Abstract
This study was conducted to investigate the individual and combined effects of xylo-oligosaccharides (XOS) and gamma-irradiated Astragalus polysaccharides (IAPS) on the growth performance and intestinal mucosal barrier function of broiler chickens. A total of 240 1-day-old Ross-308 chicks were allocated into 5 groups for 21 d: control group (basal diet), antibiotic growth promoter (AGP) group (basal diet supplemented with 50 mg/kg chlortetracycline), XOS group (basal diet supplemented with 100 mg/kg XOS), IAPS group (basal diet supplemented with 600 mg/kg IAPS), and XOS + IAPS group (basal diet supplemented with 100 mg/kg XOS and 600 mg/kg IAPS). The results showed that birds in the XOS + IAPS group showed higher ADG and lower feed-to-gain ratio than those in the control group (P < 0.05). The XOS, IAPS, and XOS + IASP treatments significantly increased villus height (VH) of all intestine segments, jejunal goblet cell numbers, and VH–to–crypt depth ratio (VH/CD) of broilers than those of the control group (P < 0.05). Birds in the XOS + IAPS group had higher jejunal VH/CD ratio and goblet cell numbers than those from the XOS or IAPS groups (P < 0.05). In addition, there was a synergy effect between XOS and IAPS on increasing duodenal goblet cell numbers and improving ileal morphology (higher VH and VH/CD ratio) (P < 0.05). The XOS, IAPS and XOS + IAPS treatments increased the mRNA expression of zonula occludens-1 and occludin of the jejunum as compared with the control group (P < 0.05). Simultaneously, birds in the XOS + IAPS group showed lower plasma D-lactic acid concentration and higher mRNA expression of claudin-1, claudin-3, and occludin in the jejunum than those in the control or IAPS groups (P < 0.05). Moreover, there was no significant difference in growth performance, intestinal morphology, and intestinal barrier function of broilers between the AGP and XOS + IAPS groups. In conclusion, the combination of XOS and IAPS had a better potential as chlortetracycline substitute for improving the growth performance, intestinal morphology, and intestinal barrier function of broilers.
Key words: broiler, xylo-oligosaccharide, gamma-irradiated Astragalus polysaccharide, growth, intestinal barrier function
Introduction
Owing to imperfect intestinal function development and low immunity, chicks are easily stimulated by external factors such as diseases, nutritional, and environmental challenges, often leading to poor health and growth performance and even high mortality. For a long time, antibiotic growth promoters (AGP), widely accepted as performance-enhancing feed additives, have been used in poultry for promoting growth and preventing disease (Alagawany et al., 2018; Broom, 2018). Although AGP are beneficial for animal health and productivity, scientific evidence suggests that abuse of AGP leads to increased problems of antibiotic resistance and the presence of antibiotics residues in feed and environment (Mehdi et al., 2018; Suresh et al., 2018). Therefore, AGP are now being phased out in most countries of the world. China has also banned the use of AGP in animal feed from July 1, 2020. However, the ban of AGP has resulted in a reduction in animal performance and an increase in economic losses for European countries (Burel, 2012). This situation may also occur in China after the ban of AGP in animal feed. Therefore, it is urgent to find effective alternatives to AGP that improve chicken health and maintain high-quality products while protecting environment and consumer health.
Xylo-oligosaccharides (XOS) is a kind of functional oligosaccharide formed by 2-7 xylose residues linked through β-(1-4)-linkages. Xylo-oligosaccharide has characteristics of acid resistance, heat resistance, and nonresistance (Carvalho et al., 2013). Supplementation of XOS in the diet can enhance the growth performance of chickens by positively boosting immune function and improving the intestinal health. As a kind of prebiotic, XOS promote the growth of gut beneficial bacteria and enhance the production of short-chain fatty acids (SCFA) at the large bowel of broilers (Pourabedin et al., 2015). Locally, SCFA are the major energy sources for gut microbiota itself and for intestinal epithelial cells (Farzi et al., 2018). Ding et al. (2018) reported that dietary XOS supplementation enhanced the intestinal health and immune function by increasing butyric acid content and the number of Bifidobacteria in the cecum of laying hen. Besides, XOS could improve humoral immunity of broilers by enhancing the titers of antibody against the avian influenza H5N1 vaccine virus (Sun et al., 2013). All the aforementioned evidence suggested that XOS are one of the potential prebiotic candidates that can be considered as a viable alternative to AGP.
Astragalus polysaccharides (APS), extracted from perennial herb Astragalus membranaceus (Fisch.) Bunge (family Fabaceae), is a kind of macromolecular polysaccharides formed from mannose, D-glucose, D-galactose, xylose, and L-arabinose (Kallon et al., 2013). Numerous studies have explored some potential biological functions of APS, such as antioxidant, growth promotion, immune regulation, and improvement of intestinal barrier function (Yuan et al., 2008; Zahran et al., 2014; Wu, 2018). Molecular weight is one of the most critical factors that mediating the biological functions of natural polysaccharides (Choi and Kim, 2013). We have previously shown that gamma irradiation modification with a proper dose decreased molecular weight and viscosity of the natural APS and increased its water solubility (Ren et al., 2018; Li et al., 2019a). More importantly, in vivo and in vitro studies have shown that gamma-irradiated APS (IAPS) have a higher immunomodulatory activity than natural APS (Ren et al., 2018; Li et al., 2019a, 2019b). Recently, Liu et al. (2020a) reported that dietary IAPS was effective in increasing the cecal beneficial bacteria and SCFA production of immunosuppressive broilers. Thus, IAPS could be exploited as a potential effective immune-enhancing agent to poultry.
Previous studies have showed that XOS and IAPS play different roles in the intestinal tract. Xylo-oligosaccharides, as a prebiotic, can promote intestinal health through improving the proliferation of beneficial bacteria and the production of SCFA (Pourabedin et al., 2015; Ding et al., 2018). However, IAPS is a kind of macromolecular polysaccharides. It is mainly delivered to the gut-associated lymphoid tissue through the endocytosis of M cells, thereby activating the intestinal immune system and enhancing the body's immunity (Liang et al., 2001; Li et al., 2019a). We thus speculate that XOS and IAPS could work together in a synergetic manner to improve broiler health and performance by enhancing broiler immunity and improving intestinal health. Therefore, the aim of this study was to explore the effects of dietary supplement with XOS, IAPS, and their combination on growth performance, intestinal morphology, and intestinal barrier function of broiler chickens.
