Abstract
The aim of this study was to explore the protective effects of squalene supplementation on growth performance, oxidative status, and liver function of diquat-challenged broilers. One hundred forty-four 1-day-old male Ross 308 broiler chicks were allocated to 3 groups, and each group consisted of 6 replicates of 8 birds each. The three groups were as follows: 1) nonchallenged broilers fed with a basal diet (control group), 2) diquat-challenged broilers fed a basal diet, and 3) diquat-challenged broilers fed with a basal diet supplemented with 1.0 g/kg of squalene. Broilers were intraperitoneally injected with 20 mg/mL of diquat solution at a dosage of 1 mL/kg of BW or an equivalent amount of saline at 20 d. Compared with the control group, weight gain and BW change rate during 24 h after injection were decreased by diquat challenge (P < 0.05), and the diquat-induced compromised growth performance was improved by squalene supplementation (P < 0.05). Diquat administration reduced plasma superoxide dismutase activity and increased malondialdehyde accumulation and glutathione peroxidase activity in both plasma and the liver (P < 0.05). In contrast, plasma glutathione peroxidase activity in diquat-challenged broilers was reduced by squalene supplementation (P < 0.05). The hepatic glutathione level was reduced by diquat administration (P < 0.05), whereas its level in plasma and the liver of diquat-challenged broilers was increased by squalene supplementation (P < 0.05). The relative liver weight of broilers was increased by diquat challenge (P < 0.05), with its value being intermediate in the squalene-supplemented group (P > 0.05). The plasma aminotransferase activities and total bilirubin concentration were increased by diquat challenge (P < 0.05), which were reduced by squalene supplementation (P < 0.05). The mRNA abundance of hepatic nuclear factor erythroid 2–related factor 2 (P < 0.05) was upregulated by diquat treatment, regardless of squalene supplementation. The mRNA abundance of hepatic glutathione peroxidase 1 and B-cell lymphoma/leukemia 2–associated X protein was upregulated by diquat challenge (P < 0.05), which was reversed by squalene administration (P < 0.05). Squalene increased NAD(P)H quinone dehydrogenase 1 mRNA abundance and decreased caspase 3 mRNA abundance in the liver of diquat-challenged broilers (P < 0.05). The results suggested that squalene can increase weight gain, improve oxidative status, and alleviate liver injury in diquat-challenged broilers.
Key words: squalene, diquat, oxidative stress, liver, broiler
Introduction
Oxidative stress is usually defined as an imbalance between the excessive generations of cellular reactive oxygen species (ROS) and the compromised capacity of living organisms to counteract ROS through antioxidant system response (Persson et al., 2014; Pisoschi and Pop, 2015). Compared with other domestic animal species, the genetic selection for fast growth rate and lean and large breast muscle in modern broiler lines makes them particularly vulnerable to oxidative stress arising from various sources (Estévez, 2015), such as diseases (Lee et al., 2019), high temperature (Belhadj Slimen et al., 2016), and exposure to toxic substances from environment, water, and feed (Wu et al., 2014). The occurrence of oxidative stress would result in inferior growth performance, disrupt cellular antioxidant defense, compromise health status, and lower product quality in broiler chickens (Lykkesfeldt and Svendsen, 2007; Estévez, 2015; Akbarian et al., 2016). The liver is responsible for synthesis, storage, metabolism, and detoxification of macromolecules and plays a key role in the clearance of foreign antigens and pathogens that invade the body from the gastrointestinal lumen (Lalor and Adams, 2002). These unique multifunctional properties, in turn, render it a main target organ attacked by oxidative stress, mainly owing to its continuous exposure to oxidative stimuli and high mitochondrial content, the main source of energy and cellular ROS (Starkov, 2008). The available literature on broiler chickens has already demonstrated that the liver is extremely sensitive to oxidative insults, and the oxidative stress–induced liver damage is directly correlated with growth retardation (Yang et al., 2010, 2016; Chen et al., 2020a). It is, hence, imperative to develop new effective interventions to protect the liver from oxidative damage to improve growth performance and health status of broilers subjected to oxidative stress.
Squalene, a natural triterpene compound, is widely available in nature, especially in shark liver oil, olive oil, and wheat germ oil (Spanova and Daum, 2011; Lou-Bonafonte et al., 2018). Squalene is an important intermediate for cholesterol biosynthesis in mammals, but only a small proportion of squalene uptake is actually metabolized and converted into cholesterol (Strandberg et al., 1990). Approximately 60–85% of an orally consumed squalene is absorbed through gastrointestinal lymphatic vessels, and it is then transported in blood by binding with lipoproteins to target organs and tissues (Strandberg et al., 1990; Gylling and Miettinen, 1994; Miettinen and Vanhanen, 1994). An in vivo study has shown that squalene can substantially accumulate in the liver, and its cellular localization in hepatocytes varies as per animal species (Martínez-Beamonte et al., 2018). Squalene is actually not susceptible to lipid peroxidation and is stable when attacked by free radicals, and its in vitro ability to scavenge free radicals is comparable with an effective lipophilic antioxidant, 3,5-di-t-butyl-4-hydroxytoluene, although belonging to a class of triterpene derivatives (Kohno et al., 1995). The role of squalene has recently drawn increasing research attention as a natural antioxidant. An in vitro cell culture experiment has demonstrated that squalene administration attenuated hydrogen peroxide–induced oxidative stress in human mammary epithelial cells in a dose-dependent pattern (Warleta et al., 2010). Likewise, the protective effects of squalene against oxidative damage have been also reported in rodent models of oxidative stress (Sabeena Farvin et al., 2004, 2007; Senthilkumar et al., 2006; Das et al., 2008; Motawi et al., 2010). Little, however, is known with regard to the administration and outcomes of squalene in broiler chickens subjected to oxidative stress.
Diquat, a redox cycler, is readily converted to a free radical in the presence of molecular oxygen and then produces superoxide anions and subsequently other redox products, which would induce cellular oxidative stress (Jones and Vale, 2000). Based on this special characteristic, diquat has been widely used as a model chemical for in vivo and in vitro studies of oxidative stress (Koch and Hill, 2017). As for poultry, diquat has been already used to establish the oxidative model in the chicken embryo, with the liver being a major target organ (Li et al., 2020). Our recent work has successfully induced oxidative stress in broiler chickens at 21 d of age by administrating diquat intraperitoneally and has found that diquat-induced oxidative stress resulted in serious liver damage, ultimately leading to inferior growth performance of broilers (Chen et al., 2020a). This study was therefore conducted to explore the protective effects of dietary squalene supplementation on growth performance, antioxidant status, and liver function of young broilers in a model of diquat-induced oxidative stress, and the acquired results would provide a valuable reference for alleviating oxidative stress that occurred in broiler production.
Materials and methods
Animals and Treatment
All experiments involving animals were carried out under the authority of Laboratory Animal Use License (SYXK-2017-0007) issued by the Jiangsu Provincial Department of Science and Technology and strictly in compliance with the protocol approved by the Nanjing Agricultural University Institutional Animal Care and Use Committee.
