Significance
Both type 1 and type 2 diabetes are associated with reduced β-cell mass or function, resulting from decreased proliferation and increased apoptosis. Understanding the signals governing β-cell survival and regeneration is critical for developing strategies to maintain healthy populations of these cells in individuals. Both forms of diabetes are associated with hyperglucagonemia and an increased plasma glucagon:insulin ratio. Glucagon excess contributes to metabolic dysregulation of the diabetic state and glucagon receptor antagonism is a potential target area for the treatment and prevention of diabetes. Our studies presented here suggest that blockade of glucagon signaling lowers glycemia in mouse models of type 1 diabetes while enhancing formation of functional β-cell mass and production of insulin-positive cells from α-cell precursors.
Keywords: insulin, glucagon, islet, regeneration, diabetes
Abstract
We evaluated the potential for a monoclonal antibody antagonist of the glucagon receptor (Ab-4) to maintain glucose homeostasis in type 1 diabetic rodents. We noted durable and sustained improvements in glycemia which persist long after treatment withdrawal. Ab-4 promoted β-cell survival and enhanced the recovery of insulin+ islet mass with concomitant increases in circulating insulin and C peptide. In PANIC-ATTAC mice, an inducible model of β-cell apoptosis which allows for robust assessment of β-cell regeneration following caspase-8–induced diabetes, Ab-4 drove a 6.7-fold increase in β-cell mass. Lineage tracing suggests that this restoration of functional insulin-producing cells was at least partially driven by α-cell-to-β-cell conversion. Following hyperglycemic onset in nonobese diabetic (NOD) mice, Ab-4 treatment promoted improvements in C-peptide levels and insulin+ islet mass was dramatically increased. Lastly, diabetic mice receiving human islet xenografts showed stable improvements in glycemic control and increased human insulin secretion.
Pancreatic islets secrete both insulin and glucagon in a manner which is tightly juxtaposed. Aberrant glucagon production correlates with diabetes and suppression of glucagon corrects the hyperglycemia of diabetes; however, glucagon’s role in the metabolic manifestations of diabetes remains a subject of debate. More recently, targeted disruption of glucagon action has gained traction as a potential treatment for diabetes. Glucagon receptor (GcgR) antagonists include small-molecule inhibitors and humanized antibodies which antagonize glucagon receptor activation. Both antisense oligonucleotides and human antibodies against GcgR show promise to promote glycemic control in patients while minimizing the side effects seen with small molecules (1). GcgR-antagonizing antibodies provide effective glucose control in rodents and primate models of type 2 diabetes (2–4). Hyperglucagonemia and excess proliferation of α-cells also result following these treatments, which phenocopy global or liver-specific GcgR−/− mice (5, 6).
More recently, our work demonstrated evidence that the blockage of glucagon action improves glycemia in type 1 diabetic rodents (7). Our observations in type 1 diabetic rodents have been replicated by others who have met these results with skepticism (8, 9). In humans, GcgR antagonism diminishes insulin requirements (10, 11). Initial clinical trials in patients with type 1 diabetes showed no serious adverse effects while improving glucose control (10).
During the severe insulin resistance promoted by insulin receptor-antagonizing peptide S961, GcgR antagonism can improve insulin production and β-cell mass over S961 alone, suggesting that GcgR antagonism may be beneficial in promoting functional β-cell mass under conditions of severe insulin resistance (12). We hypothesize that such conditions may also exist during insulin deficiency. While testing the efficacy of a similar GcgR-antagonizing antibody (Ab-4) in models of type 1 diabetes (13), we were struck by the remarkable finding that stable normoglycemia can remain following cessation of the treatment. Here, we assess the efficacy of the GcgR antagonist Ab-4 to enhance functional β-cell mass and restore stable glucose control.