Materials and methods
Preparation of IAPS, XOS, and Chlortetracycline
Natural APS was purchased from Tianjin Sainuo Pharmaceutical Co., Ltd. (Tianjin, China), and the detected polysaccharide content was 87.64%. Gamma-irradiation treatment of natural APS was carried out by using a BFT-IV cobalt-60 source irradiator at an ambient temperature of 25 ± 0.5°C in XiYue Irradiation Technology Co., Ltd. (Nanjing, China) to obtain IAPS according to Ren et al. (2018) and Li et al. (2019a). Xylo-oligosaccharides with a purity of 35% were purchased from Jiangsu Kangwei Biological Co., Ltd. (Nanjing, China), and chlortetracycline with a purity of 15% was provided by Nanjing Furunde Animal Pharmaceutical Co., Ltd. (Nanjing, China).
Birds and Experimental Design
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A total of 240 1-day-old Ross-308 chicks (BW: 44.00 ± 0.45 g) were purchased form a local hatchery (Jiangsu Shengnong Poultry Industry Development Co., Ltd., Jiangsu, China) and randomly divided to 5 groups, with 6 replicate pens per group and 8 chicks per pen. These 5 experimental groups included the following: 1) control group (basal diet), 2) AGP group (basal diet supplemented with 50 mg/kg chlortetracycline), 3) XOS group (basal diet supplemented with 100 mg/kg XOS), 4) IAPS group (basal diet supplemented with 600 mg/kg IAPS), and 5) XOS + IAPS group (basal diet supplemented with 100 mg/kg XOS and 600 mg/kg IAPS). The experiment period lasted for 21 d.
All the birds were given free access to feed and water in a temperature-controlled room at Nanjing Kangxin Poultry Industry Company (Nanjing, China) during the experimental duration. The temperature of chicken coop was set at 33°C at the age of 1 to 4 d and then reduced by 3°C per week to a final temperature of around 24°C. The composition and nutrition levels of the basal diet are presented in Table 1. The basal diet was formulated to meet or exceed the nutrient requirements of the NRC (1994) and was devoid of antibiotics.
Table 1.
Ingredient composition and calculated nutrient levels of the basal diet.
| Items | 1–21 d |
|---|---|
| Ingredient (%) | |
| Corn | 57.61 |
| Soybean meal | 31.00 |
| Corn gluten meal1 | 3.29 |
| Soybean oil | 3.11 |
| Dicalcium phosphate | 2.00 |
| Limestone | 1.20 |
| Salt | 0.30 |
| L-Lysine HCl | 0.34 |
| DL-Methionine | 0.15 |
| Premix2 | 1.00 |
| Calculated nutrient levels (%) | |
| ME (MJ/kg) | 12.56 |
| CP | 21.00 |
| Calcium | 1.00 |
| Total phosphorus | 0.70 |
| Available phosphorus | 0.46 |
| Lysine | 1.20 |
| Methionine | 0.50 |
| Methionine + cysteine | 0.85 |
The CP content was 60%.
Premix provided per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 2500 IU; vitamin E, 20 IU; menadione sodium bisulfate, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; calcium pantothenate, 10 mg; pyridoxine·HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; choline chloride, 400 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc sulfate), 60 mg; I (from calcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
Sample Collection
At 21 d of age, 2 birds per replicate (12 birds per treatment) with a BW close to the average BW of the cage were chosen and weighed. Blood samples were collected from the wing vein and immediately transferred into heparinized centrifuge tubes. Then, the centrifuge tubes were subjected to centrifugation at 3,000 × g for 15 min at 4°C to separate plasma. Separated plasma was stored at −20°C. The birds were then electrically stunned and killed by exsanguination of the left carotid artery. After being dissection, the duodenum (from ventriculus to pancreobiliary ducts), jejunum (from pancreobiliary ducts to yolk stalk), and ileum (from yolk stalk to ileocecal junction) were separated. After the contents of the small intestine were individually flushed out with cold physiological saline (0.75%, w/v), the length and weight of small intestines were measured for calculated the intestine index. Afterward, about 1-cm middle portion of the small intestine were excised and fixed in 4% paraformaldehyde at least for 24 h. Finally, the mucosa of the jejunum was scraped with sterilized slides, put into a RNAase-free tube, frozen in liquid nitrogen, and stored at −80°C for mRNA extraction.
Growth Performance and Small Intestine Index
The birds were group weighed by cage at 1 and 21 d of age, and feed consumption of 1 to 21 d was recorded by replicate to calculate ADFI, ADG, and the feed to gain ratio (F/G). The intestine index was calculated as per the following formula: small intestine index (g/kg) = the weight of small intestine (g)/BW (kg), intestine index (cm/kg) = the length of small intestine (cm)/BW (kg).
Plasma Analysis
The concentrations of plasma diamine oxidase (DAO) activity and D-lactic acid (D-LA) were measured using a DAO determination kit (UV colorimetry) and Chicken D-LA ELISA kit in accordance with the manufacturer's instructions. The aforementioned kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Small Intestine Morphological Examination
As per standard histology procedures, small intestine tissues were dehydrated in ethanol, equilibrated in xylene, and embedded in paraffin wax. About 5 μm of cross sections was prepared and stained with hematoxylin and eosin (Wuhan Servicebio Technology Co., Ltd., Wuhan, China) according to Zhang et al. (2019). Stained samples were used to assess the villus height (VH), crypt depth (CD), and VH to CD (VH/CD) ratio. The VH and CD were visually observed and captured using an Olympus XC10 digital camera (Olympus Optical Co. Ltd., Tokyo, Japan) under a light microscope at 40× magnification (Olympus BX51; Olympus Optical Co. Ltd., Tokyo, Japan). Then, the ImagePro Plus 6.0 software (Media Cybernetics, Bethesda, MD) was taken to measure the VH and CD. The VH was measured from the tip of the villus to the villus–crypt junction and CD referred to depth from this junction to the base of the crypt. Eight randomly selected well-oriented villus–crypt units from 1 slide per chicken were examined. The average value of the 8 structures per chicken was used.
Goblet Cell Counts
Using the same method as described previously, 5-μm cross sections were prepared and stained with periodic acid–Schiff staining as described by Wang et al. (2009). The reagents used were purchased from Wuhan Servicebio Technology Co., Ltd. (Wuhan, China). Stained tissue slices were observed under a light microscope with a final magnification of ×400 (Olympus BX51; Olympus Optical Co. Ltd., Tokyo, Japan). Goblet cells, which were located in the small intestinal villus of different fields from 1 intestinal cross section per bird, were counted and expressed as per 100 epithelial cells. The numbers of goblet cells of 5 villus from 1 intestinal cross section per bird were counted for statistical analysis.
