). ∗indicates significant difference compared to the control group at P < 0.05. Abbreviations: AVHGP, AV/HGP/16 premix; BHGP, bacteriostatic herbal growth promotor; mTOR, mechanistic target of rapamycin; PFA, phytogenic feed additive; RPS6KB1, ribosomal protein S6 kinase beta 1; SCP; superliv concentrate premix.
Table 3.
The effects of PFA on muscle AMPK expression in broilers1.
| Target genes | Cont. | Experimental groups2 |
SCP | BHGP |
|---|---|---|---|---|
| AVHGP | ||||
| AMPKα1 | 1 ± 0.2 | 1.02 ± 0.3 | 1.31 ± 0.3 | 1.16 ± 0.3 |
| AMPKα2 | 1 ± 0.1 | 1.65 ± 0.1∗ | 1.42 ± 0.3 | 1.65 ± 0.1∗ |
| AMPKβ1 | 1 ± 0.05 | 1.11 ± 0.09 | 1.35 ± 0.1∗ | 1.54 ± 0.2∗ |
| AMPKβ2 | 1 ± 0.1 | 1.46 ± 0.08∗ | 1.36 ± 0.1∗ | 1.54 ± 0.1∗ |
| AMPKγ1 | 1 ± 0.06 | 1.42 ± 0.09∗ | 1.37 ± 0.1∗ | 1.30 ± 0.08∗ |
| AMPKγ2 | 1 ± 0.1 | 1.03 ± 0.1 | 1.22 ± 0.2 | 1.09 ± 0.1 |
| AMPKγ3 | 1 ± 0.09 | 1.60 ± 0.1∗ | 1.40 ± 0.2 | 1.52 ± 0.1∗ |
Abbreviations: AMPK, AMP-activated protein kinase; PFA, phytogenic feed additive.
Data are means ± SEM, and ∗indicates a significant difference compared to the control group at P < 0.05.
AVHGP, AV/HGP/16; BHGP, bacteriostatic herbal growth promoter with essential oil; Cont., control; SCP, superliv concentrate premix.
Discussion
By following “raised without antibiotics” demand and the ban of their subtherapeutic use as feed additives, global research effort on identification of alternative supplements has intensified. On being fueled by consumers' changing tastes, values, and preferences for natural products, phytogenics gained considerable attention and popularity in the feed industry and have quickly become the fastest growing segments of the animal feed additives (Mehdi et al., 2018).
Phytogenic or phytobiotic feed additives, derived from plants, herbs, and spices, are used to improve animal performance. Although the underlying mechanisms are not well defined, PFA have been very successful because of their beneficial effects on growth, immune system, and stress relief response (Windisch et al., 2008; Toghyani et al., 2011; Alimohamadi et al., 2014; Ghasemi et al., 2014; Li, 2015). Recently, our group showed that PFA (AVHGP, SCP, and BHGP) improved FE in broilers by reducing feed intake while maintaining similar body weights to the control group (Flees et al., 2020). At the central level, these effects seemed to be mediated through modulation of feeding-related hypothalamic neuropeptides (Flees et al., 2020). As FE is a result of complex interaction between the central nervous system and the periphery (intermediary metabolism), which are tightly controlled not only by hypothalamic circuits but also by highly integrated peripheral signaling pathways, we sought to determine here the effects of these PFA on lipid and protein metabolism–associated pathways in 3 metabolically important tissues, namely the liver, AT, and breast muscle.
