Abstract
This study evaluated the effects of high phytase doses and soybean meal (SBM) with different CP content on growth performance, ileal nutrient digestibility, digestible energy, plasmatic myo-inositol, phosphate release in vitro, and bone composition of broiler chickens. One thousand two hundred 1-day-old broilers were distributed in a 2 × 2 completely randomized factorial arrangement, with 2 phytase doses (1,000 and 2,500 phytase units [FYT]/kg of feed) and 2 SBM with different CP concentrations (45 and 47%), totaling 4 treatments with 12 replicates of 25 birds each. The chickens received feed and water ad libitum. Diets were based on corn and SBM, with different inclusions of soybean hull used to dilute the CP content of SBM according to each treatment. The inclusion of 2,500 FYT increased weight gain from 0 to 21 d (P < 0.05), whereas growth performance from 22 to 42 d was not affected, and SBM had no effect on growth performance. At day 21, ileal digestibility of dry matter, ash, and P, and digestible energy were greater in diets with 2,500 FYT/kg (P < 0.05), as well as phosphate in vitro release (P < 0.01) compared to the lower dose. At day 42, diets with SBM 47% CP and 2,500 FYT/kg promoted greater digestibility of dry matter, ash, CP, Ca, P, and digestible energy (P < 0.001), and greater phosphate release (P < 0.05) in comparison to other treatments. myo-inositol level in the plasma at 21 and 42 d was higher with the use of 2,500 FYT compared to 1,000 FYT (P < 0.05). The higher phytase dose increased tibia ash, toe ash, and Seedor Index (P < 0.05) at day 21, and the Ca content in tibia was higher with 2,500 FYT and SBM 47% CP at day 42. In conclusion, higher phytase doses for broilers improve weight gain, myo-inositol provision, and bone mineral composition. Nutrient ileal digestibility can be enhanced by higher phytase doses when in combination with SBM of greater nutritional quality.
Key words: P, phytase, poultry, protein, soybean meal
Introduction
In poultry diets based on vegetable ingredients, up to 70% of P is present as phytate-P, bound to the phytic acid molecule, and unavailable for digestion and absorption (Angel et al., 2001). Regular levels of phytate in corn- and soybean meal (SBM)-based diets range from 2.5 to 4.0 g/kg, and can cause a negative impact on growth performance and feed efficiency (Ravindran, 1995), as phytate can form complexes with protein, amino acids, and also other minerals (Santos, 2012).
Soybean meal is the main source of protein used in poultry diets because of its relatively high CP content, good amino acids profile, and bioavailability. The CP content of SBM is still the major reason for its inclusion in nutritional matrices (Ibáñez et al., 2020), but other components are also relevant to determine the quality of SBM. According to the NRC (1994), the phytate-P/total P proportion on SBM is around 58.5%. Other antinutritional components are also common in soybean, like trypsin inhibitors and oligosaccharides - raffinose, stachyose, polysaccharides - which can impair the availability of dietary P, Ca, and other minerals, and also reduce amino acids' digestibility and energy utilization (Hurrell, 2003; Kocher et al., 2003; Choct et al., 2010; Ibánez et al., 2020).
One of the steps of SBM processing includes the removal and reincorporation of soybean hull to the meal, in order to dilute the CP content and lower costs, but this addition of hull increases the oligosaccharides and fiber content in SBM. As pointed out by Ibáñez et al. (2020), there is a negative correlation between CP and fiber content of SBM, which depends, among other factors, on the amount of hulls added to the meal. In addition, 10% of the phytate content in grains is present in the hull (Abdelrahman et al., 1984); so non-dehulled SBM may also contain more phytate. Some of the antinutritional components present in SBM are thermolabile, susceptible to the high temperatures employed during SBM processing, but phytate is thermal resistant (Park et al., 2001) and needs to be eliminated via other methods, that is utilization of exogenous phytase.
Phytases are commonly used to hydrolyze phytate into free myo-inositol and 6 molecules of inorganic phosphate. Phytate degradation and elimination from the gastrointestinal tract with the use of phytase is correlated to significant improvements in P and Ca digestibility (Cowieson et al., 2006; Kiarie et al., 2015), ash content in tibia bone (Sousa et al., 2015; Walk and Rama Rao, 2020), weight gain (WG), and feed efficiency (Broch et al., 2018), and the extent to which phytate is eliminated from the tract can be intensified with greater levels of phytase. Supplementation of broiler chicken diets with phytase levels greater than those recommended by the industry sector (≥1,500 phytase units [FYT]/kg) has been proved to enhance even further the solubility and digestibility of nutrients beyond P—protein, amino acids, and energy—leading to extra-phosphoric effects (Cowieson et al., 2017; Dersjant-Li and Kwakernaak, 2019; Walk and Rama Rao, 2020).
Considering that the CP concentration in SBM is related to different inclusions of soybean hull, which may lead to different phytate concentrations, the objective of this study was to evaluate the supplementation of broiler chicken diets with high levels of phytase and the use of SBM with different protein contents on growth performance, nutrient ileal digestibility, plasmatic myo-inositol, phosphate release in vitro, and bone mineral composition.
Materials and methods
All experimental procedures were approved by the Animal Use Ethics Committee of Federal University of Paraná.
Birds, Facilities, and Experimental Diets
One thousand two hundred 1-day-old Cobb 500 broiler chicks from a commercial hatchery were housed in 2.06 m2 experimental pens (12.1 broilers/m2) with wood shavings as litter, equipped with nipple drinkers and tube feeders.
The initial temperature was set to 32°C and gradually reduced to 18°C until day 42. During the first 10 days, incandescent light was continuously provided (24 h); thereafter, a lighting program of 9 h of darkness per day was applied. Pens were checked daily for the removal of dead birds. Feed and water were offered ad libitum throughout the experimental period.
The dietary treatments were a 2 × 2 complete factorial arrangement, which consisted of 2 SBM with different protein concentrations (45 and 47% of CP) and 2 phytase doses (1,000 and 2,500 FYT/kg of feed), totaling 4 dietary treatments with 12 replicates of 25 birds each. Diets were based on corn and SBM, offered in mashed form, and divided into 2 broiler development stages: starter stage (1–21 d; Table 1) and grower-finisher stage (22–42 d; Table 2).
Table 1.
Composition and calculated analysis of the starter (0–21 d) diets.
| SBM 45% + 1,000 FYT | SBM 47% + 1,000 FYT | SBM 45% + 2,500 FYT | SBM 47% + 2,500 FYT | |
|---|---|---|---|---|
| Ingredients (%) | ||||
| Corn | 55.963 | 60.615 | 55.963 | 60.615 |
| SBM | 30.040 | 30.050 | 30.040 | 30.050 |
| Soybean hull | 5.810 | 2.980 | 5.810 | 2.980 |
| Soybean oil | 3.650 | 1.800 | 3.650 | 1.800 |
| Limestone | 1.070 | 1.120 | 1.070 | 1.120 |
| Dicalcium phosphate | 0.970 | 0.970 | 0.970 | 0.970 |
| Sodium chloride | 0.470 | 0.470 | 0.470 | 0.470 |
| L-Lysine HCl | 0.283 | 0.292 | 0.283 | 0.292 |
| L-Threonine | 0.085 | 0.074 | 0.085 | 0.074 |
| L-Valine | 0.071 | 0.063 | 0.071 | 0.063 |
| DL-Methionine | 0.326 | 0.311 | 0.326 | 0.311 |
| Choline chloride | 0.070 | 0.070 | 0.070 | 0.070 |
| Vitamin premix1 | 0.130 | 0.130 | 0.130 | 0.130 |
| Mineral premix2 | 0.050 | 0.050 | 0.050 | 0.050 |
| Phytase3 | 0.005 | 0.005 | 0.013 | 0.013 |
| Celite4 (marker) | 1.000 | 1.000 | 1.000 | 1.000 |
| Calculated chemical composition | ||||
| Metabolizable energy (kcal) | 3,070 | 3,070 | 3,070 | 3,070 |
| Sodium (%) | 0.20 | 0.20 | 0.20 | 0.20 |
| Chlorine (%) | 0.35 | 0.35 | 0.35 | 0.35 |
| Analyzed chemical composition | ||||
| CP (%) | 21.09 | 21.13 | 21.03 | 21.60 |
| Ca (%) | 1.00 | 0.99 | 1.10 | 1.06 |
| Total P (%) | 0.60 | 0.62 | 0.59 | 0.61 |
| Ash (%) | 6.70 | 6.90 | 6.79 | 6.83 |
| Crude fiber (%) | 4.18 | 3.29 | 4.31 | 3.22 |
| Acid detergent fiber (%) | 6.12 | 4.87 | 6.09 | 4.78 |
| Neutral detergent fiber (%) | 12.65 | 11.21 | 12.55 | 11.07 |
| Analyzed composition via near-infrared reflectance spectroscopy | ||||
| Phytate-P (%) | 0.22 | 0.25 | 0.19 | 0.24 |
| Total sugars (%) | 3.68 | 3.55 | 3.64 | 3.57 |
| Digestible lysine (%) | 1.20 | 1.18 | 1.21 | 1.22 |
| Digestible methionine (%) | 0.62 | 0.58 | 0.61 | 0.64 |
| Methionine + cysteine (%) | 0.90 | 0.86 | 0.85 | 0.91 |
| Digestible threonine (%) | 0.73 | 0.74 | 0.72 | 0.79 |
| Digestible tryptophan (%) | 0.20 | 0.21 | 0.19 | 0.24 |
| Digestible valine (%) | 0.86 | 0.90 | 0.87 | 0.91 |
Abbreviations: FYT, phytase units; SBM, soybean meal.
