Abstract
The purpose of this study was to investigate the effects of Bacillus subtilis on growth performance, intestinal morphology, and cecal microbial composition of broilers. A total of 270 healthy one-day-old Arbor Acres male broiler chicks were randomly divided into 3 dietary treatment groups, with 6 replicates per group and 15 chickens per replicate. The dietary treatment groups were as follows: 1) basal diet, negative control group; 2) basal diet +250 g/t of zinc bacitracin, positive control group; and 3) basal diet +750 g/t of B. subtilis, B. subtilis group. Results of this experiment showed that compared with the negative control group, body weight at 42 d, average daily gain and European Production Efficiency Factor over the 42 d phase in the B. subtilis group and positive control group were significantly increased (P < 0.05); feed conversion rates in the B. subtilis group and positive control group were significantly decreased (P < 0.05); and average daily feed intake and mortality were not significantly different (P > 0.05). The villus height to crypt depth ratio in the ileum of the B. subtilis group was significantly higher (P < 0.05) than that of the negative control group. The results of cecal microflora at genus level were as follows. As compared with the negative control group, the abundance of Blautia, Faecalibacterium, Flavonifractor, and Hydrogenoanaerobacterium of the B. subtilis group and positive control group was significantly higher (P < 0.05), whereas the abundance of Odoribacter was significantly lower (P < 0.05). Moreover, abundance of the genera Romboutsia in the B. subtilis group was higher (P < 0.05) than that in the positive control group. The abundance of Flavonifractor, Erysipelatoclostridium, and Hydrogenoanaerobacterium were positively correlated with body weight and average daily gain by Spearman correlation analysis. In conclusion, dietary supplementation with B. subtilis improved growth performance of broilers which may be related to the increased abundance of Blautia, Faecalibacterium, Flavonifractor, Hydrogenoanaerobacterium, and Romboutsia, along with the decreased abundance of Odoribacter. In addition, the effect of B. subtilis was superior to zinc bacitracin in improving intestinal microbial composition of broilers. Therefore, B. subtilis may act as an effective antibiotic substitute in broilers.
Key words: Bacillus subtilis, 16S rDNA sequencing, growth performance, cecal microbiota, broiler
Introduction
In the production of livestock and poultry, long-term abuse of antibiotics has led to several negative consequences, such as the residue of antibiotics in livestock and poultry products, the resistance of pathogens to antibiotics, the imbalance of normal microbial flora, etc. (Barton, 2000; Bogaard et al., 2000; Sorum and Sunde, 2001). With increasing food safety awareness and the introduction of relevant laws and regulations in various countries to control the use of antibiotics, the search for antibiotic alternatives has become a research focal point in the industry. One such group of antibiotic alternatives, probiotics, have been studied for nearly 20 yr (Rolfe, 2000). Probiotics have been used in livestock and poultry production as feed additives, and Bacillus subtilis is one of the most common probiotics (Guo et al., 2006).
Bacillus subtilis is a spore-forming aerobic bacterium. Its spores are metabolically dormant during feed processing and adaptable to external conditions, such as extremely low and high temperatures as well as low and high pH (Nicholson, 2002). In addition, dietary supplementation with B. subtilis can effectively improve growth performance, immunity, and intestinal morphology of poultry (Lee et al., 2010; Korosi et al., 2011; Jeong and Kim, 2014; Nguyen et al., 2015). This growth-promoting effect of B. subtilis may be due to its influence on gut microbial populations, including increasing the number of beneficial bacteria and reducing the number of certain pathogenic bacteria (Guo et al., 2006; Molnar et al., 2011; Wu et al., 2011; Jeong and Kim, 2014; Yang et al., 2016). Studies on the substitution of B. subtilis for antibiotics have also been reported (Cavazzoni et al., 1998; Lee et al., 2014). However, few studies have analyzed how B. subtilis as an antibiotics substitute changed the intestinal microbial community of broilers to improve the performance by microbial sequencing technology.
In this study, B. subtilis was used as an antibiotic substitution to determine its effect on growth performance of broilers. In addition, 16S rDNA sequencing was used to compare the effects of B. subtilis and the antibiotic, zinc bacitracin, on the intestinal microbial community of these same broilers to better understand the impact that B. subtilis had on growth performance.
Materials and methods
Experimental Design and Animal Management
A total of 270 one-day old Arbor Acres male broiler chicks with similar weight (42.11 ± 0.10 g) were randomly divided into 3 groups, with 6 replicates per group and 15 chickens per replicate. The treatment groups were as follows: 1) basal diet, negative control group (NC); 2) basal diet + 250 g/t of zinc bacitracin, positive control group (PC); and 3) basal diet + 750 g/t of B. subtilis, B. subtilis group (BS). Zinc bacitracin and B. subtilis (LIFEGUFS-S 200) were provided by Lifecome Biochemistry co., Ltd., and the number of viable B. subtilis in the raw product was between 2 × 1010 CFU/g and 3 × 1010 CFU/g. The testing period was from 1 to 42 d of age. Two feeding phase diets were utilized: starter diet from 1 to 21 d and grower diet from 22 to 42 d (Table 1). The diets were formulated to meet the nutrient requirements recommended by the National Research Council (NRC, 1994). The chickens were raised in an experimental farm in the Institute of Poultry Science, Chinese Academy of Agricultural Science. Birds had ad libitum to feed and water and were reared in wire cages (1.2 m × 0.9 m, length × width), with 23 h of illumination per day throughout the study. The animal use protocol was approved by the Animal Care and Use Committee of the Institute of Poultry Science, Chinese Academy of Agricultural Science (Yangzhou, Jiangsu, China).
Table 1.
Composition and nutrient levels of the basal diet (air-dry basis).
| Items | Contents |
|
|---|---|---|
| Starter stage (1–21 d) | Grower stage (22–42 d) | |
| Ingredients (%) | ||
| Corn | 54.30 | 56.84 |
| Soybean oil | 3.40 | 3.98 |
| Soybean meal (43%) | 38.12 | 35.32 |
| Lysine hydrochloride (98%) | 0.15 | 0.16 |
| DL -Met | 0.25 | 0.24 |
| CaCO3 | 1.14 | 0.93 |
| CaHPO4·2H2O | 1.86 | 1.80 |
| Salt | 0.40 | 0.40 |
| Choline chloride (50%) | 0.15 | 0.10 |
| Vitamin premix1 | 0.03 | 0.03 |
| Mineral premix2 | 0.20 | 0.20 |
| Total | 100.00 | 100.00 |
| Nutrient levels (%)3 | ||
| ME (kcal/kg) | 2,950 | 3,020 |
| CP | 21.00 | 20.00 |
| Ca | 1.01 | 0.90 |
| Available phosphorus | 0.45 | 0.43 |
| DLys | 1.15 | 1.10 |
| DMet | 0.50 | 0.48 |
| DCys | 0.29 | 0.28 |
| DMet + DCys | 0.86 | 0.82 |
The vitamin premix provides the following per kg of diet: Vitamin A, 8000 IU; Vitamin D3, 1000 IU; Vitamin E, 20 IU; Vitamin K3, 0.50 mg; Vitamin B1, 2.00 mg; Vitamin B2, 8.00 mg; Vitamin B6, 3.50 mg; Vitamin B12, 0.01 mg; niacin, 35.00 mg; calcium pantothenate, 10.00 mg; folic acid, 0.55 mg; biotin, 0.18 mg.