The liver is the main site for lipogenesis in chickens, and it is also a site for fat storage. In fact, the avian liver is responsible for more than 90% of de novo fatty acid synthesis, (Goodridge and Ball, 1967; Leveille et al., 1968; Yeh and Leveille, 1971) which is controlled by several key enzymes. Acetyl-CoA carboxylase alpha, which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, is a rate-limiting enzyme in fatty acid biosynthesis (Brownsey et al., 1997; Tong 2005). As ACCα is inactivated by phosphorylation at serine 79 site (Ha et al., 1994; Abu-Elheiga et al., 2001; Fullerton et al., 2013), our data indicated that PFA reduced hepatic lipogenesis in chickens. This is supported by the significant downregulation of SCAP expression although the levels of its binding partner SREBP1 and 2 did not change. The SCAP is a key protein in the regulation of lipid metabolism, and its knockdown in the liver reduced de novo lipogenesis in mice and rhesus monkeys (Jensen et al., 2016). The increased levels of ME protein in the BHGP-fed group are intriguing as this decarboxylating enzyme is known to serve as an additional source of NADPH for lipogenesis (Wise and Ball, 1964). However, cytosolic ME enhances also anaplerosis (the replenishment of the TCA cycle) via converting malate into pyruvate, which re-enters the mitochondria via the pyruvate transporter resulting in increased ATP synthesis (Owen et al., 2002). It has been also reported that ME is involved in desaturation of polyunsaturated fatty acids (Kendrick and Ratledge, 1992), and the latter requires NADPH-dependent reductase to be β-oxidized (Tserng and Jin, 1991). Although further in-depth mechanistic studies are warranted, our data suggested that BHGP-enriched diet induced ME protein expression for β-oxidation and ATP synthesis rather than fatty acid synthesis. The upregulation of the PPARγ protein expression, transcription factor belonging to the nuclear receptor superfamily, supports our hypothesis. Peroxisome proliferator–activated receptor gamma is directly regulated by fatty acids and their derivatives (Varga et al., 2011), and its pivotal role in lipid catabolism has been described in many metabolically important tissues (Tanaka et al., 2003; Wang et al., 2003; Cheng et al., 2004). The abovementioned changes were accompanied by an upregulation of hepatic AdipoQ levels in the AVHGP- and BHGP-fed birds. AdipoQ is highly expressed in the avian liver (Maddineni et al., 2005; Mohammadpour et al., 2020) although its role is still not well elucidated. In human liver hepatocellular cells, adiponectin treatment decreased the expression of ACCα and increased that of acyl-CoA oxidase and carnitine palmitoyltransferase 1, key players in fatty acid β-oxidation (Simo et al., 2014). In chickens, Yan et al. reported that adiponectin impaired adipocyte differentiation and negatively regulated fat deposition (Yan et al., 2014). Together, these data sustain the role of PFA in avian lipid metabolism by reducing hepatic lipogenesis and inducing β-oxidation.
As a subtle balance between lipogenesis and lipolysis is a critical point for lipid metabolism homeostasis and to gain further insights into the PFAs' mode of action, we next determined the expression profile of key players controlling lipolysis in both liver and AT. Adipose triglyceride lipase and LIPC gene expression was upregulated by AVHGP in the liver and by SCP and BHGP in the AT. This suggests that the regulation of these key players by PFA is tissue-specific, which might be due to differential composition and active substances between the PFA. Adipose triglyceride lipase and LIPC both catalyze triacylglycerol hydrolysis, and their upregulation indicated an enhanced lipid catabolism (Lass et al., 2011). Furthermore, recent studies have shown that fatty acid oxidation was increased by ATGL overexpression and decreased by ATGL knockdown (Ong et al., 2011), which is in line with our aforementioned observations.
With seminal genetic progress for high growth rate over the past 80 yr, breast muscle size has dramatically increased in modern broilers. Indeed, Fleming et al. (2007) reported that the proportion of breast meat by weight at slaughter has increased by 54% since the 1970s Schmidt et al. (Schmidt et al., 2009), on the other hand, showed that the growth rate of breast muscle has increased twice as fast as the overall body growth rate. These successes are associated with high transfer efficiency of energy from feed to the breast muscle, resulting in a higher protein synthesis and lower degradation, and thereby a larger breast weight and yield (Tomas et al., 1988, 1991). One of the most widely recognized major players in controlling protein synthesis and muscle mass is mTOR; it is a serine/threonine kinase, which senses various environmental and intracellular changes including nutrient availability and energy status and coordinates diverse cellular processes including cell growth, differentiation, and survival (Laplante and Sabatini, 2012). In our experimental conditions, the increase in mTOR mRNA abundance as well as its phosphorylated and total protein suggested an enhanced protein synthesis in the PFA-fed birds. This is confirmed by the upregulation of its downstream mediator RPS6KB1 expression as well as by the upregulation of AMPK catalytic and regulatory subunits. While the role of P70 S6K in muscle protein synthesis is well established (Kawasome et al., 1998; Welle et al., 2009; Marabita et al., 2016), the function of AMPK is still not known mainly in avian species. In rodents, observations in dominant-negative AMPK or AMPKα1/α2 double knockout transgenic showed a key role for AMPK catalytic subunits in regulating basal muscle size (Lantier et al., 2010). On the other hand, muscle-specific AMPKβ1/β2 double knockout muscles were reportedly not different in size compared with wild-type muscles (O'Neill et al., 2011). Thus, not all AMPK subunits or AMPK-deficient models support the notion that AMPK controls muscle mass, and such studies are currently lacking in avian species.
