Abstract
FDG-PET has been shown to be a useful imaging modality for the assessment of cardiovascular infection and inflammatory pathologies. However, interpretation of these studies can be challenging in light of the variability of physiological myocardial uptake and, occasionally, interpreter’s lack of familiarity with the typical findings present in cardiac pathologies. In this article, we review established and emerging applications for cardiovascular infection and inflammation imaging with FDG-PET and present typical examples of representative pathologies.
Keywords: Positron emission tomography, fluorodeoxyglucose, FDG, cardiovascular, infection, inflammation
Introduction
Fluoro-deoxyglucose positron emission tomography (FDG-PET) has long been known to be useful in the assessment of systemic infectious and inflammatory processes, such as spondylodiscitis, osteomyelitis, sarcoidosis, and hardware infection [1-6]. However, application of FDG-PET for the evaluation of cardiovascular infection and inflammation has been slower to flourish, likely due to difficulties in distinguishing between pathological and physiological myocardial FDG uptake. Nonetheless, thanks to the development of reliable myocardial suppression protocols, there have been significant advances recently in the use of FDG-PET for the imaging of cardiovascular infection and inflammation.
Increased FDG uptake is a hallmark of immune inflammation as neutrophils, cells of the monocyte/macrophage family, and lymphocytes are able to express high levels of glucose transporters (in particular GLUT1 and GLUT3) and hexokinase activity [7], with uptake mediated by various cytokines. This specificity of the uptake mechanism, particularly in the tissues of the cardiovascular system, underpin the potential utility of FDG-PET for the evaluation of cardiovascular infection and inflammation.
The purpose of this article is to review both well-established and emerging applications in the use of FDG-PET for assessing cardiovascular inflammation that radiologists, nuclear medicine specialists, and cardiologists may encounter.
Cardiac metabolism and myocardial suppression techniques
Cardiovascular FDG-PET imaging differs from other FDG-PET applications due to the highly variable and unpredictable nature of physiological myocardial FDG uptake. As versatile metabolic omnivores, cardiomyocytes are able to metabolize free fatty acids, glucose, lactate, ketones, pyruvate, and even certain types of amino acids based on blood glucose levels, activity, endocrine factors, and recent diet [8]. This is reflected in a highly variable pattern of physiological FDG uptake. In order to identify pathological uptake, and thus be able to interpret cardiac FDG-PET studies, it is essential to suppress physiological glucose uptake - this is generally accomplished through a combination of fasting and dietary changes in order to convert cardiomyocyte metabolism to using free fatty acids as a substrate. In particular, patients undergoing FDG-PET for cardiovascular indications should not be prepared in the same fashion as oncology patients (i.e. 4 hr fast alone) as physiological cardiac uptake is not reliably suppressed in these patients.
Recent SNMMI-ASNC guidelines recommend the use of a combination of prolonged (12-18 hr) fasting, conversion to a high-fat, low-carbohydrate, protein-permitted diet, and intravenous unfractionated heparin (UFH, 50 IU/kg intravenous bolus of UFH approximately 15 min before 18F-FDG administration) [9]; however, even with strict adherence to such a regimen, it’s noted that complete myocardial suppression may not be achieved in up to 20% of patients. In these cases, typical patterns of incomplete suppression are often seen. Factors which have been shown to be associated with decreased cardiac FDG uptake include diabetes, levothyroxine, and bezafibrate [10]. Conversely, heart failure, valvulopathy, cardiotoxic chemotherapy, corticosteroids and the use of benzodiazepines have been shown to be associated with increased cardiac uptake [10]. Figure 1 illustrates the potential difference in the degree of myocardial FDG uptake seen following an oncological preparation versus a dedicated cardiac preparation.
Figure 1.
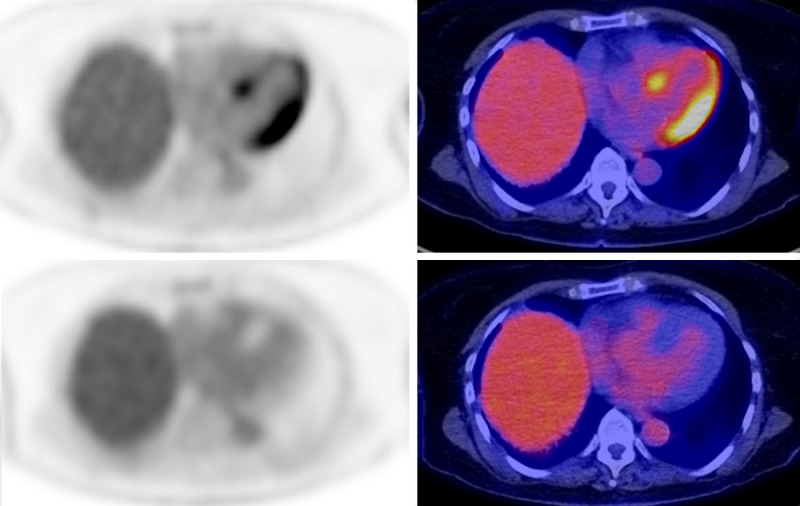
The variability of cardiac uptake on FDG-PET necessitates the use of a more stringent pre-scan patient preparation for cardiac applications then in the case of oncology. In this patient, initial PET images (upper panels) were acquired following a standard oncological protocol (4 hr fast prior to injection). Diffuse uptake is seen throughout the left ventricular myocardium, severely limiting the ability to assess for any cardiac pathology. A repeat study was acquired following a dedicated cardiac protocol. On these images, note the absence of any significant myocardial uptake.
Cardiovascular inflammatory pathology
Cardiac sarcoidosis
Sarcoidosis is a granulomatous disease of unknown etiology which can affect virtually any organ system. There is increasing recognition of the importance of identifying cardiac involvement due to the association of cardiac sarcoidosis (CS) with arrhythmias and the risk of sudden cardiac death [11] (Figure 2). Recent guidelines have appeared governing the use of FDG-PET for the assessment of patients with suspected cardiac disease [9]. The diagnosis of CS is not made on the basis of FDG-PET findings alone - rather, PET findings are incorporated into diagnostic criteria (either the Heart Rhythm Society (HRS) or Japanese Ministry of Health and Welfare (JMHW) [12,13]) with the diagnosis established by a combination of pathology, imaging, clinical and/or electrocardiogram findings.