Total RNA Extraction and Quantitative Real-Time PCR
TRIzol reagent (Takara Biotechnology Co. Ltd. Dalian, China) was used to extract the total RNA from the jejunum mucosa in accordance with the manufacturer's instructions. Then, quantity and quality of total RNA were assessed using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE), and the sample with OD260/OD280 ratio at 1.8 to 2.0 was used for subsequent PCR reactions. Finally, total RNA was reverse transcribed into cDNA using a PrimeScript RT Master Mix kit (Takara Biotechnology Co. Ltd., Dalian, China). The resulting cDNA products were stored at −20°C.
The mRNA expressions of selected genes, including claudin-1, claudin-3, occludin, zonula occludens-1 (ZO-1), were evaluated by the real-time quantitative PCR. The primers of these genes and the housekeeping gene (β-actin) were synthesized by Sangon Biotech Co., Ltd., (Shanghai, China), and the sequences are listed in Table 2. The real-time quantitative PCR assay was carried out in the CFX ConnectTM Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) using SYBR Premix Ex Taq kits (Takara Biotechnology Co. Ltd.). The reaction volume was 20 μL, containing 10 μL SYBR Premix Ex Taq II (2 ×), 0.4 μL ROX Reference Dye II (50 ×), 0.4 μL of each primer (10 μmol), 1 μL cDNA, and 7.8 μL sterilized double-distilled water. The reaction conditions were used as follows: 95°C for 5 min; 40 cycles at 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s; and 72°C for 5 min. The cycle threshold values were normalized to the expression level of β-actin. Relative mRNA expression levels of selected genes were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Primer sequences used for RT-qPCR analysis.
| Genes | Primer sequences (5′-3′) | Amplicon sizes (bp) | GenBank number |
|---|---|---|---|
| Claudin-1 | F: GACCAGGTGAAGAAGATGCGGATG | 107 | NM 001013611.2 |
| R: CGAGCCACTCTGTTGCCATACC | |||
| Claudin-3 | F: CTTCATCGGCAACAACATCGTGAC | 113 | NM 204202.1 |
| R: CCAGCATGGAGTCGTACACCTTG | |||
| ZO-1 | F: CTTCAGGTGTTTCTCTTCCTCCTC | 131 | XM 015278981.2 |
| R: CTGTGGTTTCATGGCTGGATC | |||
| Occludin | F: TCATCGCCTCCATCGTCTAC | 240 | NM 205128.1 |
| R: TCTTACTGCGCGTCTTCTGG | |||
| β-actin | F: ATCCGGACCCTCCATTGTC | 120 | NM 205518.1 |
| R: AGCCATGCCAATCTCGTCTT |
Abbreviations: RT-qPCR, real-time quantitative PCR; ZO-1, zonula occludens-1.
Statistical Analysis
Date were subjected to 1-way ANOVA using SPSS statistical software (version 20.0 for Windows; SPSS Inc., Chicago, IL) and presented as mean values and SEM. Duncan's multiple range tests was used to detect differences among 5 treatments. Besides, all data excluded AGP group were analyzed as a 2 × 2 factorial arrangement using the same SPSS statistical software, and the model included the main effects of XOS and IAPS supplementation as well as XOS × IAPS interaction. Significant differences among treatment means were determined at P ≤ 0.05. All indexes excluded growth performance were analyzed by the mean of 2 chickens per cage as a replicate (n = 6).
Results
Growth Performance
As shown in Table 3, the main effect analysis results showed that neither XOS nor IAPS treatments affected the ADFI and ADG of broilers (P > 0.05). Although there was no interaction between XOS and IAPS treatments (P > 0.05), the 1-way ANOVA analysis results showed that birds in the XOS + IAPS group showed higher ADG and lower F/G ratio as compared with those in the control group (P < 0.05). Birds in the AGP group showed higher ADG than the control group (P < 0.05), while no significant differences in ADG and F/G between the XOS + IAPS and AGP groups were observed (P > 0.05).
Table 3.
Growth performance of broilers fed diets supplementation with xylo-oligosaccharides (XOS),60Co γ-ray–irradiated Astragalus polysaccharides (IAPS), and their combination from 1 to 21 d.
| Treatments 1 | ADFI (g/day·bird) | ADG (g/day·bird) | F/G (g/g) |
|---|---|---|---|
| Control | 40.74 | 28.35b | 1.41a |
| AGP | 42.68 | 32.40a | 1.32a,b |
| XOS | 42.61 | 29.80a,b | 1.43a |
| IAPS | 41.02 | 29.59a,b | 1.39a,b |
| XOS + IAPS | 41.18 | 32.73a | 1.26b |
| SEM | 0.41 | 0.54 | 0.21 |
| P value | 0.40 | 0.03 | 0.03 |
| Main effects | |||
| XOS | |||
| − | 40.88 | 28.97 | 1.41 |
| + | 41.77 | 31.04 | 1.36 |
| IAPS | |||
| − | 41.68 | 29.08 | 1.43x |
| + | 40.98 | 30.93 | 1.33y |
| P values | |||
| XOS | 0.39 | 0.10 | 0.31 |
| IAPS | 0.50 | 0.14 | 0.03 |
| XOS × IAPS | 0.35 | 0.61 | 0.16 |
a,bSuperscripts for means belong to 1-way ANOVA among 5 treatment groups.
x,ySuperscripts for means belong to 2 × 2 factorial arrangement (XOS and IAPS), excluding the AGP group.
Means with no common superscripts within the column of each classification are significantly different (P ≤ 0.05).
Abbreviations: F/G, feed conversion ratio (feed: gain, g: g).
Control, basal diet; AGP, basal diet supplemented with 50 mg/kg chlortetracycline; XOS, basal diet supplemented with 100 mg/kg XOS; IAPS, basal diet supplemented with 600 mg/kg IAPS; XOS + IAPS, basal diet supplemented with 100 mg/kg XOS and 600 mg/kg IAPS.
Intestine Index and Intestinal Morphology
As per Table 4, there was no significant difference in small intestine indexes of birds among 5 groups (P > 0.05). However, the main effect analysis results showed that dietary XOS increased relative weight and length of the duodenum (P < 0.05). The main effect analysis results showed that both XOS and IAPS treatments increased the VH and VH/CD ratio of all intestine segments (P < 0.05; Table 5). There was a significant interaction effect between XOS and IAPS on the VH of the duodenum and ileum and VH/CD ratio of the ileum (P < 0.05). The 1-way ANOVA results showed that birds in the XOS + IAPS group showed higher VH of the duodenum and ileum and VH/CD ratio of the ileum than those in the control, as well as higher VH/CD ratio of the duodenum and jejunum than those in the XOS and IAPS groups (P < 0.05). The VH and VH/CD ratio of 3 small intestine segments of broilers in the AGP group were higher than those in the control group (P < 0.05). There was no significant difference in small intestinal morphology of birds between the AGP and XOS + IAPS groups (P > 0.05).
