Abstract
Early posthatch nutrition is important for gut health. Vitamin E (VE) and omega-3 (n-3) fatty acids can improve gut health through antioxidative and anti-inflammatory effects. The objective of this study was to identify the effects of VE, n-3 fatty acids, and combination of both during the starter phase (0–10 d) or grower phase (11–24 d) on intestinal morphology and expression of genes associated with gut health. A total of 210 Ross 708 broilers were randomly assigned into 7 treatments with 10 replicates of 3 birds each. The control group was fed a corn–soybean meal–basal diet during the entire study (0–58 d). Supplementation of VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), and combination of both were fed during the starter phase (0–10 d) or grower phase (11–24 d). All of the broilers were harvested at 58 d of age. Villus height, crypt depth, villus width, distance between villi, and number of intraepithelial lymphocytes were obtained. Expression of 21 genes was measured using NanoString analysis. Expression of solute carrier family 15 member 1 (P = 0.01) associated with peptide transport and mucin 2 (P = 0.03) related with intestinal mucus barrier was increased in the broilers supplemented with n-3 fatty acids in the grower diet compared with the control. Expression of solute carrier family 7 member 1 associated with amino acid transport was decreased in the group supplemented with n-3 fatty acids during the starter phase compared with the group supplemented with n-3 fatty acids (P = 0.01) or VE and n-3 fatty acids during the grower phase (P = 0.03). These data suggest that VE and n-3 fatty acids supplemented during the grower phase have a positive effect on improving nutrient transport with n-3 fatty acids supplementation in the grower diet showing the most beneficial effect. These findings can be used in the development of nutritional management strategies to improve broiler growth performance and meat quality.
Key words: broiler, gene expression, intestinal morphology, omega-3 fatty acid, vitamin E
Introduction
A healthy gastrointestinal system is essential for poultry growth performance and meat production (Noy and Sklan, 1998; Rinttilä and Apajalahti, 2013; Sugiharto, 2016). Various studies have suggested that early posthatch nutrition plays a key role in intestinal development (Noy et al., 2001; Batal and Parsons, 2002) and gut health (Ao et al., 2012; Jha et al., 2019). Yamauchi et al. (1996) showed that reduced villus height and enterocyte number results from delayed feeding. In contrast, enhanced intestinal development with improved intestinal morphology has been observed with sufficient early nutrient supply (Uni and Ferket, 2003). Because the early posthatch period is important for gut health (Ao et al., 2012; Jha et al., 2019) and broiler chicks are sensitive to nutritional changes during the early posthatch period (Noy et al., 2001; Batal and Parsons, 2002), early posthatch nutritional strategies can be used to enhance gut health and nutrient absorption. With good nutrient absorption and transportation, nutrients can be used better in muscle growth (Hocquette et al., 1998), contributing to improved meat production and meat quality as well as the potential to reduce development of myopathies such as wooden breast.
Nutritional interventions including vitamin E (VE) (Tappel, 1962; Burton and Traber, 1990) and omega-3 (n-3) fatty acids (Korver and Klasing, 1997; Calder, 2006; Yu et al., 2018) have the potential to lessen oxidative stress and inflammation improving gut health. Omega-3 fatty acids have been shown to reduce intestinal inflammation in humans (Calder, 2006; Yu et al., 2018) and decrease inflammatory responses and improve immune function in broilers (Korver and Klasing, 1997; Wang et al., 2000; Saleh et al., 2009; Al-Khalifa et al., 2012; El-Katcha et al., 2014). The n-3 fatty acids, including α-linolenic acid (18:3n-3), eicosapentaenoic acid (20:5n-3, and docosahexaenoic acid (22:6n-3), are polyunsaturated fatty acids (PUFA) (Reiser, 1949). The principal link between long-chain PUFA and immune function is primarily mediated by the synthesis of oxylipins from PUFA called eicosanoids (C20-derived) or docosanoids (C22-derived) (Calder, 2003). Eicosanoids are usually synthesized by arachidonic acid (C20:4n-6) and are involved in a variety of inflammatory responses (Brock and Peters-Golden, 2007). Supplementation of n-3 fatty acids decreases the concentration of arachidonic acid and therefore decreases the amount of n-6 derived eicosanoids that are produced from arachidonic acid (Calder, 2006). Alterations in dietary n-6 and n-3 PUFA can alter eicosanoid profiles, and increased dietary n-3 PUFA can modulate production of n-6 eicosanoids by increasing n-3 derived eicosanoids and docosanoids because the precursor PUFA (n-6 or n-3) use the same enzymes for synthesis. The n-3–derived eicosanoids are less biologically potent than eicosanoids synthesized from arachidonic acid and thus dampen the inflammatory effects (Calder, 2012). In this way, n-3 fatty acids can improve gut health through reducing inflammation in the gastrointestinal tract.
Vitamin E is a widely known powerful antioxidant (Tappel, 1962; Burton and Traber, 1990). Vitamin E can protect the cell membrane from oxidation as the lipid peroxidation process is terminated (Niki, 1993). DL-α-tocopherol acetate is one of the 8 forms of VE commonly used in the animal industries (Hosomi et al., 1997; Panda and Cherian, 2014). It has a high biological efficiency removing free radicals in lipid peroxidation (Hosomi et al., 1997; Panda and Cherian, 2014). Dietary VE has been shown to enhance intestinal antioxidant capacity (Cheng et al., 2017) and decrease intestinal inflammation (Pitargue et al., 2019) in broilers. In addition, VE and n-3 fatty acids work synergistically on reducing oxidative stress and improving immune function (Taulescu et al., 2011). Thus, VE and n-3 fatty acids are highly likely to improve gut health through anti-inflammatory and antioxidative effects.
Although VE (Bartov and Frigg, 1992; Rebolé et al., 2006; Lu et al., 2014) and n-3 fatty acids (Schreiner et al., 2005; Haug et al., 2007) have been evaluated in a number of studies to improve growth performance and meat quality, effects of VE and n-3 fatty acids supplementation during the early posthatch phase on gut health has not been well studied in broilers. Therefore, the objective of the present study was to identify the effects of VE, n-3 fatty acids, and combination of both during the starter phase (0–10 d) or the grower phase (11–24 d) on intestinal morphology and expression of genes associated with nutrient transport, hypoxia, oxidative stress, inflammation, extracellular matrix in broilers.
Materials and methods
Birds and Experimental Diets
All bird protocols were approved by the Institutional Animal Care and Use Committee of The Ohio State University. A total of 210 commercial Ross 708 broiler chicks were individually wing banded and placed into pens immediately after hatch. Broilers had ad libitum access to feed and water. Birds were assigned to 7 experimental groups in a completely randomized design. There were 10 pens per treatment, and each pen included 3 birds. The control group was fed a corn–soybean meal–basal diet with VE (DL-α-tocopherol acetate, 10 IU/kg) and n-3 fatty acids (n-6/n-3 ratio of 30.2:1) at a standard level during the starter (0–10 d), grower (11–24 d), and finisher phases (25–58 d). Additional supplemental VE or n-3 fatty acids were fed during the starter or grower phases. For the starter dietary supplementation, starter VE, starter n-3, and starter VE and n-3 groups were fed the basal starter diet supplemented at concentrations of 200 IU/kg diet of VE, n-3 fatty acids with a n-6/n-3 ratio of 3.2:1, or a combination of both. The grower and finisher diets were the same as the control group. For the grower dietary supplementation, grower VE, grower n-3, and grower VE and n-3 groups were fed the basal grower diets supplemented at concentrations of 200 IU/kg diet of VE, n-3 fatty acids with a n-6/n-3 ratio of 3.2:1, or a combination of both. The starter and finisher diets were the same as the control group. Diets were formulated to meet or exceed all NRC (National Research Council, 1994) nutritional requirements and recommendations in Aviagen's Ross broiler production handbook (Aviagen, 2016). Feed ingredients and nutrient composition have been previously reported in Wang et al. (2020b). At 58 d of age, all broilers were harvested in accordance with humane and commercial slaughter procedures.