Figure 2.
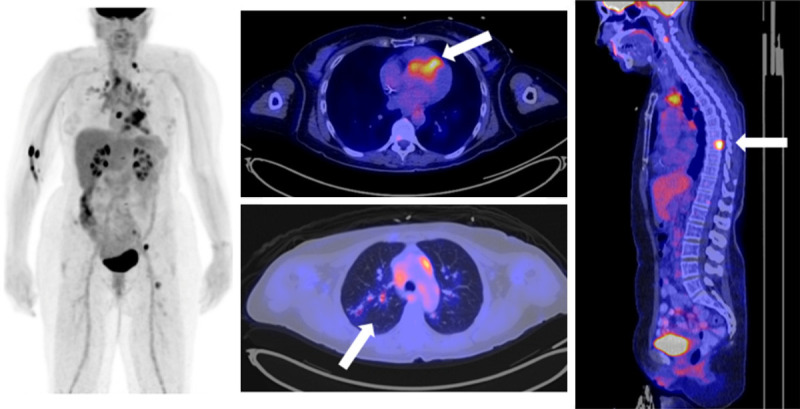
Sarcoidosis is a commonly encountered finding on FDG-PET. Cardiac involvement is challenging to assess unless the patient has undergone appropriate dietary preparation. This 61-year-old woman had an atrioventricular block on her electrocardiogram. FDG-PET revealed diffuse mediastinal lymphadenopathy (best seen on the MIP images), as well as evidence of cardiac (arrow), lung (arrow), and bone (arrow) involvement.
In a recent meta-analysis, FDG-PET was shown to have a sensitivity/specificity of 89%/78% for active CS using the JMHW criteria as gold standard [14]; however, the accuracy of this test is difficult to establish due to the lack of a true pathological gold standard. PET findings in CS include focal or patchy myocardial uptake. In addition, extra-cardiac findings such as FDG-avid lymphadenopathy are seen in the majority of patients with CS. These latter findings are helpful in supporting a diagnosis of CS as focal myocardial uptake is non-specific and can be seen in patients with myocarditis or hibernating myocardium.
Myocarditis
Myocarditis - inflammation of the myocardium - is a non-specific process which can be caused by a number of different factors, including viruses, autoimmune diseases, and various medications. Echocardiography is known to have low sensitivity when compared to cardiac magnetic resonance (CMR) [15]. On the other hand, FDG-PET has been shown to have high accuracy for the diagnosis of myocarditis [16], with good agreement when compared to CMR [15]. This is particularly advantageous in light of the fact that many patients with myocarditis can have MR contraindications (i.e. pacemaker) due to associated arrhythmias.
When compared to CS, FDG-PET findings in myocarditis generally show more diffuse uptake (Figure 3); however, the distinction can be challenging and extra-cardiac findings, as well as clinical history, can serve as important ancillary findings. Given the more diffuse uptake seen in myocarditis, distinction between inflammation and poor myocardial suppression may be challenging in some cases.
Figure 3.
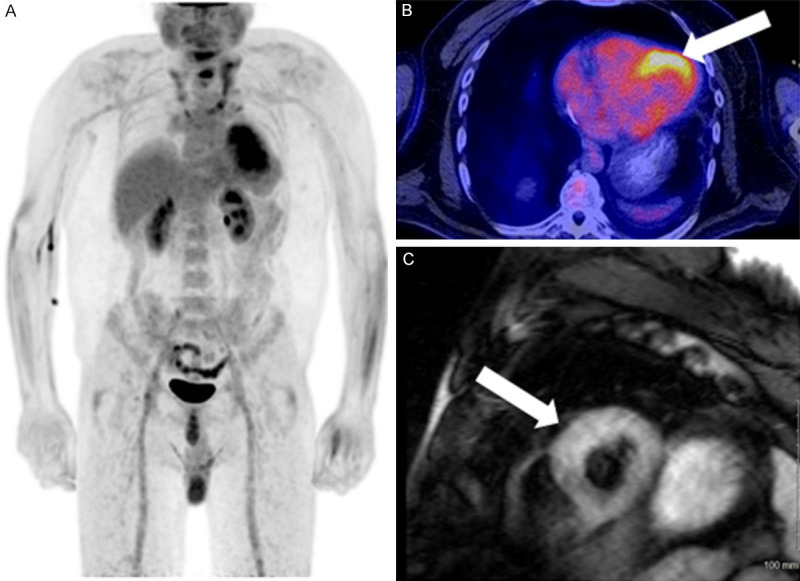
Typical findings of myocarditis in a 41-year-old male with biopsy proven lymphocytic myocarditis. Note the diffuse myocardial uptake and the lack of extracardiac findings on the MIP image (A). On axial images PET/CT images (B), diffuse uptake is seen predominantly in the anterior/anterolateral wall of the left ventricle (arrow). On CMR T2 STIR sequence (C), mild, diffuse edema is seen throughout the left ventricular wall (arrow).
Vasculitis
Vasculitis, inflammation of vessels walls, is traditionally classified by vessel size into small, medium, and large vessel disease. Vasculitides can be caused by infectious agents, autoimmune disorders, paraneoplastic phenomena, or be a reaction to chemicals or drugs. Due to the ubiquitous presence of vessels in the body and all organ systems, clinical manifestations of vasculitis are protean and diagnosis can be challenging.