Table 4.
Small intestine indexes of broilers fed diets supplementation with xylo-oligosaccharides (XOS),60Co γ-ray–irradiated Astragalus polysaccharides (IAPS), and their combination on day 21.
| Treatments 1 | Relative weight (g/kg·BW) |
Relative length (cm/kg·BW) |
||||
|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum | |
| Control | 6.35 | 16.68 | 12.76 | 34.38 | 83.83 | 80.95 |
| AGP | 6.70 | 16.67 | 12.27 | 33.49 | 77.02 | 75.93 |
| XOS | 7.67 | 15.75 | 11.62 | 36.56 | 81.54 | 78.69 |
| IAPS | 6.41 | 15.80 | 11.69 | 32.40 | 76.72 | 71.65 |
| XOS + IAPS | 6.91 | 16.87 | 11.91 | 34.04 | 77.15 | 72.39 |
| SEM | 0.17 | 0.21 | 0.22 | 0.51 | 1.38 | 1.75 |
| P value | 0.08 | 0.30 | 0.45 | 0.15 | 0.40 | 0.43 |
| Main effects | ||||||
| XOS | ||||||
| - | 6.27y | 15.90 | 12.58 | 33.02y | 79.67 | 76.52 |
| + | 7.28x | 16.18 | 11.75 | 35.30x | 78.35 | 74.56 |
| IAPS | ||||||
| - | 6.94 | 15.74 | 12.48 | 34.94 | 80.29 | 79.06 |
| + | 6.61 | 16.34 | 11.86 | 33.38 | 77.72 | 72.02 |
| P values | ||||||
| XOS | 0.03 | 0.69 | 0.18 | 0.09 | 0.65 | 0.67 |
| IAPS | 0.45 | 0.39 | 0.31 | 0.24 | 0.38 | 0.13 |
| XOS × IAPS | 0.33 | 0.27 | 0.13 | 0.46 | 0.95 | 0.55 |
x,ySuperscripts for means belong to 2 × 2 factorial arrangement (XOS and IAPS), excluding the AGP group.
Means with no common superscripts within the column of each classification are significantly different (P ≤ 0.05).
Control, basal diet; AGP, basal diet supplemented with 50 mg/kg chlortetracycline; XOS, basal diet supplemented with 100 mg/kg XOS; IAPS, basal diet supplemented with 600 mg/kg IAPS; XOS + IAPS, basal diet supplemented with 100 mg/kg XOS and 600 mg/kg IAPS.
Table 5.
Intestine morphology of broilers fed diets supplementation with xylo-oligosaccharides (XOS),60Co γ-ray–irradiated Astragalus polysaccharides (IAPS), and their combination on day 21.
| Treatments 1 | Duodenum |
Jejunum |
Ileum |
||||||
|---|---|---|---|---|---|---|---|---|---|
| VH (μm) | CD (μm) | VH/CD | VH (μm) | CD (μm) | VH/CD | VH (μm) | CD (μm) | VH/CD | |
| Control | 1,210.26b | 109.95 | 11.34c | 665.68c | 97.51 | 6.96c | 479.38b | 77.67 | 6.18b |
| AGP | 1,458.15a | 84.34 | 17.51a | 996.87a | 93.62 | 10.67a | 735.78a | 84.01 | 8.84a |
| XOS | 1,453.55a | 110.84 | 13.46c | 793.26b | 87.14 | 9.16b | 685.90a | 83.92 | 8.19a |
| IAPS | 1,534.30a | 109.07 | 14.11b,c | 918.69a | 101.20 | 9.20b | 761.39a | 84.95 | 8.98a |
| XOS + IAPS | 1,443.61a | 90.85 | 16.58a,b | 944.72a | 87.40 | 10.91a | 746.80a | 83.50 | 8.96a |
| SEM | 25.47 | 3.26 | 0.53 | 27.08 | 2.27 | 0.31 | 21.99 | 1.18 | 0.24 |
| P value | <0.01 | 0.11 | <0.01 | <0.01 | 0.21 | <0.01 | <0.01 | 0.28 | <0.01 |
| Main effects | |||||||||
| XOS | |||||||||
| - | 1,350.24y | 107.85 | 12.66y | 798.20y | 98.98x | 8.10y | 620.38y | 81.51 | 7.58y |
| + | 1,454.18x | 100.20 | 14.55x | 869.59x | 86.02y | 10.18x | 716.35x | 83.71 | 8.58x |
| IAPS | |||||||||
| - | 1,309.86y | 108.74 | 12.10y | 729.47y | 92.32 | 7.89y | 582.64y | 81.39 | 7.18y |
| + | 1,494.56x | 99.31 | 15.18x | 938.32x | 92.68 | 10.29x | 754.09x | 83.83 | 8.97x |
| P values | |||||||||
| XOS | 0.05 | 0.32 | 0.01 | 0.08 | 0.01 | <0.01 | <0.01 | 0.23 | 0.01 |
| IAPS | <0.01 | 0.22 | <0.01 | <0.01 | 0.94 | <0.01 | <0.01 | 0.18 | <0.01 |
| XOS × IAPS | <0.01 | 0.13 | 0.70 | 0.16 | 0.59 | 0.69 | <0.01 | 0.12 | 0.01 |
a-cSuperscripts for means belong to 1-way ANOVA among 5 treatment groups.
x,ySuperscripts for means belong to 2 × 2 factorial arrangement (XOS and IAPS), excluding the AGP group.
Means with no common superscripts within the column of each classification are significantly different (P ≤ 0.05).
Abbreviations: CD, crypt depth; VH, villus height; VH/CD, the ratio of villus height to crypt depth.
Control, basal diet; AGP, basal diet supplemented with 50 mg/kg chlortetracycline; XOS, basal diet supplemented with 100 mg/kg XOS; IAPS, basal diet supplemented with 600 mg/kg IAPS; XOS + IAPS, basal diet supplemented with 100 mg/kg XOS and 600 mg/kg IAPS.
Plasma DAO Activity and D-LA Concentration
There was no interaction between XOS and IAPS treatments on plasma DAO activity and D-LA concentration (P > 0.05, Table 6). Although neither XOS nor IAPS treatment affected plasma DAO activity (P > 0.05), birds fed diets supplemented with XOS had lower plasma D-LA concentration than those fed diets without XOS (P < 0.05). Moreover, the 1-way ANOVA results showed that XOS + IAPS treatment decreased plasma D-LA concentration of broilers than that in the control group (P < 0.05), to a level equal to that of the AGP group (P > 0.05).
Table 6.