Results
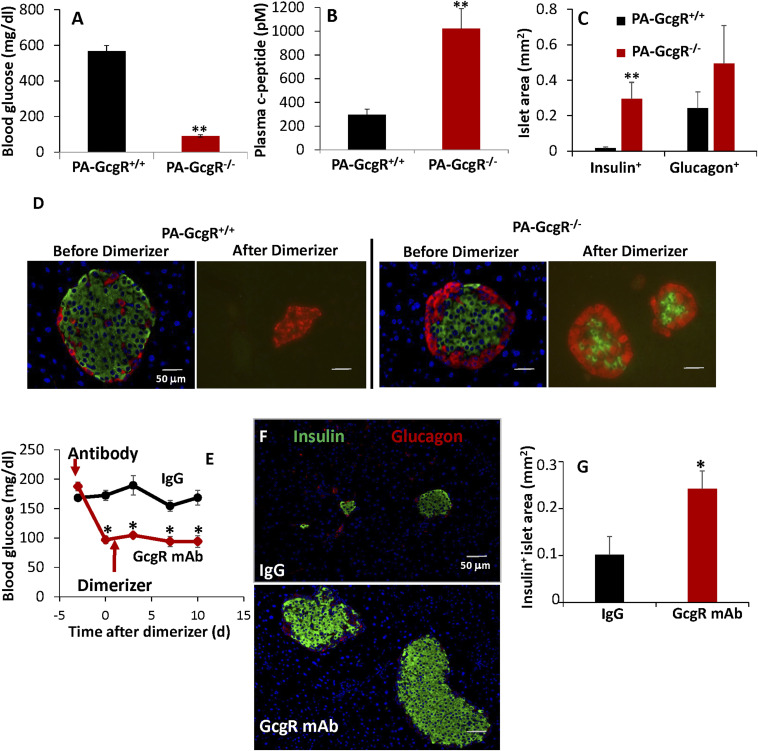
It has been previously reported by our group (14, 15) and others (16) that GcgR−/− mice are resistant to β-cell destruction. To ascertain if GcgR alters susceptibility to β-cell apoptosis, we bred GcgR−/− mice into the PANIC-ATTAC background. The PANIC-ATTAC model allows induction of caspase-8–mediated apoptosis selectively within the pancreatic β-cells (17). Not surprisingly, GcgR−/− PANIC-ATTAC mice were resistant to the onset of hyperglycemia (Fig. 1A). Following dimerizer-induced apoptosis, circulating levels of C peptide (Fig. 1B) and insulin+ islet mass (Fig. 1 C and D) were retained in GcgR−/− PANIC-ATTAC but not GcgR+/+ PANIC-ATTAC mice. Consistent with expected results, α-cell hyperplasia was strongly evident in GcgR−/− PANIC-ATTAC mice before and after (Fig. 1 C and D) dimerizer treatment. Plasma triglyceride and fatty acid concentrations were lower by 63 and 75%, respectively, in GcgR−/− PANIC-ATTAC mice (SI Appendix, Fig. S1 A and B). In order to allow for an inducible elimination of GcgR signaling, we next administered a GcgR monoclonal antibody (mAb) (mAb-4) antagonist of the glucagon receptor 3 d prior to dimerizer treatment of GcgR+/+ PANIC-ATTAC mice. GcgR inhibition lowered blood glucose prior to dimerizer (Fig. 1E). Circulating insulin and C-peptide (SI Appendix, Fig. S1 C and D) levels were higher after dimerizer treatment in GcgR mAb-treated mice. Prior to euthanasia, half of our mice were allowed to metabolize the remaining GcgR mAb, and glucose tolerance was tested by oral dosing (SI Appendix, Fig. S1E). This appeared to be due to enhanced insulin secretion following an oral glucose load after GcgR mAb treatment (SI Appendix, Fig. S1F). Coincident with these changes, GcgR mAb-treated mice had more insulin-positive surface area and larger islets remaining after the dimerizer challenge (Fig. 1 F and G). Collectively, these data suggest that glucagon antagonism promotes functional β-cell survival in PANIC-ATTAC mice.
Fig. 1.
Glucagon receptor inhibition or genetic ablation protects against caspase-8–mediated apoptosis. Blood glucose (A) and C-peptide (B) concentrations were measured from plasma 10 d after inducing apoptosis. Immunofluorescent imaging of insulin (green) and glucagon (red) was performed at multiple levels before or 10 d after inducing apoptosis (C and D). GcgR mAb or IgG control was provided 3 d prior to dimerizer (E–G). Glucose (E) was measured before antibody and 10 d after dimerizer. Pancreatic sections were visualized by immunofluorescence against insulin (green) and glucagon (red) (F), and whole-islet area was quantified from multiple nonadjacent sections (G). n = 6. *P < 0.05, **P < 0.005. Standard error shown.
