Abstract
An enriched environment can promote adaptability of animals to cope with complex environments. A total of 18-week-old 216 laying hens were randomly divided into 2 groups; of which, one group was housed in conventional battery cages (CC, n = 36), and the others were housed in furnished cages (FC, n = 180). At the end of 64 wk of age, 24 chickens of each group were selected for 4-hour transport treatment. The spleen tissues of laying hens were collected before transportation (BT), immediately after transportation, and at 48 h after transportation to detect the expression of the heat shock protective response signaling pathway and inflammatory factors. Serum samples were collected to detect the content of immune cytokines. Transport stress decreased heat shock proteins (HSP; including Small HSP, HSP27, HSP40, HSP60, HS70, HSP90, HSP110) in the CC group (P < 0.05), whereas there was no significant difference in the expression of HSP (except for Small HSP and HSP40) in the FC group (P > 0.05) immediately after transportation. At 48 h after transportation, mRNA levels of HSP (except for Small HSP and HSP40) in the FC group were upregulated, which were higher than those at BT (P < 0.05). The changes in HSP60, HSP70, and HSP90 protein levels had similar tendencies. The results showed that housing in furnished cages alleviated the inhibition of expression of HSP in the hens' spleen induced by transport stress. In addition, the hens housed in the FC group had lower expression levels of proinflammatory factors (nuclear transcription factor-kappa B, inducible nitric oxide synthase, cyclooxygenase-2, prostaglandin E synthase, inflammatory cytokines [IL-1β and IL-6], and tumor necrosis factor alpha) (P < 0.05). We suggest that the enriched environment can reduce transport stress damage in laying hens and improve resistance to transport stress by regulating expression of heat shock response proteins and inflammatory cytokines.
Key words: enriched environment, stress, heat shock response, inflammatory factor, laying hen
Introduction
Transportation is an important part of poultry and livestock management (Harris, 2001). Transportation can affect livestock and poultry to varying degrees, ranging from mild discomfort to death. Transport stress is complex, related to many factors, including loading and unloading, crowding, noise, high temperature, and lack of food and water (Smith et al., 2004). These factors affect animal welfare, carcass weight, and meat quality, causing huge economic losses (Greger, 2007, Schwartzkopf-Genswein et al., 2012). Many different approaches, such as addition of vitamins, probiotics, and antibiotics to feed, can reduce the economic lost due to transport stress (Line et al., 1997; Zulkifli et al., 2000; Sohn et al., 2013; Zhang et al., 2016).
The response of livestock and poultry to transport is not only related to transport conditions but also related to animal body size, age, and health status before transportation (Schwartzkopf-Genswein et al., 2012). Enrichment of the environment can improve the welfare level and immune function and reduce the stress response of laying hens (Rodenburg et al., 2008; Shinmura et al., 2010; Meng et al., 2015; Matur et al., 2016). It was observed that environmental enrichment significantly increased antibody production and tended to increase monocyte percentage and CD8+ cell proportion (Matur et al., 2016). Pusic et al. (2016) found that enrichment of the environment stimulated immune cell secretion of exosomes and altered immune function that supports brain health. Altan et al. (2013) demonstrated that early-enriched environmental experience could effectively reduce fear and stress response of broilers to future environmental changes. Matur et al. (2017) found that comfort behavior of laying hens in furnished cages increased and agonistic behavior decreased, compared with laying hens raised in conventional cages after transportation, indicating that enrichment of the environment was conducive to recovery of laying hens after transport stress. However, no study reported the influence of enrichment of the environment on the effects of transport stress on laying hens.
Under various stress conditions, the heat shock protective response signaling pathway was activated in body cells, and the expression of heat shock factors (HSF) and heat shock proteins (HSP) was then increased. Heat shock proteins have a protective effect on tissues under stress and maintain cellular metabolism and structural integrity (Larkins et al., 2012). Heat shock factors regulate HSP by combining heat shock elements (Pirkkala et al., 2001). Activation of nuclear transcription factor-kappa B (NF-κB) is closely related to the occurrence of inflammatory responses (Mazzarella et al., 2000). Nuclear transcription factor-kappa B controls the expression of many preinflammatory factors, including inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and prostaglandin E synthase (PTGES) (Cogswell et al., 1994), and inflammatory cytokines, such as IL, interferon gamma, and tumor necrosis factor alpha (TNF-α) (Valenzuela et al., 2005; Barber et al., 2006; Yang et al., 2016). The purpose of this study was to assess the damage of transport stress to the immune function of laying hens and determine whether the long-term experience of enriched environmental housing could enhance the tolerance of laying hens to transport stress.
Materials and methods
Ethics Statement
All experiments were approved by and conducted as per the guidelines of the Institutional Animal Care and Use Committee of Northeast Agriculture University (NEAU-[2011]-9).
Animals and Experimental Design
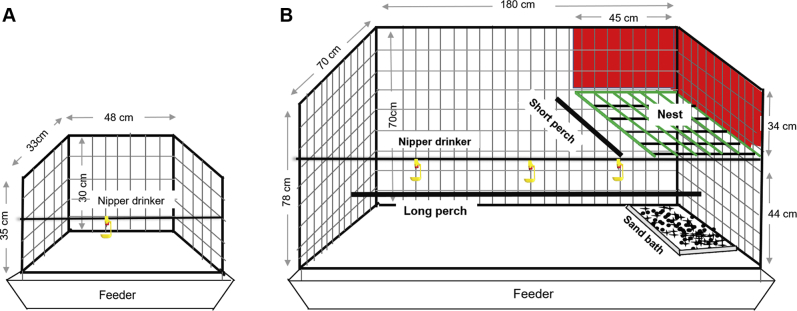
A total of 18-week-old 216 healthy laying hens were randomly divided into 2 groups, including conventional cage (CC, n = 36, 12 replicates) and furnished cage (FC, n = 180, 12 replicates) groups. In the CC group, the feeding density was 480 cm2 per bird (Figure 1A). The FC group was furnished with a nest, a sand bath, 2 perches, and a feeding density of 840 cm2 per bird (Figure 1B). The experiment was conducted in a semiclosed chicken house (temperature, 18°C–25°C; RH, 40–70%), using natural ventilation and artificial light. Artificial light was programmed for 15 h (04:30–19:30), and the light intensity was 18 to 22 lux. Immunization and disinfection were performed as per routine procedures. All laying hens had free access to feed and water. The feed came from the commercial laying hens' full price feed during the laying period (Wellhope Animal Husbandry Co., Ltd., Harbin, China). The dietary nutrition level was as follows: ME = 11.13% MJ/kg, CP = 16.08%, crude fat (CP) = 2.1%, crude fiber = 6.96%, ash content = 13.52%, calcium = 2.12 to 4.12%, total phosphorus = 0.48%, table salt = 0.29 to 0.81%, methionine = 0.31 to 0.99%, and lysine = 0.34 to 0.94%.
Figure 1.
A sample drawing of the (A) conventional cage and (B) furnished cage.