Plasma diamine oxidase activity and D-lactic acid concentration of broilers fed diets supplementation with xylo-oligosaccharides (XOS),60Co γ-ray–irradiated Astragalus polysaccharides (IAPS), and their combination on day 21.
| Treatments1 | DAO (U/L) | D-LA (nmol/mL) |
|---|---|---|
| Control | 24.62 | 23.61a |
| AGP | 23.08 | 13.64b |
| XOS | 29.13 | 18.00a,b |
| IAPS | 25.60 | 18.80a,b |
| XOS + IAPS | 26.92 | 15.39b |
| SEM | 1.10 | 1.12 |
| P value | 0.49 | 0.04 |
| Main effects | 2.74 | 2.39 |
| XOS | ||
| − | 26.39 | 21.21x |
| + | 28.03 | 16.69y |
| IAPS | ||
| − | 26.88 | 20.81 |
| + | 27.55 | 17.09 |
| P values | ||
| XOS | 0.57 | 0.08 |
| IAPS | 0.81 | 0.15 |
| XOS × IAPS | 0.32 | 0.66 |
a,bSuperscripts for means belong to 1-way ANOVA among 5 treatment groups.
x,ySuperscripts for means belong to 2 × 2 factorial arrangement (XOS and IAPS), excluding the AGP group.
Means with no common superscripts within the column of each classification are significantly different (P ≤ 0.05).
Abbreviations: DAO, diamine oxidase; D-LA, D-lactic acid.
Control, basal diet; AGP, basal diet supplemented with 50 mg/kg chlortetracycline; XOS, basal diet supplemented with 100 mg/kg XOS; IAPS, basal diet supplemented with 600 mg/kg IAPS; XOS + IAPS, basal diet supplemented with 100 mg/kg XOS and 600 mg/kg IAPS.
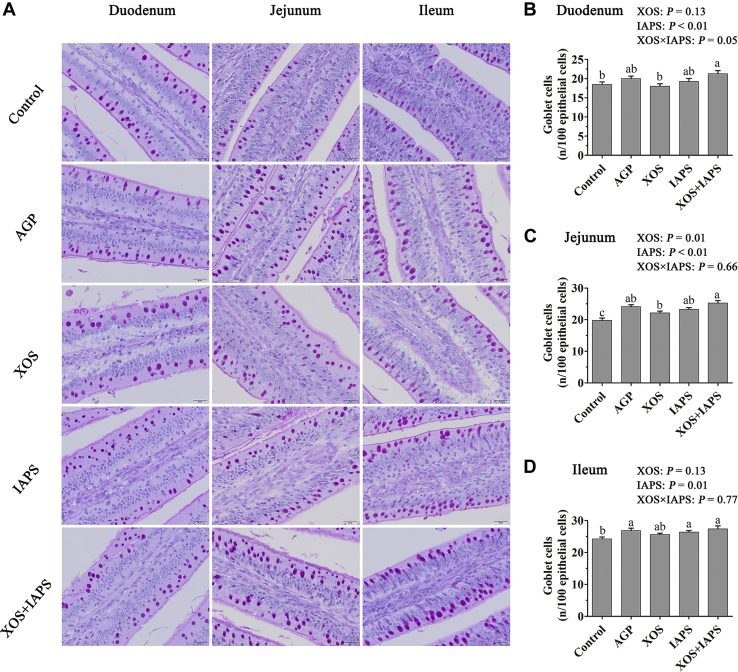
The Numbers of Goblet Cells in the Intestine
As exhibited in Figure 1, the main effect analysis results showed that birds fed diets supplemented with IAPS increased numbers of goblet cells in all 3 small intestine segments than birds fed diets without IAPS (P < 0.05). Diet supplementation with XOS also increased goblet cell numbers in the jejunum of birds when compared with diets without XOS. There was a significant interaction between XOS and IAPS treatments on goblet cell numbers of the duodenum (P = 0.05). The 1-way ANOVA results showed that birds in the XOS + IAPS group showed higher goblet cell numbers in the duodenum and jejunum than those in birds from the control and XOS groups (P < 0.05). Overall, birds from the AGP and XOS + IAPS groups showed higher goblet cell numbers in 3 small intestine segments among 5 treatments. There was no significant difference in the goblet cell number of the ejunum and ileum between the AGP and XOS + IAPS groups (P > 0.05).
Figure 1.
The number of small intestinal goblet cells of broilers fed diets supplementation with xylo-oligosaccharides (XOS), 60Co γ-ray–irradiated Astragalus polysaccharides (IAPS), and their combination on day 21. The data are represented as the mean value ± SEM of each treatment (n = 6). Means without a common letter significantly differ (P ≤ 0.05). a–cSuperscripts for means belong to 1-way ANOVA among 5 treatment groups; P values means the significant from 2 × 2 factorial analysis (excluded the AGP group), and the model included the main effects of XOS and IAPS supplementation as well as XOS × IAPS interaction. Abbreviations: Control, basal diet; AGP, basal diet supplemented with 50 mg/kg chlortetracycline; IAPS, basal diet supplemented with 600 mg/kg IAPS; XOS, basal diet supplemented with 100 mg/kg XOS; XOS + IAPS, basal diet supplemented with 100 mg/kg XOS and 600 mg/kg IAPS.
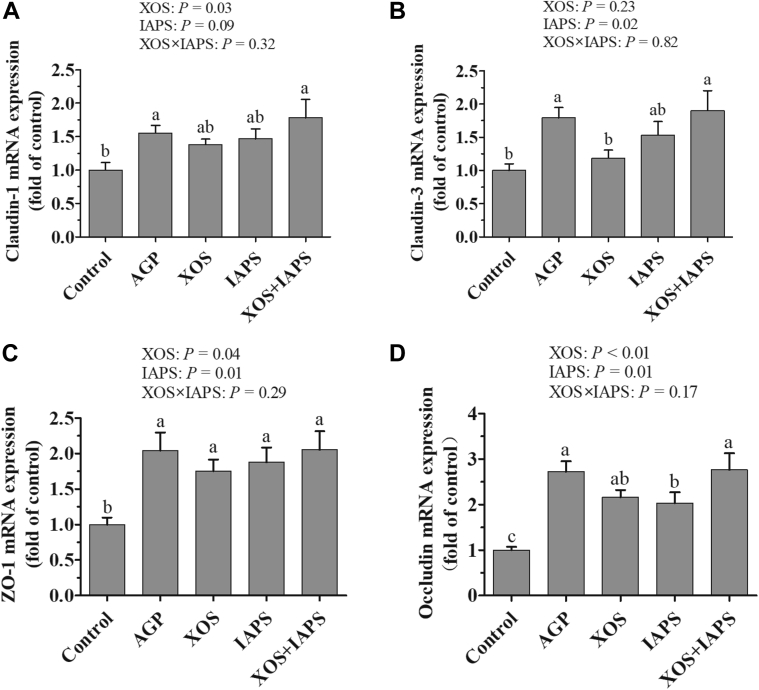
Relative mRNA Expression of Claudin-1, Claudin-3, Occludin, and ZO-1 in the Jejunum
As shown in Figure 2, the main effect analysis results showed that XOS and IAPS treatments increased the mRNA expression of claudin-1, ZO-1, and occludin of the jejunum (P < 0.05). There was no interaction between XOS and IAPS treatments on the mRNA expression of claudin-1, claudin-3, ZO-1, and occludin of the jejunum (P > 0.05). However, the 1-way ANOVA results showed that birds in the XOS + IAPS group showed higher the mRNA expression of claudin-3 and occludin than those in the XOS or IAPS groups (P < 0.05). Moreover, birds from the AGP and XOS + IAPS groups showed higher mRNA expression of claudin-1, claudin-3, ZO-1, and occludin of the jejunum than those from the control groups (P < 0.05). There were no significant differences in mRNA expression of these genes in the broiler jejunum between the AGP and XOS + IAPS groups (P > 0.05).