Supplied per kilogram of product: copper, 20 g; iron, 100 g; iodine, 2 g; manganese, 130 g; zinc, 130 g.
Supplied per kilogram of product: vitamin A, 11,000,000 UI; vitamin D3, 4,000,000 UI; vitamin E, 55,000 UI; vitamin K3, 3 g; vitamin B1, 2.3 g; vitamin B2, 7 g; pantothenic acid, 12 g; vitamin B6, 4 g; vitamin B12, 25 mg; nicotinic acid, 60 g; folic acid, 2 g; biotin, 250 mg; selenium, 300 mg.
RONOZYME HiPhos GT with 20,000 FYT/g (DSM Nutritional Products, Kaiseraugst, Switzerland).
Celite insoluble marker (Celite 400, Celite Corp., Lompoc, CA).
Table 2.
Composition and calculated analysis of the grower/finisher (22–42 d) diets.
| SBM 45% + 1,000 FYT | SBM 47% + 1,000 FYT | SBM 45% + 2,500 FYT | SBM 47% + 2,500 FYT | |
|---|---|---|---|---|
| Ingredients (%) | ||||
| Corn | 63.971 | 66.548 | 63.963 | 66.540 |
| SBM | 24.200 | 24.200 | 24.200 | 24.200 |
| Soybean hull | 4.100 | 2.320 | 4.100 | 2.320 |
| Soybean oil | 3.700 | 2.900 | 3.700 | 2.900 |
| Limestone | 0.770 | 0.790 | 0.770 | 0.790 |
| Dicalcium phosphate | 0.970 | 0.970 | 0.970 | 0.970 |
| Sodium chloride | 0.470 | 0.470 | 0.470 | 0.470 |
| L-Lysine HCl | 0.220 | 0.220 | 0.220 | 0.220 |
| L-Threonine | 0.054 | 0.048 | 0.054 | 0.048 |
| L-Valine | 0.043 | 0.038 | 0.043 | 0.038 |
| DL-Methionine | 0.247 | 0.241 | 0.247 | 0.241 |
| Choline chloride | 0.050 | 0.050 | 0.050 | 0.050 |
| Vitamin premix1 | 0.100 | 0.100 | 0.100 | 0.100 |
| Mineral premix2 | 0.100 | 0.100 | 0.100 | 0.100 |
| Phytase3 | 0.005 | 0.005 | 0.013 | 0.013 |
| Celite4 (marker) | 1.000 | 1.000 | 1.000 | 1.000 |
| Calculated chemical composition | ||||
| Metabolizable energy (kcal) | 3,220 | 3,220 | 3,220 | 3,220 |
| Sodium (%) | 0.18 | 0.18 | 0.18 | 0.18 |
| Chlorine (%) | 0.32 | 0.32 | 0.32 | 0.32 |
| Analyzed chemical composition | ||||
| CP (%) | 18.38 | 18.82 | 18.57 | 18.41 |
| Ca (%) | 0.80 | 0.73 | 0.80 | 0.79 |
| Total P (%) | 0.50 | 0.51 | 0.54 | 0.53 |
| Ash (%) | 6.09 | 6.04 | 6.03 | 5.98 |
| Crude fiber (%) | 3.61 | 2.91 | 3.55 | 2.98 |
| Acid detergent fiber (%) | 5.20 | 4.38 | 5.16 | 4.41 |
| Neutral detergent fiber (%) | 11.83 | 10.98 | 11.78 | 11.02 |
| Analyzed composition via near-infrared reflectance spectroscopy | ||||
| Phytate-P (%) | 0.23 | 0.21 | 0.24 | 0.19 |
| Total sugars (%) | 3.18 | 2.97 | 3.25 | 3.05 |
| Digestible lysine (%) | 0.95 | 1.01 | 0.97 | 0.94 |
| Digestible methionine (%) | 0.48 | 0.53 | 0.52 | 0.50 |
| Methionine + cysteine (%) | 0.80 | 0.72 | 0.79 | 0.78 |
| Digestible threonine (%) | 0.60 | 0.67 | 0.68 | 0.63 |
| Digestible tryptophan (%) | 0.19 | 0.20 | 0.17 | 0.17 |
| Digestible valine (%) | 0.74 | 0.78 | 0.75 | 0.72 |
Abbreviations: FYT, phytase units; SBM, soybean meal.
Supplied per kilogram of product: copper, 20 g; iron, 100 g; iodine, 2 g; manganese, 130 g; zinc, 130 g.
Supplied per kilogram of product: vitamin A, 11,000,000 UI; vitamin D3, 4,000,000 UI; vitamin E, 55,000 UI; vitamin K3, 3 g; vitamin B1, 2.3 g; vitamin B2, 7 g; pantothenic acid, 12 g; vitamin B6, 4 g; vitamin B12, 25 mg; nicotinic acid, 60 g; folic acid, 2 g; biotin, 250 mg; selenium, 300 mg.
RONOZYME HiPhos GT with 20,000 FYT/g (DSM Nutritional Products, Kaiseraugst, Switzerland).
Celite insoluble marker (Celite 400, Celite Corp., Lompoc, CA).
Different levels of soybean hull, coming from the same batch as the SBM, were included in the formula in order to dilute the CP concentration of the SBM according to each treatment. Geometric mean diameter of the diets with SBM 45 and 47% CP was 753 and 709 μm, respectively, and the geometric SD was 2.16 and 2.18%, respectively. The CP (method 954.01), ash (method 942.05), Ca (method 927.02), P (method 965.17), and crude fiber (method 962.10) values of diets (Tables 1 and 2) and main ingredients (Table 3) were analyzed according to the Association of the Official Analytical Chemists (AOAC, 1995). Soluble neutral detergent fiber and soluble acid detergent fiber were analyzed by using the Van Soest methodology (Van Soest et al., 1991). Phytate-P of the diets was predicted by near-infrared reflectance spectroscopy (Evonik Nutrition & Care, São Paulo, Brazil), similar to Beeson et al. (2017), and total sugars and digestible amino acids were also predicted by near-infrared reflectance spectroscopy.
Table 3.
Nutritional composition of corn, soybean meal, and soybean hull.
| Nutrient (%) | Corn | Soybean meal | Soybean hull |
|---|---|---|---|
| Analyzed chemical composition | |||
| CP | 8.30 | 50.10 | 12.40 |
| Crude fiber | 2.01 | 3.11 | 37.62 |
| Neutral detergent fiber | 10.90 | 9.02 | 61.55 |
| Acid detergent fiber | 2.92 | 5.40 | 46.93 |
| Ash | 1.11 | 5.65 | 4.49 |
| Ca | 0.03 | 0.38 | 0.36 |
| Total P | 0.23 | 0.64 | 0.13 |
| Analyzed composition via near-infrared reflectance spectroscopy | |||
| Phytate-P | 0.15 | 0.38 | 0.07 |
| Total sugars | 1.10 | 9.70 | 1.11 |
| Digestible lysine | 0.18 | 2.78 | 0.43 |
| Digestible methionine | 0.15 | 0.63 | 0.09 |
| Methionine + cysteine | 0.29 | 1.28 | 0.14 |
| Digestible threonine | 0.29 | 1.71 | 0.18 |
| Digestible tryptophan | 0.06 | 0.59 | 0.06 |
| Digestible valine | 0.31 | 2.09 | 0.29 |
The phytase product used was Ronozyme HiPhos (Ronozyme HiPhos GT, DSM Nutritional Products, Kaiseraugst, Switzerland), which is a 6-phytase originating from Citrobacter braakii and expressed in Aspergillus oryzae, with a minimum activity of 20,000 FYT/g of the product. One FYT is defined as the quantity of enzyme that liberates 1 μmol of inorganic phosphate per minute from 5.0 μmol/L sodium phytate at pH 5.5 and 37°C (Engelen et al., 1994). The activity of phytase in the supplemented diets was measured at Biopract GmbH, Berlin, Germany. Enzyme recovery was calculated as the percentage of the measured enzyme activity in the diet to the expected enzyme activity estimated from the amount and minimum activity of enzymes added to the diets.