FDG-PET has shown high sensitivity and specificity for the diagnosis of vasculitis and results in significant impact on management when compared to conventional imaging [17,18] (Figure 4); however, diagnostic performance can suffer in patients undergoing anti-inflammatory treatment [19]. Joint EANM/SNMMI/PIG guidelines on the use of FDG-PET for large vessel vasculitis and polymyalgia rheumatica imaging have recently appeared [20]. These have proposed interpretation criteria consisting of a graded approach for the assessment of vascular uptake of FDG: 0 = no uptake (≤ mediastinum); 1 = low-grade uptake (< liver); 2 = intermediate-grade uptake (= liver), 3 = high-grade uptake (> liver). Grade 2 and 3 uptake is considered possibly indicative and positive, respectively, for active large vessel vasculitis. Small studies have shown a decreased in vascular FDG uptake following successful immunosuppressive therapy [18], suggesting FDG-PET could play a role in monitoring treatment response in patients with vasculitis. Nonetheless, low grade FDG uptake may be seen several months after successful therapy with complete study normalization observed in only 20% of patients [21]. In that context, further studies are needed to assess the role of FDG-PET in the monitoring of response to therapy.
Figure 4.
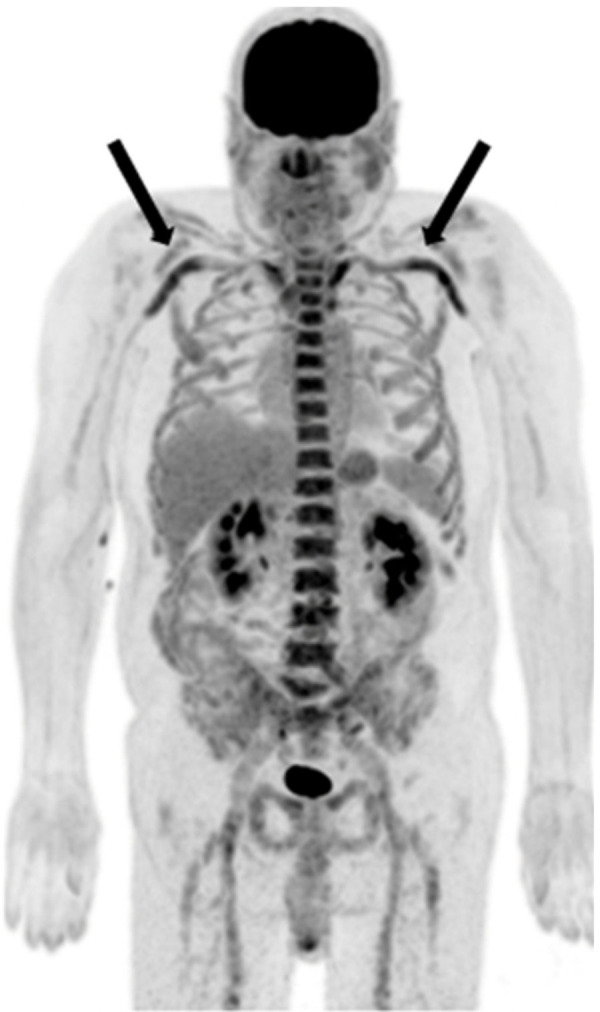
Example of large vessel vasculitis in a 74-year-old man with giant cell arteritis involving bilateral subclavian and axillary arteries. Mild involvement of the bilateral iliac and femoral arteries is also noted.
An advantage of FDG-PET over conventional imaging is the ability to identify articular/peri-articular inflammatory activity, which frequently co-exists in patients with vasculitis. These features can be useful in distinguishing between the various vasculitides.
A possible confounder for vasculitis is atherosclerosis, where FDG uptake can be seen within active plaques; however, the distribution of atherosclerotic uptake is generally patchy, compared to the smoother uptake seen in vasculitis. Finally, persistent blood pool activity may affect image interpretation. In those cases, delayed imaging acquired between 120 to 180 minutes after tracer injection may improve visualization of the vascular walls [22]. Of note, interpretation criteria have been validated for 60 min PIV imaging and have not been validated for delayed imaging [20].
Atherosclerosis
Atherosclerosis plays a key role in myocardial infarction, cerebrovascular accidents, and peripheral vascular disease. Given the key role that inflammatory cells play in the pathophysiology of atherosclerotic lesions and plaque rupture in particular, FDG-PET has a potential role in the assessment of unstable plaques. Histopathological studies using carotid endarcterectomy samples have shown excellent correlation between macrophage density and FDG uptake [23]. Potential applications of FDG-PET in this context include prognostication and assessment of therapeutic efficacy. For the former, studies have shown that FDG uptake in large vessels was a strong predictor of subsequent cardiovascular events [24]. In addition, multiple studies have shown that anti-atherosclerotic therapy resulted in decreases of FDG uptake [25]. The cardiovascular committee of the EANM have recently produced a position paper on the use of FDG-PET for atherosclerosis imaging. Amongst some of the recommendations proposed is a prolongation of the time between injection to imaging, which should be extended to 2 hours (from approximately 1 hour) in order to allow or increased clearance of blood pool activity, resulting in an increase in visibility of uptake in atherosclerotic plaque. Furthermore, the same vascular territories should be compared on sequential studies due to inherent variability within different arterial regions. Use of the target to background ratio (TBR), defined as the ratio of the SUVMax in a lesion or arterial wall to venous blood pool SUVMean, is recommended over the use of SUV; however, multiple different quantitative approaches have been developed (i.e. TBRMax, TBRMean, “most diseased segment”, “active segment analysis”) and use of a particular approach should be determined by the specific aim of the study. Overall, this is an emerging application for FDG-PET and further studies are required in order to establish its best use in this context. Numerous other non-FDG PET tracers have also demonstrate early promise for the imaging of atherosclerosis [26].