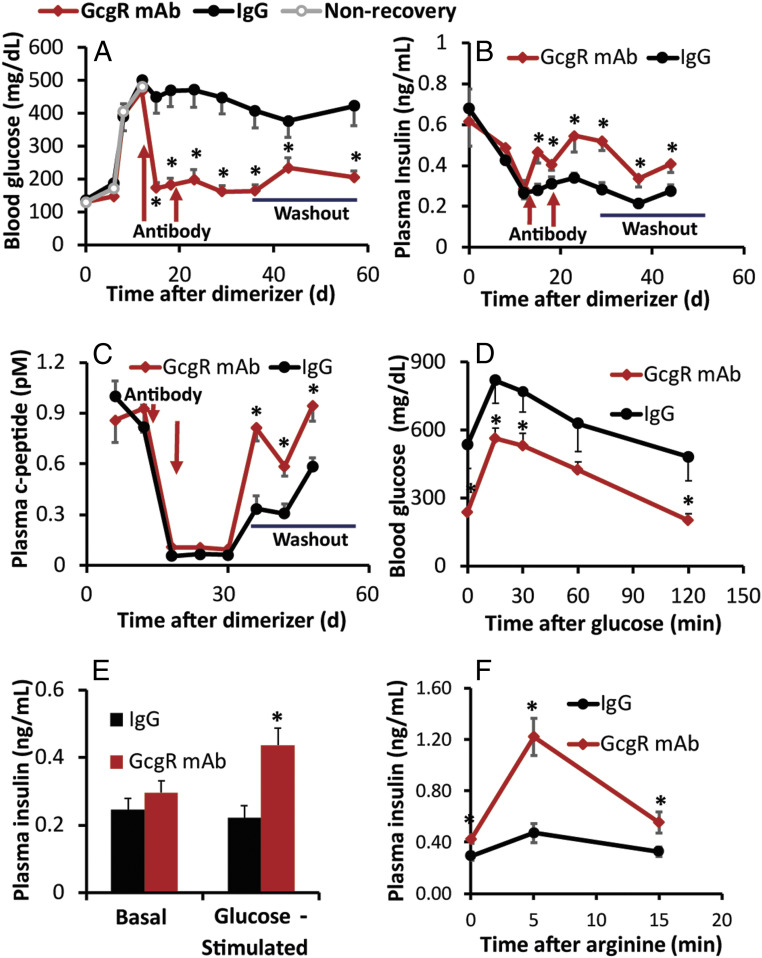
The greatest strength of the PANIC-ATTAC mouse model is its potent propensity for β-cell regeneration, which can even occur spontaneously following dimerizer-induced diabetes (17, 18). To determine the effect of GcgR antagonism on β-cell regeneration, PANIC-ATTAC mice were first allowed to become overtly hyperglycemic (Fig. 2A). Twelve days after dimerizer treatment, mice were pair-matched based on fed blood glucose and assigned for treatment with GcgR mAb (10 mg/kg) or immunoglobulin (IgG) (10 mg/kg) control, or euthanized for islet histology. Blood glucose rapidly dropped in GcgR-antagonized mice but not IgG control mice after antibody injection. Glucose remained low for 2 consecutive weeks of treatment and for an extended washout period for 40 d after the last antibody treatment. Plasma insulin levels (Fig. 2B) and C-peptide levels (Fig. 2C) continued to decline during GcgR mAb treatment, presumably due to a diminished need for insulin. Subsequently, mAbs were given time to be metabolized during a 3-wk washout period prior to metabolic testing. The half-life of mAb-4 is ∼1.9 ± 0.1 d in rodents, and it lacks any binding affinity for glucagon-like peptide-1 receptor (13). Normalization of glucagon levels indicated the return of normal glucagon signaling (SI Appendix, Fig. S2). During this time, GcgR mAb-treated mice showed increases in plasma insulin and C-peptide concentrations. Glucose tolerance remained substantially improved after withdrawal of GcgR mAb (Fig. 2D). Glucose-stimulated insulin secretion (Fig. 2E) and the rapidly secreted pool of insulin in response to arginine challenge (Fig. 2F) were greatly enhanced in mice that had received GcgR mAb treatment.
Fig. 2.
GcgR antagonism regenerates functional β-cell mass in PANIC-ATTAC mice. GcgR mAb was provided 12 and 19 d after dimerizer. Glucose (A), insulin (B), and C peptide (C) were measured at routine intervals during recovery, which included a washout period without treatment. Glucose (D) and insulin (E) were measured during an oral glucose tolerance test. Insulin was quantified following an arginine tolerance test (F). (A–C) n = 18; (D–F) n = 9. *P < 0.05. Standard error shown.