The transportation was conducted at 65 wk of age on a cloudy day at 15°C to 20°C, and the hens were fasted for 10 h before transportation without limitation of drinking water. The temperature inside the truck ranged from 24°C to 28°C. Before transport (BT), a total of 12 chickens were randomly selected from 6 replicates, with 2 birds each replicate, from the original CC group and FC group, respectively. Six chickens of 12 from each group were anesthetized using sodium pentobarbital and then slaughtered by using a sharp knife via a cut to the carotid artery to collect blood samples and spleen tissues, and the other half of the birds (6 chickens) were transported together with 6 birds selected from the other 6 replicates from the CC group and FC group, respectively, and labeled by leg rings. Two transport cages (TC) of 74 × 55.5 × 23 cm were used for the stress test. Each cage was sectioned into 6 even compartments in which 2 birds were allowed per section. A total of 12 birds from the CC group were allocated into the same TC (CC-TC, 6 replicates), and 12 birds from the FC group were allocated into another TC (FC-TC, 6 replicates). The transport treatment time was 4 h. No food and water were provided during transportation. After transportation (0 h), 6 chickens (unlabeled) were selected from each compartment in the CC-TC and FC-TC groups, respectively, for collecting samples, and six other chickens with leg rings were returned to the original house for 48 h (provided enough food and water) and sampled at 48 h after transport (48 h). All birds went through the unloading process. Chickens were killed by bleeding after anesthetizing; the spleen tissues were collected and immediately frozen in liquid nitrogen and then stored at −80°C for quantitative real-time PCR (qRT-PCR) and Western blot analysis. The blood samples were collected from the carotid artery into plastic syringes (5-mL capacity) and clotted at room temperature and then centrifuged at 2,500 rmp/min for 10 min. Serum samples were transported into new 1.5-mL Eppendorf tubes. The prepared serum was stored at −80°C for ELISA analysis.
Total RNA Extraction and Real-Time PCR Analysis
Total RNA was extracted from 100 mg of spleen tissue as per the instructions of the RNAiso Plus kit (Takara, Dalian, China). The dried RNA was resuspended in 30 μL of 1% diethyl pyrocarbonate water, and then, RNA concentration was measured by spectrophotometry, with a 260/280 ratio of 1.9–2.1 considered acceptable (Gene Quant 1300/100, GE Healthcare Bio-sciences AB, Uppsala, Sweden). RNA was reversely transcribed to synthesize cDNA as per the RR047 reverse transcription kit instructions (Takara, Dalian, China). The final cDNA samples were used for qRT-PCR.
The primers for qRT-PCR were designed using Premier 5.0 software (PREMIER Biosoft International, CA) based on the deposited sequences in GenBank and synthesized by Sangon Biotech Co. Ltd. (Shanghai, China). The primer sequences are shown in Table 1.
Table 1.
Sequence of primers used in quantitative real-time PCR.
| Gene | Reference sequence | Forward primer sequences (5'–3') | Reverse primer sequences (5'–3') |
|---|---|---|---|
| β-actin | NM 205,518.1 | CCGCTCTATGAAGGCTACGC | CTCTCGGCTGTGGTGGTGAA |
| iNOS | NM 204,961.1 | CCTGGAGGTCCTGGAAGAGT | CCTGGGTTTCAGAAGTGGC |
| NF-κB | NM 205,134.1 | TCAACGCAGGACCTAAAGACAT | GCAGATAGCCAAGTTCAGGATG |
| COX-2 | NM 0.011,67718.1 | TGTCCTTTCACTGCTTTCCAT | TTCCATTGCTGTGTTTGAGGT |
| PTGES | NM 0.011,94983.1 | GTTCCTGTCATTCGCCTTCTAC | CGCATCCTCTGGGTTAGCA |
| TNF-α | NM 204,267.1 | GCCCTTCCTGTAACCAGATG | ACACGACAGCCAAGTCAACG |
| Interferon gamma | NM 205,149.1 | GCTGACGGTGGACCTATTATTGTAGAG | TTCTTCACGCCATCAGGAAGGTTG |
| IL-2 | NM 204153.1 | CTGTATTTCGGTAGCAATG | ACTCCTGGGTCTCAGTTG |
| IL-6 | NM 204628.1 | AAATCCCTCCTCGCCAATCT | CCCTCACGGTCTTCTCCATAAA |
| IL-1β | NM 204524.1 | ACTGGGCATCAAGGGCTACA | GCTGTCCAGGCGGTAGAAGA |
| Small HSP | NM 001010842.2 | CGGCTTCATCTCCAGGTGCTTC | TTGGCTGGTTCTTCCTTCTTGGC |
| HSP27 | NM 205290.1 | ACACGAGGAGAAACAGGATGAG | ACTGGATGGCTGGCTTGG |
| HSP40 | NM 001199325.1 | GGGCATTCAACAGCATAGA | TTCACATCCCCAAGTTTAGG |
| HSP60 | NM 001012916.1 | AGCCAAAGGGCAGAAATG | TACAGCAACAACCTGAAGACC |
| HSP70 | NM 001006685.1 | CGGGCAAGTTTGACCTAA | TTGGCTCCCACCCTATCTCT |
| HSP90 | NM 001109785.1 | TCCTGTCCTGGCTTTAGTTT | AGGTGGCATCTCCTCGGT |
| HSP110 | NM 001159698.1 | GGCTCCAGTATTACGCTGCGATAG | GCACTCACAGCATTGTTCATCCAC |
| HSF1 | NM 001305256.1 | GTCGCAGGAGCAGCAGAAGC | CTGAGCCGTGTAGTGAACCAACTG |
| HSF2 | NM 001006685.1 | TTCCGCTGCTCGCATTCCTTG | GCCTCACTTGCTTCTGCCTCTG |
| HSF3 | NM 001305041.1 | AGGCAGAAGCACAGTCAACAACAG | GACGGCTGTACTTGGAAGGTGAAG |
| HSF4 | NM 001172374.1 84 | TGCCAGCCTTCCTAACCAAG | TGGTGCCATTCGTACTCCAG |
| HSP10 | NM 205067.1 | GCGCAGCAGAGACCGTAACC | TCCAACTGCTACCACTGTTGCTTG |
Abbreviations: COX-2, cyclooxygenase-2; HSF, heat shock factor; HSP, heat shock protein; iNOS, inducible nitric oxide synthase; NF-κB, nuclear transcription factor-kappa B; PTGES, prostaglandin E synthase; TNF-α, tumor necrosis factor alpha.
Quantitative real-time PCR was performed using the Roche Light Cycler 480 (Roche Life Science, Basel, Switzerland). The reaction system of qRT-PCR was 10 μL, including 5 μL of SYBR green master, 1 μL of diluted cDNA, 0.3 μL of forward primer, 0.3 μL of reverse primer, and 3.4 μL of PCR-grade water. The qRT-PCR reactions were run in triplicate in 96-well format, and the program was carried out at 95°C for 10 min; 40 cycles were repeated at 95°C for 15 s and at 60°C for 1 min. The established 2−ΔΔCt method was used to analyze the amplification curves, and β-actin was used as the reference gene (Schmittgen and Livak, 2008).