Intestinal Morphology
To evaluate intestinal morphology, a 3-cm-long section of the ileum was obtained from each broiler. Tissue samples were immediately fixed in 10% (vol/vol) buffered formalin (pH 7.0) and stored at room temperature. Histologic samples were dehydrated in a graded series of alcohols, cleared in Pro-Par Clearant (Anatech, Battle Creek, MI) and paraffin embedded as per the procedure of Jarrold et al. (1999). Paraffin blocks were cross sectioned at 5 μm, mounted on Starfrost Adhesive slides (Mercedes Medical, Sarasota, FL), and hematoxylin and eosin stained as described by Velleman et al. (2002). Each slide contained a minimum of 4 sections and imaged with a QImaging digital camera (QImaging, Burnaby, BC, Canada) attached to an Olympus IX 70 microscope (Olympus America, Mellville, NY).
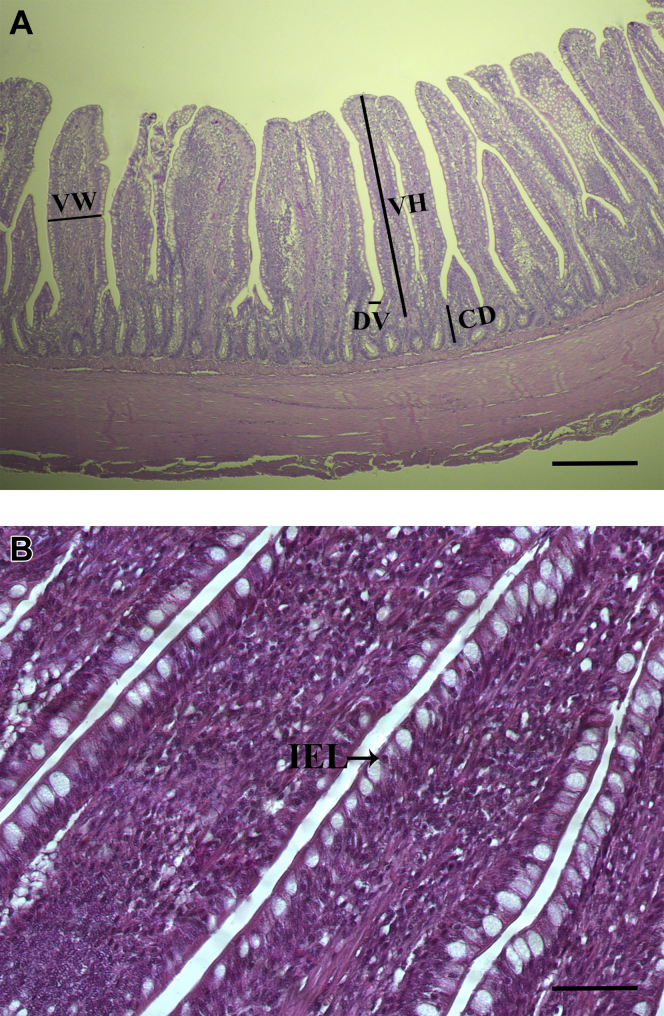
Four photomicrographs from each sample were taken for measurement of villus height, crypt depth, villus width, and distance between villi. Representative images of the ileum are presented in Figure 1. Villus height was determined as the distance between the tip of the villi and the villus–crypt junction. Crypt depth was measured as the distance of the invagination between 2 adjacent villi (Uni et al., 1999). Villus width was measured at the middle part of the villi. Distance between villi was determined as the distance between the adjacent villi at the base of the villi. Measurements were taken in 10 well-structured villi and crypts from each section of each sample using Image J 1.8.0 software (National Institutes of Health, Bethesda, MD). The ratio of villus height to crypt depth was calculated as the ratio of villus height and crypt depth. Surface area of the villi was calculated as (2π) × (villus width/2) × (villus height) (De Los Santos et al., 2007).
Figure 1.
Representative photomicrographs of the broiler ileum. Panel A shows were measurements of villus height, crypt depth, villus width, and distance between villi were taken. Villus height was determined as the distance between the tip of the villi and the villus crypt junction. Crypt depth was measured as the distance of the invagination between 2 adjacent villi. Villus width was measured at the middle part of the villi. Distance between villi was determined as the distance between the adjacent villi at the base of the villi. Measurements were taken in 10 well-structured villi and crypts from each section of each sample. Scale bar is 100 μm. Panel B shows intraepithelial lymphocytes centrally located in epithelial cells. Scale bar is 50 μm. Abbreviations: CD, crypt depth; DV, distance between villi; IEL, intraepithelial lymphocytes; VH, villus height; VW, villus width.
Intraepithelial lymphocytes (IEL) number and epithelial cells number in villi were determined in 4 photomicrographs from each sample. The IEL are small round cells with nucleus centrally located and with little cytoplasm inside (Wilson et al., 1986; Figure 1B). Epithelial cells were counted to calculate the number of IEL per 100 epithelial cells.
NanoString nCounter Gene Expression
Approximately 0.50 g of ileal mucosal scraping was isolated from the ileum and stored at −80°C until use. Total RNA was extracted from ileal mucosal scrapings using RNAzol RT (Molecular Research Center, Cincinnati, OH) as per the manufacturer's protocol. The quality and quantity of the RNA samples were checked at the Molecular and Cellular Imaging Center, The Ohio State University, Wooster, OH. A total of 96 ileal samples were randomly selected and around 10 μL RNA per sample was used for gene expression analysis by Nanostring nCounter Analysis (NanoString Technologies, Seattle, WA) following the procedure described in Geiss et al. (2008). Thirteen or 14 total RNA samples were randomly used for gene expression analysis from each treatment. Genes whose expression is associated with gut nutrient transport, hypoxia, oxidative stress, inflammation, and extracellular matrix were selected as target sequences to be measured (Table 1). Code sets containing reporter and capture probes were designed by NanoString Technologies. The RNA samples were hybridized to the code sets, incubated for 16 h, and digitally analyzed for quantification.
Table 1.
List of genes analyzed by NanoString nCounter gene expression analysis.