A total of one hundred forty-four 1-day-old male Ross 308 broiler chicks with a similar initial BW of 42.31 ± 0.31 g were used in a completely randomized design and were randomly allocated to one of the following 3 groups for a 21-d feeding experimental trial: 1) nonchallenged broilers fed with a basal diet (control group), 2) diquat-challenged broilers fed with a basal diet, and 3) diquat-challenged broilers fed with a basal diet supplemented with 1.0 g/kg of squalene. Each experimental group consisted of 6 replicates (cages) of 8 birds each. The diquat (diquat dibromide monohydrate; product no. 45422; Sigma-Aldrich, St Louis, MO) was dissolved in 0.86% physiological saline to prepare 20 mg/mL of diquat solution before injection. At 20 d of age after weighing, broiler chickens were administered intraperitoneally with the diquat solution at a dosage of 1 mL/kg of BW as previously reported by Chen et al., 2020a or an equal amount of vehicle (0.86% physiological saline, control group). The squalene sample with a purity of approximately 92% was extracted and purified from deodorizer distillate produced during rice bran oil processing, which was kindly gifted by Yichun Dahaigui Life Science Co., Ltd. (Yichun City, Jiangxi Province, People's Republic of China). The dosage of squalene (1.0 g/kg) used in this study was selected based on the results of our previous study in which dietary supplementation with 1.0 g/kg of squalene exhibited the most favorable effects in improving the growth performance and antioxidant status of broiler chickens under normal physiological conditions among its administration levels from 0.25 to 2.0 g/kg of diet (Chen et al., 2020b). Before feed preparation, the liquid squalene was first mixed thoroughly with soybean oil used in feed formulation until they were homogeneous. The basal diet was formulated as per National Research Council (1994) recommendations set for broiler chickens, and its ingredient composition and nutrient level are presented in Table 1. Broiler chickens were reared in steel cages with plastic floors (120 cm [length] × 60 cm [width] × 50 cm [height]) in a temperature-controlled room on a light schedule of 23 h of light and 1 h of dark. All birds were allowed free access to water and were fed ad libitum on mash diet except during the periods of fasting, when only water was available. The temperature of the chicken house was maintained between 32°C and 34°C during the first week after hatching, and it was then decreased by 2°C to 3°C at 1-wk interval until a final temperature of 26°C was achieved. The RH was set at around 70% during the first 3 d of age, which was then maintained at approximately 60–65% thereafter.
Table 1.
Composition and the nutrient level of basal diet.
| Ingredients, % | Content |
|---|---|
| Corn | 57.01 |
| Soybean meal | 31.50 |
| Corn gluten meal | 3.40 |
| Soybean oil | 3.10 |
| Limestone | 1.20 |
| Dicalcium phosphate | 2.00 |
| L-Lysine | 0.34 |
| DL-Methionine | 0.15 |
| Sodium chloride | 0.30 |
| Premix1 | 1.00 |
| Total | 100 |
| Calculated nutrient levels | |
| AME, MJ/kg | 12.55 |
| CP, % | 21.33 |
| Calcium, % | 1.00 |
| Total phosphorus, % | 0.68 |
| Available phosphorus, % | 0.46 |
| Lysine, % | 1.21 |
| Methionine, % | 0.50 |
| Methionine + cystine, % | 0.86 |
| Analyzed nutrient levels2 | |
| Gross energy, MJ/kg | 15.48 |
| CP, % | 20.79 |
| Calcium, % | 1.06 |
| Total phosphorus, % | 0.72 |
| Lysine, % | 1.24 |
| Methionine, % | 0.48 |
Premix provided per kilogram of diet: vitamin A (transretinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 3,000 IU; vitamin E (all-rac-α-tocopherol), 30 IU; menadione, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; choline chloride, 600 mg; calcium pantothenate, 10 mg; pyridoxine·HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8.0 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc oxide), 60 mg; I (from calcium iodate), 1.1 mg; and Se (from sodium selenite), 0.3 mg.
The results are the average values of triplicate measurements.
Sample Collection
At 21 d of age, one bird from each cage (replicate) with a BW close to the replicate mean (6 birds for each treatment in total) was selected for sampling after a 24-h period after administration of diquat. Blood samples (around 5.0 mL) were collected from the left wing vein in heparinized tubes. Plasma was then separated after centrifugation for 15 min at 2,000× g at 4°C, transferred into another tube, and immediately frozen at −20°C pending analysis. Birds were euthanized by the cervical dislocation method and necropsied after blood sampling. The liver was then dissected free from vessels and the surrounding tissues, rinsed off using an ice-cold phosphate buffer solution, surface-dried using a filter paper, and weighed to calculate absolute liver weight and relative liver weight, using the following formula: relative liver weight (g/kg) = absolute liver weight/terminal BW. After being placed on a chilled stainless steel tray, the right lobe of liver samples (approximately 6 g) was excised from the remaining fresh liver tissues, minced, and then quickly frozen and stored in liquid nitrogen for subsequent analysis.
Growth Performance Determination
At 20 d of age, all birds were weighed after a 12-h feed withdrawal period, and the cumulative intake of feed by birds was recorded on cage basis to determine average BW, ADFI, ADG, and BW gain-to-feed intake ratio (G/F) during 1 to 20 d of age. The birds were also weighed at 21 d of age to calculate ADG during the 24 h after challenge and BW change rate (the ratio between 21-d average BW and 20-d average BW).
Analysis of Biochemical Parameters in Plasma
The aminotransferase activities (aspartate aminotransferase [cat. no. C010-1] and alanine aminotransferase [cat. no. C009-2]) and the concentrations of total bilirubin (cat. no. C019-1-1), total protein (cat. no. A045-4-1), albumin (cat. no. A028-2-1), total cholesterol (cat. no. A111-1-1), triglyceride (cat. no. A110-2-1), and glucose (cat. no. F006-1-1) in plasma were determined using specific colorimetric commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, People's Republic of China) following the instructions of the manufacturer, using a MODEL 680 microplate reader (Bio-Rad Laboratories Inc., Hercules, CA).
Determination of Antioxidant-Related Parameters
Around 0.7 g of liver samples stored in liquid nitrogen was homogenized using a motor-driven homogenizer (PRO-PK-02200D; Pro Scientific, Inc., Monroe, CT) in an ice-cold water bath until no tissue particles were visible in the solution (around 40 s), using a cold (4°C) physiological saline solution as the homogenate medium at a ratio of 1:9 (wt/vol). The supernatant was then harvested after centrifugation at 4,450× g for 20 min at 4°C and was immediately stored at −80°C for further assay.