Figure 2.
The mRNA expression of zonula occludens-1 (ZO-1) (A), claudin-1 (B), claudin-3 (C), and occludin (D) in the jejunum of broilers fed diets supplementation with xylo-oligosaccharides (XOS), 60Co γ-ray–irradiated Astragalus polysaccharides (IAPS), and their combination on day 21. The data are represented as the mean value ± SEM of each treatment (n = 6). Means without a common letter significantly differ (P ≤ 0.05). a–cSuperscripts for means belong to 1-way ANOVA among 5 treatment groups; P values means the significant from 2 × 2 factorial analysis (excluded the AGP group), and the model included the main effects of XOS and IAPS supplementation as well as XOS × IAPS interaction. Abbreviations: Control, basal diet; AGP, basal diet supplemented with 50 mg/kg chlortetracycline; IAPS, basal diet supplemented with 600 mg/kg IAPS; XOS, basal diet supplemented with 100 mg/kg XOS; XOS + IAPS, basal diet supplemented with 100 mg/kg XOS and 600 mg/kg IAPS.
Discussion
In the present study, the main effect analysis results showed that XOS and IAPS treatments did not affect ADFI and ADG of birds from 1 to 21 d, but IAPS treatment significantly decreased the F/G ratio. The results of XOS treatment on growth performance of broilers were in agreement with a previous study (Suo et al., 2015). A previous study reported that dietary supplementation with 500 and 1,000 mg/kg APS significantly increased ADG of broilers from 1 to 42 d (Wu, 2018). Li et al. (2019a) found that birds receiving 600 or 900 mg/kg IAPS in diets relieved the decreased growth performance of cyclophosphamide-treated broilers. These results were inconsistent with those of the present study, which might be a result of differences in polysaccharide content (IAPS vs. APS used by Wu et al.), growth stage of broilers (1–21 d vs. 1–42 d), and physiological state of broilers (normal condition vs. immunosuppression). Although there was no significant interaction between XOS and IAPS treatments, birds in the XOS + IAPS group showed a similar ADG and F/G ratio with those in the AGP group and both ADG and F/G ratio in broilers from the XOS + IAPS and AGP groups were higher than birds from the control group. This finding indicated that the combination of XOS and IAPS had a better potential as antibiotics substitute for improving the growth performance of broilers. The growth performance of broilers was tightly associated with the digestive and absorptive capacity of the digestive organs, especially the small intestine (Gao et al., 2018b; Li et al., 2019b). Therefore, we further analyzed the morphologic development of the small intestinal mucosa of broilers.
The morphology indexes of the small intestine mucosa mainly include VH, CD, and VH/CD ratio. The VH and VH/CD ratio are the common criteria for estimating the nutrient absorptive capacity of the small intestine, and higher VH and VH/CD ratio means a higher absorptive capacity of the small intestine (Montagne et al., 2003; Tucci et al., 2011; Gomes Cairo et al., 2018). Xylo-oligosaccharides can improve intestinal morphology and structure that is associated with the beneficial effects of XOS on the intestinal microflora population and bacterial metabolites (Suo et al., 2015; Ding et al., 2018). Ding et al. (2018) revealed that diets supplemented with XOS improved the relative length, VH, and VH/CD ratio of the jejunum of laying hens. In addition, APS also has a similar effect. Li et al. (2009) revealed APS could act as a prebiotic and increase beneficial bacteria of teh intestinal tract. A previous study found that 600 or 900 mg/kg IAPS significantly increased the VH and VH/CD ratio of the jejunum of broilers treated with cyclophosphamide (Li et al., 2019b). Similar with these previous findings, the present study found that dietary supplementation with XOS, IAPS, or XOS + IAPS significantly increased VH of 3 small intestine segments and VH/CD ratio of the jejunum and ileum. In addition, we found there was a synergy between XOS and IAPS on increasing the VH and VH/CD of the ileum. Moreover, birds from teh AGP and XOS + IAPS groups exhibited a higher VH/CD ratio of the duodenum and jejunum than those in birds from the XOS and IAPS groups. These results indicated that combination of XOS and IAPS had a better effect on improving mucosal morphology of the duodenum and jejunum as compared with individual supplementation of XOS or IAPS. It was well known that the majority of the absorption of digested products (from fat, starch, and protein) is completed in the duodenum and jejunum to a large extent (Oso et al., 2019). Therefore, we speculated that the AGP and XOS + IAPS groups might improve broiler performance by more effectively affecting intestinal mucosa morphology and promote intestinal health.
Diamine oxidase is an intracellular enzyme that was mainly secreted by intestinal epithelial cells (Wu et al., 2013). D-lactic acid is a metabolic product of bacteria mainly found in the intestinal mucosa (Liu et al., 2020b). A complete intestinal mucosal barrier can prevent DAO and D-LA from entering the blood circulation (Zheng et al., 2020). Therefore, the intestinal barrier function can be assessed indirectly by assessing plasma DAO activity and D-LA concentration (Wang et al., 2020). Improving the intestinal mucosal barrier function can reduce DAO activity and D-LA concentration in blood, which has been confirmed by a previous study (Wu et al., 2013). In the present study, neither dietary supplementation with XOS nor IAPS affect the plasma D-LA concentration of broilers as compared with the control group. However, birds in the XOS + IAPS group showed obviously lower plasma D-LA concentration than that in the control group. Although these was on interaction effect between XOS and IAPS on the plasma D-LA concentration, combination of XOS and IAPS effectively enhanced the intestinal mucosal barrier function of broilers and reduced intestinal barrier permeability. The permeability of the intestinal mucosa was directly related to the intestinal mechanical barrier. Although we can indirectly know that the intestinal barrier function had been enhanced from the D-LA content in blood, the analysis of the intestinal mechanical barrier is necessary.