The mineral premix provides the following per kg of diet: Fe, 80.00 mg; Cu, 8.00 mg; Mn, 100.00 mg; Zn, 80.00 mg; I, 0.70 mg; Se, 0.30 mg.
The nutrient levels were calculated values.
Growth Performance
Daily, health status was observed, and the death and feed consumption of chickens were recorded. The birds were fasted 8 h and then weighed on day 21 and 42, to calculate the average body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), feed conversion ratio (FCR), and European Production Efficiency Factor (EPEF). The EPEF was determined as per the formula (Slizewska et al., 2020): EPEF = [(viability (%) × body weight)/(FCR × age)] × 100.
Sample Collection and Index Determination
On day 42, 6 birds (1 bird per replicate) with similar weight per group were selected and euthanized by severing the jugular vein. About 2 cm of intestinal tissue from the duodenum, jejunum, and ileum were excised, emptied of chyme, and then fixed with 4% paraformaldehyde solution. The intestinal segments were dehydrated in an ascending gradient of ethanol. These samples were then cleaned in xylene, embedded in paraffin wax, processed into slices, and stained with hematoxylin and eosin. Villus height and crypt depth were measured using a positive fluorescence microscope (DM4000B, Leica Microsystems, Wetzlar, Germany), and villus height to crypt depth ratio (VCR) was calculated. Cecal chyme was collected, immersed in liquid nitrogen, and then stored at −80°C for DNA extraction and 16S rDNA amplicon sequencing analysis by Novogene Corporation (Beijing, China).
DNA Extraction and Sequencing Library Construction
Total genomic DNA was extracted from cecal contents of each chick using the EZNA Soil DNA kit (D5625-02, Omega Bio-Tek Inc., Norcross, GA). After extraction, DNA concentration and purity were analyzed by a NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). The DNA was then stored at −20°C until further processing. DNA amplicons were amplified using primers for the V4 domain of bacterial 16S rRNA gene by polymerase chain reaction (Bergmann et al., 2011; Gao et al., 2017). The amplified products were extracted by electrophoresis with a 2% agarose gel, and the polymerase chain reaction products were mixed equally and purified with the GeneJET Gel Extraction Kit (Thermo Fisher Scientific, Waltham, MA). The library was constructed using the Ion Plus Fragment Library Kit (Thermo Fisher Scientific). After Qubit quantification (Qubit 2.0 fluorometer, Life Technology, Carlsbad) and library testing, the constructed library was sequenced using the IonS5XL sequencing platform at Novogene Bioinformatics Technology Co., Ltd. (Beijing, China).
Quality Filtering and Sequence Data Analysis
Based on the IonS5XL sequencing platform, a small fragment library was constructed using the single-end sequencing (Single-End) method. Clean data were obtained by cutting and filtering reads. Based on the clean data, the sequences were clustered into operational taxonomic units (OTU) with 97% identity, and then the OTU sequences and Silva132 database were used for species annotation analysis (Edgar, 2013). In accordance with species annotation, the differences in community structure among treatments were revealed by calculating alpha diversity and beta diversity. For alpha diversity measurements, the alpha diversity indexes were calculated based on the OTU using the Shannon, Simpson, and Chao1 (Chao, 1984; Chao and Lee, 1992) methods. For beta diversity measurements, beta diversity heatmap, principal coordinates analysis (PCoA) (Minchin, 1987), analysis of similarities (Chapman and Underwood, 1999), multiple response permutation procedure (O'Reilly and Jr, 1980), and permutation multivariate analysis of variance (Adonis) (Stat et al., 2013) were used to analyze the differences of community structure among different treatments. In addition, MetaStat (Edgar, 2004) and LEfSe analysis (Segata et al., 2011) were used to identify the biological differences between treatments. CCA-envfit function analysis (Yang et al., 2007) and Spearman correlation analysis (Segata et al., 2011) were carried out to obtain the growth performance factors which were significantly correlated with the change of community among treatments. Finally, the annotated results of the amplifier were correlated with the corresponding functional database, and functional prediction of the microbial community in the samples was carried out by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (Qin et al., 2012).
Statistical Analysis
All data were presented as the mean with pooled SEM values. Statistical analyses were carried out with SPSS 22.0 for windows (SPSS Inc., Chicago, IL). One-way ANOVA followed by LSD's multiple comparison test was used to evaluate the differences among the treatment groups. A P-value less than 0.05 was considered statistically significant.
Results
Growth Performance
Table 2 shows the growth performance and EPEF of broilers in each treatment group. Compared with the NC group, BW at 42 d (P = 0.004), ADG over the 42 d phase (P = 0.004) and EPEF (P = 0.012) in the BS group were significantly increased; and BW at 42nd day (P = 0.010), ADG over the 42-day phase (P = 0.010) and EPEF (P = 0.003) in the PC group were also significantly increased. The FCR in the BS group (P = 0.019) and PC group (P = 0.033) were significantly lower than in the NC group. There were no significant differences in ADFI and mortality during the 42-day phase among the 3 groups.
Table 2.
Effects of Bacillus subtilis on growth performance and European Production Efficiency Factor of broilers at 42 d of age.
| Items | Groups |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|
| NC | PC | BS | NC-BS | NC-PC | PC-BS | ||
| BW (g) | 2949b | 3058a | 3075a | 20 | 0.004 | 0.010 | 0.651 |
| ADG (g) | 69.2b | 71.8a | 72.2a | 0.47 | 0.004 | 0.010 | 0.651 |
| ADFI (g) | 113.3 | 114.6 | 114.9 | 0.63 | 0.321 | 0.441 | 0.817 |
| FCR | 1.637a | 1.595b | 1.590b | 0.0085 | 0.019 | 0.033 | 0.781 |
| Mortality (%) | 4.45 | 1.11 | 3.33 | 1.344 | 0.748 | 0.342 | 0.523 |
| EPEF | 410b | 451a | 444a | 6.4 | 0.012 | 0.003 | 0.555 |
a,bMeans in the same row with different superscripts are significantly different at P < 0.05. Values are expressed as means with pooled SEM values, n = 6.
EPEF = [(Viability (%) × Body weight)/(FCR × Age)] × 100.
Abbreviations: ADFI, average daily feed intake; ADG, average daily weight gain; BS, Bacillus subtilis group; BW, body weight; EPEF, European Production Efficiency Factor; FCR, feed conversion rate; NC, negative control group; PC, positive control group.