Western Blot for HSP60, HSP70, and HSP90 in Spleen Tissues of Laying Hens
Frozen spleen tissues of laying hens (100 mg) were homogenized at low temperature and then placed in 1 mL of lysis buffer containing 1 mmol phenylmethanesulfonyl (Biyuntian, Shanghai, China) and let to stand at 4°C for 2 h to lyse. The homogenates were centrifuged at 12,000× g for 5 min to collect the supernatants. The supernatant was quantified using an enhanced BCA protein kit (Biosharp, Beijing, China), and the concentration of each protein sample was diluted to 5 mg/μL. Equal amounts of spleen proteins were separated by SDS-PAGE (12% polyacrylamide) and transferred to a nitrocellulose membrane (Biosharp, Hefei, China) using a semidry transfer instrument (Liuyi, Beijing, China). After blocking nonspecific binding sites with 5% skim milk (BD, New Jersey), these were then incubated overnight at 4°C with diluted rabbit antibodies against antichicken HSP90 (1:3000), HSP70 (1:5000), HSP60 (1:3000) (Class 10,000, Hangzhou, China), and β-actin (1:7000) (Abcam, Cambridge, UK). Polyclonal antibodies (HSP70 and HSP90) were provided by Professor Shiwen Xu, Northeast Agricultural University, Harbin, China. The nitrocellulose membrane was then incubated with horseradish peroxidase–conjugated goat antirabbit immunoglobin G (IgG) (1:40,000; Bioss Antibodies, Beijing, China) for 1 h at room temperature. After eluting the unbound antibody, horseradish peroxidase was detected using the ECL chemiluminescent kit (Biosharp, Beijing, China). The gray band scanner (GeneGnomeXRQ, Cambridge, UK) was used to take photographs and analyze the protein bands. Finally, the optical density (OD) value of the protein bands was calculated using Image J software technology (National Institutes of Health, Bethesda, MD). The relative abundance of each protein is expressed as the ratio of the OD of each protein to the OD of its internal reference β-actin.
ELISA
A commercial ELISA kit (Xinle, Shanghai, China) was used to determine the levels of IL-1β, IL-2, IL-6, TNF-α, and TFN-γ in each group of serum, and all steps were strictly followed in accordance with the instructions.
Statistical Analysis
All data were analyzed using SPSS 22.0 Windows (SPSS, Inc., Chicago IL) for experimental data. The Shapiro–Wilk test was used to test the normal distribution of the data before statistical analysis. The data were analyzed by one-way ANOVA, and the Duncan method was used for multiple comparisons. The results were expressed as mean ± SE. Statistical significance was considered significant when P < 0.05.
Results
Expression Levels of HSP and HSF in the Spleen of Laying Hens
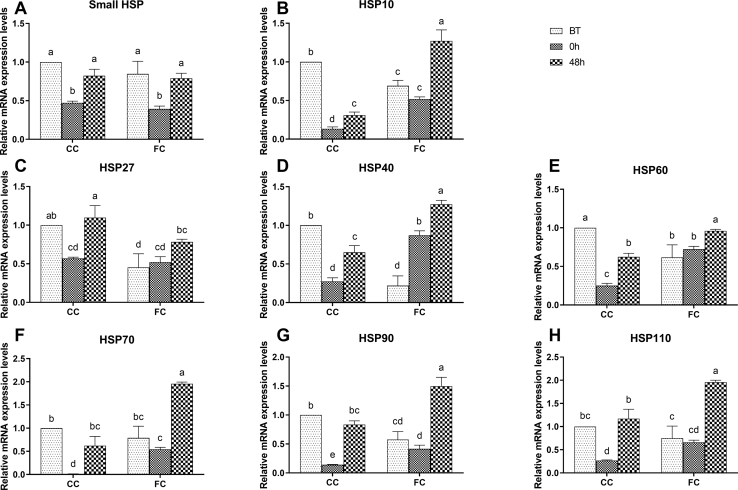
The mRNA expression levels of HSP in the spleen of laying hens are shown in Figure 2. Before transportation, the mRNA expression levels of HSP10, HSP27, HSP40, HSP60, and HSP90 in the FC group were significantly lower than those in the CC group (P < 0.05), and the mRNA expression levels of Small HSP, HSP70, and HSP110 in the FC group were not significantly different from those in the CC group (P > 0.05). Transport stress significantly reduced the mRNA expression levels of Small HSP, HSP10, HSP27, HSP40, HSP60, HSP70, HSP90, and HSP110 in the CC group (P < 0.05). In the FC group, HSP10, HSP27, HSP60, HSP70, HSP90, and HSP110 mRNA expression levels did not change significantly between those at BT and at 0 h (P > 0.05), except that expression of Small HSP decreased (P < 0.05) and HSP40 expression increased (P < 0.05) at 0 h after transportation. At 48 h after transportation, expression of Small HSP, HSP27, HSP70, HSP90, and HSP110 in the CC group recovered to the level at BT. In addition, expression of HSP10, HSP40, and HSP60 increased significantly compared with the 0 h group (P < 0.05), but did not return to the level at BT. At 48 h, the mRNA expression of HSP in the FC group significantly increased and was significantly higher than that at BT (P < 0.05), except for Small HSP, which recovered to the level at BT.
Figure 2.
Changes in (A) Small HSP, (B) HSP10, (C) HSP27, (D) HSP40, (E) HSP60, (F) HSP70, (G) HSP90, and (H) HSP110 mRNA expression levels in spleens of laying hens in the CC and FC groups before transportation (BT), immediately after transportation (0 h), and at 48 h after transportation (48 h). Lowercase letters “a, b, c” indicate significant differences (P < 0.05), and the same or nonstandard means no significant differences (P > 0.05). Abbreviations: CC, conventional cage; FC, furnished cage; HSP, heat shock protein.
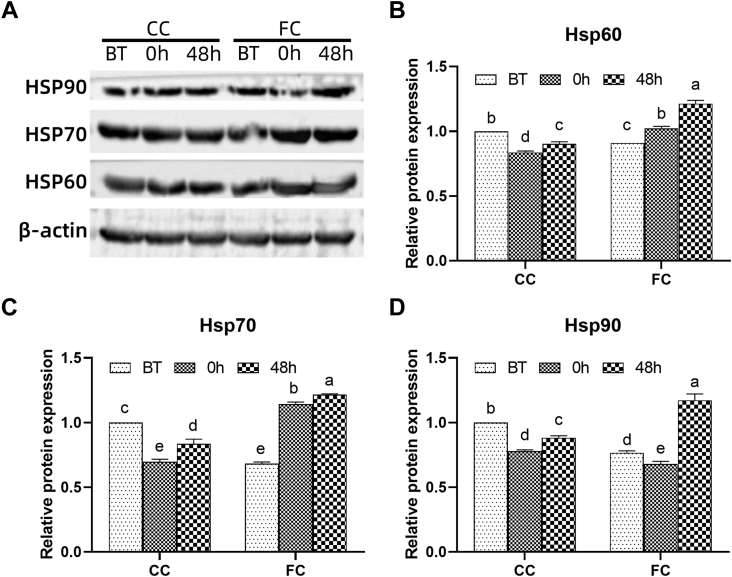
The protein levels of HSP60, HSP70, and HSP90 in the spleen of laying hens are shown in Figure 3, which were similar to the expression levels of HSP mRNAs. The expression levels of HSP60, HSP70, and HSP90 in the FC group were significantly lower than those in the CC group before transportation (P < 0.05). Expression levels of HSP60, HSP70, and HSP90 in the CC group and HSP90 protein expression levels in the FC group significantly decreased immediately after transportation (P < 0.05) and increased at 48 h after transportation (P < 0.05), but expression levels of HSP60, HSP70, and HSP90 in the CC group were not restored to the level at BT. However, the expression of HSP90 in the FC group was significantly higher than that before transportation (P < 0.05). The expression of HSP60 and HSP70 in the FC group was increased continually after transportation (at 0 h and 48 h) compared with that at BT (P < 0.05), and it was higher than that in the CC group at 0 h and 48 h (P < 0.05).