Conclusion
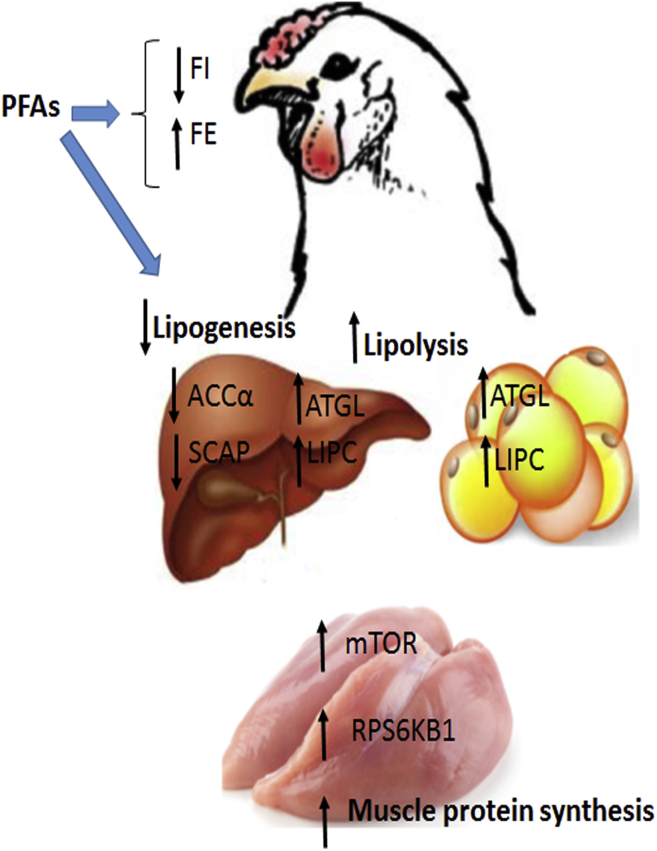
This is the first report to our knowledge using integrated approaches in shedding light on the peripheral mechanisms exhibited by PFA to improve FE in broilers. As summarized in Figure 6, PFA reduced hepatic lipogenesis, enhanced lipolysis, and stimulated muscle protein synthesis, which in turn resulted in similar body weights and a slight increase in muscle yield despite the decreased feed intake compared with the control diet (Flees et al., 2020).
Figure 6.
Schematic representation summarizing the integrated effects of PFA-enriched diets on intermediary metabolism in the broilers. The PFA reduced hepatic lipogenesis (downregulation of ACCα and SCAP), enhanced lipolysis (upregulation of ATGL and LIPC), and stimulated muscle protein synthesis via activation of mTOR pathway, which in turn resulted in similar body weights despite the decreased feed intake compared to the control diet. Abbreviations: ACC, acetyl-CoA carboxylase; ATGL, adipose triglyceride lipase; LIPC, hepatic triacylglycerol lipase; mTOR, mechanistic target of rapamycin; PFA, phytogenic feed additive; RPS6KB1, ribosomal protein S6 kinase beta 1; SCAP, sterol regulatory element–binding protein cleavage–activating protein.