Ventricular arrhythymias
Myocardial inflammation contributes to cardiomyocyte damage and the development of myocardial fibrosis which, in turn, can lead to the formation of arrhythmogenic foci. Identifying these foci, which constitute potential targets for catheter ablation, is a significant clinical challenge. FDG-PET has been used in the assessment of subjects with unexplained ventricular arrhythmias (frequent PVCs, sustained ventricular tachycardia, or ventricular fibrillation) of unknown cause. These studies showed that the majority of subjects evaluated by FDG-PET demonstrated abnormal myocardial uptake despite adherence to an appropriate suppression protocol [27]. In one study, correlation with endomyocardial biopsy results confirmed that the majority (90%) of these subjects demonstrated pathological evidence of either granulomatous (60%) or non-granulomatous (30%) inflammation [27]. Use of FDG-PET in this context represents an emerging application which may prove to be clinically useful in the near future.
Cardiovascular infection
Prosthetic valve endocarditis
Infective endocarditis (IE) is associated with significant morbidity and mortality. Diagnosis of IE is usually accomplished through the use of the modified Duke criteria [28]. FDG-PET has been shown to be a useful imaging modality for the assessment of the prosthetic valve IE [29] with higher sensitivity than leukocyte scintigraphy [30]. In fact, inclusion of FDG-PET positivity as a major criterion has been shown to significantly improve sensitivity of the modified Duke criteria [31]. In addition, extra-cardiac manifestations of IE identified on FDG-PET/CT can significantly affect management and prognosis of patients with IE [32]. Extra-cardiac findings of relevance include septic emboli found in 30-55% of cases [32]. When an FDG-PET scan is performed for the workup of suspected endocarditis, whole body images allow detection of septic emboli- a finding highly suggestive for the diagnosis of infectious endocarditis. Figure 5 shows an example of a prosthetic valve IE. Finally, increased splenic uptake has also been associated with PVE and could represent a new minor criteria, but further studies are needed to confirm this finding [31].
Figure 5.
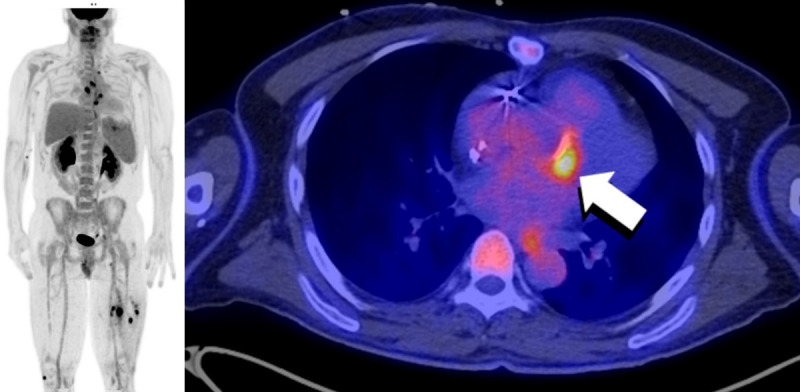
FDG PET/CT of a 51-year-old male status post aortic valve and ascending aorta replacement 10 years prior to the PET study. Multiple areas of abnormally increased FDG uptake are seen throughout on the body on the MIP images (left). Increased FDG uptake (arrow) is seen anteriorly to the aortic valve prosthesis (right). A focus of uptake in the spleen was compatible with a splenic abscess. Finally, areas of focally increased uptake are seen in both legs, with corresponding hypodensities on CT (not shown), compatible with intramuscular abscesses. The findings are compatible with an infectious endocarditis with septic emboli. Culture of the mechanical valve grew Staphylococcus capitis.
It should be noted, however, that the use of FDG-PET in this context can be associated with false-positives [31]. FDG uptake at the site of a prosthetic valve may simply reflect physiological myocardial uptake or may represent a reaction to the adhesive used during surgery (e.g. biological glue) [33], or as a foreign-body reaction to suture material [34]. Valve thrombosis has also been associated with false positive studies [35]. False-negative results can occur in the case of previous antibiotic therapy, cardiac motion, or in small vegetations.
Native valve endocarditis
The use of FDG-PET for the assessment of native valve IE has been less widely studied than for prosthetic valve IE [36]; however, studies have shown that, like in the case of prosthetic valve IE, include of FDG-PET positivity in the modified Duke criteria also results in an increase in sensitivity [37]. As in the case of prosthetic valves, limitations include physiological myocardial uptake, previous antibiotic therapy, cardiac motion, and in small vegetations. Figure 6 shows an example of native valve IE. As no physiological or inflammatory uptake should be seen in native valves, any focal uptake greater than blood pool at the valve or valve supporting structure, excluding papillary muscles, is suspicious for NVE [37]. Importantly, the sensitivity of FDG-PET has been reported to be lower in NVE compared to PVE.
Figure 6.
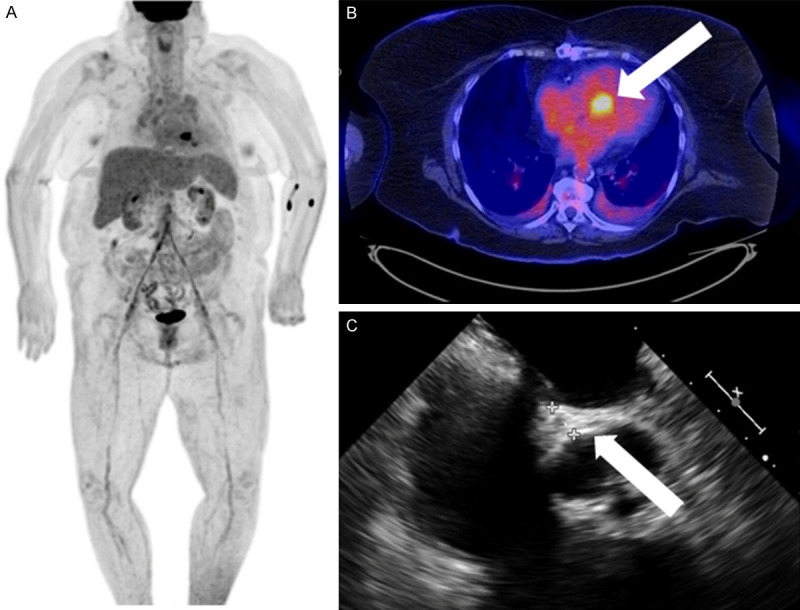
74-year-old septic female with fever and leukocytosis. FDG PET/CT with cardiac suppression revealed focal cardiac uptake on the images (A) which localizes to the aortomitral junction (arrow) on axial images (B). Transesophageal echocardiography (C) demonstrated heterogeneous thickening of the aortomitral junction with several small mobile masses attached to the aortic and mitral valves, in keeping with native valve endocarditis and an aortomitral junction phlegmon/abscess.