Intestinal Morphology
Intestinal morphology of the broilers at 42 d of age is shown in Table 3. Compared with the NC group, the VCR of the ileum in the BS group was significantly increased (P = 0.047), whereas the VCR of the duodenum and jejunum was not significantly different (P > 0.05) among the 3 groups. Moreover, there were no significant differences (P > 0.05) among any of the treatments for villus height and crypt depth of the duodenum, jejunum, and ileum.
Table 3.
Effects of Bacillus subtilis on intestinal morphology of broilers at 42 d of age.
| Items | Groups |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|
| NC | PC | BS | NC-BS | NC-PC | PC-BS | ||
| Duodenum | |||||||
| Crypt depth (μm) | 235 | 216 | 246 | 12 | 0.730 | 0.560 | 0.388 |
| Villus height (μm) | 1,279 | 1,107 | 1,342 | 57 | 0.642 | 0.243 | 0.133 |
| VCR | 5.5 | 5.3 | 5.8 | 0.33 | 0.709 | 0.781 | 0.548 |
| Jejunum | |||||||
| Crypt depth (μm) | 237 | 220 | 215 | 10 | 0.439 | 0.532 | 0.855 |
| Villus height (μm) | 1,152 | 1,221 | 1,306 | 64 | 0.369 | 0.668 | 0.618 |
| VCR | 4.9 | 5.8 | 6.3 | 0.36 | 0.156 | 0.317 | 0.617 |
| Ileum | |||||||
| Crypt depth (μm) | 202 | 186 | 150 | 15 | 0.165 | 0.659 | 0.329 |
| Villus height (μm) | 944 | 885 | 941 | 40 | 0.981 | 0.577 | 0.593 |
| VCR | 4.7b | 5.5a,b | 6.4a | 0.35 | 0.047 | 0.328 | 0.268 |
a,bMeans in the same row with different superscripts are significantly different at P < 0.05. Values are expressed as means with pooled SEM values, n = 6.
Abbreviations: BS, Bacillus subtilis group; NC, negative control group; PC, positive control group; VCR, villus height to crypt depth ratio.
Variation in Cecal Microbiota Composition
Phylum Level
Appendix Table 1 shows the relative abundance of the top 10 microorganisms at the phylum level. The cecal microbiome of each group was dominated by Bacteroidetes, Firmicutes, Proteobacteria, Tenericutes, Melainabacteria, Actinobacteria, Cyanobacteria, Acidobacteria, Gemmatimonadetes, and Chloroflexi. Among them, Bacteroidetes and Firmicutes were the most dominant bacterial groups, which together accounted for more than 80% of the total microbial community detected. By MetaStat analysis, Proteobacteria of the PC group was significantly higher (P = 0.032) than that of the BS group (Figure 1A and Appendix Table 1). However, there were no significant differences among treatments in the relative abundance of the other microorganisms in cecum of broilers at the phylum level.
Figure 1.
Significantly different taxa between different groups by MetaStat analysis (n = 6). (A) Phylum. (B) Family. (C) Genus. Abbreviations: NC, negative control group; PC, positive control group; BS, Bacillus subtilis group.
Family Level
Appendix Table 2 shows the relative abundance of the top 10 microorganisms at the family level. Compared with the NC group, the relative abundance of Ruminococcaceae (P = 0.007, P = 0.018) and Lachnospiraceae (P = 0.019, P = 0.011) in Firmicutes of the BS group and PC group were significantly increased (Figure 1B and Appendix Table 2). There was no significant difference in the relative abundance of the predominant microorganisms in cecum of broilers at the phylum level between the BS and PC groups.
Genus Level
Appendix Table 3 shows the relative abundance of the top 35 microorganisms at the genus level. Compared with the NC group, the relative abundance of Faecalibacterium (P = 0.006), Flavonifractor (P = 0.010), Hydrogenoanaerobacterium (P = 0.006) and Blautia (P = 0.009) in Firmicutes, and Rikenella (P = 0.047) in Bacteroidetes of the BS group was significantly increased, and the relative abundance of Odoribacter (P = 0.047) in Bacteroidetes of the BS group was significantly decreased (Figure 1C and Appendix Table 3). In addition, when compared with the NC group, the relative abundance of Faecalibacterium (P = 0.024), Flavonifractor (P = 0.005), Hydrogenoanaerobacterium (P = 0.027), Blautia (P = 0.004), and Erysipelatoclostridium (P = 0.005) in Firmicutes, and Parasutterella (P = 0.003) and Bilophila (P = 0.049) in Proteobacteria of the PC group were significantly increased, and the relative abundance of Odoribacter (P = 0.031) in Bacteroidetes of the PC group was significantly decreased (Figure 1C and Appendix Table 3). Compared with the PC group, the relative abundance of Romboutsia (P = 0.033) in Firmicutes and Rikenella (P = 0.035) in Bacteroidetes of the BS group was significantly increased (Figure 1C and Appendix Table 3).
Diversity of Cecal Microbiota
Alpha Diversity
lpha diversity among the NC, PC, and BS groups is presented in Table 4. Alpha diversity indexes were calculated based on the OTU using the Shannon, Simpson, and Chao1 methods. There was no significant difference in the indexes (including OTU, Shannon, Simpson, Chao1) of alpha diversity of microbiota in cecum of broilers at 42 d of age.
Table 4.
Effects of Bacillus subtilis on alpha diversity of microbiota in cecum of broilers at 42 d of age.
| Items | Groups |
SEM |
P-value |
||||
|---|---|---|---|---|---|---|---|
| NC | PC | BS | NC-BS | NC-PC | PC-BS | ||
| Observed_species | 694 | 661 | 553 | 41 | 0.179 | 0.742 | 0.300 |
| Shannon | 5.53 | 5.71 | 5.69 | 0.071 | 0.383 | 0.329 | 0.914 |
| Simpson | 0.903 | 0.924 | 0.928 | 0.0071 | 0.155 | 0.233 | 0.802 |
| Chao1 | 746 | 692 | 596 | 43 | 0.171 | 0.615 | 0.370 |
Abbreviations: NC, negative control group; PC, positive control group; BS, Bacillus subtilis group.
Beta Diversity
Beta diversity was assessed by beta diversity heatmap and PCoA using the weighted UniFrac distance method. Beta diversity heatmap showed that microbiota diversity parameters were not affected by the treatments at 42 d of age (Figure 2A). Figure 2B shows PCoA of the variation among these 3 groups, and the cecal microbiota compositions of broilers were not separated. Analysis of similarities, multiple response permutation procedure, and Adonis are a series of nonparametric methods used to test the difference of community structure among groups. There was no significant difference in any of these 3 indexes of beta diversity of microbiota in cecum of broilers at 42 d of age (Table 5). LEfSe analysis was used to find the biomarkers with statistical difference among different treatments. Figure 2C showed the species with significant differences among the NC, PC, and BS groups with LDA scores >2.5. Seven specific biomarkers were present in the NC group, 5 in the PC group and 4 in the BS group.