Figure 3.
Changes in HSP60, HSP70, and HSP90 protein expression levels in spleens of laying hens in CC and FC groups before transportation (BT), immediately after transportation (0 h), and at 48 h after the transportation (48 h). Lowercase letters “a, b, c” indicate significant differences (P < 0.05), and the same or nonstandard means no significant differences (P > 0.05). Abbreviations: CC, conventional cage; FC, furnished cage; HSP, heat shock protein.
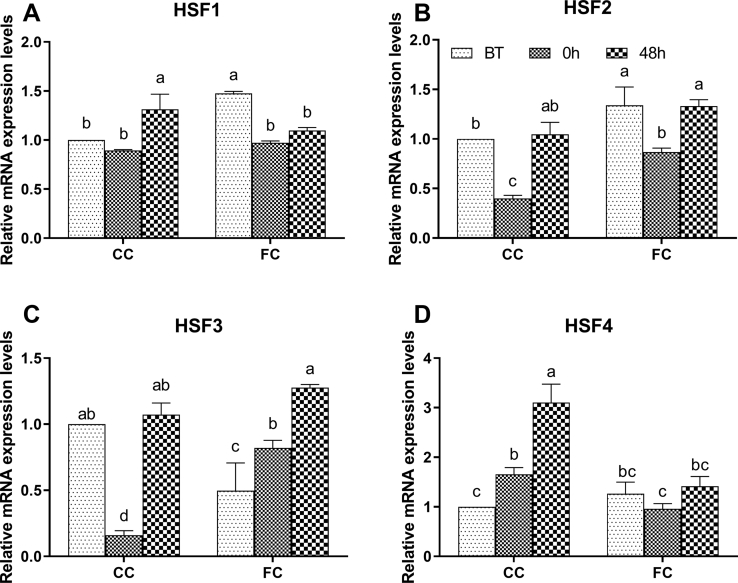
As shown in Figure 4, before transportation, the mRNA expression levels of HSF1 and HSF2 in the FC group were higher (P < 0.05). The mRNA expression levels of HSF3 were lower than those in the CC group (P < 0.05), and there was no significant difference in HSF4 expression (P > 0.05) compared with the CC group. In the CC group, HSF2 and HSF3 expression decreased significantly after transportation (P < 0.05) and then returned to the level at BT at 48 h after transportation (P < 0.05). The mRNA expression level of HSF1 did not change significantly immediately after transportation (P > 0.05) but increased to a higher level at 48 h (P < 0.05). The mRNA expression level of HSF4 continued to increase at 0 h and 48 h after transportation (P < 0.05). In the FC group, the mRNA expression levels of HSF1 and HSF2 decreased significantly immediately after transportation (P < 0.05). Expression levels of HSF3 increased significantly (P < 0.05) and HSF1 expression levels did not change significantly at 48 h compared with 0 h (P > 0.05). Expression levels of HSF2 returned to the level at BT and HSF3 expression levels continued to increase (P < 0.05). There was no significant difference in the mRNA expression level of HSF4 in the FC group at 0 h and 48 h after transportation (P > 0.05).
Figure 4.
Changes in (A) HSF1, (B) HSF2, (C) HSF3, and (D) HSF4 mRNA expression levels in spleens of laying hens in the CC and FC groups before transportation (BT), immediately after transportation (0 h), and at 48 h after transportation (48 h). Lowercase letters “a, b, c” indicate significant differences (P < 0.05), and the same or nonstandard means no significant differences (P > 0.05). Abbreviations: CC, conventional cage; FC, furnished cage; HSF, heat shock factor.
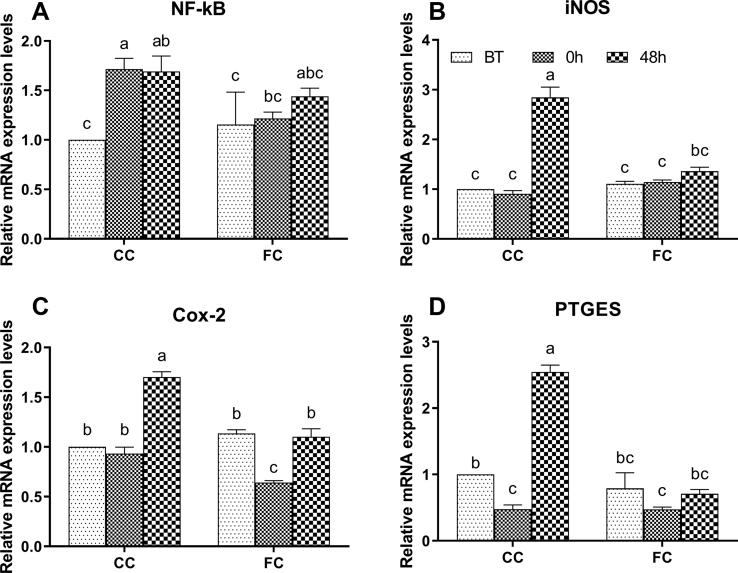
Expression Levels of Inflammatory Factors in the Spleen of Laying Hens
As shown in Figure 5, there was no significant difference between the CC group and FC group before transportation (P > 0.05). After transportation, the mRNA expression level of NF-κB increased significantly (P < 0.05), and the expression level of PTGES decreased significantly (P < 0.05) in the CC group. The mRNA expression levels of COX-2 decreased significantly in the FC group (P < 0.05). At 48 h after transportation, the mRNA expression level of NF-κB in the CC group was maintained at the level immediately after transportation (P > 0.05). The mRNA expression levels of iNOS, COX-2, and PTGES at 48 h in the CC group were significantly increased (P < 0.05) than the levels at BT and 0 h. There was no significant difference of the mRNA expression level of NF-κB at 48 h compared with that at BT in the FC group (P > 0.05).
Figure 5.
Changes in (A) NF-κB, (B) iNOS, (C) COX-2, and (D) PTGES mRNA expression levels in spleens of laying hens in the CC and FC groups before transportation (BT), immediately after transportation (0 h), and at 48 h after transportation (48 h). Lowercase letters “a, b, c” indicate significant differences (P < 0.05), and the same or non-standard means no significant differences (P > 0.05). Abbreviations: CC, conventional cage; COX-2, cyclooxygenase -2; FC, furnished cage; iNOS, inducible nitric oxide synthase; NF-κB, nuclear transcription factor-kappa B; PTGES, prostaglandin E synthase.