The intestinal mechanical barrier, mainly composed of intestinal epithelial cells and the tight junctions between the cells, plays the important roles in the absorption of nutrients and in protecting the gut from enteric pathogen invasion (Gao et al., 2018a). Goblet cells, a specialized intestinal epithelial cell, can secrete a variety of proteins (such as mucin 2, trefoil factor 2) involved in maintaining the integrity of the intestinal mucosa (Chen et al., 2017). The number of goblet cells in the small intestine can reflect the integrity of the intestinal mucosal barrier function. Li et al. (2019b) reported that the administration of 600 or 900 mg/kg IAPS elevated the number of goblet cells in the small intestine of broilers treated with cyclophosphamide. In the present study, we found that there was a significantly synergy between XOS and IAPS on increasing the goblet cell numbers of the duodenum. Meanwhile, birds from the AGP and XOS + IAPS groups showed higher number of goblet cells of the jejunum and ileum than those from the control, XOS, and IAPS groups. Generally, the higher VH/CD ratio represents a higher number of mature and functional enterocytes (Tucci et al., 2011; Gomes Cairo et al., 2018). Thereby, the direct reason of the increase in goblet cells might be owing to the increased VH/CD of the small intestine. These findings indicated that combination of XOS and IAPS had a synergistic effect for better increasing the number of goblet cells in the small intestine.
Tight junctions, mainly composed of integral membrane proteins (such as occludin and claudins) and ZO-1, are directly involved in the intestinal epithelial barriers against the paracellular penetration of endotoxins, exogenous pathogens, and toxic luminal antigens (Tsukita et al., 1999; Wang et al., 2013). The expression of tight junction proteins in the intestine is important in maintaining the integrity of intestinal epithelium as well as in retaining the intestinal permeability (Ahluwalia et al., 2017). The current results suggested that dietary XOS, IAPS, or XOS + IAPS supplementation significantly upregulated the mRNA expression of occludin and ZO-1 in the jejunum. This demonstrated that XOS, IAPS, and XOS + IAPS could improve intestinal barrier integrity. Yin et al. (2019) reported that dietary XOS significantly enhanced the mRNA expression of ZO-1 in the ileum of weaned piglets, thereby improving the intestinal barrier function of weaned piglets. Similar with our present findings, it is reported that IAPS administration enhanced mRNA expressions of occludin and ZO-1 of Caco2 cells (Ren et al., 2018). In this study, we found that there was no significant interaction between XOS and IAPS on the mRNA expression of tight junction proteins of the jejunum. However, birds from XOS + IAPS treatment had higher mRNA expression of jejunal claudin-1, claudin-3, and occludin than birds from the control, XOS, or IAPS groups, to a level equal to that of the AGP group, indicating that combination effect of XOS and IAPS was superior to the individual use of XOS and IAPS for maintaining the integrity of intestinal epithelium and reducing the intestinal permeability.
Conclusion
Taken together, although XOS or IAPS treatments could enhance intestinal barrier function of broilers, their combination treatment had better effects on improving growth performance, increasing intestinal mucosal morphology, and intestinal mucosal barrier function of broilers than those in individual XOS or IAPS treatments. Moreover, birds receiving diets with XOS + IAPS supplementation showed similar effect on improving intestinal mechanical barrier function as birds receiving diets with chlortetracycline supplementation. These results suggested that combination of XOS and IAPS may be a potential natural alternative to antibiotics.
Acknowledgments
This work was financed by the National Key Research and Development Program of China (2017YFD0500505), the National Natural Science Foundation of China (31601957), the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2020]407), and Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents (2018).
Disclosures
The authors declare no conflicts of interest.
References
- Ahluwalia B., Magnusson M.K., Ohman L. Mucosal immune system of the gastrointestinal tract: maintaining balance between the good and the bad. Scand. J. Gastroenterol. 2017;52:1185–1193. doi: 10.1080/00365521.2017.1349173. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Hack M.E.A., Farag M.R., Sachan S., Karthik K., Dhama K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018;25:10611–10618. doi: 10.1007/s11356-018-1687-x. [DOI] [PubMed] [Google Scholar]
- Broom L.J. Gut barrier function: effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 2018;97:1572–1578. doi: 10.3382/ps/pey021. [DOI] [PubMed] [Google Scholar]
- Burel C. Alternatives to antimicrobial growth promoters (AGPs) in animal feed. In: FinkGremmels J., editor. Animal Feed Contamination: Effects on Livestock and Food Safety. Woodhead Publ Ltd, Abington Hall Abington; Cambridge, Cambs, UK: 2012. pp. 432–448. [Google Scholar]
- Carvalho A.F.A., Neto P.D., Da Silva D.F., Pastore G.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013;51:75–85. [Google Scholar]
- Chen Y.P., Cheng Y.F., Li X.H., Yang W.L., Wen C., Zhuang S., Zhou Y.M. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult. Sci. 2017;96:405–413. doi: 10.3382/ps/pew240. [DOI] [PubMed] [Google Scholar]
- Choi J.I., Kim H.J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 2013;97:358–362. doi: 10.1016/j.carbpol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Ding X.M., Li D.D., Bai S.P., Wang J.P., Zeng Q.F., Su Z.W., Xuan Y., Zhang K.Y. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 2018;97:874–881. doi: 10.3382/ps/pex372. [DOI] [PubMed] [Google Scholar]
- Farzi A., Frohlich E.E., Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics. 2018;15:5–22. doi: 10.1007/s13311-017-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Zhao M., Zhang L., Li J., Yu L., Gao F., Zhou G. In ovo feeding of l-arginine regulates intestinal barrier functions of posthatch broilers by activating the mTOR signaling pathway. J. Sci. Food Agric. 2018;98:1416–1425. doi: 10.1002/jsfa.8609. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao M.M., Li Y.J., Zhang L., Li J.L., Yu L.L., Gao F., Zhou G.H. Effects of in ovo feeding of L-arginine on the development of digestive organs, intestinal function and post-hatch performance of broiler embryos and hatchlings. J. Anim. Physiol. Anim. Nutr. 2018;102:E166–E175. doi: 10.1111/jpn.12724. [DOI] [PubMed] [Google Scholar]