Growth Performance
All the birds and feed leftovers were weighted at 1, 21, and 42 d of age to calculate feed intake, WG, and feed conversion ratio (FCR) from 1 to 21 and 22 to 42 d, corrected to the weight of dead birds.
Digestibility Assay
At 21 and 42 d, 240 broilers on each day (60 per treatment) were randomly selected and euthanized by cervical dislocation, according to the method described by Ludtke et al. (2010), and the ileal content was collected for digestibility analyses. The birds were eviscerated, and an ileal fraction was separated for content removal, defined as 4 cm below Meckel's diverticulum and 4 cm above the ileum-cecum-colon junction. The ileal content of all 5 birds from each replicate was pooled, placed in identified plastic containers, and frozen at −18°C.
Samples were subsequently thawed to room temperature and dried in a forced ventilation oven at 55°C until constant weight. Feed and ileal samples were then ground to a particle size of 0.5 mm. The DM content was obtained by oven drying the samples at 105°C for 16 h, and CP (method 954.01), ash (method 942.05), Ca (method 927.02), and P (method 965.17) contents were analyzed according to the methodology described by AOAC (1995). Gross energy of the samples was determined in a calorimetric bomb (Ika Werke C2000 Control Oxygen Bomb Calorimeter, Ika-Werke GmbH & Co., Staufen, Germany). Acid-insoluble ash (AIA) was used as an insoluble marker compound in the digestibility calculations, and AIA content in the samples was determined according to methodology used by Scott and Boldaji (1997).
Based on the results, the coefficient of apparent ileal digestibility (CAID) was calculated according to the following formula:
where IF (indigestibility factor) is the ratio between the AIA content in the diet and the AIA in the excreta or ileal digesta. Ileal digestible energy (IDE) was calculated according to the following formula:
Plasmatic myo-inositol
At 21 and 42 d, 2 birds per replicate were randomly chosen to collect 3 to 5 mL of blood, held in tubes with heparin (Vacutainer Plus, BD, Franklin Lakes, NJ). Immediately after blood collection, the tubes were centrifuged at 3,000 rpm for 10 min to separate the plasma from blood. After centrifugation, the obtained plasma was held in graduated microtubes and frozen at −20°C before being used for analysis. Concentration of myo-inositol (MYO) in the plasma was determined by mass spectrometry using an UPLC system (ACQUITY UPLC System, Waters, Milford, MA) according to the method described by Leung et al. (2011).
Phosphate (PO4−3) Release In Vitro
Twelve feed samples of each treatment from each phase (starter and grower/finisher, totaling 24 samples of each treatment) were separated for in vitro tests, which were conducted according to the methodology described by Zyla et al. (1999). For the in vitro incubation model, 2.25 g of each sample were weighted and incubated at 40°C with 12.75 g of acid and alkaline solutions (15% DM) for 15 min at pH 3.0 ± 0.25, followed by 4 h incubation at pH 6.5 ± 0.25 with stirring (25 rpm, rotisserie agitation), to simulate the pH and temperature of crop and intestinal digestion phases, respectively. Hydrochloric acid solution (0.065–0.085 M) was used to reach pH 3.0 ± 0.25, after which a 0.75 M sodium bicarbonate solution was added to adjust the reaction to pH 6.5 ± 0.25. The reaction was carried out without digestive enzymes (pepsin or pancreatin) in order to assess only the effect of exogenous phytase in the diets.
Once the incubation time was finalized, supernatants were collected, filtered, and diluted. High performance anion-exchange chromatography with conductivity detection (HPAEC-CD) was used to quantify the amount of soluble phosphate (PO4−3) using standard solution series with fluoride, chloride, nitrite, sulfate, bromide, nitrate, and phosphate in 6 concentration levels (0.0025–0.075 g/L). PO4−3 was quantified on an Dionex ICS-5000+ Capillary HPIC System (Thermo Fisher Scientific, Waltham, MA) equipped with an IonPac AS11-HC column (250 × 4 mm) and an IonPac AS11-HC guard column (4 × 50 mm). Potassium hydroxide (30 mM) mobile phase was used with a 1.5 mL/min flow.
Bone Mineral Composition
At 21 and 42 d of age, 2 broilers per replicate, randomly selected from the 5 broilers euthanized for ileal content collection on the same sampling day, had their left legs manually removed. Tibial bones were severed from the leg without boiling and cleaned of litter and excrement. Bones were then cleaned with ether to remove any remnants of fat and muscle, and oven-dried at 105°C for 12 h. Bone length and weight measurements were then noted by using a digital caliper and a digital scale to the nearest 0.0001 g, and used in the calculation of the Seedor Index (SI), by dividing the bone weight by its length, as an indicative of bone mineral density (Seedor, 1993). Dried bones were ashed in a muffle furnace at 600°C and ash (method 942.05), Ca (method 927.02), and P (method 965.17) contents were analyzed according to AOAC (1995). Toes were also removed from the same 21-d-old broiler chickens euthanized for tibia collection. The middle toe was clipped and cleaned; skin, flesh, and toenail of the middle toe were kept intact; toes were ashed to determine ash content (method 942.05).
Experimental Design and Statistical Analysis
All collected data were tested for residue normality by Shapiro-Wilk test, and after a normal distribution was detected, data were used for a two-way ANOVA using the linear model of ExpDes package (Experimental Designs Package, E. B. Ferreira et al., Belo Horizonte, Minas Gerais, Brazil) Ferreira et al., 2013, including 2 main factors and their interaction, on R program (R Foundation for Statistical Computing, Vienna, Austria). When significant interactions were observed, their deployment was submitted to Tukey test at 5% probability for mean comparison.
Results and discussion
The analysis of phytase recovery in the experimental diets showed a low variation between expected and analyzed values (Table 4), which certifies that the enzyme activity was in line with each dietary treatment proposal.
Table 4.
Expected and analyzed1 phytase activity recovered in feed samples.
| Treatment | Phytase2 (FYT/kg) |
|||
|---|---|---|---|---|
| Starter diet |
Grower-finisher diet |
|||
| Expected | Analyzed | Expected | Analyzed | |
| SBM 45% + 1,000 FYT | 1,000 | 938 | 1,000 | 987 |
| SBM 47% + 1,000 FYT | 1,000 | 1,040 | 1,000 | 1,023 |
| SBM 45% + 2,500 FYT | 2,500 | 2,426 | 2,500 | 2,332 |
| SBM 47% + 2,500 FYT | 2,500 | 2,359 | 2,500 | 2,864 |
Abbreviations: FYT, phytase units; SBM, soybean meal.
Recovery analysis performed by Biopract GmbH, Berlin, Germany.
Enzyme activity is expressed as the quantity of product added in the feed.
Growth Performance
No interaction between factors was observed (P > 0.05) for growth performance variables from 0 to 21 d and 22 to 42 d (Table 5). When evaluating the main effects, the inclusion of 2,500 FYT/kg of feed increased WG from 0 to 21 d (P < 0.05) by 3.11% compared to broiler chickens fed diets containing 1,000 FYT/kg. The effects of phytase on growth performance are well known, and there seems to be an even further response with higher doses. In a recent study, Broch et al. (2018) evaluated increasing levels of phytase up to 3,000 FYT for 21-day-old broiler diets and reported a linear response for all growth performance measurements. Kiarie et al. (2015) also observed that phytase inclusion linearly increased growth performance of broilers fed a low Ca and P diet supplemented with 2,000 FTU, as WG improved by 20% and FCR by 7.4% in comparison to birds fed the same diet without phytase. According to Walk et al. (2014), the benefits of phytase on WG and FCR of poultry are associated with greater phytate destruction and provision of inositol rather than excess intake of Ca and P. High phytase doses tested by the authors resulted in almost complete hydrolysis of IP6, increasing inositol concentration in the gizzard and improving growth performance.