Superoxide dismutase (SOD, cat. no. A001-1-1), glutathione peroxidase (GPX, cat. no. A005-1-2), and catalase (CAT, no. A007-1-1) activities and the reduced glutathione (GSH, cat. no. A006-1) and malondialdehyde (MDA, cat. no. A003-1) concentrations in both plasma and liver samples were colorimetrically quantified using the commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, People's Republic of China), strictly following a standardized protocol as per the recommendations of the manufacturer. The hydroxylamine method (Kono, 1978) was used to measure SOD activity at 550 nm, and one unit of its activity was calculated as the amount of this enzyme that was required to produce 50% inhibition of the rate of nitrite production per milliliter of plasma or per milligram protein of the liver in 40 min at 37°C. The measurement of GPX activity and GSH concentration was performed using the 5,5′-dithiobis (2-nitrobenzoic acid) method (Owens and Belcher, 1965) at 412 nm, and one unit of GPX was measured as the amount of enzyme depleting 1.0 μmol of GSH per 0.1 mL of plasma or 1.0 mg of liver protein in 5 min at 37°C. The ammonium molybdate method (Góth, 1991) was adopted for assaying CAT activity at 405 nm, one unit of which was defined as the amount of enzyme decomposing 1.0 μmol of hydrogen peroxide per milliliter of plasma or per milligram protein of liver in 1 min at 37°C. Malondialdehyde accumulation was quantified using the thiobarbituric acid method at 532 nm (Placer et al., 1966). The total protein concentration of liver samples was determined by the Bradford assay (Kruger, 1994), using crystalline BSA as a reference standard. All acquired results of liver samples were normalized against the corresponding total protein concentration from the same sample before comparisons.
Isolation and Quantification of mRNA
Total cellular RNA was extracted from the liquid nitrogen-frozen liver samples using a commercial TRIzol reagent kit (TaKaRa Biotechnology, Dalian, Liaoning Province, People's Republic of China), according to the kit manufacturer's instructions. The assessment of extracted RNA integrity was performed by 1.5% agarose gel electrophoresis containing 0.5 mg/mL of ethidium bromide. Its purity and concentration were measured using a NanoDrop ND-1000 UV spectrophotometer (Nano Drop Technologies, Wilmington, DE) based on the ratio of absorbance at 260/280 nm wavelength, an indicator of RNA purity. The quantified RNA was then dissolved in diethyl pyrocarbonate–treated water (Biosharp Co., Ltd., Hefei, Anhui Province, People's Republic of China) and was adjusted to a final concentration of 0.5 μg/μL. The commercial PrimeScript RT reagent kit (TaKaRa Biotechnology, Dalian, Liaoning Province, China) was used to reverse transcribe 1.0 μg of extracted total RNA into the complementary deoxyribonucleic acid in the presence of random and oligo(dT) primers, following the manufacturer's guidelines. The reverse transcription conditions were 15 min at 37°C and 5 s at 85°C. The primer sequences of the target genes along with the internal reference genes (B-cell lymphoma/leukemia 2-associated X protein [Bax], B-cell lymphoma/leukemia 2 [Bcl-2], caspase 3 [CASP3], heme oxygenase 1, glutathione peroxidase 1 [GPX1], kelch-like ECH-associated protein 1 [Keap1], NAD(P)H quinone dehydrogenase 1 [NQO1], nuclear factor erythroid 2–related factor 2 [Nrf2], superoxide dismutase 1, X-linked inhibitor of apoptosis protein, and β-actin) were designed using Primer-Blast (http://www.ncbi.nlm.nih.gov) and are presented in Table 2. The real-time PCR was performed using an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Grand Island, NY). The PCR reaction mixture consisted of 2.0 μL of diluted complementary deoxyribonucleic acid, 0.4 μL of each primer, 10 μL of SYBR Premix Ex Taq (TaKaRa Biotechnology, Dalian, Liaoning Province, People's Republic of China), 0.4 μL of ROX reference dye (TaKaRa Biotechnology, Dalian, Liaoning Province, People's Republic of China), and 6.8 μL of double-distilled water. The PCR thermal conditions were set as follows: preheating for denaturation at 95°C for 30 s, 40 cycles of denaturation at 95°C for 5 s, and annealing at 60°C for 30 s. The melting curve analysis of the amplification products was performed at the end of each PCR reaction following the conditions: 1 cycle of denaturation at 95°C for 10 s, followed by an increase in temperature from 65 to 95°C with a temperature change velocity at 0.5 °C/s. Fold changes were calculated by normalizing relative expression of target genes to that of the reference gene (β-actin), as per the 2−ΔΔCT method as previously described by Livak and Schmittgen, 2001.
Table 2.
Sequences of real-time PCR primers.
| Gene | Gene Bank ID | Primer sequence, sense/antisense | Length |
|---|---|---|---|
| Nrf2 | XM_015289381.2 | CCCGCACCATGGAGATCGAG | 180 |
| GGAGCTGCTCTTGTCTTTCCT | |||
| Keap1 | XM_015274015 | GCATCACAGCAGCGTGGAGAG | 117 |
| GCGTACAGCAGTCGGTTCAGC | |||
| HO1 | NM_205344.1 | GTCGTTGGCAAGAAGCATCC | 106 |
| GGGCCTTTTGGGCGATTTTC | |||
| NQO1 | NM_001277619.1 | AACCTCTTTCAACCACGCCA | 113 |
| GTGAGAGCACGGCATTGAAC | |||
| SOD1 | NM_205064.1 | GGCAATGTGACTGCAAAGGG | 133 |
| CCCCTCTACCCAGGTCATCA | |||
| GPX1 | HM590226 | AACCAATTCGGGCACCAG | 122 |
| CCGTTCACCTCGCACTTCTC | |||
| Bcl-2 | NM_205339.2 | GCTGCTTTACTCTTGGGGGT | 128 |
| CTTCAGCACTATCTCGCGGT | |||
| Bax | XM_422067 | GGTGACAGGGATCGTCACAG | 108 |
| TAGGCCAGGAACAGGGTGAAG | |||
| CASP3 | NM_204725.1 | TGGTGGAGGTGGAGGAGC | 183 |
| TGTCTGTCATCATGGCTCTTG | |||
| XIAP | NM_204588.2 | AACCTGGTGATCGAGCTTGG | 71 |
| GTCCCGACCCAGGACAAAAA | |||
| β-actin | NM_205518.1 | TTGGTTTGTCAAGCAAGCGG | 100 |
| CCCCCACATACTGGCACTTT |
Abbreviations: Bax, B-cell lymphoma/leukemia 2-associated X protein; Bcl-2, B-cell lymphoma/leukemia 2; CASP3, caspase 3; GPX1, glutathione peroxidase 1; HO1, heme oxygenase 1; Keap1, kelch-like ECH-associated protein 1; NQO1, NAD(P)H quinone dehydrogenase 1; Nrf2, nuclear factor erythroid 2–related factor 2; SOD1, superoxide dismutase 1; XIAP, X-linked inhibitor of apoptosis protein.
Statistical Analysis
Data were analyzed by one-way ANOVA using SPSS (2008) statistical software (version 16.0 for Windows, SPSS Inc., Chicago, IL). A cage (replicate) was the experimental unit for the growth performance data, whereas an individual bird from each cage was the experimental unit for other measured parameters (antioxidant-related parameters, plasma biochemical indices, organ weight, and gene expression). Differences among the 3 experimental groups were examined using Tukey's multiple range test. The differences were considered statistically significant when P < 0.05. The results were presented as means with their pooled standard errors.
Results
Growth Performance
Before diquat challenge (1–20 d, Table 3), the growth performance (BW at 20 d of age, ADG, ADFI, and G/F, P > 0.05) of broiler chickens did not differ among groups. Although 21-d BW (P > 0.05) of broilers was similar between these 3 treatments, diquat challenge decreased ADG (P < 0.05) and BW change rate (P < 0.05) during 24 h after administration in broilers, when compared with the control group. In contrast, dietary supplementation with squalene increased ADG (P < 0.05) and BW change rate (P < 0.05) of diquat-challenged broilers in comparison with their counterparts receiving a basal diet only, but which were still lower than those of the control group (P < 0.05).