Acknowledgments
The authors would like to thank Drs. Elizabeth Greene, Alissa Welsher, and Phuong Nguyen for their technical assistance. This work was supported by grant from Ayurvet Ltd. (to SD). Ayurvet Ltd. had no role in conducting the research, generating the data, interpreting the results, or writing the manuscript. All animal experiments were approved by the University of Arkansas Animal Care and Use Committee (protocol number 16084) and were in accordance with the recommendations in NIH's G. for the Care and Use of Laboratory Animals. Availability of data and materials: All data generated or analyzed during this study are included in this article.
Disclosures
Author Bhaskar Ganguly is employed by company Ayurvet Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Ayurvet Ltd. had no role in conducting the research, generating the data, interpreting the results, or writing the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.12.060.
Supplementary data
References
- Abu-Elheiga L., Matzuk M.M., Abo-Hashema K.A., Wakil S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Alimohamadi K., Taherpour K., Ghasemi H.A., Fatahnia F. Comparative effects of using black seed (Nigella sativa), cumin seed (Cuminum cyminum), probiotic or prebiotic on growth performance, blood haematology and serum biochemistry of broiler chicks. J. Anim. Physiol. Anim. Nutr. 2014;98:538–546. doi: 10.1111/jpn.12115. [DOI] [PubMed] [Google Scholar]
- Amad A.A., Manner K., Wendler K.R., Neumann K., Zentek J. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult. Sci. 2011;90:2811–2816. doi: 10.3382/ps.2011-01515. [DOI] [PubMed] [Google Scholar]
- Blankenship K., Gilley A., Piekarski A., Orlowski S., Greene E., Bottje W., Anthony N., Dridi S. Differential expression of feeding-related hypothalamic neuropeptides in the first generation of quails divergently selected for low or high feed efficiency. Neuropeptides. 2016;58:31–40. doi: 10.1016/j.npep.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Bongaarts J. Human population growth and the demographic transition. Philosophical Transactions R. Soc. Lond. Ser. B, Biol. Sci. 2009;364:2985–2990. doi: 10.1098/rstb.2009.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsey R.W., Zhande R., Boone A.N. Isoforms of acetyl-CoA carboxylase: structures, regulatory properties and metabolic functions. Biochem. Soc. Trans. 1997;25:1232–1238. doi: 10.1042/bst0251232. [DOI] [PubMed] [Google Scholar]
- Bruce A. Critical role of animal science research in food security and sustainability. Food Sec. 2016;8:299–300. [PubMed] [Google Scholar]
- Castanon J.I. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Cheng L., Ding G., Qin Q., Huang Y., Lewis W., He N., Evans R.M., Schneider M.D., Brako F.A., Xiao Y., Chen Y.E., Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat. Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- Ferver A., Greene E., Dridi S. Hormonal regulation of visfatin gene in avian Leghorn male hepatoma (LMH) cells. Comp. Biochem. Physiol. Part A. Mol. Integr. Physiol. 2020;240:110592. doi: 10.1016/j.cbpa.2019.110592. [DOI] [PubMed] [Google Scholar]
- Flees J., Greene E., Ganguly B., Dridi S. Phytogenic feed- and water-additives improve feed efficiency in broilers via modulation of (an)orexigenic hypothalamic neuropeptide expression. Neuropeptides. 2020;81:102005. doi: 10.1016/j.npep.2020.102005. [DOI] [PubMed] [Google Scholar]
- Flees J., Rajaei-Sharifabadi H., Greene E., Beer L., Hargis B.M., Ellestad L., Porter T., Donoghue A., Bottje W.G., Dridi S. Effect of Morinda citrifolia (Noni)-Enriched diet on hepatic heat Shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 2017;8:919. doi: 10.3389/fphys.2017.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, E. C., Fisher, C., McAdam, J. 2007. Genetic progress in broiler traits—implications for body composition. Page 067 in Proc. Br. Soc. Anim. Sci. Southport, UK.