Vascular graft infection
Vascular graft infection (VGI) has been reported in up to 15% of patients [38]. Accurate and timely diagnosis of VGI is essential yet often challenging as the symptoms can be vague. A recent meta-analysis has shown that the use of FDG-PET offers high accuracy for the detection of vascular graft infection [39]. As for endocarditis, the criteria for positivity include both qualitative and quantitative approaches: in addition to the use of SUVmax or TBR, focal and graded uptake scales have been proposed [40]. An example of an infected aortic root graft is shown in Figure 7.
Figure 7.
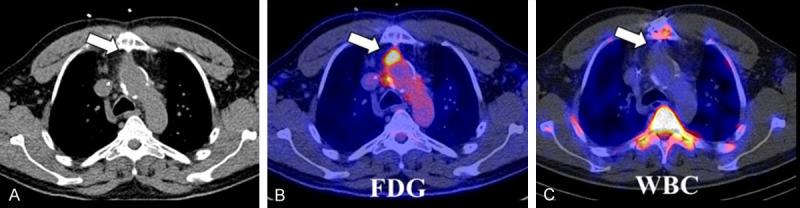
Example of an infected aortic graft. A 48-year-old male underwent a Bentall procedure (graft replacement of the aortic value, root, and ascending aorta) three years prior to the PET study. On the low-dose, unenhanced CT (A), a soft-tissue density (arrow) is visible anterior to the aortic graft which demonstrated focally increased FDG uptake (arrow) (B), but no significant activity (arrow) on a radiolabelled white blood cell (WBC) study (C). Despite this, imaging findings were compatible with an aortic graft infection. Cultures of this collection grew Kingella kingae. The low virulence of the pathogen and the chronicity of this process may explain the lack of WBC uptake.
Beyond diagnosis, some studies have suggested that FDG-PET may play a role in guiding therapeutic response in patients with VGI [41]. Interestingly, a correlation between CRP and SUV is reported to be present in only a subset of patients, suggesting that SUV could potentially serve as an independent biomarker to assess therapy response in patients with VGI.
Some limitations in the use of FDG-PET for the assessment of VGI need to be recognized. In particular, it has been shown that the use of adhesives on prosthetic grafts can result in areas of focal or patchy uptake which can be mistaken for areas of infection [42]. Furthermore, prosthetic vascular grafts can demonstrate diffuse FDG uptake even in the absence of infection, with the degree of uptake persisting for years following surgery [43].
Cardiac implantable electronic device (CIED) infection
CIED infections can occur at the level of the pocket, leads, or both. Management is usually dependent on the severity and location of the infection with either deep pocket infections or involvement of the leads requiring surgical extraction of the device, while more superficial infections can typically be treated with antibiotic therapy. FDG-PET has been shown to be accurate in the diagnosis of CIED infection [44]. FDG-PET can also assess for concomitant involvement of the valves, although its sensitivity in that context has been reported to be lower compared to PVE [45].
While FDG uptake in the pocket and along the leads is expected in the immediate post-implantation period, the degree of uptake should be mild and diffuse, involving the entirety of the pocket. Focal or intense uptake is considered suspicious for the presence of infection. Evaluation of the leads is more challenging than the pocket (likely related to their small size) reflected in the relatively low sensitivity (60%) for lead infection [44]. Focal or inhomogeneous uptake along the leads is suspicious for lead infection (Figure 8).
Figure 8.
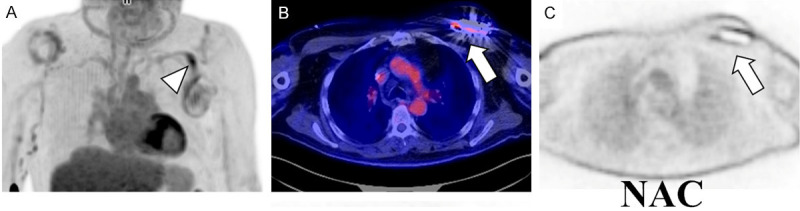
FDG PET/CT of a 73-year-old male with pain at the site of a pacemaker installed 2 years prior to the study. Focally increased FDG uptake is seen along the lead (arrowhead on the MIP image (A). Furthermore, increased FDG uptake is seen surrounding the generator/battery (arrow) on both the fused (B) and non-attenuation corrected images (C). Uptake greater than lung activity on the non-attenuation corrected images suggests infection. These findings are compatible with pacemaker infection involving the pocket and lead. The patient underwent pacemaker extraction and replacement. Culture obtained during the procedure confirmed infection of the lead and pocket with Staphylococcus scheleiferi.
LVAD infection
Left ventricular assist devices (LVADs) are increasingly being used in patients with heart failure, both as bridge to transplant and as destination therapy. Unfortunately, a complication of LVADs is concomitant infection of the driveline, pump, cannula, or peri-LVAD soft tissues. It has been shown that FDG-PET can be useful for the diagnosis of early LVAD infection [46]. An example of an LVAD infection is shown in Figure 9.
Figure 9.