Table 3.
Effects of dietary squalene supplementation on the growth performance of diquat-challenged broilers.
| Items1 | Treatments |
SEM2 | P-value | ||
|---|---|---|---|---|---|
| CON | DQ | DQ + SQ | |||
| Before challenge (1–20 d) | |||||
| BW at 1 d of age, g | 42.16 | 42.34 | 42.44 | 0.31 | 0.192 |
| BW at 20 d of age, g | 621.03 | 622.30 | 623.30 | 7.95 | 0.994 |
| ADG, g/d/bird | 28.95 | 29.00 | 28.99 | 0.40 | 0.998 |
| ADFI, g/d/bird | 43.71 | 43.58 | 42.62 | 0.70 | 0.806 |
| G/F, g/g | 0.66 | 0.67 | 0.68 | 0.01 | 0.522 |
| After challenge (20–21 d) | |||||
| BW at 21 d of age, g | 669.08 | 643.50 | 656.92 | 8.79 | 0.522 |
| ADG, g/d/bird | 48.05a | 21.20c | 33.63b | 2.89 | <0.001 |
| BW change rate, g/g | 1.077a | 1.034c | 1.054b | 0.005 | <0.001 |
a–cMeans within a row with different superscripts are different at P <0.05.
Abbreviations: CON, nonchallenged broilers fed with a basal diet; DQ, diquat-challenged broilers fed with a basal diet; DQ + SQ, diquat-challenged broilers fed with a basal diet supplemented with 1.0 g/kg of squalene.
G/F: BW gain-to-feed intake ratio; BW change rate is calculated as 21-d BW divided by 20-d BW.
n = 6.
Antioxidant Status in Plasma and the Liver
Compared with the control group (Table 4), diquat challenge decreased plasma SOD activity (P < 0.05) and increased MDA accumulation (P < 0.05) in plasma and the liver of broiler chickens, and the values of these parameters were intermediate in the squalene-supplemented group, similar to normal controls and those subjected to diquat challenge only (P > 0.05). The GPX activity (P < 0.05) in plasma and the liver of broilers was both increased by diquat treatment, when compared with the control group. Dietary squalene administration decreased plasma GPX activity (P < 0.05) of diquat-challenged broilers, although its activity in the squalene-supplemented group was still higher than that of the control group (P < 0.05). However, squalene did not alter hepatic GPX activity (P > 0.05) in broilers challenged with diquat, although hepatic GPX activity (P > 0.05) in the squalene group was intermediate among the 3 experimental groups. The plasma GSH level (P < 0.05) of diquat-challenged broilers was increased by squalene supplementation, and there was no difference between the normal controls and diquat-challenged broilers fed with a basal diet supplemented with or without squalene (P > 0.05). The hepatic GSH concentration (P < 0.05) was decreased in broilers subjected to diquat challenge when compared with the control group. On the contrary, squalene administration increased hepatic GSH concentration (P < 0.05) of diquat-challenged broilers to a level comparable with the control group (P > 0.05). Treatments did not affect CAT activity in both plasma and the liver or hepatic SOD activity of broiler chickens (P > 0.05).
Table 4.
Effects of dietary squalene supplementation on the antioxidant status in the plasma and liver of diquat-challenged broilers.
| Items | Treatments |
SEM1 | P-value | ||
|---|---|---|---|---|---|
| CON | DQ | DQ + SQ | |||
| Plasma | |||||
| SOD, U/mL | 246.05a | 155.34b | 191.79a,b | 12.70 | 0.005 |
| GPX, U/mL | 462.48c | 683.34a | 591.30b | 24.88 | <0.001 |
| CAT, U/mL | 1.11 | 1.03 | 1.08 | 0.13 | 0.972 |
| MDA, nmol/mL | 1.00b | 1.63a | 1.20a,b | 0.10 | 0.012 |
| GSH, mg/L | 10.07a,b | 7.09b | 14.20a | 1.02 | 0.007 |
| Liver | |||||
| SOD, U/mg protein | 76.60 | 72.72 | 76.96 | 0.99 | 0.156 |
| GPX, U/mg protein | 37.19b | 46.89a | 41.30a,b | 1.64 | 0.041 |
| CAT, U/mg protein | 5.21 | 5.27 | 5.43 | 0.22 | 0.925 |
| MDA, nmol/mg protein | 0.59b | 0.85a | 0.73a,b | 0.04 | 0.015 |
| GSH, mg/g protein | 8.60a | 5.82b | 8.24a | 0.43 | 0.007 |
a,b,cMeans within a row with different superscripts are different at P <0.05.
Abbreviations: CAT, catalase; CON, nonchallenged broilers fed with a basal diet; DQ, diquat-challenged broilers fed with a basal diet; DQ + SQ, diquat-challenged broilers fed with a basal diet supplemented with 1.0 g/kg of squalene; GPX, glutathione peroxidase; GSH, reduced glutathione; MDA, malondialdehyde; SOD, superoxide dismutase.
n = 6.
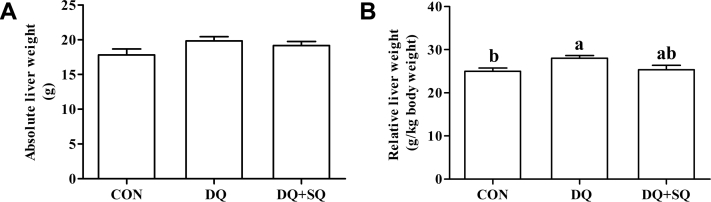
Liver Weight and Plasma Biochemical Parameters
The absolute liver weight (Figure 1, P > 0.05) did not differ among treatments, although it was numerically increased by diquat challenge. The relative liver weight (P < 0.05) in diquat-challenged broilers fed with a basal diet was higher than that of normal controls, and its value was intermediate in the squalene-supplemented group (P > 0.05). Compared with the control group (Table 5), diquat challenge increased alanine aminotransferase and aspartate aminotransferase activities (P < 0.05) in plasma of broilers, which were similar to control levels when feeding squalene (P < 0.05). In parallel, diquat administration increased plasma total bilirubin concentration (P < 0.05). The total bilirubin level (P < 0.05) in plasma of diquat-challenged broilers was decreased by squalene supplementation, but its level in the squalene-supplemented group was higher than that of the control group (P < 0.05). However, treatments did not alter plasma total protein, albumin, glucose, triglyceride, or total cholesterol concentration in broiler chickens (P > 0.05).
Figure 1.
Effects of dietary squalene supplementation on the (A) absolute liver weight and (B) relative liver weight of diquat-challenged broilers. The column and its bar represented the means value and standard error (n = 6), respectively. a,bMeans with different letters are different at P <0.05. Abbreviations: CON, nonchallenged broilers fed with a basal diet; DQ, diquat-challenged broilers fed with a basal diet; DQ + SQ, diquat-challenged broilers fed with a basal diet supplemented with 1.0 g/kg of squalene.
Table 5.