| Accession number | Symbol | Gene full name |
|---|---|---|
| Gut nutrient transport | ||
| NM_204365 | SLC15A1 | Solute carrier family 15 member 1 |
| XM_004935370.3 | SLC3A1 | Solute carrier family 3 member 1 |
| NM_001145490.1 | SLC7A1 | Solute carrier family 7 member 1 |
| XM_425011.5 | SLC1A3 | Solute carrier family 1 member 3 |
| NM_001293240.1 | SLC5A1 | Solute carrier family 5 member 1 |
| NM_207178.1 | SLC2A2 | Solute carrier family 2 member 2 |
| XM_025142667 | SLC2A5 | Solute carrier family 2 member 5 |
| NM_001007923.1 | FABP2 | Fatty acid binding protein 2 |
| XM_015284386.2 | GALNT2 | Polypeptide N-acetylgalactosaminyltransferase 2 |
| Gut hypoxia, oxidative stress and inflammation | ||
| NM_001318434.1 | MUC2 | Mucin 2 |
| NM_205149.1 | IFNG | Interferon gamma |
| NM_204267.1 | LITAF | Lipopolysaccharide induced TNF factor |
| NM_001282432.1 | CXCR1 | C-X-C motif chemokine receptor 1 |
| NM_001123031.1 | CRH | Corticotrophin releasing hormone |
| XM_427836.6 | HSPB7 | Heat shock protein family B (small) member 7 |
| NM_001163245.1 | GPX7 | Glutathione peroxidase 7 |
| NM_001115017.4 | SELENOO | Selenoprotein O |
| NM_001277411.1 | CA3A | Carbonic anhydrase 3A |
| NM_204524.1 | IL1B | Interleukin 1, beta |
| XM_025153162 | SELE | Selectin E |
| Extracellular matrix | ||
| NM_001162399.3 | COL4A1 | Collagen type 4 alpha 1 chain |
| Housekeeping genes | ||
| NM_204902.2 | HMGB1 | High mobility group box 1 |
| NM_204861.1 | ANPEP | Alanyl aminopeptidase, membrane |
| NM_001007479.1 | RPL4 | Ribosomal protein L4 |
| XM_424881.6 | FNTA | Farnesyltransferase, CAAX box, alpha |
Statistical Analysis
Intestinal morphologic attributes were analyzed as a completely randomized design using PROC MIXED procedure of SAS, version 9.4, software (SAS Institute INC., Cary, NC). Individual pen was identified as the experimental unit. Dietary treatments were used as a fixed effect. Least square means were estimated with the LSMEANS procedure and separated with the PDIFF option. Significance was accepted at P ≤ 0.05. Gene expression was analyzed with NanoString nSolver, version 4.0, software (Nanostring Technologies, Seattle, WA). Fold change for each gene was calculated as the ratio between each dietary treatment and the control group. If ratio was higher than 1, fold change was equal to the ratio. If ratio was lower than 1, fold change was the negative inverse of the ratio. Fold differences of gene expression among the dietary treatments were calculated by the fold changes. If one of the fold changes was positive and another was negative, the fold difference of gene expression between the 2 treatments was calculated as the percentage of the multiplication of their fold changes subtracted from 100%. If the fold changes were both positive or both negative, the fold difference of gene expression between the 2 treatments was calculated as the percentage of the division of their fold changes subtracted from 100%. A heatmap was generated with RStudio, version 3.5.2, with the R pheatmap package (RStudio INC., Boston, MA). Intestinal gene expression from NanoString nCounter gene expression analysis was used for correlation coefficients analysis with ileal morphology. Final BW and pectoralis major muscle weight from Wang et al. (2020b) and pectoralis major muscle morphology and gene expression from Wang et al. (2020a) were used for correlation coefficients analysis with ileal morphology and gene expression. The final BW, pectoralis major muscle weight, morphology, and gene expression were collected from the same broilers as the present study. Pearson correlation coefficients were determined with the CORR procedure of SAS. The P ≤ 0.05 was considered as a significant difference, and P ≤ 0.10 was considered as a trend toward significance.
Results
Intestinal Morphology
Ileal morphological attributes are shown in Table 2. There was no significant dietary effect on villus height, crypt depth, the ratio of villus height to crypt depth, villus width, surface area of villi, or IEL (P > 0.05). However, there was a trend that distance between villi was different among the treatments (P = 0.08). Broilers supplemented with n-3 fatty acids in the grower diet had a 26.0% decrease of distance between villi compared with the broilers fed dietary n-3 fatty acids in the starter diet (P = 0.01).
Table 2.
Effect of vitamin E and omega-3 fatty acids on ileal morphology of broilers.
| Treatments1 |
SEM | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Starter VE | Starter n-3 | Starter VE and n-3 | Grower VE | Grower n-3 | Grower VE and n-3 | |||
| Villus height (μm) | 1,241.12 | 1,241.73 | 1,263.05 | 1,235.35 | 1,239.9 | 1,262.41 | 1,253.86 | 20.86 | 0.99 |
| Crypt depth (μm) | 276.36 | 274.87 | 275.80 | 289.37 | 271.76 | 286.25 | 303.21 | 3.26 | 0.19 |
| Villus/crypt2 | 4.60 | 4.62 | 4.64 | 4.50 | 4.70 | 4.53 | 4.22 | 0.07 | 0.71 |
| Villus width (μm) | 281.83 | 275.88 | 278.40 | 287.85 | 261.50 | 258.79 | 263.02 | 3.22 | 0.21 |
| Surface area (mm2) | 1.10 | 1.09 | 1.12 | 1.13 | 1.02 | 1.06 | 1.04 | 0.02 | 0.79 |
| Distance between villi (μm) | 54.34 | 55.56 | 63.96 | 56.01 | 60.07 | 50.78 | 57.27 | 1.08 | 0.08 |
| IEL3 | 25.85 | 24.69 | 24.36 | 25.09 | 21.65 | 24.61 | 21.29 | 0.61 | 0.24 |
Broilers in control group were fed diets with standard level of vitamin E (VE; 10 IU/kg) and omega-3 (n-3) fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Supplementation of dietary VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both were fed during the starter phase (0–10 d) or grower phase (11–24 d).
Villus/crypt = Ratio of villus height to crypt depth.
IEL: Number of intraepithelial lymphocytes per 100 epithelial cells.
NanoString nCounter Gene Expression
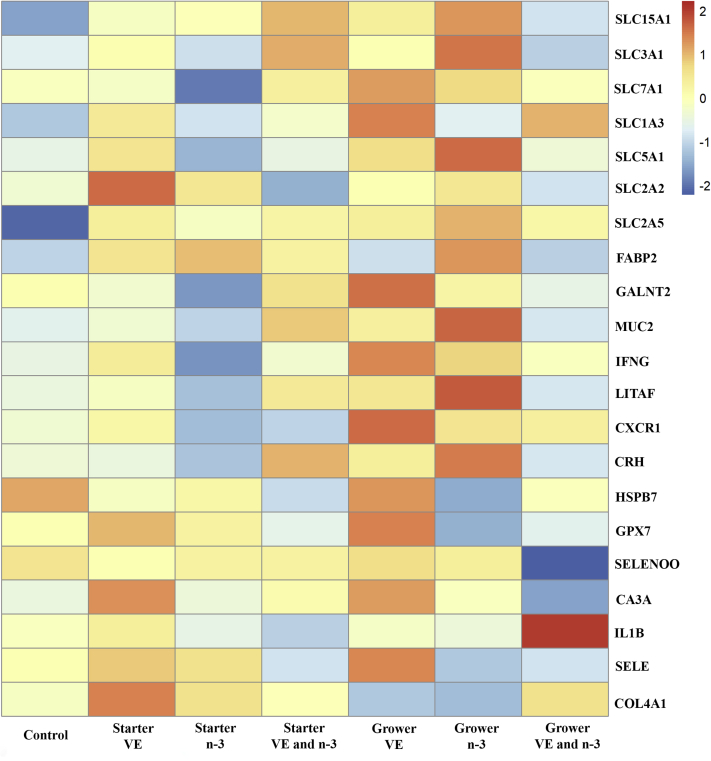
Heatmap analysis of gene expression from RNA extracted from ileal mucosal scrapings in different dietary treatments is shown in Figure 2. These data revealed differential gene expression among the treatments. Normalized gene expression abundance is color-coded as per the legend. Redness represents upregulation of genes, while blueness represents downregulation of genes compared with the control group. Table 3 contains the gene fold changes. In terms of genes related with gut nutrient transport, expression of solute carrier family 15 member 1 (SLC15A1; P = 0.01) had a 52% increase in the broilers fed dietary n-3 fatty acids in the grower diet compared with the control group. Expression of solute carrier family 7 member 1 (SLC7A1) was significantly decreased in the group supplemented with n-3 fatty acids during the starter phase compared with the group of n-3 fatty acids supplementation in the grower diet (P = 0.01) and the group of VE and n-3 fatty acids supplementation in the grower diet (P = 0.03). Supplementation of VE in the grower diet had a 15.6% increase in expression of polypeptide N-acetylgalactosaminyltransferase 2 (GALNT2) compared with n-3 fatty acids supplementation in the starter diet (P = 0.04).
Figure 2.
Heatmap for gene expression in ileal mucosal scrapings from broilers with different early posthatch dietary treatments. The heatmap shows the expression of each gene (in rows) and treatments (in columns). The normalized expression levels are color-coded as per the legend. Redness represents upregulation of gene expression, while blueness represents downregulation of gene expression compared with the control group. Broilers in the control group were fed diets with a standard level of vitamin E (VE; 10 IU/kg) and omega-3 (n-3) fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Supplementation of dietary VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both were fed during the starter phase (0–10 d) or grower phase (11–24 d). Ileal mucosal scrapings were collected when the broilers were harvested at 58 d of age.