Table 5.
Effect of protein concentration of SBM and phytase doses on FI, WG, and FCR of broiler chickens.
| SBM (% CP) | Phytase (FYT/kg) | 0–21 d |
22–42 d |
||||
|---|---|---|---|---|---|---|---|
| FI (g) | WG (g) | FCR (g/g) | FI (g) | WG (g) | FCR (g/g) | ||
| SBM × phytase | |||||||
| 45 | 1,000 | 855.33 | 782.45 | 1.097 | 3,640.51 | 2,226.10 | 1.635 |
| 47 | 866.00 | 784.27 | 1.093 | 3,699.28 | 2,274.33 | 1.626 | |
| 45 | 2,500 | 881.24 | 808.40 | 1.083 | 3,638.52 | 2,215.35 | 1.642 |
| 47 | 881.68 | 808.47 | 1.085 | 3,633.67 | 2,245.28 | 1.618 | |
| SEM | 11.135 | 6.744 | 0.005 | 37.085 | 22.060 | 0.004 | |
| Effect of SBM (% CP) | |||||||
| 45 | 868.28 | 795.42 | 1.090 | 3,639.49 | 2,220.65 | 1.640 | |
| 47 | 873.84 | 796.37 | 1.089 | 3,666.39 | 2,259.74 | 1.622 | |
| Effect of phytase (FYT/kg) | |||||||
| 1,000 | 866.28 | 783.36 | 1.095 | 3,669.84 | 2,250.01 | 1.631 | |
| 2,500 | 881.46 | 808.43 | 1.084 | 3,636.05 | 2,230.22 | 1.630 | |
| P-values | |||||||
| SBM | 0.620 | 0.888 | 0.880 | 0.472 | 0.081 | 0.062 | |
| Phytase | 0.078 | 0.006 | 0.135 | 0.367 | 0.370 | 0.971 | |
| Interaction | 0.648 | 0.896 | 0.678 | 0.395 | 0.680 | 0.386 | |
Abbreviations: FCR, feed conversion ratio; FI, feed intake; FYT, phytase units; SBM, soybean meal; WG, weight gain.
The protein concentration of SBM had no significant effect (P > 0.05) on growth performance variables in both the evaluated age groups, contrary to other studies. Park et al. (2001), for instance, obtained higher WG and feed efficiency in broilers fed SBM with 48.3% CP compared to an SBM with 45% CP. Gerber et al. (2006) also observed greater WG and FCR on 21-day-old broilers fed SBM with 48% CP compared to 44% CP. These reported improvements in growth performance can be associated to the nutritional composition of SBM, as SBM with greater CP content, or less hull, contains relatively less fiber and higher metabolizable energy than non-dehulled SBM (Swick, 1998; Ibáñez et al., 2020). However, the alteration of SBM CP content in this study with the different additions of hull was presumably not sufficient to cause significant effects on growth performance or even to generate an interaction with the supplemented phytase doses on performance variables.
Digestibility
No interaction was observed between factors for CAID variables at 21 d (P > 0.05; Table 6). When analyzing the main effects separately, protein concentration of SBM had a significant effect on the CAID of P (P < 0.05), as the SBM with 45% CP resulted in a 3.92% higher P digestibility compared to SBM 47%. Phytase supplementation had significant effects on CAID of DM, ash, P, and also IDE (P < 0.01) which were, respectively, increased by 2.13, 2.77, 8.1%, and 175 kcal with the inclusion of 2,500 FYT in comparison to the 1,000 FYT dose. Similarly, Cowieson et al. (2006) and Kiarie et al. (2015) demonstrated that high doses of phytase (1,200 and 2,000 FTU/kg, respectively) improved apparent and total P digestibility compared with lower doses of phytase, which is duly expected since more phytate-P is released and absorbed.
Table 6.
Effect of protein concentration of SBM and phytase doses on the CAID of DM, CP, ash, Ca, and P, and IDE of broiler chickens at 21 d.
| SBM (% CP) | Phytase (FYT/kg) | DM (%) | CP (%) | Ash (%) | Ca (%) | P (%) | IDE (kcal) |
|---|---|---|---|---|---|---|---|
| SBM × phytase | |||||||
| 45 | 1,000 | 70.79 | 81.89 | 51.62 | 63.05 | 71.53 | 3,472 |
| 47 | 69.65 | 81.39 | 50.16 | 59.69 | 65.03 | 3,431 | |
| 45 | 2,500 | 71.55 | 81.71 | 52.40 | 63.42 | 77.06 | 3,616 |
| 47 | 73.25 | 82.88 | 54.92 | 62.50 | 75.70 | 3,639 | |
| SEM | 0.754 | 0.594 | 1.130 | 1.880 | 1.526 | 30.100 | |
| Effect of SBM (% CP) | |||||||
| 45 | 71.17 | 81.80 | 52.01 | 63.23 | 74.29 | 3,544 | |
| 47 | 71.45 | 82.13 | 52.54 | 61.10 | 70.37 | 3,535 | |
| Effect of phytase (FYT/kg) | |||||||
| 1,000 | 70.27 | 81.64 | 50.89 | 61.37 | 68.28 | 3,452 | |
| 2,500 | 72.40 | 82.30 | 53.66 | 62.96 | 76.38 | 3,627 | |
| P-values | |||||||
| SBM | 0.716 | 0.577 | 0.641 | 0.261 | 0.013 | 0.770 | |
| Phytase | 0.006 | 0.277 | 0.018 | 0.403 | <0.001 | <0.001 | |
| Interaction | 0.070 | 0.165 | 0.086 | 0.519 | 0.100 | 0.297 | |
Abbreviations: CAID, coefficient of apparent ileal digestibility; FYT, phytase units per kg of feed; IDE, ileal digestible energy; SBM, soybean meal.
Although the CAID of CP and Ca were affected neither by SBM protein concentration nor by phytase inclusion at 21 d (P > 0.05), an interaction was observed for ileal digestibility of all the evaluated nutrients and IDE at 42 d (P < 0.05; Table 7). The highest CAID of DM, CP, ash, Ca, and P, as well as IDE, were obtained from broilers fed SBM with 47% CP and supplemented with 2,500 FYT when compared to treatments with lower protein concentration (45%), and lower phytase dose (1,000 FYT). Regarding Ca, higher dietary levels of this mineral can contribute to the formation of stable complexes between Ca and phytate, inhibiting the latter hydrolysis (Amerah et al., 2014), but Ravindran et al. (2008) demonstrated that phytase supplementation (500 FTU/kg) can efficiently improve the ileal availability of Ca and other minerals. Similar to our results, Santos et al. (2008) verified that Ca digestibility was not affected by phytase in 21-day-old broilers, but was rather improved with the use of 1,000 FTU in older broiler diets (35-day-old). The authors linked this result to the higher Ca:P ratio in their starter diets, although in the current study the Ca:P ratio was kept at 2:1 in both diets. The lack of effect on Ca digestibility, and also CP digestibility at 21 d is more likely related to age factors that affect phytase efficacy, such as gut development (Babatunde et al., 2019).
Table 7.