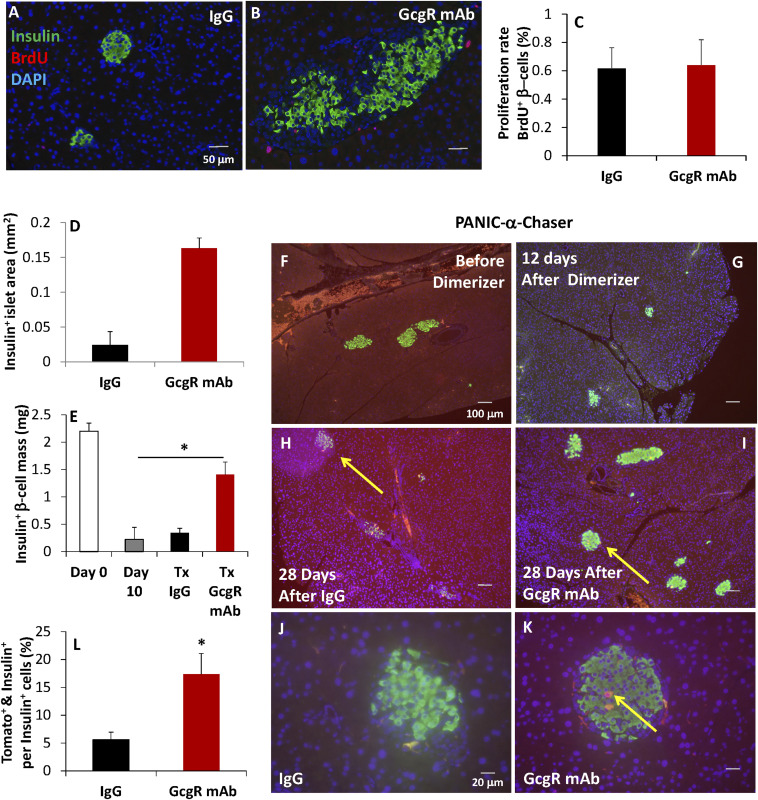
Treatment with IgG control failed to increase insulin+ area (Fig. 3 A and G); however, treatment with GcgR mAb increased insulin+ area by 6.7-fold (Fig. 3 B, D, and I). To assess rates of β-cell proliferation, bromodeoxyuridine (BrdU) was given to a portion of these mice at day 18 postdimerizer (1 d after a second dose of GcgR mAb or IgG) (Fig. 3B, red); however, BrdU incorporation was not significantly increased in β-cells (Fig. 3C). Histologic assessment confirmed that dimerizer induced a 91% reduction in insulin+ β-cell mass by day 12, prior to antibody treatments (Fig. 3 E and G). In parallel, we also assessed α-cell→β-cell conversion by pulse labeling α-cells via the genetic knockin of td-tomato in response to the brief addition of a doxycycline-containing diet prior to the induction of diabetes; this uses the “PANIC α-chaser” method that we described previously (19). In this system, we see a substantial abundance of insulin+ cells that were preproglucagon-positive prior to the induction of diabetes (Fig. 3 K and L). In summary, these studies suggest that a substantial amount of the newly formed functional β-cell mass induced by GcgR antagonism in PANIC-ATTAC mice derives from enhanced α-cell→β-cell conversion. Our data do not preclude contributions from β-cell proliferation or other cell sources, as BrdU only captures a brief window of time during regeneration.
Fig. 3.
β-Cell regeneration and α-cell-to-β-cell conversion are increased after GcgR mAb treatment of PANIC-ATTAC mice. (A–C) BrdU was given 48 h prior to euthanasia, after 10 d of treatment with IgG (A) or GcgR mAb (B). n = 6 per group. (C) BrdU-positive β-cells were counted and expressed as percentage of insulin+ cells. (D) Average insulin+ pancreatic area was quantified. n = 18 per group. (E–K) td-Tomato expression was knocked-in to preproglucagon-expressing cells (using a doxycycline-inducible system) prior to the onset of diabetes. n = 8 per group. The formation of insulin+ (green) and td-Tomato+ (red) cells is seen in yellow, indicating insulin production from α-cell precursor cells. Insulin-positive islet mass was quantified from multiple nonadjacent sections following euthanasia. Yellow arrows indicate islets containing Tomato+ & Insulin+ cells. (L) Tomato+ & Insulin+ cells were counted and expressed as a percentage of Insulin+ cells. Standard error shown.