- Gomes Cairo P.L., Gois F.D., Sbardella M., Silveira H., de Oliveira R.M., Allaman I.B., Cantarelli V.S., Costa L.B. Effects of dietary supplementation of red pepper (Schinus terebinthifolius Raddi) essential oil on performance, small intestinal morphology and microbial counts of weanling pigs. J. Sci. Food Agric. 2018;98:541–548. doi: 10.1002/jsfa.8494. [DOI] [PubMed] [Google Scholar]
- Kallon S., Li X., Ji J., Chen C., Xi Q., Chang S., Xue C., Ma J., Xie Q., Zhang Y. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. J. Anim. Sci. Biotechnol. 2013;4:22. doi: 10.1186/2049-1891-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ren L., Zhu X., Li J., Zhang L., Wang X., Gao F., Zhou G. Immunomodulatory effect of gamma-irradiated Astragalus polysaccharides on immunosuppressed broilers. Anim. Sci. 2019;90:117–127. doi: 10.1111/asj.13133. [DOI] [PubMed] [Google Scholar]
- Li S., Wang X.F., Ren L.N., Li J.L., Zhu X.D., Xing T., Zhang L., Gao F., Zhou G.H. Protective effects gamma-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019;98:6400–6410. doi: 10.3382/ps/pez478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.P., Zhao X.J., Wang J.Y. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009;88:519–525. doi: 10.3382/ps.2008-00365. [DOI] [PubMed] [Google Scholar]
- Liang E., Kabcenell A.K., Coleman J.R., Robson J., Ruffles R., Yazdanian M. Permeability measurement of macromolecules and assessment of mucosal antigen sampling using in vitro converted M cells. J. Pharmacol. Toxicol. Methods. 2001;2:93–101. doi: 10.1016/s1056-8719(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Liu Y.S., Li S., F Wang X., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing g-irradiated Astragalus polysaccharides. Poult. Sci. 2020;100:273–281. doi: 10.1016/j.psj.2020.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang D., Liu Y., Zhou D., Yang H., Zhang K., Zhang D. Circular RNA circ_0001105 protects the intestinal barrier of septic rats by inhibiting inflammation and oxidative damage and YAP1 expression. Gene. 2020;755:144897. doi: 10.1016/j.gene.2020.144897. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mehdi Y., Letourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Cote C., Ramirez A.A., Godbout S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003;108:95–117. [Google Scholar]
- National Research Council . 9th rev. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Oso A.O., Suganthi R.U., Reddy G.B.M., Malik P.K., Thirumalaisamy G., Awachat V.B., Selvaraju S., Arangasamy A., Bhatta R. Effect of dietary supplementation with phytogenic blend on growth performance, apparent ileal digestibility of nutrients, intestinal morphology, and cecal microflora of broiler chickens. Poult. Sci. 2019;98:4755–4766. doi: 10.3382/ps/pez191. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Guan L., Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome. 2015;3:15. doi: 10.1186/s40168-015-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Wang X., Li S., Li J., Zhu X., Zhang L., Gao F., Zhou G. Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. Int. J. Biol. Macromol. 2018;120:641–649. doi: 10.1016/j.ijbiomac.2018.08.138. [DOI] [PubMed] [Google Scholar]
- Sun Z., Lv W., Yu R., Li J., Liu H., Sun W., Wang Z., Li J., Shan Z., Qin Y. Effect of a straw-derived xylooligosaccharide on broiler growth performance, endocrine metabolism, and immune response. Can. J. Vet. Res.-Rev. Can. Rech. Vet. 2013;77:105–109. [PMC free article] [PubMed] [Google Scholar]
- Suo H., Lu L., Xu G., Xiao L., Chen X., Xia R., Zhang L., Luo X. Effectiveness of dietary xylo-oligosaccharides for broilers fed a conventional corn-soybean meal diet. J. Integr. Agric. 2015;14:2050–2057. [Google Scholar]
- Suresh G., Das R.K., Brar S.K., Rouissi T., Ramirez A.A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M. Structural and signalling molecules come together at tight junctions. Curr. Opin. Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Tucci F.M., Thomaz M.C., Nakaghi L.S.O., Hannas M.I., Scandolera A.J., Budino F.E.L. The effect of the addition of trofic agents in weaned piglet diets over the structure and ultra-structure of small intestine and over performance. Arq Bras Med. Vet. Zootec. 2011;63:931–940. [Google Scholar]
- Wang Y., An Y., Ma W., Yu H., Lu Y., Zhang X., Wang Y., Liu W., Wang T., Xiao R. 27-Hydroxycholesterol contributes to cognitive deficits in APP/PS1 transgenic mice through microbiota dysbiosis and intestinal barrier dysfunction. J. Neuroinflamm. 2020;17:199. doi: 10.1186/s12974-020-01873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Ma W., She R., Sun Q., Liu Y., Hu Y., Liu L., Yang Y., Peng K. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult. Sci. 2009;88:967–974. doi: 10.3382/ps.2008-00533. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang S., Li Y., Wang F., Yang X., Yao J. Sulfated Astragalus polysaccharide can regulate the inflammatory reaction induced by LPS in Caco2 cells. Int. J. Biol. Macromol. 2013;60:248–252. doi: 10.1016/j.ijbiomac.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Zhou Y.M., Wu Y.N., Zhang L.L., Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet. Immunol. Immunopathol. 2013;153:70–76. doi: 10.1016/j.vetimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Yin J., Li F., Kong X., Wen C., Guo Q., Zhang L., Wang W., Duan Y., Li T., Tan Z., Yin Y. Dietary xylo-oligosaccharide improves intestinal functions in weaned piglets. Food Funct. 2019;10:2701–2709. doi: 10.1039/c8fo02485e. [DOI] [PubMed] [Google Scholar]
- Yuan C., Pan X., Gong Y., Xia A., Wu G., Tang J., Han X. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio L. Int. Immunopharmacol. 2008;8:51–58. doi: 10.1016/j.intimp.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Zahran E., Risha E., AbdelHamid F., Mahgoub H.A., Ibrahim T. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2014;38:149–157. doi: 10.1016/j.fsi.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y.N., Xu R.S., Min L., Ruan D., Kim H.Y., Hong Y.G., Chen W., Wang S., Xia W.G., Luo X., Xie C.Y., Shang X.G., Zheng C.T. Effects of L-methionine on growth performance, carcass quality, feather traits, and small intestinal morphology of Pekin ducks compared with conventional DL-methionine. Poult. Sci. 2019;98:6866–6872. doi: 10.3382/ps/pez438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Yang P., Dai J., Yu G., Ou W., Xu W., Mai K., Zhang Y. Dynamics of intestinal inflammatory cytokines and tight junction proteins of turbot (Scophthalmus maximus L.) during the development and recovery of enteritis induced by dietary beta-conglycinin. Front. Mar. Sci. 2020;7:198. [Google Scholar]