Table 3.
Effect of vitamin E and omega-3 fatty acids on ileal relative gene expression (fold change1).
| Item | Treatments2 |
||||||
|---|---|---|---|---|---|---|---|
| Starter VE | Starter n-3 | Starter VE and n-3 | Grower VE | Grower n-3 | Grower VE and n-3 | ||
| Gut nutrient transport | |||||||
| SLC15A1 | Solute carrier family 15 member 1 | 1.26 | 1.28 | 1.47 | 1.35 | 1.52 | 1.13 |
| SLC3A1 | Solute carrier family 3 member 1 | 1.08 | −1.03 | 1.19 | 1.07 | 1.23 | −1.04 |
| SLC7A1 | Solute carrier family 7 member 1 | −1.01 | −1.21 | 1.04 | 1.13 | 1.08 | 1.00 |
| SLC1A3 | Solute carrier family 1 member 3 | 1.24 | 1.04 | 1.13 | 1.37 | 1.07 | 1.32 |
| SLC5A1 | Solute carrier family 5 member 1 | 1.11 | −1.09 | 1.01 | 1.12 | 1.22 | 1.02 |
| SLC2A2 | Solute carrier family 2 member 2 | 1.53 | 1.23 | −1.41 | 1.1 | 1.24 | −1.16 |
| SLC2A5 | Solute carrier family 2 member 5 | 1.16 | 1.13 | 1.15 | 1.16 | 1.21 | 1.15 |
| FABP2 | Fatty acid binding protein 2 | 1.14 | 1.18 | 1.12 | 1.01 | 1.2 | −1.00 |
| GALNT2 | Polypeptide N-acetylgalactosaminyltransferase 2 | −1.01 | −1.08 | 1.02 | 1.07 | 1.01 | −1.03 |
| Gut hypoxia, oxidative stress and inflammation | |||||||
| MUC2 | Mucin 2, oligomeric mucus/gel-forming | 1.06 | −1.08 | 1.27 | 1.17 | 1.41 | −1.03 |
| IFNG | Interferon gamma | 1.11 | −1.17 | 1.03 | 1.22 | 1.14 | 1.04 |
| LITAF | Lipopolysaccharide-induced TNF factor | 1.02 | −1.06 | 1.07 | 1.08 | 1.17 | −1.03 |
| CXCR1 | C-X-C motif chemokine receptor 1 | 1.03 | −1.07 | −1.05 | 1.12 | 1.06 | 1.04 |
| CRH | Corticotrophin-releasing hormone | −1.01 | −1.13 | 1.19 | 1.11 | 1.25 | −1.06 |
| HSPB7 | Heat-shock protein family B member 7 | −1.2 | −1.14 | −1.38 | 1.02 | −1.53 | −1.18 |
| GPX7 | Glutathione peroxidase 7 | 1.06 | 1.01 | −1.04 | 1.08 | −1.09 | −1.04 |
| SELENOO | Selenoprotein O | −1.02 | −1.01 | −1.01 | 1 | −1.01 | −1.09 |
| CA3A | Carbonic anhydrase 3A | 1.29 | 1.01 | 1.09 | 1.27 | 1.06 | −1.21 |
| IL1B | IL 1, beta | 1.05 | −1.05 | −1.12 | −1.01 | −1.03 | 1.24 |
| SELE | Selectin E | 1.08 | 1.06 | −1.09 | 1.13 | −1.13 | −1.09 |
| Extracellular matrix | |||||||
| COL4A1 | Collagen type 4 alpha 1 chain | 1.62 | 1.29 | 1.06 | −1.68 | −1.83 | 1.30 |
The fold change for each gene was calculated as the ratio between treatments and the control group. If the ratio was higher than 1, fold change is equal to the ratio. If the ratio was lower than 1, fold change is the negative inverse of the ratio.
Broilers in the control group were fed diets with standard level of vitamin E (VE; 10 IU/kg) and omega-3 (n-3) fatty acids (n-6/n-3 ratio of 30.2:1) during the entire study (0–58 d). Supplementation of dietary VE (200 IU/kg), n-3 fatty acids (n-6/n-3 ratio of 3.2:1), or combination of both were performed during the starter phase (0–10 d) or grower phase (11–24 d).
For genes associated with gut hypoxia, oxidative stress, and inflammation, expression of mucin 2 (MUC2) was increased when the broilers were fed dietary n-3 fatty acids in the grower diet compared with the broilers fed with the control diet (P = 0.03) and n-3 fatty acids in the starter diet (P = 0.01). Broilers supplemented with VE during the starter phase had a 29.9% increase in expression of interferon gamma (IFNG) compared with the broilers supplemented with n-3 fatty acids during the starter phase (P = 0.01). Vitamin E supplementation in the starter diet (P = 0.01) and grower diet (P = 0.03) decreased expression of carbonic anhydrase 3A (CA3A) in the broilers compared with VE and n-3 fatty acids supplementation during the grower phase. Supplemental VE in the grower diet had a 56.1% increase in expression of heat-shock protein family B (small) member 7 (HSPB7) compared with the group of n-3 fatty acids supplementation in the grower diet (P = 0.04). Expression of collagen type 4 alpha 1 chain (COL4A1), an indicator of structure of the basement membrane, had a 196% increase in the broilers fed dietary VE in the starter diet compared with the broilers supplemented with n-3 fatty acids in the grower diet (P = 0.03).
Correlation Coefficient of Morphology and Gene Expression
Significant correlations between ileal morphology and ileal gene expression are shown in Table 4. Expression of SLC15A1 (r = 0.36, P < 0.01), SLC5A1 (r = 0.23, P = 0.03), and MUC2 (r = 0.30, P < 0.01) was positively correlated with villus height. Expression of HSPB7 was positively correlated with crypt depth (r = 0.26, P = 0.01). Expression of SLC15A1 (r = 0.31, P < 0.01), SLC5A1 (r = 0.26, P = 0.01), GALNT2 (r = 0.25, P = 0.01), and MUC2 (r = 0.30, P < 0.01) was positively correlated, and HSPB7 (r = −0.35, P < 0.01) was negatively correlated with the ratio of villus height to crypt depth. There was a trend of positive correlation between surface area and expression of SLC15A1 (r = 0.17, P = 0.09). Meanwhile, IEL were positively correlated with expression of IFNG (r = 0.25, P = 0.02).
Table 4.
Correlation coefficients for ileal morphology and gene expression1.
| SLC15A12 | SLC5A13 | GALNT24 | MUC25 | IFNG6 | HSPB77 | |
|---|---|---|---|---|---|---|
| Villus height (μm) | ||||||
| Pearson | 0.36 | 0.23 | 0.17 | 0.30 | 0.16 | −0.19 |
| P-value8 | <0.01 | 0.03 | 0.10 | <0.01 | 0.14 | 0.07 |
| Crypt depth (μm) | ||||||
| Pearson | 0.07 | 0.04 | −0.11 | 0.02 | 0.17 | 0.26 |
| P-value | 0.48 | 0.69 | 0.29 | 0.83 | 0.09 | 0.01 |
| Villus/crypt9 | ||||||
| Pearson | 0.31 | 0.26 | 0.25 | 0.30 | 0.17 | −0.35 |
| P-value | <0.01 | 0.01 | 0.01 | <0.01 | 0.10 | <0.01 |
| Surface area (mm2) | ||||||
| Pearson | 0.17 | 0.11 | −0.04 | 0.11 | 0.04 | −0.11 |
| P-value | 0.09 | 0.30 | 0.67 | 0.28 | 0.69 | 0.30 |
| IEL10 | ||||||
| Pearson | 0.07 | 0.14 | 0.06 | 0.04 | 0.25 | 0.04 |
| P-value | 0.49 | 0.18 | 0.56 | 0.67 | 0.02 | 0.68 |
Pearson correlation coefficient for ileal morphology (in rows) and expression level of differentially expressed genes (in columns).