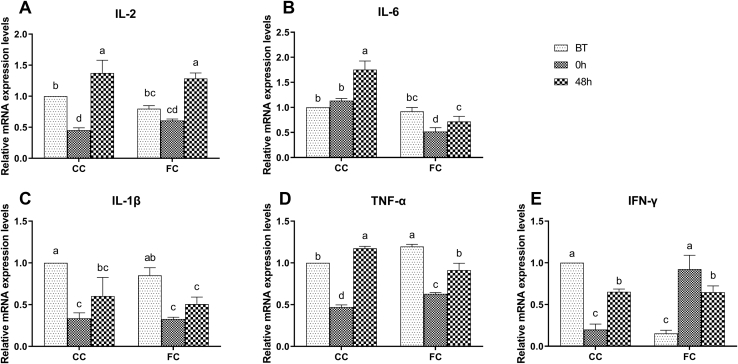
Expression Levels of Inflammatory Cytokines in the Spleen of Laying Hens
As shown in Figure 6, there was no significant difference in the expression of inflammatory factors between the FC group and CC group, except for TNF-α and interferon gamma before transportation (P > 0.05). In the CC group, the mRNA expression levels of IL-1β, IL-2, TNF-α, and interferon gamma decreased (P < 0.05), and there was no significant change in the mRNA expression level of IL-6 at 0 h compared with that at BT (P > 0.05). In the FC group, the mRNA expression levels of IL-1β, IL-2, IL-6, and TNF-α at 0 h decreased significantly than those at BT (P < 0.05). On the other hand, expression levels of interferon gamma increased significantly (P < 0.05). Expressions of all cytokines in the CC group and FC group were recovered to normal at 48 h after transportation, except for the mRNA expression level of IL-6 in the CC group (P < 0.05). The mRNA expression levels of IL-1β and interferon gamma at 48 h in the CC group and the mRNA expression levels of IL-1β and TNF-α at 48 h in the FC group were lower than those at BT (P < 0.05). The mRNA expression levels of IL-2 and TNF-α at 48 h in the CC group and the expression of IL-2 at 48 h in the FC group were higher than those at BT (P < 0.05).
Figure 6.
Changes in (A) IL-2, (B) IL-6, (C) IL-1β, (D) TNF-α, and (E) interferon gamma mRNA expression levels in spleens of laying hens in the CC and FC groups before transportation (BT), immediately after transportation (0 h), and at 48 h after transportation (48 h). Lowercase letters “a, b, c” indicate significant differences (P < 0.05), and the same or nonstandard means no significant differences (P > 0.05). Abbreviations: CC, conventional cage; FC, furnished cage; TNF-α, tumor necrosis factor alpha.
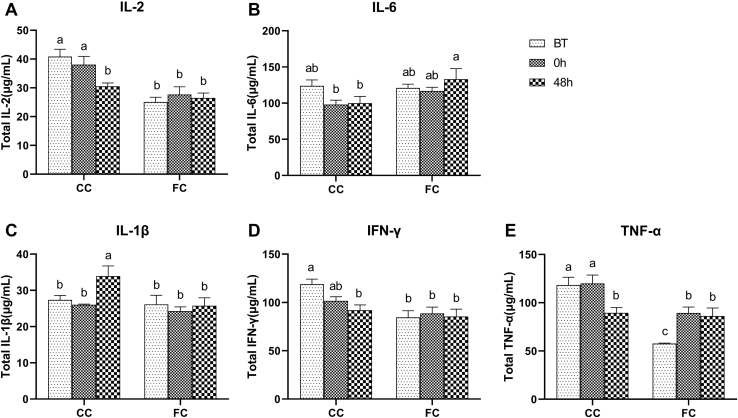
Determination of Serum Cytokines
The change of levels of serum cytokines is shown in Figure 7. Transport stress showed no significant difference in expression levels of serum cytokines in laying hens in the CC and FC groups, except for TNF-α in the FC group (Figures 7A-7D) (P > 0.05). At 48 h after transportation, the expression level of IL-2 and IL-1β in the serum of the CC group had significantly changed compared with the level at 0 h. The expression level of IL-2 was lower, whereas the expression level of IL-1β was higher than that at 0 h (P < 0.05). When compared with the level at BT, expression levels of IL-2 and TNF-α were significantly lower, and the expression level of IL-1β was significantly higher (P < 0.05). At 48 h after transportation, there were no significant differences in the expression level of IL-2, IL-6, IL-1β, interferon gamma, and TNF-α in the serum of the FC group compared with that at 0 h (P > 0.05). Compared with the expression level at BT, there was no significant change in expression levels of IL-2, IL-6, IL-1β, and interferon gamma (P > 0.05), except for TNF-α, which was significantly increased (P < 0.05). Generally, transport stress had no significant influence in the CC and FC groups, whereas there was a continuous downward trend in the CC group. Before transportation, serum cytokines in the FC group had the same level of expression as that of IL-6 and IL-1β (Figures 7B and 7C, P > 0.05), or a lower level of IL-2, interferon gamma, and TNF-α expression (Figures 7A, 7D and 7E, P < 0.05) was observed than that in the CC group.
Figure 7.
Changes in (A) IL-2, (B) IL-6, (C) IL-1β, (D) interferon gamma, and (E) TNF-α expression levels in serum of laying hens in the CC and FC groups before transportation (BT), immediately after transportation (0 h), and at 48 h after transportation (48 h). Lowercase letters “a, b, c” indicate significant differences (P < 0.05), and the same or nonstandard means no significant differences (P > 0.05). Abbreviations: CC, conventional cage; FC, furnished cage; TNF-α, tumor necrosis factor alpha.
Discussion
Transportation can adversely affect the health of chickens. Chickens may be exposed to many potential stressors during transportation, including human handling, fasting, noise, vibration, high temperature, social disruption, congestion, and restricted movement (Nicol and Scott, 1990). Transport stress decreases weight and quality of meat and increases susceptibility to disease with tissue damage and immunity weakening (Ge et al., 2017; Li et al., 2017; Xing et al., 2017). The enriched environment has the potential to enhance the immunity of laying hens and can promote chickens' adaptability to complex environments via mild stimulation (Campbell et al., 2019). This study indicates that long-term housing in an enriched environment can enhance tolerance of laying hens to transport stress.