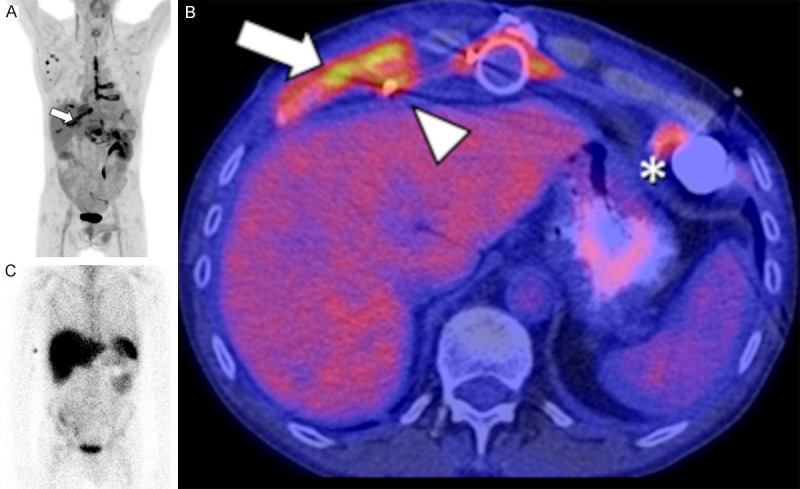
FDG PET/CT and WBC scans of a 54-year-old male post LVAD (HeartMate II) insertion presenting with skin drainage at the level of the xiphoid. On the PET MIP image (A), increased uptake is seen on the sternotomy line extending along several ribs, with corresponding cartilaginous destruction on CT (B, arrow), compatible with sternal osteitis involving several ribs. Uptake along the driveline (arrowhead), associated with emphysema on CT (not shown), and along the outflow tract is also compatible with infection. Activity around the inflow tract at the apex (B, *) is normal, can persist for several months, and likely represents inflammation. The WBC scan (C) shows a complete absence of white cell uptake in the sternum, rib, and around the device. Extensive infection of the sternum, ribs, and device with Aspergillus flavus was confirmed following surgical drainage.
A recent meta-analysis examined the diagnostic accuracy of FDG-PET in the assessment of LVAD infection [47]. The authors proposed a diagnostic algorithm for the workup of suspected LVAD infection recommending that PET be used in cases with persistent diagnostic uncertainty following a conventional workup, or in patients in whom surgical exploration is deemed high risk. Furthermore, these authors also stressed the importance of reviewing the non-attenuation corrected images as attenuation corrected images can result in false-positives due to over-correction of the PET images due to the proximity with metal densities. It was found that PET sensitivity for LVAD infection was 92% with a slightly lower specificity of 83%. A subsequent meta-analysis, which included a greater number of studies and patients, reported an improved diagnostic performance (sensitivity/specificity of 95%/91%) [48].
FDG-PET vs WBC
Infection imaging with radiolabeled (either with In111 or Tc99m) white blood cells (WBCs) has been shown to be highly accurate for the detection of many different types of infections (e.g. osteomyelitis, periprosthetic infection); however, the literature supporting it’s use for cardiovascular infections is more limited. Furthermore, the literature comparing radiolabeled WBCs with FDG-PET imaging for cardiovascular infection is even more limited; nonetheless, studies have suggested that FDG-PET is superior to WBC imaging in the evaluation of infective endocarditis [49] and LVAD infection [50].
A possible explanation for the superiority of FDG over WBC imaging may be related to the greater spatial resolution afforded by the use of a PET camera, rather than a conventional gamma camera. Certainly, the non-specific mechanism of uptake of FDG (in which radiotracer can accumulate in any metabolically active tissue) would seem to offer high sensitivity at the expense of specificity. In comparison, WBCs are known to accumulate only at sites of infection/inflammation, in addition to the reticuloendothelial system, and should offer both high sensitivity and specificity - unfortunately, to date, this does not appear to have translated to better diagnostic performance. Overall, additional comparative studies are required in order to conclusively determine. In centers with PET availability, WBC imaging is often reserved for equivocal cases on FDG-PET/CT or when false positive FDG-PET/CT are suspected.
Conclusion
FDG-PET has already shown itself to be useful in many different applications in cardiovascular infection and inflammation imaging and continues to evolve. While its role is well-established in various conditions such as sarcoidosis, LVV, and prosthetic valve IE, and evidence suggests that FDG-PET may play a growing role in other emerging applications such as NVE and LVAD infections. With its exquisite sensitivity and unique ability to monitor response to therapy, FDG-PET may find applications earlier in the investigational algorithms of patients with suspected cardiovascular infection or inflammation. Furthermore, the combination of FDG-PET with contrast-enhanced CT (PET/CT+) will likely further crystalize those applications, by enhancing the diagnostic properties of FDG-PET and providing a one-stop-shop imaging modality for conditions such as IE and LVV. Importantly, optimized patient preparation as well as knowledge of the typical appearance of pathologies is essential in order to provide an accurate FDG-PET interpretation.
Acknowledgements
M.P.-G. is supported by a grant from les Fonds la recherche en santé du Quebec (FRSQ), and by la Fondation de l’Institut de Cardiologie de Montréal.
Disclosure of conflict of interest
None.