Effect of protein concentration of SBM and phytase doses on CAID of DM, CP, ash, Ca, and P, and IDE of broiler chickens at 42 d.
| SBM (% CP) | Phytase (FYT/kg) | DM (%) | CP (%) | Ash (%) | Ca (%) | P (%) | IDE (kcal) |
|---|---|---|---|---|---|---|---|
| SBM × phytase1 | |||||||
| 45 | 1,000 | 67.61b | 80.54b | 49.77b | 59.49b | 75.90b | 3,155c |
| 47 | 67.34b | 80.63b | 46.08c | 52.67c | 70.45c | 3,160c | |
| 45 | 2,500 | 68.59b | 79.85b | 46.00c | 52.61c | 74.79b | 3,345b |
| 47 | 76.42a | 84.08a | 54.03a | 64.82a | 81.22a | 3,641a | |
| SEM | 0.654 | 0.352 | 0.606 | 1.183 | 0.843 | 31.271 | |
| Effect of SBM (% CP) | |||||||
| 45 | 69.10 | 80.19 | 47.88 | 56.05 | 75.34 | 3,250 | |
| 47 | 71.80 | 82.30 | 50.06 | 58.74 | 75.83 | 3,401 | |
| Effect of phytase (FYT/kg) | |||||||
| 1,000 | 67.47 | 80.58 | 47.92 | 56.08 | 73.17 | 3,158 | |
| 2,500 | 72.50 | 81.96 | 50.00 | 58.71 | 78.01 | 3,493 | |
| P-values | |||||||
| SBM | <0.001 | 0.647 | 0.001 | 0.313 | 0.845 | 0.030 | |
| Phytase | 0.061 | 0.837 | 0.008 | 0.070 | 0.006 | <0.001 | |
| Interaction | 0.001 | 0.027 | <0.001 | <0.001 | <0.001 | <0.001 | |
Abbreviations: CAID, coefficient of apparent ileal digestibility; FYT, phytase units per kg of feed; IDE, ileal digestible energy; SBM, soybean meal.
Means followed by superscripted letters (a, b, c) differ in the same column (P < 0.05).
Extra-phosphoric effects were observed in this study, as DM, Ca, and CP ileal digestibility, and also energy utilization were improved by the higher phytase dose. Other studies have highlighted the effect of high doses of phytase on increasing dietary energy, digestibility, and solubility of nutrients other than P in broiler diets (Walk et al., 2013; Cowieson et al., 2017; Dersjant-Li and Kwakernaak, 2019; Walk and Rama Rao, 2020). As stated by Beeson et al. (2017), greater concentrations of phytase in the diet can intensify the dephosphorylation of phytate, contributing to the elimination of IP6 and lesser phytate esters (IP5-IP1) from the tract and therefore increasing the availability of the previously phytate-bound nutrients, in addition to improving the overall nutrient solubility as the antinutritional effects caused by the presence of phytate are hindered.
The digestibility results obtained from the interaction between phytase and protein concentration of SBM are possibly related to the different inclusions of soybean hull to the diets, because, as mentioned before, dehulled SBM has a better nutritional quality than non-dehulled SBM (Swick, 1998; Ibáñez et al., 2020). Studies have demonstrated that greater presence of indigestible carbohydrates in SBM, such as fiber and oligosaccharides, can hinder the utilization of dietary protein, amino acids, and energy in diets for poultry (Kocher et al., 2003; Gerber et al., 2006; Choct et al., 2010; Singh et al., 2019), and that is because enzymes will have limited access to substrates held in the cell wall. Other aspects of SBM also have an influence on the nutritional value, for example source and genotype, which in turn affects the digestibility of SBM nutrients (Coca-Sinova et al., 2008), and its response to exogenous enzymes (Singh et al., 2019). In addition, 10% of phytate content in grains are located in the hull (Abdelrahman et al., 1984), so a greater inclusion of hull would also increase the presence of phytate in the diet. According to Cowieson et al. (2016), the concentration of phytate in a diet required to elicit a response of high phytase dosing is not known, although there seems to be a particular threshold over which phytase responses are elevated, or higher phytase inclusion concentrations are justified. In this study, the use of an SBM with greater concentration of CP (47%), or less inclusion of hull, together with a higher dose of phytase (2,500 FYT/kg), led to improvements in nutrient and energy utilization at 42 d.
myo-inositol
No interactions were observed for plasma MYO (P > 0.05; Table 8) at 21 and 42 d. This variable was not affected by SBM protein content at either of the evaluated ages (P > 0.05), but plasma MYO of both 21 and 42-day-old broiler chickens was, respectively, 26.76 and 9.97% higher with the use of 2,500 FYT compared to the lower dose (P < 0.01). By potentializing phytate hydrolysis, the use of higher doses of phytase also increases the concentration and absorption of myo-inositol, which results from the complete dephosphorylation of phytate, as has been verified by other studies (Cowieson et al., 2015; Sommerfeld et al., 2018; Walk and Olukosi, 2019). Once absorbed, the MYO molecule seems to take part in many metabolic functions, such as glycose transportation processes by acting as an insulin-mimetic, lipid metabolism, osmotic balance in specific tissues, mineral absorption, and antioxidant functions (Gonzalez-Uarquin et al., 2020). For this reason, greater cellular concentrations of MYO are often associated to better growth performance and nutrient availability for broiler chickens (Pirgozliev et al., 2017; Sommerfeld et al., 2018; Pirgozliev et al., 2019). In the current study, however, even though plasma MYO was increased by the higher phytase dose at 42 d, it apparently had no effect on growth performance from 22 to 42 d.
Table 8.
Effect of protein concentration of SBM and phytase doses on the plasmatic levels of MYO in broiler chickens at 21 and 42 d of age.
| SBM (% CP) | Phytase (FYT/kg) | MYO (μmol/L) |
|
|---|---|---|---|
| 21 d | 42 d | ||
| SBM × phytase | |||
| 45 | 1,000 | 243.42 | 252.00 |
| 47 | 209.33 | 240.25 | |
| 45 | 2,500 | 286.25 | 266.83 |
| 47 | 307.00 | 276.75 | |
| SEM | 16.188 | 11.487 | |
| Effect of SBM (% CP) | |||
| 45 | 264.83 | 259.39 | |
| 47 | 258.17 | 258.44 | |
| Effect of phytase (FYT/kg) | |||
| 1,000 | 226.37 | 246.05 | |
| 2,500 | 296.33 | 271.78 | |
| P-values | |||
| Soybean | 0.682 | 0.936 | |
| Phytase | 0.001 | 0.030 | |
| Interaction | 0.104 | 0.350 | |
Abbreviations: FYT, phytase units per kg of feed; MYO, myo-inositol; SBM, soybean meal.
PO4−3 Release In Vitro
The determination of PO4−3 in chemical samples has been carried out by many studies with ion chromatography methods (Kaiser et al., 2001; DeBorba et al., 2004; Geng et al., 2008). Talamond et al. (2000) proposed the use of HPAEC-CD as a technique to determine the presence of phytic acid in food samples, and the authors proved that it was capable of quantifying this analyte from different sources, such as cereals and oilseeds. In the current study, the HPAEC-CD technique was used to quantify PO4−3 from feed samples, by measuring the conductivity generated by PO4−3 groups released from IP6 hydrolysis and comparing it with previously defined standard curves.
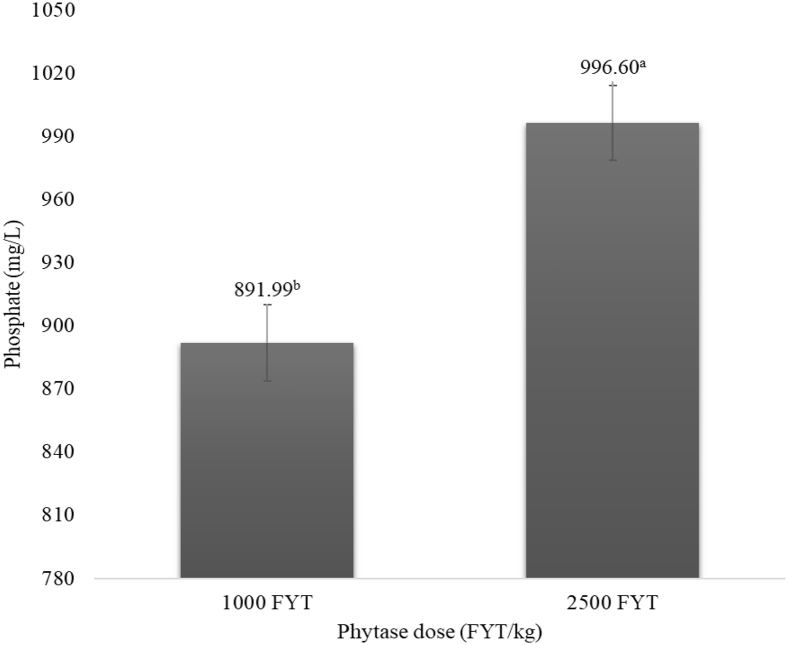
In the starter phase diets, PO4−3 availability was greater in feed containing 2,500 FYT/kg rather than 1,000 FYT/kg (P < 0.01; Figure 1), as in vitro PO4−3 release was increased by 11.07% with the higher phytase dose. This result is an outcome of a greater rate of phytate dephosphorylation, as the PO4−3 groups are being released from the IP6 myo-inositol ring. Other studies also used ion chromatography to evaluate the efficacy of phytase in vitro, but with different phosphate or sugar phosphate detection methods, that is quantifying phytate lower esters (IP5-IP1) rather than the presence of PO4−3 groups. Menezes-Blackburn et al. (2015) compared the efficacy of 7 commercial phytases in an in vitro simulation of poultry gastrointestinal tract and observed that the use of increasing doses up to 1,000 FTU resulted in greater presence of myo-inositol phosphate esters in wheat samples compared to lower doses of the enzymes. Zeller et al. (2015) also detected greater in vitro concentrations of IP5-IP1 on corn-SBM complete diets with the addition of 3 commercial phytases (1 Aspergillus niger and 2 Escherichia coli) at the same doses (∼450 phytase units/kg) compared to the same diet without phytase. Similarly, Hirvonen et al. (2019) reported a reduction of feed phytate and accumulation of lower phosphate esters in corn, SBM, and complete corn-SBM diets, as IP6 from corn and SBM samples, for example, was 100% hydrolyzed with the addition of 1,500 and 2,000 FTU/kg doses. Although the detection method of phosphates in these previous studies differs from the one applied in the current study, they equally prove the enzyme efficacy in hydrolyzing phytate, and how this reaction can be further potentialized with the use of greater doses, above 1,000 FYT/kg.