Effects of dietary squalene supplementation on the plasma biochemical parameters of diquat-challenged broilers.
| Items | Treatments |
SEM1 | P-value | ||
|---|---|---|---|---|---|
| CON | DQ | DQ + SQ | |||
| Alanine aminotransferase, U/L | 7.62b | 11.09a | 7.98b | 0.41 | <0.001 |
| Aspartate aminotransferase, U/L | 14.87b | 28.61a | 21.12b | 1.67 | <0.001 |
| Total bilirubin, μmol/L | 3.93c | 30.64a | 17.09b | 2.88 | <0.001 |
| Total protein, g/L | 33.22 | 36.08 | 37.91 | 1.56 | 0.492 |
| Albumin, g/L | 17.06 | 19.47 | 19.03 | 0.75 | 0.401 |
| Glucose, mmol/L | 13.74 | 14.62 | 14.61 | 0.40 | 0.605 |
| Total cholesterol, mmol/L | 4.96 | 5.30 | 5.80 | 0.26 | 0.439 |
| Triglyceride, mmol/L | 0.543 | 0.510 | 0.803 | 0.060 | 0.088 |
a–cMeans within a row with different superscripts are different at P < 0.05.
Abbreviations: CON, nonchallenged broilers fed with a basal diet; DQ, diquat-challenged broilers fed with a basal diet; DQ + SQ, diquat-challenged broilers fed with a basal diet supplemented with 1.0 g/kg of squalene.
n = 6.
Hepatic Gene Expression
Compared with the control group (Table 6), mRNA abundance of Nrf2 (P < 0.05) was upregulated by diquat challenge, regardless of squalene supplementation, and there was no significant difference between these 2 diquat-challenged groups (P > 0.05). The mRNA expression levels of GPX1 (P < 0.05) and Bax (P < 0.05) were increased after challenge with diquat, and their expression levels in diquat-challenged birds were reduced by squalene administration (P < 0.05), with their levels in the squalene-supplemented group being similar to those in the control group (P > 0.05). The diquat-challenged broilers receiving a basal diet exhibited a lower mRNA level of NQO1 (P < 0.05) and a higher mRNA abundance of CASP3 (P < 0.05) than their counterparts receiving squalene, but their levels did not differ between normal controls and diquat-challenged broilers fed with a basal diet (P > 0.05). Moreover, mRNA abundance of Bcl-2 in broilers subjected to diquat challenge tended to increase by dietary squalene administration (P = 0.066). However, treatments did not alter mRNA abundance of Keap1, heme oxygenase 1, superoxide dismutase 1, or X-linked inhibitor of apoptosis protein (P > 0.05).
Table 6.
Effects of dietary squalene supplementation on the hepatic gene expression level of diquat-challenged broilers.
| Items | Treatments |
SEM1 | P-value | ||
|---|---|---|---|---|---|
| CON | DQ | DQ + SQ | |||
| Nrf2 | 1.00b | 1.80a | 1.96a | 0.16 | 0.015 |
| Keap1 | 1.00 | 0.94 | 0.95 | 0.07 | 0.942 |
| HO1 | 1.00 | 0.82 | 0.92 | 0.06 | 0.540 |
| NQO1 | 1.00a,b | 0.90b | 1.62a | 0.13 | 0.034 |
| GPX1 | 1.00b | 1.89a | 0.94b | 0.17 | 0.021 |
| SOD1 | 1.00 | 1.10 | 1.34 | 0.09 | 0.344 |
| Bcl-2 | 1.00 | 0.98 | 1.46 | 0.10 | 0.066 |
| Bax | 1.00b | 1.62a | 0.97b | 0.11 | 0.015 |
| CASP3 | 1.00a,b | 1.55a | 0.79b | 0.11 | 0.010 |
| XIAP | 1.00 | 1.02 | 1.05 | 0.06 | 0.947 |
a,bMeans within a row with different superscripts are different at P < 0.05.
Abbreviations: Bax, B-cell lymphoma/leukemia 2-associated X protein; Bcl-2, B-cell lymphoma/leukemia 2; CASP3, caspase 3; CON, nonchallenged broilers fed with a basal diet; DQ, diquat-challenged broilers fed with a basal diet; DQ + SQ, diquat-challenged broilers fed with a basal diet supplemented with 1.0 g/kg of squalene; GPX1, glutathione peroxidase 1; HO1, heme oxygenase 1; Keap1, kelch-like ECH-associated protein 1; NQO1, NAD(P)H quinone dehydrogenase 1; Nrf2, nuclear factor erythroid 2–related factor 2; SOD1, superoxide dismutase 1; XIAP, X-linked inhibitor of apoptosis protein.
n = 6.
Discussion
The diquat-induced oxidative stress would disrupt antioxidant defense (Wang et al., 2013; Liu et al., 2019), damage organs especially the liver and small intestine (Mao et al., 2014; Zheng et al., 2017), trigger acute inflammation (Deng et al., 2010; Xu et al., 2018), inhibit nutrient absorption (Yin et al., 2015) and alter its allocation in favor of acute response (Lv et al., 2012; Zheng et al., 2017), and impair intestinal integrity and barrier function (Cao et al., 2018, 2019), eventually leading to inferior growth performance of animals subjected to diquat challenge (Deng et al., 2010; Mao et al., 2014; Yin et al., 2015; Chen et al., 2020a). Until date, available findings with regard to the harmful consequences of diquat-induced oxidative stress on the growth performance of domestic animals mainly focus on piglets. In this research, i.p. administration with diquat reduced ADG and BW change rate during the 24 h after challenge in broiler chickens. In agreement with our finding, Lv et al. (2012) and Yin et al. (2015) have found that diquat treatment decreased weight gain of piglets. Moreover, a recent study conducted on broilers challenged with diquat has also reported a reduction in the BW change ratio during the 24 h after injection (Chen et al., 2020a). The literature concerning the effects of squalene administration on the growth performance of poultry and other domestic animal species is scarce, but the squalene-rich feed supplements such as olive and palm oil have been shown to possess growth-promoting effects in broiler chickens (Zhang et al., 2013; Long et al., 2019). However, growth performance of broilers did not differ among the 3 experimental groups before diquat challenge, and this may be owing to the normal physiological status of broilers and the healthy feeding environment in this study. In a rodent model of oxidative stress induced by cyclophosphamide, oral administration with squalene for 7 d increased final BW and weight gain of male Wistar rats (Motawi et al., 2010). Dietary squalene supplementation also increased ADG and BW change rate in broilers subjected to diquat-induced oxidative status in the present study. This improved growth performance can be explained by the antioxidant properties of squalene as previously summarized (Spanova and Daum, 2011; Kim and Karadeniz, 2012; Lou-Bonafonte et al., 2018).