SLC15A1 = Solute carrier family 15 member 1.
SLC5A1 = Solute carrier family 5 member 1.
GALNT2 = Polypeptide N-acetylgalactosaminyltransferase 2.
MUC2 = Mucin 2, oligomeric mucus/gel-forming.
IFNG = Interferon gamma.
HSPB7 = Heat-shock protein family B member 7.
P-value for each Pearson correlation coefficient.
Villus/crypt = Ratio of villus height to crypt depth.
IEL = Number of intraepithelial lymphocytes per 100 epithelial cells.
Correlation coefficients between ileal morphology and gene expression and broiler final BW, pectoralis major muscle weight, morphology, and gene expression are shown in Table 5. Broiler final BW, pectoralis major muscle weight, and pectoralis major muscle fiber width were positively correlated with villus height, the ratio of villus height to crypt depth, and surface area (P ≤ 0.05). Broiler final BW (r = −0.21, P = 0.04) and pectoralis major muscle weight (r = −0.20, P = 0.05) were negatively correlated with ileal HSPB7 expression. The pectoralis major muscle fiber width was positively correlated with ileal COL4A1 expression (r = 0.23, P = 0.02) and MUC2 expression (r = 0.20, P = 0.05). Morphology score, which was obtained with higher score representing more well-structured muscle fibers, was negatively correlated with IEL (r = −0.21, P = 0.04) and positively correlated with MUC2 expression (r = 0.21, P = 0.04). Expression of COL4A1 in pectoralis major muscle was negatively correlated with ileal IEL (r = −0.21, P = 0.05) and ileal HSPB7 expression (r = −0.23, P = 0.02).
Table 5.
Correlation coefficients for ileal morphology and gene expression, and broiler final BW, pectoralis major muscle weight, morphology, and gene expression1.
| Final BW | Pectoralis major muscle weight | Pectoralis major muscle fiber width | Morphology score2 | COL4A13 | |
|---|---|---|---|---|---|
| Villus height | |||||
| Pearson | 0.32 | 0.22 | 0.24 | −0.16 | −0.13 |
| P-value4 | <0.01 | 0.03 | 0.02 | 0.12 | 0.21 |
| Villus/crypt5 | |||||
| Pearson | 0.30 | 0.23 | 0.28 | −0.19 | −0.10 |
| P-value | <0.01 | 0.02 | 0.01 | 0.06 | 0.35 |
| Surface area | |||||
| Pearson | 0.28 | 0.22 | 0.27 | −0.15 | −0.01 |
| P-value | 0.01 | 0.03 | 0.01 | 0.15 | 0.96 |
| IEL6 | |||||
| Pearson | 0.08 | 0.07 | 0.17 | −0.21 | −0.21 |
| P-value | 0.46 | 0.50 | 0.10 | 0.04 | 0.05 |
| COL4A1 | |||||
| Pearson | −0.16 | −0.19 | 0.23 | 0.02 | −0.14 |
| P-value | 0.12 | 0.07 | 0.02 | 0.84 | 0.17 |
| HSPB77 | |||||
| Pearson | −0.21 | −0.20 | 0.02 | 0.17 | −0.23 |
| P-value | 0.04 | 0.05 | 0.86 | 0.09 | 0.02 |
| MUC28 | |||||
| Pearson | 0.04 | 0.02 | 0.20 | 0.21 | 0.10 |
| P-value | 0.72 | 0.86 | 0.05 | 0.04 | 0.34 |
Pearson correlation coefficient for ileal morphology and differentially expressed genes (in rows) and pectoralis major muscle (pectoralis major muscle; breast muscle) weight, morphology, and differentially expression genes (in columns). The broiler final BW and pectoralis major muscle weight from the study by Wang et al. (2020b) and pectoralis major muscle morphology and gene expression from the study Wang et al. (2020a) were used for correlation coefficients analysis with ileal morphology and gene expression. The BW, pectoralis major muscle weight, morphology and gene expression were obtained from the same broilers with the present study.
Scoring scale of 1 to five was used for pectoralis major muscle morphology evaluation. Samples with limited or no perimysial or endomysial connective tissue space, and excessive myofiber degradation were given a score of one. Samples with morphology score of five have ample perimysial and endomysial connective tissue spacing, and well-structured muscle fibers. Score of 2 to 4 are intermediate.
COL4A1 = Collagen type 4 alpha 1 chain.
P-value for each Pearson correlation coefficient.
Villus/crypt = Ratio of villus height to crypt depth.
IEL = Number of intraepithelial lymphocytes per 100 epithelial cells.
HSPB7 = Heat-shock protein family B member 7.
MUC2 = Mucin 2, oligomeric mucus/gel-forming.
Discussion
The early posthatch period has been shown to be essential for intestinal development (Noy et al., 2001; Uni et al., 2003). Broiler chicks are sensitive to nutrition during this period in which sufficient early nutrient supply improves intestinal morphology and enhances intestinal development (Noy et al., 2001; Batal and Parsons, 2002; Ao et al., 2012; Jha et al., 2019). With good intestinal development, broilers have improved nutrient absorptive functions and gut health promoting animal growth including muscle growth and potentially to decrease the occurrence of muscle myopathies (Jha et al., 2019) such as wooden breast. Nutritional interventions targeting reducing oxidative stress, such as VE, and reducing inflammation, such as n-3 fatty acids, during the early posthatch period will potentially influence gut health as well as alter nutrient absorption in the small intestine. Thus, in the present study, intestinal morphology and expression of genes associated with gut nutrient transport, hypoxia, oxidative stress, inflammation, and extracellular matrix in the ileal mucosa of broilers supplemented with VE and n-3 fatty acids independently or in combination during the starter phase (0–10 d) or grower phase (11–24 d) were investigated.
Intestinal morphology plays a crucial role as an indicator of broiler gut health. Villus height, crypt depth, villus width, surface area of the villi, and distance between villi can be used to evaluate the integrity and nutrient absorption of the gastrointestinal system (Wright, 1981; Xu et al., 2003). There was no significant difference in most of the morphologic attributes. However, supplemental n-3 fatty acids in the grower diet had a trend to decrease distance between adjacent villi compared to the group of n-3 fatty acids supplementation during the starter phase. Decreased distance between villi represents improved intestinal morphology (Uni et al., 2001; Azevedo et al., 2020), which could be more effective for nutrient absorption owing to a shorter distance of nutrients traveling and diffusion of the nutrients. Along with the intestinal morphology, expression of SLC7A1 and MUC2 was higher in the group supplemented with n-3 fatty acids in the grower diet than in the starter diet. The SLC7A1, also called cationic amino acid transporter-1, is essential in transferring cationic amino acids from enterocytes to the blood for circulation (Devés and Boyd, 1998). The mucus layer in the intestinal epithelium is mainly composed of mucin, which is synthesized by goblet cells (Deplancke and Gaskins, 2001). Mucin 2 is the main gel-forming mucin providing a protective barrier to the ileal epithelium against antigens (Velcich et al., 2002). It is widely recognized as a marker of gut health in poultry (Forder et al., 2012; Wei et al., 2012; Li et al., 2015). Absorption and immune function has been shown to be affected by mucin deficiency (Uni et al., 2003). In addition, n-3 fatty acids supplementation during the grower phase increased expression level of SLC15A1 and MUC2 compared with the control group. Solute carrier family 15 member 1, a peptide transporter, is a key transporter of dipeptides and tripeptides in the enterocytes (Osmanyan et al., 2018). Expression level of SLC15A1 is closely related with protein synthesis and degradation (Gaildrat et al., 2005). Therefore, decreased distance between villi and increased gene expression levels indicate that supplementation of n-3 fatty acids during the grower phase showed a more beneficial effect on improving gut health than supplementation during the starter phase. The intestinal mucus barrier and nutrient transport would be enhanced by n-3 fatty acids supplementation in the grower diet.
