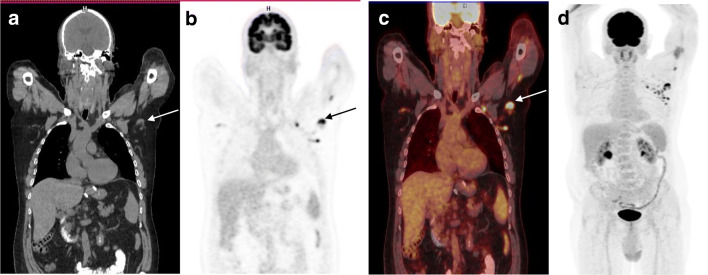
A 57-year-old male with BRAF mutant melanoma of the right thigh with lung, inguinal lymph node, and spleen metastases reached complete remission under combined treatment with targeted therapy (dabrafenib and trametinib) and anti-PD-1 (pembrolizumab). 18F-FDG-PET/CT and abdominal MRI from September 2020 showed no active disease.
Two weeks after the most recent dose of pembrolizumab, a routine PET-FDG showed newly enlarged multiple lymph nodes in the left axilla, retro pectoral space, and proximal arm, with high FDG uptake (up to SUV 9.4); no other foci were identified (see Figure). Upon questioning, the patient reported that he had received two doses of the Pfizer COVID-19 vaccine in his left arm, the last of which was given 6 days prior to imaging acquisition.
Axillary lymphadenopathy has been reported in 0.3% of BNT162b2 mRNA COVID-19 vaccine recipients and was usually resolved within 10 days post-injection [1]. Indeed, FDG-avid lymphadenopathy was described after COVID-19 vaccination [2]. Similar findings are also reported after influenza vaccination [3]. In this case, the occurrence of post vaccination lymphadenopathy is described in a melanoma patient receiving immunotherapy. The contribution of immune checkpoint inhibitors targeting the PD-1/PD-L1 axis has not yet been recorded, but the intensity of adenopathy may be attributed, at least in part, to the enhancing effects of immunotherapy. While PD-1 blockade does not appear to affect the severity of COVID-19 infection [4], it significantly enhances the emerging immune response by vaccines such as influenza [5].
With widespread deployment of COVID-19 vaccines and in-line with recent endorsement of vaccination in oncological patients under active anti-tumor treatment, similar findings to those we describe are likely to be encountered widely. It is important to keep this etiology for axillary lymphadenopathy in mind when interpreting PET scans in oncological patients.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
The patient gave written consent regarding publishing their data and images.
Footnotes
This article is part of the Topical Collection on Image of the month.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. NEJM. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eifer M, Eshet E. Imaging of COVID-19 vaccination at FDG PET/CT. Images Radiology. 2021. 10.1148/radiol.2020210030. [DOI] [PMC free article] [PubMed]
- 3.Shirone N, Shinkai T, Yamane T, Uto F, Yoshimura H, Tamai H, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10(8):1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang CK, Kim HR, Song KH, Keam B, Choi SJ, Choe PG, et al. Cell-mediated immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. J Infect Dis. 2020;222(11):1902–1909. doi: 10.1093/infdis/jiaa291. [DOI] [PubMed] [Google Scholar]