Heat shock proteins as molecular chaperones can help other proteins to correctly fold, assemble, and mediate the degradation of misfolded proteins (Lindquist and Craig, 1988; Parsell and Lindquist, 1993). Heat shock proteins have a protective effect on tissues under stress and maintain cellular metabolism and structural integrity (Larkins et al., 2012). In our study, the expression levels of HSP10, HSP27, HSP40, HSP60, and HSP90 at BT in the FC group were significantly lower than those in the CC group, indicating that the enriched environment has the potential to reduce stress levels in laying hens. Sun et al. (2018) showed that the mRNA expression levels of most HSP in the heart tissues of chickens were upregulated at 2 h and decreased significantly after 4 h for transportation. Similarly, HSP60 and HSP70 mRNA expression levels in the heart increased significantly when the cold stimulus was less than 3 h and decreased significantly after 3 h (Zhao et al., 2013). Different expression levels of HSP may be related to exposure time. Heat shock protein mRNA expression levels are upregulated in a short period to protect the tissue. Long-term exposure disrupts the heat shock protective response signaling pathway, which reduces HSP mRNA expression levels and causes damage to the tissue. In our study, consistent with the previous reports, the HSP mRNA expression levels in the CC group were significantly reduced immediately after transportation, whereas expression levels of HSP10, HSP40, and HSP60 showed no return to the level observed at BT at 48 h after transportation in the CC group. However, the HSP mRNA expression levels at 0 h in the FC group remained unchanged, contrasting with those at BT, except for small HSP and HSP40, and those at 48 h were increased significantly compared with those at 0 h and higher than those at BT, except for Small HSP. Therefore, the expression levels of HSP in the CC group were more strongly suppressed during transportation, indicating that the hens in the FC group may have a stronger tolerance to transport stress, which was also supported by the Western blotting results.
Heat shock factors regulate the expression of HSP. To further determine the effects of transport stress on HSP expression levels, mRNA expressions of HSF were examined before and immediately after transportation. Multiple inducing factors can induce HSF binding to heat shock elements, thereby mediating transcription of HSP and leading to accumulation of HSP (Morimoto, 1998). Heat shock factor 1 and HSF3 regulate the expression of HSP during stress, and the effects are synergistic (Tanabe et al., 1998; Pirkkala et al., 2001). Heat shock factor 2 is involved in expressing HSP in cells under nonstress conditions (Pirkkala et al., 2001). The expression of HSF4 is tissue specific, and the overexpression of HSF4 can reduce endogenous HSP27, HSP70, and HSP90 gene expression (Nakai et al., 1997). Therefore, downregulation of HSP expression in the CC group at 0 h after transportation may be due to the decreased expression of HSF3 and the overexpression of HSF4. The HSP expression in the FC group changed immediately after mild transportation, which may be the result of the combined effects of the decreased HSF1 expression level and increased HSF3 expression level. The rapid recovery expression of HSP to the level of before transportation in the FC group at 48 h after transportation may presumably be related to the overexpression of HSF3.
Nuclear transcription factor-kappa B is an important transcription factor in the inflammatory response and can be rapidly activated by environmental stress (Mazzarella et al., 2000). Activation of NF-κB can increase the expression of proinflammatory factors, such as COX-2 and PTGES (Ben-Neriah, 2002; Ko et al., 2017). Toll-like receptor 4 recognizes HSP60, HSP70, and HSPgp96 and activates the expression of related inflammatory cytokines through the NF-κB signaling pathway (Knapp, 2010; Yanai et al., 2012). Therefore, the HSP-activating process could mediate the responses of the NF-κB inflammation pathway. IL is a cytokine produced by white blood cells, mediating cell–cell interactions. It plays an important role in transmitting information, activating and regulating immune cells, mediating T- and B-cell activation, adjusting cell proliferation and differentiation, and participating in inflammatory responses (Lillehoj et al., 2001). Matur et al. (2015) found that hens housed in furnished cages exhibited higher innate immune responses than those reared in traditional battery cages, and Matur et al. (2016) showed that the weight of immune organs (spleen, thymus, and bursa) of birds had not been affected by the enriched environment and that the antibody response of birds was higher. However, the effects of the transport stress on the expression of inflammation-related factors have not been fully understood. In our study, the expression of NF-κB was upregulated in the CC group at 0 h and remained at a high expression level at 48 h. The expression of PTGES decreased significantly immediately after transportation. At 48 h after transportation, the expression levels of COX-2 and PTGES significantly increased. It is speculated that acute stress during the transportation phase causes expression of proinflammatory factors of COX-2 and PTGES, which promotes accumulation of NF-κB and induces persistence of inflammation. There were no significant changes in the expression of inflammatory factors of NF-κB and PTGES in the FC group in this study. Sun et al. (2018) showed that long-term transportation (more than 4 h) inhibited the expression of HSP in chicken cardiomyocytes and caused heart damage in chickens. In this study, compared with the FC group, the expression of HSP in the CC group was more strongly inhibited under transport stress, which may cause spleen damage in the CC group via activating the NF-κB inflammation pathway.
Acute stress suppresses immunoglobulin expression in the spleen and reduces white blood cell aggregation, which contributes to stress-induced immunosuppression (Zalcman and Anisman, 1993). Under stress, leukocytes accumulate at the site of inflammation, and the amount of aggregation is determined as per the severity of the injury site and reaches a peak within 24 to 48 h (Viswanathan and Dhabhar, 2005). Wein et al. (2017) demonstrated that laying hens had been acutely stressed during the collection and loading stages. During transportation, the level of inflammatory factors is suppressed and then increases, and during the recovery of transportation stress, it continues to rise to a peak and then returns to normal levels. IL-1β, IL-2, and IL-6 are proinflammatory factors, and IL-4 and IL-10 can inhibit the inflammatory response (Gordon, 2003). Tumor necrosis factor alpha can maintain survival of various cells, such as T lymphocytes, B lymphocytes, and tissue-derived fibroblasts and epithelial cells, thereby promoting the inflammatory response (Croft et al., 2013). In this study, the mRNA expression levels of inflammatory factors IL-1β, IL-2, and IL-6 in spleens had no significant difference between the CC group and FC group immediately after transportation, and the mRNA expression level of TNF-α in the FC group was higher than that in the CC group. Except for the serum concentrations of IL-2, interferon gamma, and TNF-α in the FC group, which are lower than those in the CC group, the expression levels of some detected inflammation factors (IL-6 and IL-1β) in the FC group were not significantly different from those in the CC group when subjected to housing in furnished cages or traditional cages. This is consistent with the finding of the study by Chen et al. (2014) and Meng et al. (2015), who had found that the effects of traditional cages and furnished cages with different structures were not significantly related to inflammatory factors in laying hens, which may be due to adaptation of hens to the corresponding environment for a long term to achieve a similar and stable state. The mRNA expression levels of inflammatory factors in the CC group were not consistent at 0 h after transportation because inflammatory factors were in the rising phase after acute stress. At 48 h after transportation, they were in the recovery phase. In addition to IL-6, inflammatory factors (IL-2, IL-1β, TNF-α, and interferon gamma) in the CC group recovered toward the levels at BT. The mRNA expression of inflammatory cytokines in the FC group was inhibited immediately after transportation, possibly because the FC group had a stronger tolerance to transport stress; at 0 h after transportation, the body was not injured, so the level of inflammatory cytokines (IL-6, IL-1β, and interferon gamma) increased toward the level observed at BT at 48 h. These changes may be associated with the overexpression of HSP and non-activation of NF-κB pathway in the FC group. Interferon gamma is an important proinflammatory factor and plays a protective role in some autoimmune diseases and chronic inflammation (Chu et al., 2007; Lees et al., 2008). Owing to space problems in traditional chicken cages, laying hens have been under chronic stress for a long time (Baxter, 1994; McAdie et al., 2005; Savory, 2010). To protect the body, the interferon gamma mRNA expression level in the CC group was significantly higher than that in the FC group.