- Fullerton M.D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z.P., O'Neill H.M., Ford R.J., Palanivel R., O'Brien M., Hardie D.G., Macaulay S.L., Schertzer J.D., Dyck J.R., van Denderen B.J., Kemp B.E., Steinberg G.R. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Ghasemi H.A., Kasani N., Taherpour K. Effects of black cumin seed (nigella sativa L.), a probiotic, a prebiotic and a synbiotic on growth performance, immune response and blood characteristics of male broilers. Livest. Sci. 2014;164:128–134. [Google Scholar]
- Goodridge A.G., Ball E.G. Lipogenesis in the pigeon: in vivo studies. Am. J. Physiol. 1967;213:245–249. doi: 10.1152/ajplegacy.1967.213.1.245. [DOI] [PubMed] [Google Scholar]
- Greene E., Flees J., Dhamad A., Alrubaye A., Hennigan S., Pleimann J., Smeltzer M., Murray S., Kugel J., Goodrich J., Robertson A., Wideman R., Rhoads D., Dridi S. Double-stranded RNA is a novel molecular target in Osteomyelitis Pathogenesis: a Translational avian model for human bacterial Chondronecrosis with Osteomyelitis. Am. J. Pathol. 2019;189:2077–2089. doi: 10.1016/j.ajpath.2019.06.013. [DOI] [PubMed] [Google Scholar]
- Ha J., Daniel S., Broyles S.S., Kim K.H. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J. Biol. Chem. 1994;269:22162–22168. [PubMed] [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- Jensen K.K., Tadin-Strapps M., Wang S.P., Hubert J., Kan Y., Ma Y., McLaren D.G., Previs S.F., Herath K.B., Mahsut A., Liaw A., Wang S., Stout S.J., Keohan C., Forrest G., Coelho D., Yendluri S., Williams S., Koser M., Bartz S., Akinsanya K.O., Pinto S. Dose-dependent effects of siRNA-mediated inhibition of SCAP on PCSK9, LDLR, and plasma lipids in mouse and rhesus monkey. J. Lipid Res. 2016;57:2150–2162. doi: 10.1194/jlr.M071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasome H., Papst P., Webb S., Keller G.M., Johnson G.L., Gelfand E.W., Terada N. Targeted disruption of p70(s6k) defines its role in protein synthesis and rapamycin sensitivity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5033–5038. doi: 10.1073/pnas.95.9.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick A., Ratledge C. Desaturation of polyunsaturated fatty acids in Mucor circinelloides and the involvement of a novel membrane-bound malic enzyme. Eur. J. Biochem. 1992;209:667–673. doi: 10.1111/j.1432-1033.1992.tb17334.x. [DOI] [PubMed] [Google Scholar]
- Kim D.K., Lillehoj H.S., Lee S.H., Jang S.I., Bravo D. High-throughput gene expression analysis of intestinal intraepithelial lymphocytes after oral feeding of carvacrol, cinnamaldehyde, or Capsicum oleoresin. Poult. Sci. 2010;89:68–81. doi: 10.3382/ps.2009-00275. [DOI] [PubMed] [Google Scholar]
- Kim D.K., Lillehoj H.S., Lee S.H., Lillehoj E.P., Bravo D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 2013;109:76–88. doi: 10.1017/S0007114512000530. [DOI] [PubMed] [Google Scholar]
- Lantier L., Mounier R., Leclerc J., Pende M., Foretz M., Viollet B. Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. FASEB J. 2010;24:3555–3561. doi: 10.1096/fj.10-155994. [DOI] [PubMed] [Google Scholar]
- Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A., Zimmermann R., Oberer M., Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille G.A., O'Hea E.K., Chakbabarty K. In vivo lipogenesis in the domestic chicken. Proc. Soc. Exp. Biol. Med. 1968;128:398–401. doi: 10.3181/00379727-128-33022. [DOI] [PubMed] [Google Scholar]
- Li H.L., Zhao P.Y., Lei Y., Hossain M.M., Kim I.H. Phytoncide, phytogenic feed additive as an alternative to conventional antibiotics, improved growth performance and decreased excreta gas emission without adverse effect on meat quality in broiler chickens. Livest. Sci. 2015;181:1–6. [Google Scholar]