In terms of differentially expressed genes in the VE and n-3 fatty acids combined supplementation group, expression of SLC7A1 was increased when broilers were fed dietary Vitamin E and n-3 fatty acids in the grower phase is compared with the n-3 fatty acids supplementation group during the starter phase. In addition, expression of CA3A was decreased in the VE and n-3 fatty acids group supplemented during the grower phase compared with the VE group supplemented during the starter phase or the grower phase. Most carbonic anhydrases are efficient enzymes catalyzing the reversible hydration reaction of carbon dioxide (Breton, 2001). The CA3A has also shown an effect on fatty acids metabolism converting acetyl-CoA into malonyl-CoA (Alver et al., 2004). Lipid metabolism has been found to modulate macrophage response by triggering proinflammatory activities (Riera-Borrull et al., 2017). Therefore, supplementation of VE and n-3 fatty acids during the grower phase may be associated with improved nutrient absorption, amino acid transport, altered lipid synthesis, and reduced proinflammatory activities in small intestines.
Another differentially expressed gene is COL4A1, which has an increased expression in the broilers supplemented with VE in the starter diet compared with n-3 fatty acids supplementation during the grower phase. Collagen type IV is a critical component of gut basement membrane (Zhang et al., 2003). Basement membrane has sheet-like structure acting as a barrier separating extracellular matrix and epithelial cells (Glanville, 1987). Epithelial dysfunction could be produced when the basement membrane is altered (Groulx et al., 2011). Higher expression of COL4A1 in the group supplemented with VE during the starter phase indicates improved integrity of basement membrane.
Correlation coefficient analysis comparing intestinal morphology to intestinal gene expression showed that SLC15A1, SLC5A1, GALNT2, and MUC2 were positively correlated with villus height or the ratio of villus height to crypt depth. Although the correlations were not strong, these correlations could be beneficial for understanding the relationship between intestinal morphology and gene expression during development. The SLC15A1 is responsible for dipeptides and tripeptides transport in the enterocytes (Osmanyan et al., 2018). The SLC5A1 is a sodium glucose cotransporter transporting glucose from the intestine (Wright, 2013). The GALNT2 encodes polypeptide N-acetylgalactosaminyltransferase 2, which transfers N-acetylgalactosamine to serine or threonine residue during the biosynthesis of O-linked oligosaccharide (Ten Hagen et al., 2003). The positive correlation between ileal morphology and expression of the gene involved in nutrient transport suggests that intestinal nutrient transport are closely associated with ileal structure. Meanwhile, GALNT2 is also involved in glycosylation of mucin (Ten Hagen et al., 2003), which is consistent with the present study that GALNT2 and MUC2 has similar correlation pattern with the ileal morphology. There was a positive correlation between expression of IFNG and IEL. Both IFNG and IEL can indicate inflammatory state in the gastrointestinal system. The IFNG can be produced by T cells, macrophages, natural killer cells, and mucosal epithelial cells (Adams, 1989; Schroder et al., 2004). It plays a variety of roles in innate and adaptive immune responses (Young, 1996; Bach et al., 1997). In addition, HSPB7 was negatively correlated with the ratio of villus height to crypt depth. The HSPB7 is a member of heat-shock protein family (Vos et al., 2009). It is involved in responses to oxidative stress by activating nuclear factor erythroid 2–related factor 2 signaling pathway (Sun et al., 2019). These genes associated with oxidative stress and inflammation were correlated with ileal morphology, indicating that intestinal oxidative stress and inflammation status can affect intestinal structure and function.
Ileal morphology and gene expression were correlated with broiler final BW, pectoralis major muscle weight, morphology, and gene expression. Ileal villus height, the ratio of villus height to crypt depth, and surface area were positively correlated with broiler final BW, pectoralis major muscle weight, and pectoralis major muscle fiber width. Ileal COL4A1 and MUC2 were positively correlated with pectoralis major muscle fiber width. These could be suggestive that improved intestinal structure would have positive influence on intestinal nutrient absorption, thereby have beneficial effect on growth performance and breast muscle growth. This is consistent with Sugiharto (2016) that a well-functioned gastrointestinal system is an important factor to promote broiler growth performance. On the other hand, HSPB7, the expression of which would be upregulated in responses to oxidative stress (Sun et al., 2019), was negatively correlated with final BW and pectoralis major muscle weight. This showed that intestinal oxidative stress would detrimentally affect broiler BW and breast muscle growth. The positive correlation between ileal MUC2 and pectoralis major muscle morphology score indicates that intestinal structure is closely related with pectoralis major muscle structure. The pectoralis major muscle morphology score and expression of COL4A1 in pectoralis major muscle were negatively correlated with ileal IEL. The IEL serve as a critical indicator of the intestinal inflammatory state, and IEL have a variety of immune functions (Yamamoto et al., 1998; Kakar et al., 2003). They are in the epithelial layer of the intestine and facilitate intestinal immune surveillance to sense antigenic challenges in the gut (Kakar et al., 2003; Rieger et al., 2015). In addition, expression of COL4A1 in pectoralis major muscle was negatively correlated with ileal HSPB7. The pectoralis major muscle morphology score evaluation used a 1-to-5 scoring scale with score of 1 representing limited or no perimysial or endomysial connective tissue space and excessive myofiber degradation and a score of 5 having ample perimysial and endomysial connective tissue spacing and well-structured muscle fibers. The negative correlations suggest that broilers with lower level of ileal oxidative stress and inflammation would have more well-structured breast muscle fibers as well as improved breast muscle basement membrane. The pectoralis major muscle growth could be closely related with intestinal inflammation and oxidative stress levels.
In conclusion, supplementation of VE and n-3 fatty acids independently and in combination showed a more beneficial effect on improving intestinal morphology during the grower phase than the starter phase. Genes involved in gut nutrient transport, oxidative stress, and inflammation were differentially expressed in the broilers supplemented with VE, n-3 fatty acids, or combination of both during the grower phase. Intestinal morphologic results were consistent with changes in gene expression implying a positive effect of VE and n-3 fatty acids supplementation during the grower phase on improving gut health and nutrient transport, with supplementation of n-3 fatty acids during the grower phase showing the most beneficial effects. Future research needs to be focused on determining the most beneficial supplementation concentration and administration period to improve gut health in the broilers.
Acknowledgments
This study was supported by US Poultry and Egg grant (project No. 710) to SGV and SKJ and the China Scholarship Council (No. 201706350026) to JW. The authors would like to thank Janet McCormick for technical assistance.
Disclosures
The authors declare no conflicts of interest.