References
- 1.Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–68. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier-Galarneau M, Martineau P, Zuckier LS, Pham X, Lambert R, Turpin S. 18F-FDG-PET/CT imaging of thoracic and extrathoracic tuberculosis in children. Semin Nucl Med. 2017;47:304–18. doi: 10.1053/j.semnuclmed.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Stumpe KDM, Dazzi H, Schaffner A, von Schulthess GK. Infection imaging using whole-body FDG-PET. Eur J Nucl Med. 2000;27:822–32. doi: 10.1007/s002590000277. [DOI] [PubMed] [Google Scholar]
- 4.Kagna O, Srour S, Melamed E, Militianu D, Keidar Z. FDG PET/CT imaging in the diagnosis of osteomyelitis in the diabetic foot. Eur J Nucl Med Mol Imaging. 2012;39:1545–50. doi: 10.1007/s00259-012-2183-z. [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, Ohkawa M. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–6. [PubMed] [Google Scholar]
- 6.Treglia G, Pascale M, Lazzeri E, van der Bruggen W, Delgado Bolton RC, Glaudemans AWJM. Diagnostic performance of 18F-FDG PET/CT in patients with spinal infection: a systematic review and a bivariate meta-analysis. Eur J Nucl Med Mol Imaging. 2020;47:1287–301. doi: 10.1007/s00259-019-04571-6. [DOI] [PubMed] [Google Scholar]
- 7.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–80. [PubMed] [Google Scholar]
- 8.Kolwicz SC Jr, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–16. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL, Birnie DH, Chen ES, Cooper LT, Tung RH, White ES. Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med. 2017;58:1341–53. doi: 10.2967/jnumed.117.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Israel O, Weiler-Sagie M, Rispler S, Bar-Shalom R, Frenkel A, Keidar Z, Bar-Shalev A, Strauss HW. PET/CT quantitation of the effect of patient-related factors on cardiac 18F-FDG uptake. J Nucl Med. 2007;48:234–9. [PubMed] [Google Scholar]
- 11.Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–21. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 12.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Nielsen JC, Patel AR. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1304–23. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Hiraga H, Yuwai K, Hiroe M. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102. [Google Scholar]
- 14.Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, Gulenchyn KY, Dekemp RA, DaSilva J, Birnie D, Wells GA. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–8. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- 15.Goitein O, Matetzky S, Beinart R, Di Segni E, Hod H, Bentancur A, Konen E. Acute myocarditis: noninvasive evaluation with cardiac mri and transthoracic echocardiography. Am J Roentgeno. 2009;192:254–8. doi: 10.2214/AJR.08.1281. [DOI] [PubMed] [Google Scholar]
- 16.Nensa F, Kloth J, Tezgah E, Poeppel TD, Heusch P, Goebel J, Nassenstein K, Schlosser T. Feasibility of FDG-PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol. 2018;25:785–94. doi: 10.1007/s12350-016-0616-y. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs M, Briel M, Daikeler T, Walker UA, Rasch H, Berg S, Ng QK, Raatz H, Jayne D, Kötter I, Blockmans D. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39:344–53. doi: 10.1007/s00259-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 18.Pelletier-Galarneau M, Ruddy TD. PET/CT for diagnosis and management of large-vessel vasculitis. Curr Cardiol Rep. 2019;21:34. doi: 10.1007/s11886-019-1122-z. [DOI] [PubMed] [Google Scholar]
- 19.Soussan M, Nicolas P, Schramm C, Katsahian S, Pop G, Fain O, Mekinian A. Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine. 2015;94:14. doi: 10.1097/MD.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slart RHJA. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45:1250–69. doi: 10.1007/s00259-018-3973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Boysson H, Aide N, Liozon E, Lambert M, Parienti JJ, Monteil J, Huglo D, Bienvenu B, Manrique A, Aouba A. Repetitive 18F-FDG-PET/CT in patients with large-vessel giant-cell arteritis and controlled disease. Eur J Intern Med. 2017;46:66–70. doi: 10.1016/j.ejim.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Rodríguez I, del Castillo-Matos R, Quirce R, Jiménez-Bonilla J, de Arcocha-Torres M, Ortega-Nava F, Rubio-Vassallo A, Amador NM, Bravo SI, Carril JM. Comparison of early (60 min) and delayed (180 min) acquisition of 18F-FDG PET/CT in large vessel vasculitis. Rev Esp Med Nucl Imagen Mol. 2013;32:222–6. doi: 10.1016/j.remn.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–24. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 24.Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, Dolan E, Moroney J, Murphy S, O’Rourke K, O’Malley K. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71:709–18. doi: 10.1002/ana.23553. [DOI] [PubMed] [Google Scholar]
- 25.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–17. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 26.Régis C, Martineau P, Harel F, Pelletier-Galarneau M. Personalized cardiac imaging with new PET radiotracers. Curr Cardiovasc Imaging Rep. 2020;13:11. [Google Scholar]
- 27.Tung R, Bauer B, Schelbert H, Lynch III JP, Auerbach M, Gupta P, Schiepers C, Chan S, Ferris J, Barrio M, Ajijola O. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–98. doi: 10.1016/j.hrthm.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoen B, Duval X. Infective endocarditis. N Engl J Med. 2013;368:1425–33. doi: 10.1056/NEJMcp1206782. [DOI] [PubMed] [Google Scholar]
- 29.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 30.Rouzet F, Chequer R, Benali K, Lepage L, Ghodbane W, Duval X, Iung B, Vahanian A, Le Guludec D, Hyafil F. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med. 2014;55:1980–5. doi: 10.2967/jnumed.114.141895. [DOI] [PubMed] [Google Scholar]