- Maddineni S., Metzger S., Ocon O., Hendricks G., 3rd, Ramachandran R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005;146:4250–4256. doi: 10.1210/en.2005-0254. [DOI] [PubMed] [Google Scholar]
- Marabita M., Baraldo M., Solagna F., Ceelen J.J.M., Sartori R., Nolte H., Nemazanyy I., Pyronnet S., Kruger M., Pende M., Blaauw B. S6K1 is required for increasing skeletal muscle Force during hypertrophy. Cell Rep. 2016;17:501–513. doi: 10.1016/j.celrep.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi Y., Letourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Cote C., Ramirez A.A., Godbout S. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen M.M., Neale C.M.U., Ray C., Erickson G.E., Hoekstra A.Y. Water productivity in meat and milk production in the US from 1960 to 2016. Environ. Int. 2019;132:105084. doi: 10.1016/j.envint.2019.105084. [DOI] [PubMed] [Google Scholar]
- Mohammadpour F., Darmani-Kuhi H., Mohit A., Sohani M.M. Obesity, insulin resistance, adiponectin, and PPAR-gamma gene expression in broiler chicks fed diets supplemented with fat and green tea (Camellia sinensis) extract. Domest. Anim. Endocrinol. 2020;72:106440. doi: 10.1016/j.domaniend.2020.106440. [DOI] [PubMed] [Google Scholar]
- Mulder R.W. Safe poultry meat production in the next century. Acta Vet. Hung. 1997;45:307–315. [PubMed] [Google Scholar]
- Nadkarni A.K. 3rd ed. Vol. I. Popular Prakashan; Mumbai, India: 2005. Pages 21–22 in Dr. K.M. Nadkarni's Indian Materia Medica. [Google Scholar]
- Nguyen P., Greene E., Ishola P., Huff G., Donoghue A., Bottje W., Dridi S. Chronic Mild Cold conditioning modulates the expression of hypothalamic neuropeptide and intermediary metabolic-related genes and improves growth performances in Young chicks. PloS One. 2015;10:e0142319. doi: 10.1371/journal.pone.0142319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill H.M., Maarbjerg S.J., Crane J.D., Jeppesen J., Jorgensen S.B., Schertzer J.D., Shyroka O., Kiens B., van Denderen B.J., Tarnopolsky M.A., Kemp B.E., Richter E.A., Steinberg G.R. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc. Nat. Acad. Sci. USA. 2011;108:16092–16097. doi: 10.1073/pnas.1105062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K.T., Mashek M.T., Bu S.Y., Greenberg A.S., Mashek D.G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski S., Flees J., Greene E.S., Ashley D., Lee S.O., Yang F.L., Owens C.M., Kidd M., Anthony N., Dridi S. Effects of phytogenic additives on meat quality traits in broiler chickens1. J. Anim. Sci. 2018;96:3757–3767. doi: 10.1093/jas/sky238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O.E., Kalhan S.C., Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- Piekarski A., Nagarajan G., Ishola P., Flees J., Greene E.S., Kuenzel W.J., Ohkubo T., Maier H., Bottje W.G., Cline M.A., Dridi S. AMP-activated protein kinase mediates the effect of Leptin on avian Autophagy in a tissue-specific Manner. Front. Physiol. 2018;9:541. doi: 10.3389/fphys.2018.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaei-Sharifabadi H., Greene E., Piekarski A., Falcon D., Ellestad L., Donoghue A., Bottje W., Porter T., Liang Y., Dridi S. Surface wetting strategy prevents acute heat exposure-induced alterations of hypothalamic stress- and metabolic-related genes in broiler chickens. J. Anim. Sci. 2017;95:1132–1143. doi: 10.2527/jas.2016.1290. [DOI] [PubMed] [Google Scholar]
- Reynnells R.D. Effective poultry programming in the next century. Introduction. Poult. Sci. 1999;78:646–648. doi: 10.1093/ps/78.5.646. [DOI] [PubMed] [Google Scholar]