References
- Adams D.O. Molecular interactions in macrophage activation. Immunol. Today. 1989;10:33–35. doi: 10.1016/0167-5699(89)90298-3. [DOI] [PubMed] [Google Scholar]
- Al-Khalifa H., Givens D.I., Rymer C., Yaqoob P. Effect of n-3 fatty acids on immune function in broiler chickens. Poult. Sci. 2012;91:74–88. doi: 10.3382/ps.2011-01693. [DOI] [PubMed] [Google Scholar]
- Alver A., Uçar F., Keha E.E., Kalay E., Ovali E. Effects of leptin and insulin on CA III expression in rat adipose tissue. J. Enzyme Inhib. Med. Chem. 2004;19:279–281. doi: 10.1080/14756360410001720445. [DOI] [PubMed] [Google Scholar]
- Ao Z., Kocher A., Choct M. Effects of dietary additives and early feeding on performance, gut development and immune status of broiler chickens challenged with Clostridium perfringens. Asian-Australas. J. Anim. Sci. 2012;25:541–551. doi: 10.5713/ajas.2011.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen . Aviagen; Huntsville, AL: 2016. Ross Broiler Management Manual. [Google Scholar]
- Azevedo K.S.P., Cavalcante D.T., Campos P.H.R.F., Rocha G.C., Borges S.O., do Vale B.G., de Souza Miranda J.V., Calderano A.A. Prebiotic effect on performance and intestinal morphometry of broilers chickens. RBAS. 2020;10:38–44. [Google Scholar]
- Bach E.A., Aguet M., Schreiber R.D. The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- Bartov I., Frigg M. Effect of high concentrations of dietary vitamin E during various age periods on performance, plasma vitamin E and meat stability of broiler chicks at 7 weeks of age. Br. Poult. Sci. 1992;33:393–402. doi: 10.1080/00071669208417477. [DOI] [PubMed] [Google Scholar]
- Batal A.B., Parsons C.M. Effect of fasting versus feeding Oasis after hatching on nutrient utilization in chicks. Poult. Sci. 2002;81:853–859. doi: 10.1093/ps/81.6.853. [DOI] [PubMed] [Google Scholar]
- Breton S. The cellular physiology of carbonic anhydrases. JOP. 2001;2:159–164. [PubMed] [Google Scholar]
- Brock T.G., Peters-Golden M. Activation and regulation of cellular eicosanoid biosynthesis. ScientificWorld Journal. 2007;7:1273–1284. doi: 10.1100/tsw.2007.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G.W., Traber M.G. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu. Rev. Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- Calder P.C. n-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Calder P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- Cheng K., Zhang M., Huang X., Zheng X., Song Z., Zhang L., Wang T. An evaluation of natural and synthetic vitamin E supplementation on growth performance and antioxidant capacity of broilers in early age. Can. J. Anim. Sci. 2017;98:187–193. [Google Scholar]
- De Los Santos F.S., Donoghue A.M., Farnell M.B., Huff G.R., Huff W.E., Donoghue D.J. Gastrointestinal maturation is accelerated in Turkey poults supplemented with a mannan-oligosaccharide yeast extract (Alphamune) Poult. Sci. 2007;86:921–930. doi: 10.1093/ps/86.5.921. [DOI] [PubMed] [Google Scholar]
- Deplancke B., Gaskins H.R. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- Devés R., Boyd C.A.R. Transporters for cationic amino acids in animal cells: Discovery, structure, and function. Physiol. Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- El-Katcha M.I., El-Kholy M.E., Soltan M.A., El-Gayar A.H. Effect of dietary omega-3 to omega-6 ratio on growth performance, immune response, carcass traits and meat fatty acids profile of broiler chickens. Poult. Sci. J. 2014;2:71–94. [Google Scholar]
- Forder R.E.A., Nattrass G.S., Geier M.S., Hughes R.J., Hynd P.I. Quantitative analyses of genes associated with mucin synthesis of broiler chickens with induced necrotic enteritis. Poult. Sci. 2012;91:1335–1341. doi: 10.3382/ps.2011-02062. [DOI] [PubMed] [Google Scholar]
- Gaildrat P., Møller M., Mukda S., Humphries A., Carter D.A., Ganapathy V., Klein D.C. A novel pineal-specific product of the oligopeptide transporter PepT1 gene. J. Biol. Chem. 2005;280:16851–16860. doi: 10.1074/jbc.M414587200. [DOI] [PubMed] [Google Scholar]
- Geiss G.K., Bumgarner R.E., Birditt B., Dahl T., Dowidar N., Dunaway D.L., Fell H.P., Ferree S., George R.D., Grogan T., James J.J., Maysuria M., Mitton J.D., Oliveri P., Osborn J.L., Peng T., Ratcliffe A.L., Webster P.J., Davidson E.H., Hood L., Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Glanville R.W. Structure and Function of Collagen Types. Academic Press Inc.; Orlando, FL: 1987. Type IV collagen; pp. 43–79. [Google Scholar]
- Groulx J.F., Gagné D., Benoit Y.D., Martel D., Basora N., Beaulieu J.F. Collagen VI is a basement membrane component that regulates epithelial cell–fibronectin interactions. Matrix Biol. 2011;30:195–206. doi: 10.1016/j.matbio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Haug A., Eich-Greatorex S., Bernhoft A., Wold J.P., Hetland H., Christophersen O.A., Sogn T. Effect of dietary selenium and omega-3 fatty acids on muscle composition and quality in broilers. Lipids Health Dis. 2007;6:29. doi: 10.1186/1476-511X-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocquette J.F., Ortigues-Marty J., Pethick D., Herpin P., Fernandez X. Nutritional and hormonal regulation of energy metabolism in skeletal muscles of meat-producing animals. Livest. Prod. Sci. 1998;56:115–143. [Google Scholar]
- Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O., Arai H., Inoue K. Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- Jarrold B.B., Bacon W.L., Velleman S.G. Expression and localization of the proteoglycan decorin during the progression of cholesterol induced atherosclerosis in Japanese quail: implications for interaction with collagen type I and lipoproteins. Atherosclerosis. 1999;146:299–308. doi: 10.1016/s0021-9150(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Jha R., Singh A.K., Yadav S., Berrocoso J.F.D., Mishra B. Early nutrition programming (in ovo and post-hatch feeding) as a strategy to modulate gut health of poultry. Front. Vet. Sci. 2019;6:82. doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar S., Nehra V., Murray J.A., Dayharsh G.A., Burgart L.J. Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am. J. Gastroenterol. 2003;98:2027–2033. doi: 10.1111/j.1572-0241.2003.07631.x. [DOI] [PubMed] [Google Scholar]
- Korver D.R., Klasing K.C. Dietary fish oil alters specific and inflammatory immune responses in chicks. J. Nutr. 1997;127:2039–2046. doi: 10.1093/jn/127.10.2039. [DOI] [PubMed] [Google Scholar]
- Li C., Guo S., Gao J., Guo Y., Du E., Lv Z., Zhang B. Maternal high-zinc diet attenuates intestinal inflammation by reducing DNA methylation and elevating H3K9 acetylation in the A20 promoter of offspring chicks. J. Nutr. Biochem. 2015;26:173–183. doi: 10.1016/j.jnutbio.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Lu T., Harper A.F., Zhao J., Dalloul R.A. Effects of a dietary antioxidant blend and vitamin E on growth performance, oxidative status, and meat quality in broiler chickens fed a diet high in oxidants. Poult. Sci. 2014;93:1649–1657. doi: 10.3382/ps.2013-03826. [DOI] [PubMed] [Google Scholar]
- National Research Council . Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirement of Poultry: Ninth Revised Edition. [Google Scholar]
- Niki E., Noguchi N., Gotoh N. Dynamics of lipid peroxidation and its inhibition by antioxidants. Biochem. Soc. Trans. 1993;21:313–317. doi: 10.1042/bst0210313. [DOI] [PubMed] [Google Scholar]
- Noy Y., Geyra A., Sklan D. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci. 2001;80:912–919. doi: 10.1093/ps/80.7.912. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Metabolic responses to early nutrition. Appl. Poult. Sci. 1998;7:437–451. [Google Scholar]