Stress can be acute or chronic. Importantly, cognitive mechanisms regulate the perception, response, and control of stress, and psychosocial factors (e.g., social support) are key factors that determine the duration and intensity of physiologically driven physiological stress responses (Shini et al., 2010; Dhabhar, 2011). The concepts of allostasis and the allostatic load were introduced by Sterling and Eyer (1988). Arroyo et al. (2019) suggested that housing conditions could change the way pigs cope with unknown conditions. It is speculated that hens in the FC group may have a stronger tolerance to transport stress. Therefore, transport stress is chronic stress to hens in the FC group, or allostasis of the chickens in the FC group is not destroyed. Thus, the expression of interferon gamma in the spleen at 0 h in the FC group increased to protect the body from damage and decreased at 48 h. In contrast to acute stress, chronic stress induces immunosuppression by inhibiting the redistribution of white blood cells in blood (Dhabhar and Mcewen, 1997). Therefore, after transportation, the expression of inflammatory factors in the serum in the FC group was in a stable state and recovered after 48 h. However, further studies are needed whether transport stress is a chronic stress in laying hens reared in furnished cages. Therefore, the enriched environment enhances the tolerance of laying hens to immune damage during transportation.
Conclusion
In summary, the experience of hens in furnished cages, compared with rearing in traditional cages, alleviated the inhibition of HSF–HSP expression caused by transport stress and reduced the expression of proinflammatory factors (NF-κB, iNOS, COX-2, and PTGES) and inflammatory cytokines (IL-1β, IL-6 and TNF-α). Therefore, an enriched environment may enhance laying hens' resistance to transport stress.
Acknowledgments
The authors thank the members of animal behavior and welfare laboratory at the College of Animal Science and Technology in Northeast Agricultural University. This work was supported by the National Natural Science Foundation of China (grant number: 31672466 and 31501992), the Young Talents Project of Northeast Agricultural University (grant number: 18QC36), and the Natural Science Foundation of Heilongjiang Province of China (grant number: C2017027).
Disclosures
The authors confirm that there are no conflicts of interest.
References
- Altan O., Seremet C., Bayraktar H. The effects of early environmental enrichment on performance, fear and physiological responses to acute stress of broiler. Arch. Geflugelkd. 2013;77:23–28. [Google Scholar]
- Arroyo L., Valent D., Carreras R., Peña R., Sabrià J., Velarde A., Bassols A. Housing and road transport modify the brain neurotransmitter systems of pigs: Do pigs raised in different conditions cope differently with unknown environments? PLoS One. 2019;14:e0210406. doi: 10.1371/journal.pone.0210406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D.L., Wherry E.J., Masopust D., Zhu B., P Allison J., Sharpe A.H., Freeman G.J., Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Baxter M. The welfare problems of laying hens in battery cages. Vet. Rec. 1994;134:614–619. doi: 10.1136/vr.134.24.614. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat. Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]
- Campbell D.L.M., De Haas E.N., Lee C. A review of environmental enrichment for laying hens during rearing in relation to their behavioral and physiological development. Poult. Sci. 2019;98:9–28. doi: 10.3382/ps/pey319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.H., Bao J., Meng F.Y., Wei C.B. Choice of perch characteristics by laying hens in cages with different group size and perching behaviours. Appl. Anim. Behav. Sci. 2014;150:37–43. [Google Scholar]
- Chu C.Q., Swart D., Alcorn D., Tocker J., Elkon K.B. Interferon-γ regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- Cogswell J.P., Godlevski M.M., Wisely G.B., Clay W.C., Leesnitzer L.M., Ways J.P., Gray J.G. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J. Immunol. 1994;153:712–723. [PubMed] [Google Scholar]
- Croft M., Benedict C.A., Ware C.F. Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discov. 2013;12:147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F.S. Effects of stress on immune function: implications for immunoprotection and immunopathology. In: Contrada R.J., Baum A., editors. The Handbook of Stress Science: Biology, Psychology, and Health. Springer Publishing Company; Dordrecht, The Netherlands: 2011. pp. 47–63. [Google Scholar]
- Dhabhar F.S., Mcewen B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Ge J., Li H., Sun F., Li X.N., Lin J., Xia J., Zhang C., L Li J. Transport stress–induced cerebrum oxidative stress is not mitigated by activating the Nrf2 antioxidant defense response in newly hatched chicks. Anim. Sci. J. 2017;95:2871–2878. doi: 10.2527/jas.2017.1559. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Greger M. The long haul: risks associated with livestock transport. Biosecur. Bioterror. 2007;5:301–312. doi: 10.1089/bsp.2007.0028. [DOI] [PubMed] [Google Scholar]
- Harris T. The history and development of European and North American transport regulations and international trade issues. Anim. Sci. J. 2001;79:E73–E85. [Google Scholar]
- Knapp S. Update on the role of toll-like receptors during bacterial infections and sepsis. Wien. Med. Wochenschr. 2010;160:107–111. doi: 10.1007/s10354-010-0765-6. [DOI] [PubMed] [Google Scholar]
- Ko E.Y., Cho S.H., Kwon S.H., Eom C.Y., Jeong M.S., Lee W.W., Kim S.Y., Heo, Ahn G., Lee K.P., Jeon Y.J., Kim K.N. The roles of NF-κB and ROS in regulation of pro-inflammatory mediators of inflammation induction in LPS-stimulated zebrafish embryos. Fish. Shellfish Immunol. 2017;68:525–529. doi: 10.1016/j.fsi.2017.07.041. [DOI] [PubMed] [Google Scholar]
- Larkins N.T., Murphy R.M., Lamb G.D. Influences of temperature, oxidative stress, and phosphorylation on binding of heat shock proteins in skeletal muscle fibers. Am. J. Physiol. Cell. Physiol. 2012;303:C654–C665. doi: 10.1152/ajpcell.00180.2012. [DOI] [PubMed] [Google Scholar]
- Lees J.R., Golumbek P.T., Sim J., Dorsey D., Russell J.H. Regional CNS responses to IFN-γ determine lesion localization patterns during EAE pathogenesis. J. Exp. Med. 2008;205:2633–2642. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.Y., Lin J., Sun F., Li H., Xia J., Li X.N., Ge J., Zhang C., Li J.L. Transport stress induces weight loss and heart injury in chicks: disruption of ionic homeostasis via modulating ion transporting ATPases. Oncotarget. 2017;8:24142. doi: 10.18632/oncotarget.15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H.S., Min W., Kang D.C., Babu U.S., Lillehoj E.P. Molecular, cellular, and functional characterization of chicken cytokines homologous to mammalian IL-15 and IL-2. Vet. Immunol. Immunopathol. 2001;82:229–244. doi: 10.1016/s0165-2427(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E.A. The heat-shock proteins. Annu. Rev. Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Line J., Bailey J., Cox N., Stern N. Yeast treatment to reduce Salmonella and Campylobacter populations associated with broiler chickens subjected to transport stress. Poult. Sci. 1997;76:1227–1231. doi: 10.1093/ps/76.9.1227. [DOI] [PubMed] [Google Scholar]