Figure 2.
Beta diversity of the microbiome residing in the cecal chyme of broilers at 42 d of age. (A) Beta diversity heatmap. (B) PCoA plot. (C) LDA distribution histogram (LDA scores > 2.5). Abbreviations: NC, negative control group; PC, positive control group; BS, Bacillus subtilis group. p, phylum; c, class; o, order; f, family; g, genus; s, species.
Table 5.
Anosim, MRPP and Adonis P-values based on microbial community between treatment groups.
| VS. groups | Anosim P-value | MRPP P-value | Adonis P-value |
|---|---|---|---|
| NC-BS | 0.311 | 0.377 | 0.320 |
| NC-PC | 0.311 | 0.446 | 0.500 |
| PC-BS | 0.968 | 0.936 | 0.882 |
Abbreviations: Adonis, permutation multivariate analysis of variance; Anosim, analysis of similarities; BS, Bacillus subtilis group; MRPP, multiple response permutation procedure; NC, negative control group; PC, positive control group.
Microbiome Responding to Growth Performance
The correlation between the dominant taxon of cecal microbiota at the genus level relative to growth performance of broilers was assessed by Spearman correlation analysis (Figure 3). The BW (Pr = 0.019), ADG (Pr = 0.019), FCR (Pr = 0.033), and EPEF (Pr = 0.038) screened by the CCA-envfit function analysis (Appendix Table 4) were the growth performance factors that had the most significant impact on the bacterial community. Flavonifractor, Erysipelatoclostridium, and Hydrogenoanaerobacterium in Firmicutes were positively correlated with BW and ADG. Sphingomonas in Proteobacteria was positively correlated with FCR, whereas Bilophila and Parasutterella in Proteobacteria were negatively correlated with FCR. Unidentified_Clostridiales in Firmicutes was negatively correlated with EPEF.
Figure 3.
Correlation between the most abundant taxa of the cecal microbiota at the genus level and growth performance of broilers at 42 d of age. Color legend on the right indicates correlation coefficient values by color. The value corresponding to the intermediate heat map is Spearman correlation coefficient r, which is between −1 and +1. When r < 0, it is negative correlation; and when r > 0, it is positive correlation. The significance test results are P < 0.05 or P < 0.01 (marked with ∗ or ∗∗ respectively). Abbreviations: BW, body weight; ADG, average daily weight gain; FCR, feed conversion rate; EPEF, European Production Efficiency Factor; VCR, villus height to crypt depth ratio.
Functional Prediction of Cecal Microbiota
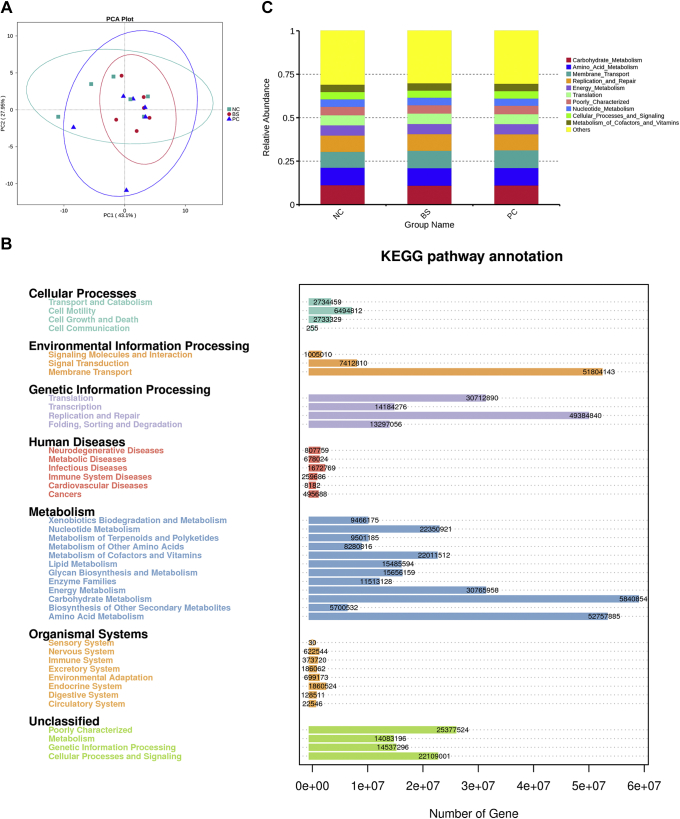
Cecal microbiota functional predictions due to dietary treatments were examined by PICRUSt. Principal component analysis revealed that the 3 dietary treatments clustered together, indicating that their functional compositions were similar (Figure 4A). Based on the Kyoto Encyclopedia of Genes and Genome, the abundant functional annotations of cecal microbiota were those corresponding to carbohydrate metabolism, amino acid metabolism, membrane transport, replication and repair, energy metabolism, translation, poorly characterized, nucleotide metabolism, cellular processes, and signaling and metabolism of cofactors and vitamins (Figure 4B). However, the predominant functions (top10) were not significantly different among the 3 groups (Figure 4C and Appendix Table 5).
Figure 4.
Impact of dietary treatment on cecal microbiota functional predictions by PICRUSt. (A) PCA plot. (B) KEGG pathway annotation. (C) The predominant functions (top10) of cecal microbiota based on KEGG. Abbreviations: KEGG, Kyoto Encyclopedia of Genes and Genome; NC, negative control group; PC, positive control group; BS, Bacillus subtilis group.
Discussion
Growth Performance
In the poultry industry, it is very important to find safe and effective antibiotic substitutes that also may provide economic benefits. Growth performance characteristics (including BW, ADG, ADFI, and FCR) are some of the most important factors used to evaluate the economic benefits of broiler production. The EPEF is a comprehensive measure of broiler production which reflects various measures of boiler performance, including BW, survival rate, FCR, production management, and so on. It is also a profitability index. The larger the index, the more profitable the birds are. In recent years, EPEF has gradually been recognized by practitioners and gradually become an important evaluation method of poultry production (Bhamare et al., 2016; Slizewska et al., 2020). Studies have shown that B. subtilis as a dietary additive can significantly promote the growth performance of broilers (Jeong and Kim, 2014; Park and Kim, 2014; Nguyen et al., 2015). Consistent with previous studies, our results showed that B. subtilis or zinc bacitracin can significantly increase BW and ADG and reduce FCR during the first 42 d of production. The EPEF in the BS and PC groups was also increased as compared with the NC group, indicating that economic benefits were improved. However, some studies have reported that B. subtilis does not affect growth performance of broiler chickens (Lee et al., 2014), which may be related to the type and additive amount of B. subtilis. In our study, the growth promoting effect and economic benefit of B. subtilis on broilers was similar to that of zinc bacitracin according to the growth performance including the BW, ADG, FCR, and EPEF.