- Osmanyan A.K., Ghazi Harsini S., Mahdavi R., Fisinin V.I., Arkhipova A.L., Glazko T.T., Kovalchuk S.N., Kosovsky G.Y. Intestinal amino acid and peptide transporters in broiler are modulated by dietary amino acids and protein. Amino Acids. 2018;50:353–357. doi: 10.1007/s00726-017-2510-6. [DOI] [PubMed] [Google Scholar]
- Panda A.K., Cherian G. Role of vitamin E in counteracting oxidative stress in poultry. J. Poult. Sci. 2014;51:109–117. [Google Scholar]
- Pitargue F.M., Kim J.H., Goo D., Reyes J.D., Kil D.Y. Effect of vitamin E sources and inclusion levels in diets on growth performance, meat quality, alpha-tocopherol retention, and intestinal inflammatory cytokine expression in broiler chickens. Poult. Sci. 2019;98:4584–4594. doi: 10.3382/ps/pez149. [DOI] [PubMed] [Google Scholar]
- Rebolé A., Rodriguez M.L., Ortiz L.T., Alzueta C., Centeno C., Viveros A., Brenes A., Arija I. Effect of dietary high-oleic acid sunflower seed, palm oil and vitamin E supplementation on broiler performance, fatty acid composition and oxidation susceptibility of meat. Br. Poult. Sci. 2006;47:581–591. doi: 10.1080/00071660600939727. [DOI] [PubMed] [Google Scholar]
- Reiser R. Fatty acid changes in egg yolk of hens on a fat-free and a cottonseed oil ration. J. Nutr. 1949;40:429–440. [Google Scholar]
- Rieger J., Janczyk P., Hünigen H., Neumann K., Plendl J. Intraepithelial lymphocyte numbers and histomorphological parameters in the porcine gut after Enterococcus faecium NCIMB 10415 feeding in a Salmonella Typhimurium challenge. Vet. Immunol. Immunopathol. 2015;164:40–50. doi: 10.1016/j.vetimm.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Riera-Borrull M., Cuevas V.D., Alonso B., Vega M.A., Joven J., Izquierdo E., Corbí Á.L. Palmitate conditions macrophages for enhanced responses toward inflammatory stimuli via JNK Activation. J. Immunol. 2017;199:3858–3869. doi: 10.4049/jimmunol.1700845. [DOI] [PubMed] [Google Scholar]
- Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites—implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Saleh H., Rahimi Sh., Karimi Torshizi M.A. The effect of diet that contained fish oil on performance, serum parameters, the immune system and the fatty acid composition of meat in broilers. Int. J. Vet. Res. 2009;2:69–75. [Google Scholar]
- Schreiner M., Hulan H.W., Razzazi-Fazeli E., Böhm J., Moreira R.G. Effect of different sources of dietary omega-3 fatty acids on general performance and fatty acid profiles of thigh, breast, liver and portal blood of broilers. J. Sci. Food Agric. 2005;85:219–226. [Google Scholar]
- Schroder K., Hertzog P., Ravasi T., Hume D.A. Interferon gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi. Soc. 2016;15:99–111. [Google Scholar]
- Sun X., Li X., Jia H., Loor J.J., Bucktrout R., Xu Q., Wang Y., Shu X., Dong J., Zuo R., Yang L., Liu G., Li X. Effect of heat-shock protein B7 on oxidative stress in adipocytes from preruminant calves. J. Dairy Sci. 2019;102:5673–5685. doi: 10.3168/jds.2018-15726. [DOI] [PubMed] [Google Scholar]
- Tappel A.L. Vitamins & Hormones. Academic Press Inc.; Orlando, FL: 1962. Vitamin E as the biological lipid antioxidant; pp. 493–510. [Google Scholar]
- Taulescu C., Mihaiu M., Bele C., Matea C., Dan S.D., Mihaiu R., Lapusan A. Antioxidant effect of vitamin E and selenium on omega-3 enriched poultry meat. Vet. Med. 2011;68:293–300. [Google Scholar]
- Ten Hagen K.G., Fritz T.A., Tabak L.A. All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003;13:1–16. doi: 10.1093/glycob/cwg007. [DOI] [PubMed] [Google Scholar]
- Uni, Z., and Ferket, P. R. 2003. Enhancement of development of oviparous species by in ovo feeding. North Carolina State University, Raleigh, NC, and Yissum Research Development Company of Hebrew University of Jerusalem, Jerusalem, IL. US Pat. No. 6,592,878.
- Uni Z., Gal-Garber O., Geyra A., Sklan D., Yahav S. Changes in growth and function of chick small intestine epithelium due to early thermal conditioning. Poult. Sci. 2001;80:438–445. doi: 10.1093/ps/80.4.438. [DOI] [PubMed] [Google Scholar]
- Uni Z., Noy Y., Sklan D. Posthatch development of small intestinal function in the poult. Poult. Sci. 1999;78:215–222. doi: 10.1093/ps/78.2.215. [DOI] [PubMed] [Google Scholar]
- Uni Z., Smirnov A., Sklan D. Pre- and posthatch development of goblet cells in the broiler small intestine: effect of delayed access to feed. Poult. Sci. 2003;82:320–327. doi: 10.1093/ps/82.2.320. [DOI] [PubMed] [Google Scholar]
- Velcich A., Yang W.C., Heyer J., Fragale A., Nicholas C., Viani S., Kucherlapati R., Lipkin M., Yang K., Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Coy C.S., Anderson J.W., Patterson R.A., Nestor K.E. Effect of selection for growth rate on embryonic breast muscle development in turkeys. Poult. Sci. 2002;81:1113–1121. doi: 10.1093/ps/81.8.1113. [DOI] [PubMed] [Google Scholar]
- Vos M.J., Kanon B., Kampinga H.H. HSPB7 is a SC35 speckle resident small heat shock protein. Biochim. Biophys. Acta. 2009;1793:1343–1353. doi: 10.1016/j.bbamcr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wang J., Clark D.L., Jacobi S.K., Velleman S.G. Effect of early post-hatch supplementation of vitamin E and omega-3 fatty acids on the severity of wooden breast, breast muscle morphological structure, and gene expression in the broiler breast muscle. Poult. Sci. 2020;99:5925–5935. doi: 10.1016/j.psj.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Clark D.L., Jacobi S.K., Velleman S.G. Effect of vitamin E and omega-3 fatty acids early posthatch supplementation on reducing the severity of wooden breast myopathy in broilers. Poult. Sci. 2020;99:2108–2119. doi: 10.1016/j.psj.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.W., Field C.J., Sim J.S. Dietary polyunsaturated fatty acids alter lymphocyte subset proportion and proliferation, serum immunoglobulin G concentration, and immune tissue development in chicks. Poult. Sci. 2000;79:1741–1748. doi: 10.1093/ps/79.12.1741. [DOI] [PubMed] [Google Scholar]
- Wei X., Yang Z., Rey F.E., Ridaura V.K., Davidson N.O., Gordon J.I., Semenkovich C.F. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe. 2012;11:140–152. doi: 10.1016/j.chom.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.D., Stokes C.R., Bourne F.J. Morphology and functional characteristics of isolated porcine intraepithelial lymphocytes. Immunology. 1986;59:109–113. [PMC free article] [PubMed] [Google Scholar]
- Wright N.A. The experimental analysis of changes in proliferative and morphological status in studies on the intestine. Scand. J. Gastroenterol. Suppl. 1981;74:3–10. [PubMed] [Google Scholar]
- Wright E.M. Glucose transport families SLC5 and SLC50. Mol. Asp. Med. 2013;34:183–196. doi: 10.1016/j.mam.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Kawabata K., McGhee J.R., Kiyono H. A mucosal intranet: intestinal Epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J. Immunol. 1998;160:2188–2196. [PubMed] [Google Scholar]
- Yamauchi K., Kamisoyama H., Isshiki Y. Effects of fasting and refeeding on structures of the intestinal villi and epithelial cells in White Leghorn hens. Br. Poult. Sci. 1996;37:909–921. doi: 10.1080/00071669608417922. [DOI] [PubMed] [Google Scholar]
- Young H.A. Regulation of interferon-γ gene expression. J. Interferon Cytokine Res. 1996;16:563–568. doi: 10.1089/jir.1996.16.563. [DOI] [PubMed] [Google Scholar]
- Yu C., Tan S., Wang Z., Yu Z., Zhuang S. Omega-3 polyunsaturated fatty acids reduce intestinal inflammation and enhance intestinal motility associated with reduced nitric oxide production in chronic kidney disease. Clin. Nutr. 2018;37:S93. S93. [Google Scholar]
- Zhang J., Li W., Sanders M.A., Sumpio B.E., Panja A., Basson M.D. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB J. 2003;17:926–928. doi: 10.1096/fj.02-0663fje. [DOI] [PubMed] [Google Scholar]