- Schmidt C.J., Persia M.E., Feierstein E., Kingham B., Saylor W.W. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult. Sci. 2009;88:2610–2619. doi: 10.3382/ps.2009-00055. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Settle T., Leonard S.S., Falkenstein E., Fix N., Van Dyke K., Klandorf H. Effects of a phytogenic feed additive versus an antibiotic feed additive on oxidative stress in broiler chicks and a Possible mechanism determined by Electron Spin Resonance. Int. J. Poult. Sci. 2014;13:62–69. doi: 10.3923/ijps.2014.62.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo R., Saez-Lopez C., Lecube A., Hernandez C., Fort J.M., Selva D.M. Adiponectin upregulates SHBG production: molecular mechanisms and potential implications. Endocrinology. 2014;155:2820–2830. doi: 10.1210/en.2014-1072. [DOI] [PubMed] [Google Scholar]
- Stevanovic Z.D., Bosnjak-Neumuller J., Pajic-Lijakovic I., Raj J., Vasiljevic M. Essential oils as feed additives-future Perspectives. Molecules. 2018;23:1717–1736. doi: 10.3390/molecules23071717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Yamamoto J., Iwasaki S., Asaba H., Hamura H., Ikeda Y., Watanabe M., Magoori K., Ioka R.X., Tachibana K., Watanabe Y., Uchiyama Y., Sumi K., Iguchi H., Ito S., Doi T., Hamakubo T., Naito M., Auwerx J., Yanagisawa M., Kodama T., Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Nat. Acad. Sci. USA. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K.L., Caffrey N.P., Nobrega D.B., Cork S.C., Ronksley P.E., Barkema H.W., Polachek A.J., Ganshorn H., Sharma N., Kellner J.D., Ghali W.A. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. The Lancet. 2017;1:e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toghyani M., Toghyani M., Gheisari A., Ghalamkari G., Eghbalsaied S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest. Sci. 2011;138:167–173. [Google Scholar]
- Tomas F.M., Jones L.M., Pym R.A. Rates of muscle protein breakdown in chickens selected for increased growth rate, food consumption or efficiency of food utilisation as assessed by N tau-methylhistidine excretion. Br. Poult. Sci. 1988;29:359–370. doi: 10.1080/00071668808417061. [DOI] [PubMed] [Google Scholar]
- Tomas F.M., Pym R.A., Johnson R.J. Muscle protein turnover in chickens selected for increased growth rate, food consumption or efficiency of food utilisation: effects of genotype and relationship to plasma IGF-I and growth hormone. Br. Poult. Sci. 1991;32:363–376. doi: 10.1080/00071669108417361. [DOI] [PubMed] [Google Scholar]
- Tong L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell Mol. Life Sci. 2005;62:1784–1803. doi: 10.1007/s00018-005-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserng K.Y., Jin S.J. NADPH-dependent reductive metabolism of cis-5 unsaturated fatty acids. A revised pathway for the beta-oxidation of oleic acid. J. Biol. Chem. 1991;266:11614–11620. [PubMed] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Nat. Acad. Sci. USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T., Czimmerer Z., Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.X., Lee C.H., Tiep S., Yu R.T., Ham J., Kang H., Evans R.M. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Welle S., Burgess K., Mehta S. Stimulation of skeletal muscle myofibrillar protein synthesis, p70 S6 kinase phosphorylation, and ribosomal protein S6 phosphorylation by inhibition of myostatin in mature mice. Am. J. Physiol. Endocrinol. Metab. 2009;296:E567–E572. doi: 10.1152/ajpendo.90862.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Wise E.M., Jr., Ball E.G. Malic enzyme and lipogenesis. Proc. Nat. Acad. Sci. USA. 1964;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Yang H., Gan L., Sun C. Adiponectin-impaired adipocyte differentiation negatively regulates fat deposition in chicken. J. Anim. Physiol. Anim. Nutr. 2014;98:530–537. doi: 10.1111/jpn.12107. [DOI] [PubMed] [Google Scholar]
- Yeh Y.Y., Leveille G.A. In vitro and in vivo restoration of hepatic lipogenesis in fasted chicks. J. Nutr. 1971;101:803–809. doi: 10.1093/jn/101.6.803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.