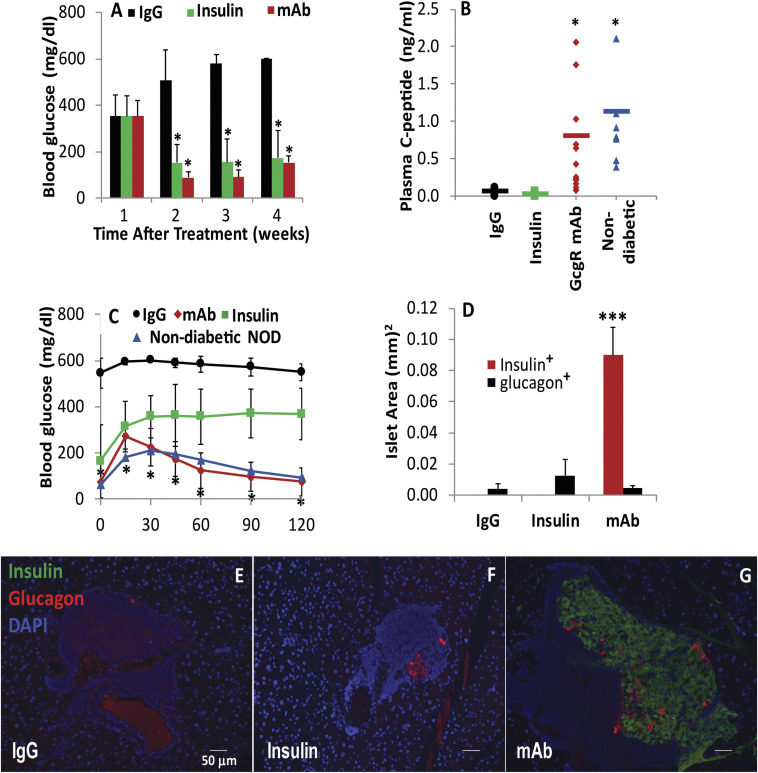
To determine if the protective effect of GcgR antagonism on β-cell health may be relevant in the context of a continual immune attack, we have also evaluated efficacy in female nonobese diabetic (NOD) mice. Mice were monitored weekly until the confirmed onset of diabetes with documented hyperglycemia (>250 mg/dL) for two consecutive measurements. At such time, mice were pair-matched for blood glucose and assigned treatment with insulin, IgG, or GcgR mAb. Both insulin and GcgR mAb treatment achieved similar degrees of glycemic lowering (Fig. 4A); however, C-peptide production was only restored in mice receiving the GcgR mAb (Fig. 4B). Glycemic control was restored during glucose tolerance tests (Fig. 4C). Histologically, the islets were well-preserved by treatment with GcgR mAb, as a greater abundance of insulin+ area is restored (Fig. 4 D and G) as compared with IgG treatment (Fig. 4E) or insulin treatment alone (Fig. 4F). Treatment with GcgR mAb prevented weight loss and loss of body fat (SI Appendix, Fig. S3 A and B), further supporting the antidiabetic effects of the treatment. Plasma ketones, fatty acids, and alanine aminotransferase levels were decreased by GcgR mAb treatment (SI Appendix, Fig. S3 C–E).
Fig. 4.
GcgR inhibition enhances β-cell mass in NOD mice. After 1 wk of confirmed hyperglycemia in female NOD mice, GcgR mAb, IgG, or insulin was provided for 4 wk. Glucose (A) was measured at routine intervals during recovery. C-peptide (B) and oral glucose tolerance (C) were assessed during the fourth week of treatment. (D) Insulin+ and glucagon+ islet area was quantified as islet architecture was evaluated by immunofluorescence. IgG and GcgR mAb, n = 15; insulin, n = 5; nondiabetic n = 8. *P < 0.05, ***P < 0.0005 versus IgG. (E–G) Representative images of IgG (E), insulin (F) and GcgR mAb (G) treated NOD mice. Insulin (green); glucagon (red); DAPI (blue). Standard error shown.