Cellular respiration is a set of metabolic reactions and processes by which chemical energy is released during the oxidation of organic molecules, and the ROS are formed as unavoidable by-products of this core life process in animals (Covarrubias et al., 2008; Hill, 2014). Intracellular ROS generation at a low level is crucial for normal cellular activities and various physiological processes, but oxidative stress will occur and then cause detrimental damage to cell macromolecules when its overproduction overwhelms the intrinsic antioxidant capacity (Nita and Grzybowski, 2016). Aerobic organisms have evolved antioxidant defense to counteract oxidative stress, including various antioxidant enzymes (e.g., SOD, GPX, and CAT) and low-molecular-weight antioxidants (e.g., GSH) (Pisoschi and Pop, 2015). The superoxide anions are dismutated by SOD to yield hydrogen peroxide, which is then eliminated by catalyzing it into water in the presence of GPX and CAT (Fukai and Ushio-Fukai, 2011). The GSH is a ubiquitous thiol-containing tripeptide, and its intracellular level is a sensitive indicator of cellular redox state (Forman et al., 2009). The toxic chemical, diquat, can undergo an enzymatic one-electron reduction mediated by flavoenzymes and subsequently lead to radical cation generation, which can react rapidly with molecular oxygen in aerobic conditions, forming superoxide anion; the produced superoxide anion can further generate hydrogen peroxide and other highly toxic hydroxyl radicals in the presence of redox-active transition metals (Jones and Vale, 2000; Fussell et al., 2011). Previous studies have shown that the diquat-induced oxidative stress decreased SOD and GPX activities and increased MDA accumulation (an important end product of lipid peroxidation) in plasma and the liver of piglets (Lv et al., 2012; Mao et al., 2014). As for chickens, diquat administration has been found to increase nitric oxide and protein carbonyl generations, but did not alter cellular antioxidant enzyme activities (GPX, CAT, and SOD) in the liver of the chicken embryo (Li et al., 2020). In this study, i.p. diquat administration reduced plasma SOD activity and hepatic GSH concentration and increased GPX activity and MDA accumulation in both plasma and the liver, suggesting that diquat challenge impaired the antioxidant system of broiler chickens. These results are partially in agreement with a previous study in which diquat treatment increases SOD and GPX activities as well as MDA accumulation and decreases GSH concentration in the liver of broilers at an early age (Chen et al., 2020a). Moreover, Doan et al. (2020) have recently reported that diquat challenge reduced plasma SOD activity and increased GPX activity in plasma and the liver of nursery pigs. The key nuclear transcription factor Nrf2 plays a vital role in cellular redox homeostasis through regulating the expression of defensive genes encoding various antioxidant proteins and detoxifying enzymes in a keap1-dependent or keap1-independent pattern (Bryan et al., 2013). In the present study, diquat challenge upregulated hepatic Nrf2 expression, independent of keap1 and its downstream target gene, GPX1, and this can be considered as an acute response to diquat-induced oxidative stress, which is in agreement with the finding of Wang et al. (2020) in a diquat-induced oxidative stress model in mice. It is necessary to mention that the measured SOD and GPX activity exhibited a completely different response to diquat stimulation in this study, which may be attributed to their difference in biological function and enzyme kinetic variation. The antioxidant characteristic of squalene is closely associated with its special and stable triterpene structure that enables it to effectively scavenge toxic free radicals (Kohno et al., 1995). In a cell culture experiment, squalene can prevent hydrogen peroxide–induced injury to human mammary epithelial cells by directly scavenging ROS in a dose-dependent manner (Warleta et al., 2010). The protective effects of squalene against oxidative damage have been previously reported in rodent animals (Sabeena Farvin et al., 2004, 2007; Senthilkumar et al., 2006; Motawi et al., 2010). Oral administration with squalene can improve redox status of rats subjected to cyclophosphamide-induced oxidative stress by increasing GPX activity and GSH concentration and by inhibiting MDA generation in the heart, testicle, and urinary bladder (Motawi et al., 2010). Similarly, squalene has been reported to normalize the alterations in antioxidant enzyme activities (SOD, GPX, and CAT) in the heart and hemolysate of red blood cells and the reductions in plasma vitamin E and vitamin C concentrations in a rat model of oxidative stress induced by cyclophosphamide (Senthilkumar et al., 2006). Moreover, prior administration with squalene can alleviate isoproterenol-induced oxidative stress in rats by blocking the induction of lipid peroxidation and maintaining the levels of nonenzymatic free radical scavenger and GSH in heart tissue (Sabeena Farvin et al., 2004, 2007). In current research, the administration of squalene totally or partially reversed the alterations in antioxidant enzyme activities (SOD in plasma and GPX in both plasma and the liver), increased the GSH level in plasma and the liver, and reduced MDA accumulation in plasma and the liver of diquat-challenged broilers to a level comparable with their normal counterparts. These results together suggest that the diquat-induced oxidative stress in broilers is effectively alleviated after squalene administration. The Nrf2 signal is involved in the protective effects of squalene against diquat-induced oxidative stress in broilers in this research, as evident by the increase in the mRNA expression level of hepatic Nrf2 and its target genes, NQO1 and GPX1. Squalene has been reported to attenuate lipopolysaccharide-induced inflammatory response in murine macrophages and human monocytes and neutrophils by reducing intracellular ROS levels and proinflammatory cytokine secretion; the Nrf2 signaling activation and Toll-like receptor 4 signaling inactivation are the possible underlying mechanisms responsible for its protective function (Cárdeno et al., 2015). Aside from directly inducing free radical generation, diquat-induced mitochondrial function disorder is another important reason for occurrence of oxidative stress (Cao et al., 2018). It has been demonstrated that squalene can improve hepatic mitochondrial function of aged rats by maintaining activities of enzymes involved in the tricarboxylic acid cycle and improving cellular energy status and by increasing mitochondrial antioxidant capacity (Buddhan et al., 2007), which may also account for the improved oxidative status of broilers subjected to diquat challenge observed in this study.
The liver is usually a major organ attacked by oxidative stress mainly owing to its multiple biological functions, especially its detoxifying function, and it has been reported that the augmented generations of ROS during oxidative stress will cause detrimental liver injury, such as exacerbated apoptosis, acute inflammation, metabolic disorder, and the development and progression of hepatic diseases (Cichoż-Lach and Michalak, 2014). The diquat challenge increased relative liver weight of broiler chickens in the present study, and this abnormal liver weight may suggest that oxidative stress induced by diquat impairs liver function. An increase in relative liver weight resulting from diquat challenge has also been reported in piglets (Yin et al., 2015) and broiler chickens (Chen et al., 2020a). The aspartate aminotransferase and alanine aminotransferase are 2 important metabolic enzymes existing in the cytoplasm of hepatocytes, and it is generally thought that the elevations in their activities in the blood are due to hepatocyte damage and disruption (Giannini et al., 2005). Total bilirubin in blood includes the conjugated and unconjugated (free) forms and, if elevated, is usually indicative of liver damage (Fevery, 2008). In parallel with an increase in relative liver weight, the circulating aspartate aminotransferase and alanine aminotransferase activities and especially total bilirubin concentration were increased by diquat challenge in this study, indicating that diquat-induced oxidative stress impairs liver normal function, which is further supported by the disrupted antioxidant system in the liver of broilers subjected to diquat challenge. The elevated aminotransferase activities induced by diquat challenge have also been found in mice (Qiao et al., 2020) and broiler chickens (Chen et al., 2020a). Chen et al. (2020a) have reported that diquat administration induced a greater number of apoptotic hepatocytes, upregulated hepatic apoptotic gene expression (CASP3), and decreased hepatic antiapoptotic gene (Bcl-2) expression in the liver of broiler chickens. In a model of diquat-induced acute oxidative stress of the chicken embryo, diquat challenge also increases the apoptotic index of liver cells (Li et al., 2020). Our results also showed that diquat upregulated 2 apoptotic gene expression levels (CASP3 and Bax) in the liver of broilers, which may provide a possible explanation for the elevated plasma biochemical parameters. In agreement with the improved redox status, the administration with squalene decreased circulating aspartate aminotransferase and alanine aminotransferase activities and total bilirubin concentration and normalized the expression levels of apoptotic genes in the liver of diquat-challenged broilers. Moreover, the relative liver weight of diquat-challenged broilers treated with squalene was reduced to a value similar to that of the normal controls. Taken together, these results indicate that squalene administration exhibits liver-protective effects on diquat-challenged broiler chickens. Its antioxidant characteristic could, at least in part, account for these effects. Mitochondria are the main source of both energy and cellular ROS, which renders them to be continuously exposed to free radical attack and therefore susceptible to oxidative stress. The oxidative damage to the bioenergetic machinery can inhibit adenosine triphosphate generation and trigger mitochondrial-dependent apoptosis (Brookes et al., 2004). A previous study has shown that diquat-induced liver impairment of broilers was due to the disrupted mitochondrial function, as supported by the reduced generation of adenosine triphosphate and the altered expression pattern of genes responsible for mitochondrial biogenesis (Chen et al., 2020a). The protective effects of dietary squalene administration on mitochondrial function in the liver have been previously reported in rats (Buddhan et al., 2007), and this finding may also provide an explanation to the improved liver function in diquat-challenged broilers receiving squalene administration.