- 31.Philip M, Tessonier L, Mancini J, Mainardi JL, Fernandez-Gerlinger MP, Lussato D, Attias D, Cammilleri S, Weinmann P, Hagege A, Arregle F. Comparison between ESC and duke criteria for the diagnosis of prosthetic valve infective endocarditis. JACC Cardiovasc Imaging. 2020;13:2605–15. doi: 10.1016/j.jcmg.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Holle SL, Andersen MH, Klein CF, Bruun NE, Tønder N, Haarmark C, Loft A, Høilund-Carlsen PF, Bundgaard H, Iversen KK. Clinical usefulness of FDG-PET/CT for identification of abnormal extra-cardiac foci in patients with infective endocarditis. Int J Cardiovasc Imaging. 2020;36:939–946. doi: 10.1007/s10554-020-01787-8. [DOI] [PubMed] [Google Scholar]
- 33.Laura R, Schouten A, Verberne HJ, Bouma BJ, Berthe L, van Eck-Smit F, Barbara J, Mulder M. Surgical glue for repair of the aortic root as a possible explanation for increased F-18 FDG uptake. J Nucl Cardiol. 2008;15:146–7. doi: 10.1016/j.nuclcard.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Lazzeri E, Signore A, Erba PA, Prandini N, Versari A, D’Errico G, Mariani G. Radionuclide imaging of infection and inflammation: a pictorial case-based atlas. Milan: Springer-Verlag; 2013. [DOI] [PubMed] [Google Scholar]
- 35.Pizzi MN, Roque A, Fernández-Hidalgo N, Cuéllar-Calabria H, Ferreira-González I, Gonzàlez-Alujas MT, Oristrell G, Gracia-Sánchez L, González JJ, Rodríguez-Palomares J, Galiñanes M. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluordeoxyglucose positron emission tomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation. 2015;132:1113–26. doi: 10.1161/CIRCULATIONAHA.115.015316. [DOI] [PubMed] [Google Scholar]
- 36.Salomäki SP, Saraste A, Kemppainen J, Bax JJ, Knuuti J, Nuutila P, Seppänen M, Roivainen A, Airaksinen J, Pirilä L, Oksi J. 18F-FDG positron emission tomography/computed tomography in infective endocarditis. J Nucl Cardiol. 2017;24:195–206. doi: 10.1007/s12350-015-0325-y. [DOI] [PubMed] [Google Scholar]
- 37.Abikhzer G, Martineau P, Grégoire J, Finnerty V, Harel F, Pelletier-Galarneau M. [18F]FDG-PET CT for the evaluation of native valve endocarditis. J Nucl Cardiol. 2020 doi: 10.1007/s12350-020-02092-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Ratliff CR, Strider D, Flohr T, Moses D, Rovnyak V, Armatas J, Johnson J, Okerlund A, Baldwin M, Lawson M, Fuhrmeister S, Tracci MC, Upchurch GR, Cherry KJ. Vascular graft infection: incidence and potential risk factors. J Wound Ostomy Continence Nurs. 2017;44:524–7. doi: 10.1097/WON.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 39.Rojoa D, Kontopodis N, Antoniou SA, Ioannou CV, Antoniou GA. 18F-FDG PET in the diagnosis of vascular prosthetic graft infection: a diagnostic test accuracy meta-analysis. Eur J Vasc Endovasc Surg. 2019;57:292–301. doi: 10.1016/j.ejvs.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 40.Pelletier-Galarneau M, Juneau D. Vascular graft infection: improving diagnosis with functional imaging. J Nucl Cardiol. 2020 doi: 10.1007/s12350-020-02269-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Husmann L, Ledergerber B, Anagnostopoulos A, Stolzmann P, Sah BR, Burger IA, Pop R, Weber A, Mayer D, Rancic Z, Hasse B. The role of FDG PET/CT in therapy control of aortic graft infection. Eur J Nucl Med Mol Imaging. 2018;45:1987–97. doi: 10.1007/s00259-018-4069-1. [DOI] [PubMed] [Google Scholar]
- 42.Bowles H, Ambrosioni J, Mestres G, Hernández-Meneses M, Sánchez N, Llopis J, Yugueros X, Almela M, Moreno A, Riambau V, Fuster D. Diagnostic yield of 18F-FDG PET/CT in suspected diagnosis of vascular graft infection: a prospective cohort study. J Nucl Cardiol. 2020;27:294–302. doi: 10.1007/s12350-018-1337-1. [DOI] [PubMed] [Google Scholar]
- 43.Keidar Z, Pirmisashvili N, Leiderman M, Nitecki S, Israel O. 18F-FDG uptake in noninfected prosthetic vascular grafts: incidence, patterns, and changes over time. J Nucl Med. 2014;55:392–5. doi: 10.2967/jnumed.113.128173. [DOI] [PubMed] [Google Scholar]
- 44.Bensimhon L, Lavergne T, Hugonnet F, Mainardi JL, Latremouille C, Maunoury C, Lepillier A, Le Heuzey JY, Faraggi M. Whole body [18F] fluorodeoxyglucose positron emission tomography imaging for the diagnosis of pacemaker or implantable cardioverter defibrillator infection: a preliminary prospective study. Clin Microbiol Infect. 2011;17:836–44. doi: 10.1111/j.1469-0691.2010.03312.x. [DOI] [PubMed] [Google Scholar]
- 45.Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J. 2019;40:3222–32. doi: 10.1093/eurheartj/ehz620. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Feller ED, Chen W, Liang Y, Dilsizian V. FDG PET/CT for early detection and localization of left ventricular assist device infection: impact on patient management and outcome. JACC Cardiovasc Imaging. 2019;12:722–729. doi: 10.1016/j.jcmg.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 47.Tam MC, Patel VN, Weinberg RL, Hulten EA, Aaronson KD, Pagani FD, Corbett JR, Murthy VL. Diagnostic accuracy of FDG PET/CT in suspected LVAD infections: a case series, systematic review, and meta-analysis. JACC Cardiovasc Imaging. 2019;13:1191–202. doi: 10.1016/j.jcmg.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ten Hove D, Treglia G, Slart RH, Damman K, Wouthuyzen-Bakker M, Postma DF, Gheysens O, Borra RJ, Mecozzi G, van Geel PP, Sinha B. The value of 18F-FDG PET/CT for the diagnosis of device-related infections in patients with a left ventricular assist device: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:241–253. doi: 10.1007/s00259-020-04930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauridsen TK, Iversen KK, Ihlemann N, Hasbak P, Loft A, Berthelsen AK, Dahl A, Dejanovic D, Albrecht-Beste E, Mortensen J, Kjær A, Bundgaard H, Bruun NE. Clinical utility of 18F-FDG positron emission tomography/computed tomography scan vs. 99mTc-HMPAO white blood cell single-photon emission computed tomography in extra-cardiac work-up of infective endocarditis. Int J Cardiovasc Imaging. 2017;33:751–60. doi: 10.1007/s10554-016-1047-1. [DOI] [PubMed] [Google Scholar]
- 50.de Vaugelade C, Mesguich C, Nubret K, Camou F, Greib C, Dournes G, Debordeaux F, Hindie E, Barandon L, Tlili G. Infections in patients using ventricular-assist devices: comparison of the diagnostic performance of 18F-FDG PET/CT scan and leucocyte-labeled scintigraphy. J Nucl Cardiol. 2019;26:42–55. doi: 10.1007/s12350-018-1323-7. [DOI] [PubMed] [Google Scholar]