Intestinal Morphology
It is well known that intestinal morphology is an important indicator of intestine health, and villus height and crypt depth are the main indicators of intestinal digestion and absorption function as well as cell maturity rate, respectively (Paiva et al., 2014). Increased VCR can provide an intestinal environment conducive to digestion and absorption of nutrients (Montagne et al., 2003). Lee et al. (2010) showed that a diet supplemented with B. subtilis could promote the growth of intestinal epithelial cells, increase villus height of the small intestine, and improve absorption of nutrients. However, in our study, villus height and crypt depth of the duodenum, jejunum, and ileum were not affected by the addition of B. subtilis or zinc bacitracin. Nevertheless, VCR of the ileum in the BS group was higher than the NC group, indicating that B. subtilis was beneficial to intestinal health.
Variation in Cecal Microbiota Composition
The composition of intestinal microflora is significant for maintaining intestinal homeostasis and health of host (Zhang et al., 2018). Cecal microflora plays an important role in chicken health and growth performance, affecting food transformation, disease resistance, and pathogen colonization (Stanley et al., 2014; Awad et al., 2016). Bacillus subtilis is a kind of aerobic bacterium, which can grow in the intestinal tract and consume oxygen to maintain anaerobic environment and inhibit the growth of harmful aerobic bacteria (Hong et al., 2005). Many studies have shown that B. subtilis supplementation caused a significant decrease in the numbers of Escherichia coli and Salmonella, whereas the numbers of Lactobacillus and Bifidobacterium increased in the cecum (Wu et al., 2011; Jeong and Kim, 2014; Yang et al., 2016). Based on the results of species annotation, we analyzed the changes of cecal microbial composition at phylum, family, and genus levels to partially elucidate the growth promoting mechanisms of B. subtilis.
At the phylum level, Bacteroidetes and Firmicutes were the dominant bacterial groups, which together accounted for more than 80% of the total microbial community detected in our study. This is consistent with previous studies in which Bacteroidetes and Firmicutes constitute most microbial communities in chickens at the phylum level, and these bacteria are known to play a role in energy production and metabolism (Ahir et al., 2010; Oakley et al., 2014; Pandit et al., 2018). It is worth mentioning that some reports found that the dominant phylum of the cecal community is Firmicutes in chickens (Awad et al., 2016; Mancabelli et al., 2016), but others have reported that the dominant phylum is Bacteroidetes (Mohd et al., 2015; Pandit et al., 2018). The results of our study showed that Bacteroidetes is the dominant phylum of the cecal community in 42-day-old broilers in our trial. However, the dominant phylum may change due to the age, breed, and regional differences of selected chickens. What we assessed was final colonization of broiler chickens in the later stage of the production cycle. In addition, the abundance of Proteobacteria caused by supplemental zinc bacitracin was higher than that of supplemental B. subtilis which is similar to the previous reports (Salaheen et al., 2017; Hu et al., 2020), that is, the addition of antibiotics increased the abundance of Proteobacteria. Salaheen et al. (2017) and Hu et al. (2020) also reported that supplementing broilers with antibiotic growth promoters (tylosin, neomycin sulfate, bacitracin, erythromycin, and oxytetracycline or virginiamycin) increased the abundance of Proteobacteria. It is important to note that Proteobacteria include some zoonotic pathogens, such as Escherichia, Salmonella, Campylobacter, and other notable pathogenic genera (Salaheen et al., 2017; Clavijo and Florez, 2018).
At the family level, the abundance of Ruminococcaceae and Lachnospiraceae in Firmicutes was influenced by addition of B. subtilis or zinc bacitracin in our study. Lachnospiraceae is a microorganism producing n-butyric acid in the intestine, which may be related to host energy regulation and intestinal mucosal integrity (Lin et al., 2018). Ruminococcaceae and Lachnospiraceae contain the genera producing short-chain fatty acids (SCFA) (Nava and Stappenbeck, 2011; Zeng et al., 2019), and SCFA could inhibit the growth and reproduction of enteropathogenic bacteria by affecting intestinal pH value, which may ultimately yield good growth performance in broilers. Therefore, the increased abundance of Ruminococcaceae and Lachnospiraceae in the BS and PC groups may be related to the improvement of ADG in broilers. This finding is supported by Ma et al. (2018) who also reported that the increased abundance of Ruminococcaceae due to B. subtilis addition was associated with increased ADG and BW.
At the genus level, the dominant bacteria species of the gut were reshaped by B. subtilis or zinc bacitracin addition in the present study. The genus Blautia is a gram-positive bacterium, which can degrade different types of carbohydrates to produce metabolites such as acetic acid and lactic acid (Liu et al., 2008), and Faecalibacterium is an important butyrate-producing bacterium in the chicken cecum (Duncan et al., 2002). Both of these bacteria can provide energy for the body and reduce inflammation, and their increased abundance is indicative of intestinal health of the host (Biddle et al., 2013; Yang et al., 2016; Abaidullah et al., 2019). Therefore, the improved growth performance of the BS and PC groups in our study may be related to the increased abundance of Blautia and Faecalibacterium, which are beneficial to intestinal health. Flavonifractor belongs to the family Ruminococcaceae in Firmicutes and contributes to butyrate production (Meng et al., 2019). It is well known that adding butyrate in the animal diet is beneficial in improving feed conversion efficiency and growth performance. Hydrogenoanaearobacterium is a proteolytic bacterium that produces sulfides through the degradation of sulfur-containing amino acids and can break the aromatic ring of plant compounds to produce SCFA (Li et al., 2017). In addition, Hydrogenoanaearobacterium has been reported to be closely related to obesity phenotypes (Jung et al., 2016). In the present study, Spearman correlation analysis showed Flavonifractor and Hydrogenoanaerobacterium in Firmicutes were positively correlated with BW and ADG. Therefore, the improved growth performance of broilers in the BS and PC groups may be due to the increased abundance of Flavonifractor and Hydrogenoanaearobacterium. Liu et al. (2019) reported that proteoglycan induced mice with Ankylosing spondylitis exhibited notably increased relative abundances of Odoribacter, and Han et al. (2020) also thought Odoribacter was positively correlated with the inflammatory state. In our study, addition of B. subtilis or zinc bacitracin both reduced the abundance of Odoribacter, but there was no difference between the PC and BS groups. Taken together, the increased abundances of Blautia, Faecalibacterium, Flavonifractor, and Hydrogenoanaerobacterium, along with the decreased abundance of Odoribacter in the gut of the BS and PC groups could have contributed to the improved growth performance of the broilers.