In conclusion, the acquired results in this study suggested that dietary supplementation with squalene can improve growth performance, antioxidant status in plasma and the liver, and liver function of broilers subjected to diquat-induced oxidative stress.
Acknowledgments
This study was funded by the Natural Science Foundation of Jiangsu Province (grant no. BK20190537) and the China Postdoctoral Science Foundation (grant no. 2017M621765).
Disclosures
All authors declare no conflict of interest.
References
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhadj Slimen I., Najar T., Ghram A., Abdrrabba M. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016;100:401–412. doi: 10.1111/jpn.12379. [DOI] [PubMed] [Google Scholar]
- Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Bryan H.K., Olayanju A., Goldring C.E., Park B.K. The Nrf2 cell defence pathway: keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Buddhan S., Sivakumar R., Dhandapani N., Ganesan B., Anandan R. Protective effect of dietary squalene supplementation on mitochondrial function in liver of aged rats. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76:349–355. doi: 10.1016/j.plefa.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Cao S., Shen Z., Wang C., Zhang Q., Hong Q., He Y., Hu C. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets. Food Funct. 2019;10:344–354. doi: 10.1039/c8fo02091d. [DOI] [PubMed] [Google Scholar]
- Cao S., Wu H., Wang C., Zhang Q., Jiao L., Lin F., Hu C.H. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018;96:1795–1805. doi: 10.1093/jas/sky104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdeno A., Aparicio-Soto M., Montserrat-de la Paz S., Bermudez B., Muriana F.J.G., Alarcón-de-la-Lastra C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Func. Food. 2015;14:779–790. [Google Scholar]
- Chen Y., Chen Y., Zhang H., Wang T. Pterostilbene as a protective antioxidant attenuates diquat-induced liver injury and oxidative stress in 21-day-old broiler chickens. Poult. Sci. 2020;99:3158–3167. doi: 10.1016/j.psj.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Gu Y., Zhao H., Zhang H., Zhou Y. Effects of graded levels of dietary squalene supplementation on the growth performance, plasma biochemical parameters, antioxidant capacity, and meat quality in broiler chickens. Poult. Sci. 2020;99:5915–5924. doi: 10.1016/j.psj.2020.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichoż-Lach H., Michalak A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Hernández-García D., Schnabel D., Salas-Vidal E., Castro-Obregón S. Function of reactive oxygen species during animal development: passive or active? Dev. Biol. 2008;320:1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- Das B., Antoon R., Tsuchida R., Lotfi S., Morozova O., Farhat W., Malkin D., Koren G., Yeger H., Baruchel S. Squalene selectively protects mouse bone marrow progenitors against cisplatin and carboplatin-induced cytotoxicity in vivo without protecting tumor growth. Neoplasia. 2008;10:1105–1119. doi: 10.1593/neo.08466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Xu J., Yu B., He J., Zhang K., Ding X., Chen D. Effect of dietary tea polyphenols on growth performance and cell-mediated immune response of post-weaning piglets under oxidative stress. Arch. Anim. Nutr. 2010;64:12–21. doi: 10.1080/17450390903169138. [DOI] [PubMed] [Google Scholar]
- Doan N., Liu Y., Xiong X., Kim K., Wu Z., Bravo D.M., Blanchard A., Ji P. Organic selenium supplement partially alleviated diquat-induced oxidative insults and hepatic metabolic stress in nursery pigs. Br. J. Nutr. 2020;124:23–33. doi: 10.1017/S0007114520000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez E. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Fevery J. Bilirubin in clinical practice: a review. Liver Int. 2008;28:592–605. doi: 10.1111/j.1478-3231.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai T., Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussell K.C., Udasin R.G., Gray J.P., Mishin V., Smith P.J., Heck D.E., Laskin J.D. Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radic. Biol. Med. 2011;50:874–882. doi: 10.1016/j.freeradbiomed.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini E.G., Testa R., Savarino V. Liver enzyme alteration: a guide for clinicians. Can. Med. Assoc. J. 2005;172:367–369. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta. 1991;196:143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- Gylling H., Miettinen T.A. Post absorptive metabolism of dietary squalene. Atherosclerosis. 1994;106:169–178. doi: 10.1016/0021-9150(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Hill G.E. Cellular respiration: the nexus of stress, condition, and ornamentation. Integr. Comp. Biol. 2014;54:645–657. doi: 10.1093/icb/icu029. [DOI] [PubMed] [Google Scholar]
- Jones G.M., Vale J.A. Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J. Toxicol. Clin. Toxicol. 2000;38:123–128. doi: 10.1081/clt-100100926. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Karadeniz F. Biological importance and applications of squalene and squalane. Adv. Food Nutr. Res. 2012;65:223–233. doi: 10.1016/B978-0-12-416003-3.00014-7. [DOI] [PubMed] [Google Scholar]
- Koch R.E., Hill G.E. An assessment of techniques to manipulate oxidative stress in animals. Funct. Ecol. 2017;31:9–21. [Google Scholar]
- Kohno Y., Egawa Y., Itoh S., Nagaoka S., Takahashi M., Mukai K. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim. Biophys. Acta. 1995;1256:52–56. doi: 10.1016/0005-2760(95)00005-w. [DOI] [PubMed] [Google Scholar]
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978;186:189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- Kruger N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- Lalor P.F., Adams D.H. The liver: a model of organ-specific lymphocyte recruitment. Expert Rev. Mol. Med. 2002;4:1–16. doi: 10.1017/S1462399402004155. [DOI] [PubMed] [Google Scholar]
- Lee M.T., Lin W.C., Lee T.T. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals-A review. Asian-Australas. J. Anim. Sci. 2019;32:309–319. doi: 10.5713/ajas.18.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Jiang L., Wang J., Xia L., Zhao R., Cai C., Wang P., Zhan X., Wang Y. Maternal dietary supplementation with different sources of selenium on antioxidant status and mortality of chicken embryo in a model of diquat-induced acute oxidative stress. Anim. Feed Sci. Technol. 2020;261 114369. [Google Scholar]