- Matur E., Akyazi B., Eraslan E., Ergul Ekiz E., Eseceli H., Keten M., Metiner K., Aktaran Bala D. The effects of environmental enrichment and transport stress on the weights of lymphoid organs, cell-mediated immune response, heterophil functions and antibody production in laying hens. Anim. Sci. J. 2016;87:284–292. doi: 10.1111/asj.12411. [DOI] [PubMed] [Google Scholar]
- Matur E., Ekiz E.E., Akyzi I., Eraslan E., Ergen E., Mert E., Polat B.A., Özcan M. The effects of furnished cages on the behaviour of laying hens in the post-stress adaptation period. J. Istanbul. Vet. Sci. 2017;1:76–83. [Google Scholar]
- Mazzarella G., Bianco A., Catena E., Palma R.D., Abbate G.F. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55:6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- McAdie T.M., Keeling L.J., Blokhuis H.J., Jones R.B. Reduction in feather pecking and improvement of feather condition with the presentation of a string device to chickens. Appl. Anim. Behav. Sci. 2005;93:67–80. [Google Scholar]
- Meng F., Chen D., Li X., Li J., Bao J. Effects of large or Small furnished cages on performance, welfare and egg quality of laying hens. Anim. Prod. Sci. 2015;55:793–798. [Google Scholar]
- Morimoto R.I. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes. Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Nakai A., Tanabe M., Kawazoe Y., Inazawa J., Morimoto R.I., Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell. Biol. 1997;17:469–481. doi: 10.1128/mcb.17.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol C.J., Scott G.B. Pre-slaughter handling and transport of broiler chickens. Appl. Anim. Behav. 1990;28:57–73. [Google Scholar]
- Parsell D., Lindquist s. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993;27:437–497. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pirkkala L., Nykänen P., Sistonen L.E.A. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB. J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- Pusic K.M., Pusic A.D., Kraig R.P. Environmental enrichment Stimulates immune cell secretion of exosomes that promote CNS Myelination and may regulate inflammation. Cell. Mol. Neurobiol. 2016;36:313–325. doi: 10.1007/s10571-015-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg T., Tuyttens F., De Reu K., Herman L., Zoons J., Sonck B. Welfare assessment of laying hens in furnished cages and non-cage systems: an on-farm comparison. Anim. Welfare. 2008;17:355–361. [Google Scholar]
- Savory J. Nutrition, feeding and drinking behaviour, and welfare. In: Duncan I., Hawkins P., editors. Springer Publishing Company; Dordrecht, The Netherlands: 2010. pp. 165–187. (The Welfare of Domestic Fowl and Other Captive Birds). [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 2008;3:1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schwartzkopf-Genswein K.S., Faucitano L., Dadgar S., Shand P., González L.A., Crowe T.G. Road transport of cattle, swine and poultry in North America and its impact on animal welfare, carcass and meat quality: a review. Meat. Sci. 2012;92:227–243. doi: 10.1016/j.meatsci.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Shini S., Huff G., Shini A., Kaiser P. Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010;89:841–851. doi: 10.3382/ps.2009-00483. [DOI] [PubMed] [Google Scholar]
- Shinmura T., Eguchi Y., Uetake K., Tanaka T. Behavioral changes in laying hens after introduction to battery cages, furnished cages and an aviary. Anim. Sci. J. 2010;77:242–249. [Google Scholar]
- Smith G.C., Grandin T., Friend T.H., Don Lay J.R., Swanson J.C. 2004. Effect of Transport on Meat Quality and Animal Welfare of Cattle, Pigs, Sheep, Horses, Deer, and Poultry. Accessed Mar. 2010. http://www.grandin.com/behaviour/effect.of.transport.html. [Google Scholar]
- Sohn S.H., Cho E.J., Jang I.S., Moon Y.S. The effects of dietary supplementation of vitamin C and E on the growth performance and the stress response in broiler chickens. Korean J. Poult. Sci. 2013;40:31–40. [Google Scholar]
- Sterling P., Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S., Reasoned J., editors. Handbook of Life Stress, Cognition and Health. John Wiley and Sons, Inc; New York, NY: 1988. pp. 629–649. [Google Scholar]
- Sun F., Zuo Y.Z., Ge J., Xia J., Li X.N., Lin J., Zhang C., Xu H.L., Li J.L. Transport stress induces heart damage in newly hatched chicks via blocking the cytoprotective heat shock response and augmenting nitric oxide production. Poult. Sci. 2018;97:2638–2646. doi: 10.3382/ps/pey146. [DOI] [PubMed] [Google Scholar]
- Tanabe M., Kawazoe Y., Takeda S., Morimoto R.I., Nagata K., Nakai A. Disruption of the HSF3 gene results in the severe reduction of heat shock gene expression and loss of thermotolerance. EMBO. J. 1998;17:1750–1758. doi: 10.1093/emboj/17.6.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela J.O., Hammerbeck C.D., Mescher M.F. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J. Immunol. 2005;174:600–604. doi: 10.4049/jimmunol.174.2.600. [DOI] [PubMed] [Google Scholar]
- Viswanathan K., Dhabhar F.S. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. P. Natl. Acad. Sci. USA. 2005;102:5808–5813. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wein Y., Geva Z., Bar-Shira E., Friedman A. Transport-related stress and its resolution in Turkey pullets: activation of a pro-inflammatory response in peripheral blood leukocytes. Poult. Sci. 2017;96:2601–2613. doi: 10.3382/ps/pex076. [DOI] [PubMed] [Google Scholar]
- Xing T., Wang M., Han M., Zhu X., Xu X., Zhou G. Expression of heat shock protein 70 in transport-stressed broiler pectoralis major muscle and its relationship with meat quality. Animal. 2017;11:1599. doi: 10.1017/S1751731116002809. [DOI] [PubMed] [Google Scholar]
- Yang D., Tan X., Lv Z., Liu B., Baiyun R., Lu J., Zhang Z. Regulation of Sirt1/Nrf2/TNF-α signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci. Rep. 2016;6:37157. doi: 10.1038/srep37157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai H., Ban T., Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends. Immunol. 2012;33:12. doi: 10.1016/j.it.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Zalcman S., Anisman H. Acute and chronic stressor effects on the antibody response to sheep red blood cells. Pharmacol. Biochem. Behav. 1993;46:445–452. doi: 10.1016/0091-3057(93)90377-6. [DOI] [PubMed] [Google Scholar]
- Zhang X.Q., Tong X.M., Wen W.Y. Effects of Compound Codonopsis oral liquids on immune Molecules with Immunological stress in layer chickens. China Anim. Husband. 2016;43:2066–2071. [Google Scholar]
- Zhao F.Q., Zhang Z.W., Wang C., Zhang B., Yao H.D., Li S., Xu S.W. The role of heat shock proteins in inflammatory injury induced by cold stress in chicken hearts. Cell. Stress Chaperon. 2013;18:773–783. doi: 10.1007/s12192-013-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkifli I., Abdullah N., Azrin N.M., Ho Y.M. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br. Poult. Sci. 2000;41:593–597. doi: 10.1080/713654979. [DOI] [PubMed] [Google Scholar]