Figure 1.
Effect of different phytase doses on phosphate in vitro release of corn- and soybean meal-based starter diets of broiler chickens (P < 0.01; SEM = 18.012). Means followed by different superscripted letters (a, b) differ (P < 0.05). Abbreviation: FYT, phytase units.
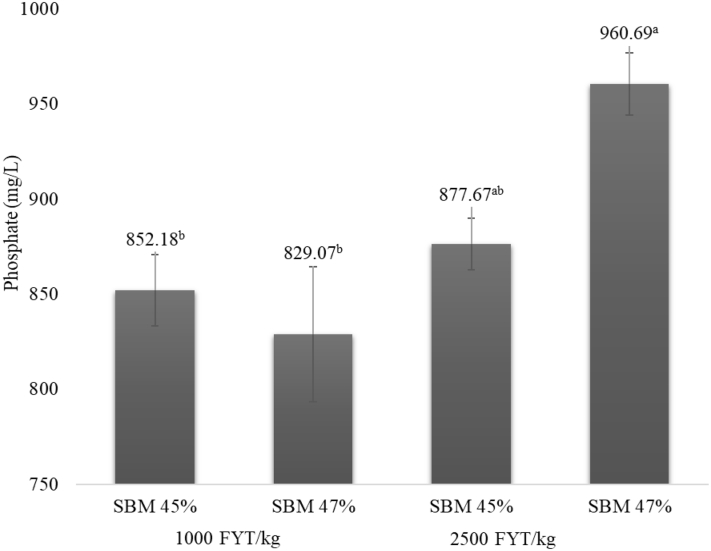
In the grower/finisher diets, there was an interaction between phytase doses and protein content of SBM (P > 0.05; Figure 2), as the diet with SBM 47% CP and 2,500 FYT/kg resulted in a greater in vitro release of PO4−3 compared to other treatments. This outcome can be compared to the observed values with nutrient ileal digestibility at 42 d, in which this same dietary treatment had the best results for nutrient CAID and IDE. Again, this greater PO4−3 release could be linked to the better nutritional quality of the SBM (less hull inclusion; less phytate and higher concentration of CP), which, together with a greater phytase dose, led to a more efficient phytase activity.
Figure 2.
Effect of protein concentration of SBM and different phytase doses on phosphate in vitro release of corn- and SBM-based grower/finisher diets of broiler chickens (P < 0.05; SEM = 22.670). Means followed by different superscripted letters (a, b) differ (P < 0.05). Abbreviations: FYT, phytase units; SBM, soybean meal.
Bone Mineral Composition and Toe Ash
Adequate bone mineralization is a reflection of good bone quality, associated to high animal growth performance, substantial to support muscular development (Cardoso et al., 2010), and is commonly used as an indicator of adequate dietary P availability and absorption (Watkins, 1992); hence its importance in studies involving phytase supplementation. In this study, an effect of interaction (P < 0.01) was observed for Ca content in the tibia bone on day 21 (Table 9). Broiler chickens fed the diet with SBM 45% and 1,000 FYT showed a lower bone Ca content than other treatments. When increasing the phytase dose to 2,500 FYT, bone Ca content was statistically similar for both SBM protein concentrations. Possibly, the dietary treatment with lower phytase dose and inferior nutritional quality of SBM (i.e., higher addition of soybean hull) enabled the formation of Ca-phytate complexes that impaired Ca availability (Amerah et al., 2014), which could explain the less Ca retention in the bone, although Ca digestibility at 21 days was not significantly affected by the dietary treatments. On day 42, an interaction was observed for Ca content as well (P < 0.05). Using SBM 47% and 2,500 FYT provided greater Ca content in the tibia. Ca digestibility for the same dietary treatment on day 42 was also greater than the other treatments, which helps explain the increased Ca content found in the tibia. Treatments had no effect on the tibia mineral composition variables (ash, P, and SI) on day 42.
Table 9.
Effect of protein concentration of SBM and phytase doses on ash, Ca, and P percentage and SI of tibia bone and toe ash of 21-day-old broiler chickens and tibia bone of 42-day-old broiler chickens.
| SBM (% CP) | Phytase (FYT/kg) | 21 d |
42 d |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ash (%) | Ca (%) | P (%) | SI (mg/mm) | Toe ash (%) | Ash (%) | Ca (%) | P (%) | SI (mg/mm) | ||
| Interaction1 | ||||||||||
| 45 | 1,000 | 56.06 | 19.81b | 10.02 | 25.74 | 42.44 | 44.39 | 15.64a,b | 8.42 | 73.22 |
| 47 | 56.58 | 22.03a | 10.13 | 25.31 | 43.70 | 44.36 | 15.41a,b | 8.26 | 74.79 | |
| 45 | 2,500 | 58.23 | 22.82a | 10.28 | 27.42 | 44.12 | 43.89 | 15.17b | 8.37 | 73.04 |
| 47 | 58.56 | 21.50a | 10.14 | 27.66 | 44.14 | 44.83 | 15.85a | 8.63 | 75.23 | |
| SEM | 0.291 | 0.332 | 0.051 | 0.267 | 0.388 | 0.654 | 0.188 | 0.142 | 1.948 | |
| Effect of SBM (% CP) | ||||||||||
| 45 | 57.14 | 21.32 | 10.15 | 26.58 | 43.28 | 44.14 | 15.46 | 8.41 | 73.13 | |
| 47 | 57.57 | 21.75 | 10.13 | 26.48 | 43.91 | 44.60 | 15.69 | 8.45 | 75.01 | |
| Effect of phytase (FYT/kg) | ||||||||||
| 1,000 | 56.30 | 20.90 | 10.07 | 25.52 | 42.44 | 44.38 | 15.92 | 8.36 | 74.00 | |
| 2,500 | 58.40 | 22.15 | 10.21 | 27.53 | 44.13 | 44.36 | 15.23 | 8.50 | 74.14 | |
| P-values | ||||||||||
| SBM | 0.352 | 0.456 | 0.836 | 0.882 | 0.112 | 0.980 | 0.921 | 0.343 | 0.945 | |
| Phytase | <0.001 | 0.040 | 0.155 | 0.004 | 0.008 | 0.505 | 0.242 | 0.842 | 0.339 | |
| Interaction | 0.837 | 0.003 | 0.161 | 0.619 | 0.116 | 0.479 | 0.020 | 0.120 | 0.874 | |
Abbreviations: FYT, phytase units per kg of feed; SBM, soybean meal; SI, Seedor Index.
Means followed by superscrpited letters (a, b, c) differ in the same column (P < 0.05).