Erysipelatoclostridium belongs to the family Erysipelotrichaceae in Firmicutes. It has been reported that high abundance of Erysipelatoclostridium may be related to reducing feed/egg ratio of laying hens (Guo et al., 2018), but Erysipelatoclostridium is also considered to be an opportunistic pathogen (Zhao et al., 2019), which may be associated with diseases such as metabolic syndrome and gout (Smith et al., 2016; Shao et al., 2017). In the present study, the genera Parasutterella and Bilophila in Proteobacteria were very abundant in cecum of broilers in the PC group, which were significantly negatively correlated with FCR. In addition, the genera Parasutterella and Bilophila have been reported to be associated with intestinal inflammation and injury (Chen et al., 2018; Cheng et al., 2018). In our study, zinc bacitracin did not exert a positive inhibiting effect on Erysipelatoclostridium, Parasutterella, or Bilophila. Although the genera Blautia, Faecalibacterium, Flavonifractor, and Hydrogenoanaearobacterium in the PC group may have yielded improved growth performance of broilers, there is also a potential risk of certain diseases due to the enrichment of the genera Erysipelatoclostridium, Parasutterella and Bilophila in the intestine of the PC group. In addition, the genus Romboutsia is a valuable intestinal biomarker because it plays a key role in maintaining health of the host (Mangifesta et al., 2018). In our study, Romboutsia of the BS group was higher than that of the PC group, further indicating that B. subtilis was superior to zinc bacitracin in improving intestinal microbial composition in broilers.
Diversity of Cecal Microbiota
The diversity of gut microbiota is important for maintaining gastrointestinal homeostasis and is beneficial to host health (Zhang et al., 2018). In our study, dietary treatments yielded several changes to the cecal microbial composition of broilers, and LEfSe analysis further identified the species with significant differences among treatments. However, B. subtilis or zinc bacitracin addition failed to modify the overall diversity of cecal microbiota at 42 d of age. These results are consistent with previous reports. Ma et al. (2018) reported that supplemental B. subtilis also did not affect the diversity of cecal microbiota in broilers. In addition, and even more like the results of the present study, Pedroso et al. (2006) described that dietary bacitracin had no significant effect on overall microbial diversity but did alter the composition of intestinal bacterial microbiota in chickens. However, Li et al. (2019) reported that B. subtilis addition improved the diversity of jejunal microbiota at 21 d but had little effect by 42 d of age. It may be that the dynamic diversity of intestinal microbiota, which is a very complex ecosystem, shifts with the change of diet and age (Isaacson and Kim, 2012). In fact, Ballou et al. (2016) suggested microbiota were affected more by age than treatment. In addition, dietary B. subtilis can improve overall microbial diversity of chickens infected by pathogenic bacteria such as Salmonella (Oh et al., 2017; Khan and Chousalkar, 2020). Therefore, it is possible that B. subtilis can restore microbial diversity in chickens infected by pathogenic bacteria but have little effect on overall microbial diversity of healthy chickens. However, as indicated in the previous section, B. subtilis did affect the abundance of some intestinal microorganisms.
Functional Prediction of Cecal Microbiota
The PICRUSt analysis was used to infer the effect of B. subtilis or zinc bacitracin on the metabolic pathways of cecal microbiota in broilers. Based on Kyoto Encyclopedia of Genes and Genome prediction, the abundant functional annotations of cecal microbiota were those corresponding to carbohydrate metabolism, amino acid metabolism, membrane transport, replication and repair, energy metabolism, translation, poorly characterized, nucleotide metabolism, cellular processes and signaling, and metabolism of cofactors and vitamins. These predicted functions of cecal microbiota in broilers were similar to those predicted by other studies (Ma et al., 2018; Hu et al., 2020). However, the addition of B. subtilis or zinc bacitracin had little effect on the predicted functions of cecal microflora in broilers. Consistent with previous studies, Ma et al. (2018) also reported that the addition of B. subtilis DSM 32315 exerted little impact on the predicted functions of cecal microbiota in broilers. These results suggest that the functional abilities of cecal microflora are stable in broilers.
Conclusion
In conclusion, dietary supplementation with B. subtilis improved growth performance including the BW, ADG, FCR, and EPEF of broilers which may be related to the increased abundance of Blautia, Faecalibacterium, Flavonifractor, Hydrogenoanaerobacterium, and Romboutsia, and the decreased abundance of Odoribacter. Moreover, the effect of B. subtilis may be superior to zinc bacitracin in improving intestinal microbial composition of broilers, which may be related to the increased abundance of Romboutsia that plays a key role in maintaining health of host.
Acknowledgements
This research was supported by the Agricultural Science and Technology Independent Innovation Fund Project of Jiangsu Province [No. CX(17)3033], the National Key R&D Program of Intergovernmental Key Projects of China (Grant No: 2018YFE0101700).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.12.032.
Disclosures
The authors declare that they have no conflicts of interest. The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence the work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this article.
Supplementary data
References
- Abaidullah M., Peng S., Kamran M., Song X., Yin Z. Current findings on gut microbiota Mediated immune Modulation against Viral diseases in chicken. Viruses. 2019;11:1–14. doi: 10.3390/v11080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahir V.B., Koringa P.G., Bhatt V.D., Ramani U.V., Tripathi A.K., Singh K.M., Dhagat U.M., Patel J.S., Patel M.M., Katudia K.H., Sajnani M.R., Jakhesara S.J., Joshi C.G. Metagenomic analysis of poultry gut microbes. Indian J. Poult. Sci. 2010;45:111–114. [Google Scholar]
- Awad W.A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., Hess M. Age-related differences in the Luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 2016;6:154. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how Early Exposure influences Future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M.D. Antibiotic use in animal feed and its impact on human healt. Nutr. Res. Rev. 2000;13:279–299. doi: 10.1079/095442200108729106. [DOI] [PubMed] [Google Scholar]
- Bergmann G.T., Bates S.T., Eilers K.G., Lauber C.L., Caporaso J.G., Walters W.A., Knight R., Fierer N. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol. Biochem. 2011;43:1450–1455. doi: 10.1016/j.soilbio.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhamare K.S., Dildeep V., Senthil M., Chavan S.J. Nutritive evaluation of cashew apple waste in broilers. Intern. J. Sci. Nat. 2016;7:629–632. [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the Genetic basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. [Google Scholar]
- Bogaard A.E., London N., Stobberingh E.E. Antimicrobial resistance in pig faecal samples from The Netherlands (five abattoirs) and Sweden. J. Antimicrob. Chemother. 2000;45:663–671. doi: 10.1093/jac/45.5.663. [DOI] [PubMed] [Google Scholar]
- Cavazzoni V., Adami A., Castrovilli C. Performance of broiler chickens supplemented with Bacillus coagulans as probiotic. Br. Poult. Sci. 1998;39:526–529. doi: 10.1080/00071669888719. [DOI] [PubMed] [Google Scholar]
- Chao A. Nonparametric estimation of the number of classes in a population. Scand. J. Statist. 1984;11:265–270. [Google Scholar]
- Chao A., Lee S.M. Estimating the number of classes via sample coverage. J. Am. Stat. Assoc. 1992;87:210–217. [Google Scholar]