- Liu J., Zhang Y., Li Y., Yan H., Zhang H. L-tryptophan enhances intestinal integrity in diquat-challenged piglets associated with improvement of redox status and mitochondrial function. Animals. 2019;9:266. doi: 10.3390/ani9050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long G.L., Hao W.X., Bao L.F., Li J.H., Zhang Y., Li G.H. Effects of dietary inclusion levels of palm oil on growth performance, antioxidative status and serum cytokines of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2019;103:1116–1124. doi: 10.1111/jpn.13108. [DOI] [PubMed] [Google Scholar]
- Lou-Bonafonte J.M., Martínez-Beamonte R., Sanclemente T., Surra J.C., Herrera-Marcos L.V., Sanchez-Marco J., Arnal C., Osada J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018;62:1800136. doi: 10.1002/mnfr.201800136. [DOI] [PubMed] [Google Scholar]
- Lv M., Yu B., Mao X.B., Zheng P., He J., Chen D.W. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal. 2012;6:928–934. doi: 10.1017/S1751731111002382. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J., Svendsen O. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet. J. 2007;173:502–511. doi: 10.1016/j.tvjl.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Mao X., Lv M., Yu B., He J., Zheng P., Yu J., Wang Q., Chen D. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J. Anim. Sci. Biotechnol. 2014;5:49. doi: 10.1186/2049-1891-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Beamonte R., Alda O., Sanclemente T., Felices M.J., Escusol S., Arnal C., Herrera-Marcos L.V., Gascón S., Surra J.C., Osada J., Rodríguez-Yoldi M.J. Hepatic subcellular distribution of squalene changes according to the experimental setting. J. Physiol. Biochem. 2018;74:531–538. doi: 10.1007/s13105-018-0616-2. [DOI] [PubMed] [Google Scholar]
- Miettinen T.A., Vanhanen H. Serum concentration and metabolism of cholesterol during rapeseed oil and squalene feeding. Am. J. Clin. Nutr. 1994;59:356–363. doi: 10.1093/ajcn/59.2.356. [DOI] [PubMed] [Google Scholar]
- Motawi T.M., Sadik N.A., Refaat A. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: an experimental study on rat myocardium, testicles and urinary bladder. Food Chem. Toxicol. 2010;48:2326–2336. doi: 10.1016/j.fct.2010.05.067. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell Longev. 2016;2016 doi: 10.1155/2016/3164734. 3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens C.W.I., Belcher R.V. A colorimetric micro-method for the determination of glutathione. Biochem. J. 1965;94:705–711. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson T., Popescu B.O., Cedazo-Minguez A. Oxidative stress in Alzheimer's disease: why did antioxidant therapy fail ? Oxid. Med. Cell Longev. 2014;2014 doi: 10.1155/2014/427318. 427318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Placer Z.A., Cushman L.L., Johnson B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Qiao L., Dou X., Yan S., Zhang B., Xu C. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity. Food Funct. 2020;11:3020–3031. doi: 10.1039/d0fo00132e. [DOI] [PubMed] [Google Scholar]
- Sabeena Farvin K.H., Anandan R., Hari Senthil Kumar S., Shiny K.S., Sankar T.V., Thankappan T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004;50:231–236. doi: 10.1016/j.phrs.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Sabeena Farvin K.H., Hari Senthil Kumar S., Anandan R., Mathew S., Sankar T.V., Viswanathan Nair P.G. Supplementation of squalene attenuates experimentally induced myocardial infarction in rats. Food Chem. 2007;105:1390–1395. [Google Scholar]
- Senthilkumar S., Yogeeta S.K., Subashini R., Devaki T. Attenuation of cyclophosphamide induced toxicity by squalene in experimental rats. Chem. Biol. Interact. 2006;160:252–260. doi: 10.1016/j.cbi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Spanova M., Daum G. Squalene–biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011;113:1299–1320. [Google Scholar]
- SPSS . SPSS Inc.; Chicago, IL: 2008. SPSS 16. 0 for Windows. [Google Scholar]
- Starkov A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg T.E., Tilvis R.S., Miettinen T.A. Metabolic variables of cholesterol during squalene feeding in humans: comparison with cholestyramine treatment. J. Lipid Res. 1990;21:1637–1643. [PubMed] [Google Scholar]
- Wang A.N., Cai C.J., Zeng X.F., Zhang F.R., Zhang G.L., Thacker P.A., Wang J.J., Qiao S.Y. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J. Appl. Microbiol. 2013;114:1582–1591. doi: 10.1111/jam.12188. [DOI] [PubMed] [Google Scholar]
- Wang M., Huang H., Hu Y., Huang J., Yang H., Wang L., Chen S., Chen C., He S. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. J. Anim. Sci. 2020;98 doi: 10.1093/jas/skaa112. skaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warleta F., Campos M., Allouche Y., Sánchez-Quesada C., Ruiz-Mora J., Beltrán G., Gaforio J.J. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. 2010;48:1092–1100. doi: 10.1016/j.fct.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Wu Q.H., Wang X., Yang W., Nüssler A.K., Xiong L.Y., Kuča K., Dohnal V., Zhang X.J., Yuan Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update. Arch. Toxicol. 2014;88:1309–1326. doi: 10.1007/s00204-014-1280-0. [DOI] [PubMed] [Google Scholar]
- Xu Y.Q., Xing Y.Y., Wang Z.Q., Yan S.M., Shi B.L. Pre-protective effects of dietary chitosan supplementation against oxidative stress induced by diquat in weaned piglets. Cell Stress Chaperones. 2018;23:703–710. doi: 10.1007/s12192-018-0882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Yang L., Yu Z., Hou J., Deng Y., Zhou Z., Zhao Z., Cui J. Toxicity and oxidative stress induced by T-2 toxin and HT-2 toxin in broilers and broiler hepatocytes. Food Chem. Toxicol. 2016;87:128–137. doi: 10.1016/j.fct.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Yin J., Liu M., Ren W., Duan J., Yang G., Zhao Y., Fang R., Chen L., Li T., Yin Y. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS One. 2015;10:e0122893. doi: 10.1371/journal.pone.0122893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.F., Zhou T.X., Kim I.H. Effects of dietary olive oil on growth performance, carcass parameters, serum characteristics, and fatty acid composition of breast and drumstick meat in broilers. Asian-australas. J. Anim. Sci. 2013;26:416–422. doi: 10.5713/ajas.2012.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Yu B., He J., Yu J., Mao X., Luo Y., Luo J., Huang Z., Tian G., Zeng Q., Che L., Chen D. Arginine metabolism and its protective effects on intestinal health and functions in weaned piglets under oxidative stress induced by diquat. Br. J. Nutr. 2017;117:1495–1502. doi: 10.1017/S0007114517001519. [DOI] [PubMed] [Google Scholar]