Considering the main effects observed on day 21, supplementation with a higher phytase dose (2,500 FYT) resulted in a 2.10% greater ash content in the bone (P < 0.001), greater SI (P < 0.01), and 1.69% greater toe ash (P < 0.01) in comparison to the lower dose (1,000 FYT). Greater bone ash is associated with an increase in the amount of available P (Mitchell and Edwards, 1996), and is also described as an efficient parameter to estimate the amount of released phytate-P in corn- and SBM-based diets (Pereira et al., 2012). Many studies have similarly reported greater ash content in the tibia bone of broilers fed phytase-supplemented diets (Camden et al., 2001; Han et al., 2009; Sousa et al., 2015; Walk and Rama Rao, 2020). Toe ash is also considered to be equivalent to tibia bone ash as a means of determining bone quality (Fritz and Roberts, 1968; Yoshida and Hoshii, 1983; Yan et al., 2005), and was also increased with the use of a higher phytase dose. SI takes into consideration bone volume, and a greater SI value means greater bone mineral density (Seedor, 1993), possibly a reflection of higher bone ash content.
SBM protein concentration had no significant effect on bone mineral composition variables (P > 0.05), and treatments did not affect bone P content (P > 0.05) in either of the age groups. Because ash content was significantly greater with the use of a higher phytase dose, P content in the bone was expected to increase as well. Walk and Rama Rao (2020) have shown, however, that random variabilities or differences in Ca and P content in the bone matrix could explain variabilities regarding bone mineral composition results.
Conclusions
The supplementation of a higher phytase dose (2,500 FYT/kg) in both starter and grower/finisher stages promoted positive effects on ileal nutrient digestibility, digestible energy, and bone mineralization, in addition to greater myo-inositol provision, and phosphate in vitro release. Even though the CP concentration of SBM had no significant effects per se, the detected interactions showed that the use of an SBM of superior nutritional quality combined with higher phytase doses enhances diet digestibility, energy utilization, and phytate hydrolysis, although growth performance was not altered by the dietary treatments.
Disclosures
The authors wish to state that there are no conflicts of interest.
References
- Abdelrahman A., Hoseney R.C., Varriano-Marston E. The proportions and chemical compositions of hand-dissected anatomical parts of pearl millet. J. Cereal Sci. 1984;2:127–133. [Google Scholar]
- Amerah A.M., Plumstead P.W., Barnard L.P., Kumar A. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets1. Poult. Sci. 2014;93:906–915. doi: 10.3382/ps.2013-03465. [DOI] [PubMed] [Google Scholar]
- Angel R., Dhanhu S.D., Applegate T.J., Christman M. Addressing Anim. Prod. Environ. Issues. Research Triangle Park; Durham, NC: 2001. Non-phytin phosphorus requirement of broilers fed a four-phytase feeding program; pp. 416–427. [Google Scholar]
- AOAC International . 16th ed. 1995. Official and Tentative Methods of Analysis. AOAC Int., Arlington, VA. [Google Scholar]
- Babatunde O.O., Cowieson A.J., Wilson J.W., Adeola O. Influence of age and duration of feeding low-phosphorus diet on phytase efficacy in broiler chickens during the starter phase. Poult. Sci. 2019;98:2588–2597. doi: 10.3382/ps/pez014. [DOI] [PubMed] [Google Scholar]
- Beeson L.A., Walk C.L., Bedford M.R., Olukosi O.A. Hydrolysis of phytate to its lower esters can influence the growth performance and nutrient utilization of broilers with regular or super doses of phytase. Poult. Sci. 2017;96:2243–2253. doi: 10.3382/ps/pex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broch J., Nunes R.V., Eyng C., Pesti G.M., Souza C., Sangallia G.G., Fascina V., Teixeira L. Effect of dietary phytase superdosing on broiler performance. Anim. Feed. Sci. Tech. 2018;2018:56–65. [Google Scholar]
- Camden B.J., Morel P.C.H., Thomas D.V., Ravindran V., Bedford M.R. Effectiveness of exogenous microbial phytase in improving the bioavailabilities of phosphorus and other nutrients in maize-soya-bean meal diets for broilers. Anim. Sci. 2001;73:2089–2297. [Google Scholar]
- Cardoso A., Jr., Rodrigues P.B., Bertechini A.G., Freitas R.T.F.D., Lima R.R.D., Lima G.F.R. Levels of available phosphorus and calcium for broilers from 8 to 35 days of age fed rations containing phytase. Rev. Bras. Zoot. 2010;39:1237–1245. [Google Scholar]
- Choct M., Dersjant-Li Y., McLish J., Peisker M. Soy oligosaccharides and soluble non-starch polysaccharides: a review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australas. J. Anim. Sci. 2010;23:1386–1398. [Google Scholar]
- Coca-Sinova A., Valencia D.G., Jiménez-Moreno E., Lázaro R., Mateos G.G. Apparent ileal digestibility of energy, nitrogen, and amino acids of soybean meals of different origin in broilers. Poult. Sci. 2008;87:2613–2623. doi: 10.3382/ps.2008-00182. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Aureli R., Guggenbuhl P., Fru-Nji F. Possible involvement of myo-inositol in the physiological response of broilers to high doses of microbial phytase. Anim. Prod. Sci. 2015;55:710–719. [Google Scholar]
- Cowieson A.J., Ruckebusch J.P., Knap I., Guggenbuhl P., Fru-Nji F. Phytate-free nutrition: a new paradigm in monogastric animal production. Anim. Feed. Sci. Tech. 2016;222:180–189. [Google Scholar]
- Cowieson A.J., Acamovic T., Bedford M.R. Phytic acid and phytase: Implications for protein utilization by poultry. Poult. Sci. 2006;85:878–885. doi: 10.1093/ps/85.5.878. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Ruckebusch J.P., Sorbara J.O.B., Wilson J.W., Guggenbuhl P., Roos F.F. A systemic view on the effect of phytase on ileal amino acid digestibility in broilers. Anim. Feed. Sci. Tech. 2017;225:182–194. [Google Scholar]
- DeBorba B.M., Rohrer J.S., Bhattacharyya L. Development and validation of an assay for citric acid/citrate and phosphate in pharmaceutical dosage forms using ion chromatography with suppressed conductivity detection. J. Pharm. Biomed. 2004;36:517–524. doi: 10.1016/j.jpba.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Dersjant-Li Y., Kwakernaak C. Comparative effects of two phytases versus increasing the inorganic phosphorus content of the diet, on nutrient and amino acid digestibility in boilers. Anim. Feed Sci. Tech. 2019;253:166–180. [Google Scholar]
- Engelen A.J., Van Der Heeh F.C., Randsdorp P.G.H., Smtt E.L.C. Simple and rapid determination of phytase activity. J. AOAC Int. 1994;77:760–764. [PubMed] [Google Scholar]
- Fritz J.C., Roberts T. Use of toe ash as a measure of calcification in the chick. J. Assoc. Official Agr. Chem. 1968;51:591–594. [Google Scholar]
- Geng X., Zhang S., Wang Q., Zhao Z.K. Determination of organic acids in the presence of inorganic anions by ion chromatography with suppressed conductivity detection. J. Chromatogr. A. 2008;1192:187–190. doi: 10.1016/j.chroma.2008.03.073. [DOI] [PubMed] [Google Scholar]
- Gerber L.F.P., Penz Júnior A.M., Ribeiro A.M.L. Effect of soybean meal composition on broiler performance and metabolism. R. Bras. Zootec. 2006;35:1359–1365. [Google Scholar]
- Gonzalez-Uarquin F.M., Rodehutscord, Huber K. Myo-inositol: its metabolism and potential implications for poultry nutrition – a review. Poult. Sci. 2020;99:893–905. doi: 10.1016/j.psj.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.C., Yang X.D., Qu H.X., Xu M., Zhang T., Li W.L., Yao J.H., Liu Y.R., Shi B.J., Zhou Z.F., Feng X.Y. Evaluation of equivalency values of microbial phytase to inorganic phosphorus in 22- to 42-day-old broilers. J. Appl. Poult. Res. 2009;18:707–715. [Google Scholar]
- Hirvonen J., Liljavirta J., Saarinen M.T., Lehtinen M.J., Ahonen I., Nurminen P. Effect of phytase on in vitro hydrolysis of phytate and the formation of myo-inositol phosphate esters in various feed materials. J. Agric. Food Chem. 2019;67:11396–11402. doi: 10.1021/acs.jafc.9b03919. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F. Influence of vegetable protein sources on trace element and mineral bioavailability. J. Nutr. 2003;133:2973–2977. doi: 10.1093/jn/133.9.2973S. [DOI] [PubMed] [Google Scholar]