- Chapman M.G., Underwood A.J. Ecological patterns in multivariate assemblages: information and interpretation of negative values in ANOSIM tests. Mar. Ecol. Prog. Ser. 1999;180:257–265. [Google Scholar]
- Chen Y.J., Wu H., Wu S.D., Lu N., Wang Y.T., Liu H.N., Dong L., Liu T.T., Shen X.Z. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 2018;33:1844–1852. doi: 10.1111/jgh.14281. [DOI] [PubMed] [Google Scholar]
- Cheng C., Wei H., Xu C., Xie X., Jiang S., Peng J. Maternal Soluble Fiber diet during Pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal Permeability in Piglets. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.01047-18. 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo V., Florez M. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.H., Hold G.L., Harmsen H., Stewart C.S., Flint H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Guo J.R., Dong X.F., Liu S., Tong J.M. High-throughput sequencing reveals the effect of Bacillus subtilis CGMCC 1.921 on the cecal microbiota and gene expression in ileum mucosa of laying hens. Poult. Sci. 2018;97:2543–2556. doi: 10.3382/ps/pey112. [DOI] [PubMed] [Google Scholar]
- Guo X., Li D., Lu W., Piao X., Chen X. Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Anton. Leeuwenhoek J. Microbiol. 2006;90:139–146. doi: 10.1007/s10482-006-9067-9. [DOI] [PubMed] [Google Scholar]
- Gao Y., Wang C., Zhang W., Di P., Yi N., Chen C. Vertical and horizontal assemblage patterns of bacterial communities in a eutrophic river receiving domestic wastewater in southeast China. Environ. Pollut. 2017;230:469–478. doi: 10.1016/j.envpol.2017.06.081. [DOI] [PubMed] [Google Scholar]
- Han L., Zhao L.H., Zhang M.L., Li H.T., Gao Z.Z., Zheng X.J., Wang X.M., Wu H.R., Zheng Y.J., Jiang X.T., Ding Q.Y., Yang H.Y., Jia W.P., Tong X.L. A Novel Antidiabetic Monomers Combination alleviates Insulin resistance through bacteria-Cometabolism-inflammation responses. Front. Microbiol. 2020;11:173. doi: 10.3389/fmicb.2020.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H.A., Duc L.H., Cutting S.M. The use of bacterial spore formers as probiotics. Fems. Microbiol. Rev. 2005;29:813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hu Y., Wang L., Shao D., Wang Q., Wu Y., Han Y., Shi S. Selectived and reshaped Early dominant microbial community in the cecum with similar Proportions and better Homogenization and species diversity due to organic acids as AGP alternatives Mediate their effects on broilers growth. Front. Microbiol. 2020;10:2948. doi: 10.3389/fmicb.2019.02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R., Kim H.B. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012;13:100–109. doi: 10.1017/S1466252312000084. [DOI] [PubMed] [Google Scholar]
- Jeong J.S., Kim I.H. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 2014;93:3097–3103. doi: 10.3382/ps.2014-04086. [DOI] [PubMed] [Google Scholar]
- Jung M.J., Lee J., Shin N.R., Kim M.S., Hyun D.W., Yun J.H., Kim P.S., Whon T.W., Bae J.W. Chronic Repression of mTOR complex 2 Induces changes in the gut microbiota of diet-induced obese mice. Sci. Rep. 2016;6:30887. doi: 10.1038/srep30887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Chousalkar K.K. Salmonella Typhimurium infection disrupts but continuous feeding of Bacillus based probiotic restores gut microbiota in infected hens. J. Anim. Sci. Biotechnol. 2020;11:29. doi: 10.1186/s40104-020-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi M.A., Podmaniczky B., Kurti P., Glavits R., Virag G., Szabo Z., Farkas Z. Effect of different concentrations of Bacillus subtilis on immune response of broiler chickens. Probiotics Antimicro. 2011;3:8–14. doi: 10.1007/s12602-011-9063-x. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lee S.H., Lillehoj H.S., Li G.X., Jang S.I., Babu U.S., Park M.S., Kim D.K., Lillehoj E.P., Neumann A.P., Rehberger T.G., Siragusa G.R. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 2010;89:203–216. doi: 10.3382/ps.2009-00418. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jang S.I., Lee S.H. Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Res. Vet. Sci. 2014;97:304–308. doi: 10.1016/j.rvsc.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Li D., Chen H., Mao B., Yang Q., Zhao J., Gu Z., Zhang H., Chen Y.Q., Chen W. Microbial Biogeography and Core microbiota of the Rat digestive tract. Sci. Rep. 2017;8:45840. doi: 10.1038/srep45840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.L., Wang J., Zhang H.J., Wu S.G., Hui Q.R., Yang C.B., Fang R.J., Qi G.H. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken Model. Front. Physiol. 2019;9:1968. doi: 10.3389/fphys.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Ye W., Zu X., Xie H., Li H., Li Y., Zhang W. Integrative metabolic and microbial profiling on patients with Spleen-yang-deficiency syndrome. Sci. Rep. 2018;8:6619. doi: 10.1038/s41598-018-24130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Finegold S.M., Song Y., Lawson P.A. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and description of Blautia wexlerae sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008;58:1896–1902. doi: 10.1099/ijs.0.65208-0. [DOI] [PubMed] [Google Scholar]
- Liu B., Yang L., Cui Z., Zheng J., Huang J., Zhao Q., Su Z., Wang M., Zhang W., Liu J., Wang T., Li Q., Lu H. Anti-TNF-alpha therapy alters the gut microbiota in proteoglycan-induced ankylosing spondylitis in mice. Microbiologyopen. 2019;8:e927. doi: 10.1002/mbo3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang W., Zhang H., Wang J., Zhang W., Gao J., Wu S., Qi G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancabelli L., Ferrario C., Milani C., Mangifesta M., Turroni F., Duranti S., Lugli G.A., Viappiani A., Ossiprandi M.C., van Sinderen D., Ventura M. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016;18:4727–4738. doi: 10.1111/1462-2920.13363. [DOI] [PubMed] [Google Scholar]
- Mangifesta M., Mancabelli L., Milani C., Gaiani F., De'Angelis N., De'Angelis G.L., van Sinderen D., Ventura M., Turroni F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018;8:13974. doi: 10.1038/s41598-018-32413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q., Sun S., Luo Z., Shi B., Shan A., Cheng B. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019;10:5626–5643. doi: 10.1039/c9fo00637k. [DOI] [PubMed] [Google Scholar]
- Minchin P.R. An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio. 1987;69:89–107. [Google Scholar]
- Mohd S.M., Sieo C.C., Chong C.W., Gan H.M., Ho Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015;7:4. doi: 10.1186/s13099-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A.K., Podmaniczky B., Kurti P., Tenk I., Glavits R., Virag G., Szabo Z. Effect of different concentrations of Bacillus subtilis on growth performance, carcase quality, gut microflora and immune response of broiler chickens. Br. Poult. Sci. 2011;52:658–665. doi: 10.1080/00071668.2011.636029. [DOI] [PubMed] [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Tech. 2003;108:95–117. [Google Scholar]
- Nava G.M., Stappenbeck T.S. Diversity of the autochthonous colonic microbiota. Gut Microbes. 2011;2:99–104. doi: 10.4161/gmic.2.2.15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A.T., Nguyen D.V., Tran M.T., Nguyen L.T., Nguyen A.H., Phan T.N. Isolation and characterization of Bacillus subtilis CH16 strain from chicken gastrointestinal tracts for use as a feed supplement to promote weight gain in broilers. Lett. Appl. Microbiol. 2015;60:580–588. doi: 10.1111/lam.12411. [DOI] [PubMed] [Google Scholar]
- Nicholson W.L. Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. 2002;59:410–416. doi: 10.1007/s00018-002-8433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly F.J., P W M Jr Asymptotic normality of MRPP statistics from invariance principles of u-statistics. Commun. Stat-theor. M. 1980;9:629–637. [Google Scholar]
- NRC. 1994. Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. Press, Washington, DC.
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. Fems Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Oh J.K., Pajarillo E., Chae J.P., Kim I.H., Kang D.K. Protective effects of Bacillus subtilis against Salmonella infection in the microbiome of Hy-Line Brown layers. Asian Australas. J. Anim. Sci. 2017;30:1332–1339. doi: 10.5713/ajas.17.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva D., Walk C., McElroy A. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poult. Sci. 2014;93:2752–2762. doi: 10.3382/ps.2014-04148. [DOI] [PubMed] [Google Scholar]
- Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Thakkar J.R., Shah T.M., Limon G., Psifidi A., Guitian J., Hume D.A., Tomley F.M., Rank D.N., Raman M., Tirumurugaan K.G., Blake D.P., Joshi C.G. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Kim I.H. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult. Sci. 2014;93:2054–2059. doi: 10.3382/ps.2013-03818. [DOI] [PubMed] [Google Scholar]
- Pedroso A.A., Menten J.F., Lambais M.R., Racanicci A.M., Longo F.A., Sorbara J.O. Intestinal bacterial community and growth performance of chickens fed diets containing antibiotics. Poult. Sci. 2006;85:747–752. doi: 10.1093/ps/85.4.747. [DOI] [PubMed] [Google Scholar]
- Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., Peng Y., Zhang D., Jie Z., Wu W., Qin Y., Xue W., Li J., Han L., Lu D., Wu P., Dai Y., Sun X., Li Z., Tang A., Zhong S., Li X., Chen W., Xu R., Wang M., Feng Q., Gong M., Yu J., Zhang Y., Zhang M., Hansen T., Sanchez G., Raes J., Falony G., Okuda S., Almeida M., LeChatelier E., Renault P., Pons N., Batto J.M., Zhang Z., Chen H., Yang R., Zheng W., Li S., Yang H., Wang J., Ehrlich S.D., Nielsen R., Pedersen O., Kristiansen K., Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Rolfe R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000;130:396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- Salaheen S., Kim S.W., Haley B.J., Van Kessel J., Biswas D. Alternative growth promoters Modulate broiler gut microbiome and Enhance body weight gain. Front. Microbiol. 2017;8:2088. doi: 10.3389/fmicb.2017.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao T., Shao L., Li H., Xie Z., He Z., Wen C. Combined Signature of the Fecal microbiome and Metabolome in patients with gout. Front. Microbiol. 2017;8:268. doi: 10.3389/fmicb.2017.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slizewska K., Markowiak-Kopec P., Zbikowski A., Szeleszczuk P. The effect of synbiotic preparations on the intestinal microbiota and her metabolism in broiler chickens. Sci. Rep. 2020;10:4281. doi: 10.1038/s41598-020-61256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Brown P., Morrison M., Krause L., Davies P.S. Dairy and plant based food intakes are associated with altered faecal microbiota in 2 to 3 year old Australian children. Sci. Rep. 2016;6:32385. doi: 10.1038/srep32385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorum H., Sunde M. Resistance to antibiotics in the normal flora of animals. Vet. Res. 2001;32:227–241. doi: 10.1051/vetres:2001121. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Stat M., Pochon X., Franklin E.C., Bruno J.F., Casey K.S., Selig E.R., Gates R.D. The distribution of the thermally tolerant symbiont lineage (Symbiodinium clade D) in corals from Hawaii: correlations with host and the history of ocean thermal stress. Ecol. Evol. 2013;3:1317–1329. doi: 10.1002/ece3.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B.Q., Zhang T., Guo L.Q., Lin J.F. Effects of Bacillus subtilis KD1 on broiler intestinal flora. Poult. Sci. 2011;90:2493–2499. doi: 10.3382/ps.2011-01529. [DOI] [PubMed] [Google Scholar]
- Yang J., Bindels L.B., Segura M.R., Martinez I., Walter J., Ramer-Tait A.E., Rose D.J. Disparate metabolic responses in mice fed a high-Fat diet supplemented with Maize-Derived non-Digestible Feruloylated Oligo- and Polysaccharides are Linked to changes in the gut microbiota. PLoS One. 2016;11:e146144. doi: 10.1371/journal.pone.0146144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Qian K., Zhang W., Xu Y., Wu Y. Effects of chromium-enriched bacillus subtilis KT260179 supplementation on chicken growth performance, plasma lipid parameters, tissue chromium levels, cecal bacterial composition and breast meat quality. Lipids Health Dis. 2016;15:188. doi: 10.1186/s12944-016-0355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.L., Zhang J., Xu X.J. Influence of the three Gorges Dam on downstream delivery of sediment and its environmental implications, Yangtze river. Geophys. Res. Lett. 2007;34:1–5. [Google Scholar]
- Zeng X., Gao X., Peng Y., Wu Q., Zhu J., Tan C., Xia G., You C., Xu R., Pan S., Zhou H., He Y., Yin J. Higher risk of Stroke is correlated with increased opportunistic pathogen Load and reduced levels of butyrate-producing bacteria in the gut. Front. Cell. Infect. Microbiol. 2019;9:4. doi: 10.3389/fcimb.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wu W., Lee Y.K., Xie J., Zhang H. Spatial Heterogeneity and Co-occurrence of mucosal and Luminal microbiome across Swine intestinal tract. Front. Microbiol. 2018;9:48. doi: 10.3389/fmicb.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li K., Luo H., Duan L., Wei C., Wang M., Jin J., Liu S., Mehmood K., Shahzad M. Comparison of the intestinal microbial community in Ducks reared differently through high-throughput sequencing. Biomed. Res. Int. 2019;2019:1–14. doi: 10.1155/2019/9015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.