- Ibáñez M.A., de Blas C., Cámara L., Mateos G.G. Chemical composition, protein quality and nutritive value of commercial soybean meals produced from beans from different countries: a meta-analytical study. Anim. Feed Sci. Tech. 2020;267:1–15. [Google Scholar]
- Kaiser E., Rohrer J.S., Jensen D. Determination of trace anions in high-nitrate matrices by ion chromatography. J. Chromatogr. A. 2001;920:127–133. doi: 10.1016/s0021-9673(01)00699-9. [DOI] [PubMed] [Google Scholar]
- Kiarie E., Woyengo T., Nyachoti C.M. Efficacy of new 6-phytase from Buttiauxella spp. on growth performance and nutrient retention in broiler chickens fed corn soybean meal-based diets. Asian Australas. J. Anim. Sci. 2015;28:1479–1487. doi: 10.5713/ajas.15.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher A., Choct M., Ross G., Broz J., Chung T.K. Effects of enzyme combinations on apparent metabolizable energy of corn-soybean meal-based diets in broilers. J. Appl. Poult. Res. 2003;12:275–283. [Google Scholar]
- Leung K.Y., Mills K., Burren K.A., Copp A.J., Greene N.D.E. Quantitative analysis of myo-inositol in urine, blood and nutritional supplements by high-performance liquid chromatography tandem mass spectrometry. J. ChromatogrB Analyt. Technol. Biomed. Life Sci. 2011;879:2759–2763. doi: 10.1016/j.jchromb.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke C.B., Ciocca J.R.P., Dandin T., Barbalho P.C., Vilela J.A. 1st ed. World Society for the Protection of Animals; Copacabana, Rio de Janeiro, Brazil: 2010. Abate humanitário de aves. [Google Scholar]
- Menezes-Blackburn D., Gabler S., Greiner R. Performance of seven commercial phytases in an in vitro simulation of poultry digestive tract. J. Agric. Food Chem. 2015;63:6142–6149. doi: 10.1021/acs.jafc.5b01996. [DOI] [PubMed] [Google Scholar]
- Mitchell R.D., Edwards H.M., Jr. Effects of phytase and 1,25-dihydroxycholecalciferol on phytate utilization and the quantitative requirement for calcium and phosphorus in young broiler chickens. Poult. Sci. 1996;75:95–110. doi: 10.3382/ps.0750095. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Park Y.H., Kim H.K., Kim H.S., Lee1 H.S., Shinl I.S., Whang K.Y. Effects of three different soybean meal sources on layer and broiler performance. J. Anim. Sci. 2001;15:254–265. [Google Scholar]
- Pereira R., Menten J.F.M., Romano G.G., Silva C.L.S., Zavarize K.C., Barbosa N.A.A. Efficiency of a bacterial phytase to release phytate phosphorus in broiler chicken diets. Arq. Bras. Med. Vet. Zootec. 2012;64:137–144. [Google Scholar]
- Pirgozliev V.R., Bedford M.R., Rose S.P., Whiting I.M., Oluwatosin O.O., Oso A.O., Oke F.O., Ivanova S.G., Staykova G.P. Phosphorus utilisation and growth performance of broiler chicken fed diets containing graded levels of supplementary myo-inositol with and without exogenous phytase. J. World Poult. Res. 2017;7:1–7. [Google Scholar]
- Pirgozliev V., Brearley C.A., Rose S.P., Mansbridge S.C. Manipulation of plasma myo-inositol in broiler chickens: effect on growth performance, dietary energy, nutrient availability, and hepatic function. Poult. Sci. 2019;98:260–268. doi: 10.3382/ps/pey341. [DOI] [PubMed] [Google Scholar]
- Ravindran V. Phytases in poultry nutrition. An overview. Poult. Sci. 1995;7:135–139. [Google Scholar]
- Ravindran V., Cowieson A.J., Selle P.H. Influence of dietary electrolyte balance and microbial phytase on growth performance, nutrient utilization, and excreta quality of broiler chickens. Poult. Sci. 2008;87:677–688. doi: 10.3382/ps.2007-00247. [DOI] [PubMed] [Google Scholar]
- Scott T.A., Boldaji F. Comparison of inert markers [chromic oxide or insoluble ash (CeliteTM)] for determining apparent metabolizable energy of wheat- or barley- based broiler diets with or without enzymes. Poult. Sci. 1997;76:594–598. doi: 10.1093/ps/76.4.594. [DOI] [PubMed] [Google Scholar]
- Santos F.R., Hruby M., Pierson E.E.M., Remus J.C., Sakomura N.K. Effect of phytase supplementation in diets on nutrient digestibility and performance in broiler chicks. J. Appl. Poult. Res. 2008;17:191–201. [Google Scholar]
- Santos T.T. Phytate: anti-nutrient for poultry and swine. Feedstuffs. 2012;84:1–3. [Google Scholar]
- Seedor J.G. The biophosphanate alendronate (MK-217) inhibit bone loss due to ovariectomy in rats. J. Bone Min. Res. 1993;4:265–270. doi: 10.1002/jbmr.5650060405. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Tiwari U.P., Berrocoso J.D., Dersjant-Li Y., Awati A., Jha R. Effects of a combination of xylanase, amylase and protease, and probiotics on major nutrients including amino acids and non-starch polysaccharides utilization in broilers fed different level of fibers. Poult. Sci. 2019;98:5571–5581. doi: 10.3382/ps/pez310. [DOI] [PubMed] [Google Scholar]
- Sommerfeld V., Künzel S., Schollenberger M., Kühn I., Rodehutscord M. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 2018;97:920–929. doi: 10.3382/ps/pex390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa J.P.L., Albino L.F.T., Vaz R.G.M.V., Rodrigues K.F., Silva G.F., Renno L.N., Barros V.R.S.M., Kaneko I.N. The effect of dietary phytase on broiler performance and digestive, bone, and blood biochemistry characteristics. Rev. Bras. Ciênc. Avíc. 2015;17:69–76. [Google Scholar]
- Swick R.A. ASA; Singapore: 1998. US Soybean Meal: Present Quality and Future Trends. ASA Technical Bulletin. MITA No. 096/11/97. AN14- 1998. [Google Scholar]
- Talamond P., Doulbeau S., Rochette I., Guyot J., Treche S. Anion-exchange high-performance liquid chromatography with conductivity detection for the analysis of phytic acid in food. J. Chromatogr. A. 2000;871:7–12. doi: 10.1016/s0021-9673(99)01226-1. [DOI] [PubMed] [Google Scholar]
- Van Soest P.J., Robertston J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Bedford M.R., Santos T.S., Paiva D., Bradley J.R., Wladecki H., Honaker C., McElroy A.P. Extra-phosphoric effects of superdoses of a novel microbial phytase. Poult. Sci. 2013;92:719–725. doi: 10.3382/ps.2012-02727. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Olukosi O.A. Influence of graded concentrations of phytase in high-phytate diets on growth performance, apparent ileal amino acid digestibility, and phytate concentration in broilers from hatch to day 28 post-hatch. Poult. Sci. 2019;98:1–10. doi: 10.3382/ps/pez106. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Santos T.T., Bedford M.R. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 2014;93:1172–1177. doi: 10.3382/ps.2013-03571. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Rama Rao S.V. Dietary phytate has a greater anti-nutrient effect on feed conversion ratio compared to body weight gain and greater doses of phytase are required to alleviate this effect as evidenced by prediction equations on growth performance, bone ash and phytate degradation in broilers. Poult. Sci. 2020;99:246–255. doi: 10.3382/ps/pez469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins B.A. Factors involved in the local regulation of bone growth. In: Whitehead C.C., editor. Bone Biology and Skeletal Disorders in Poultry. Carfax Publishers; Oxfordshire, UK: 1992. pp. 67–86. [Google Scholar]
- Yan F., Keen C.A., Zhang K.Y., Waldroup P.W. Comparison of methods to evaluate bone mineralization. J. Appl. Poult. Res. 2005;14:492–498. [Google Scholar]
- Yoshida M., Hoshii H. Relationship between ash contents of the tibia bone and the toe of chicks. Jpn. Poult. Sci. 1983;20:51–54. [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 2015;4:1–12. doi: 10.1017/jns.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyla K., Gogol D., Koreleski J., Swiatkiewicz S., Ledoux D.R. Simultaneous application of phytase and xylanase to broiler feeds based on wheat: in vitro measurements of phosphorus and pentose release from wheats and wheat-based feeds. J. Sci. Food Agric. 1999;79:1832–1840. [Google Scholar]