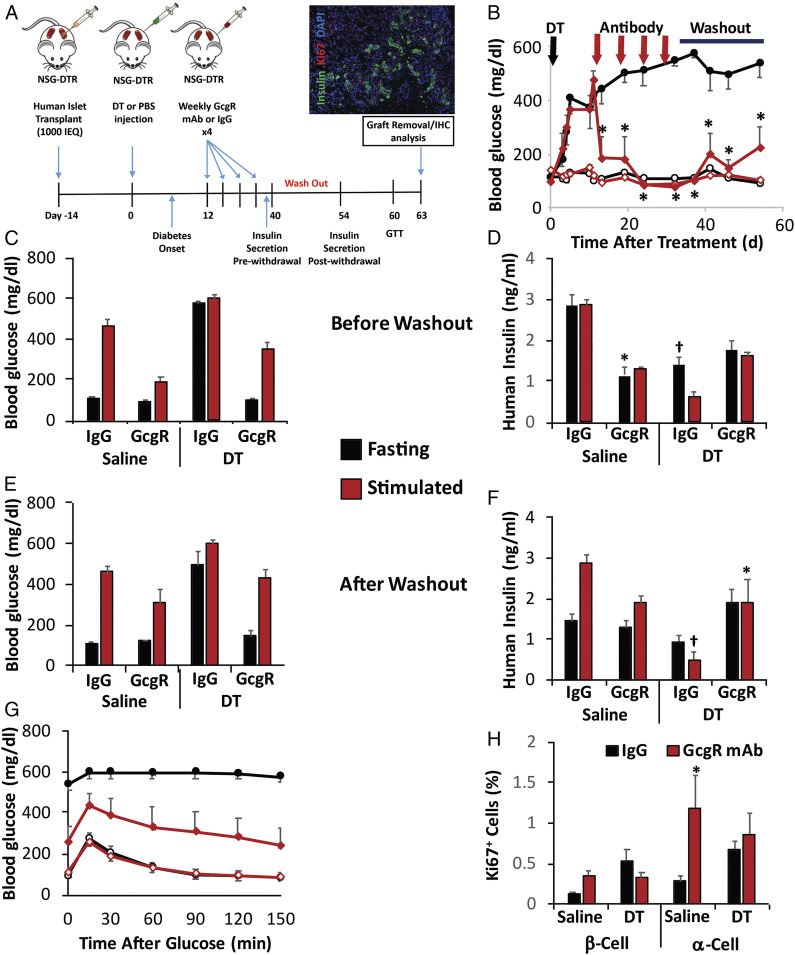
The ultimate goal of this work is to stimulate β-cell regeneration in human diabetic patients. To this aim, we have begun testing the efficacy of GcgR antagonism to enhance functional β-cell mass in human islets, using cohorts of NSG-DTR [NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(Ins2-HBEGF)6832Ugfm/SzJ] mice for engraftment of human islets into the kidney capsule. This minimal islet mass model, which we have described previously (6), allows for engraftment to take place in the nondiabetic mouse prior to induction of diabetes with low-dose diphtheria toxin (DT), which selectively eliminates the murine β-cell mass (Fig. 5A). After diabetic onset, mice were pair-matched by glucose and treated with IgG control or GcgR mAb for four consecutive weekly injections. GcgR mAb rapidly lowered blood glucose and maintained lower blood glucose for 3 wk while mAbs were metabolized and cleared during a washout period (Fig. 5B). Prior to the washout period, blood glucose (Fig. 5C) and serum insulin (Fig. 5D) were assessed during fasting conditions and after simultaneous stimulation with arginine and glucose. DT treatment clearly increased fasting hyperglycemia in IgG control mice; however, GcgR mAb-treated mice had normal fasting blood glucose and had lower blood glucose following glucose/arginine challenge. Insulin levels were diminished by DT treatment, and did not differ between IgG controls and GcgR mAb-treated mice. This suggests that under the treated conditions, glycemia is improved by GcgR mAb by diminishing the need for insulin (at least in part). Thus, mAb treatments were ceased to evaluate glycemic control by the remaining islet mass in the absence of GcgR antagonism.
Fig. 5.
GcgR mAb treatment promotes stable euglycemia in diabetic mice with human islet xenografts. A schematic of the study design using NSG-DTR islet recipients which received 1,000 IEQs of healthy human islets beneath the kidney capsule prior to induction of diabetes with diphtheria toxin to ablate endogenous mouse B cells (A). IHC, immunohistochemistry. Glycemic control (B–D) and insulin secretory responses (E and F) were assessed longitudinally (B), prior to cessation of mAb treatment (C and E), or following the metabolism of mAb after a washout period (D and F). Blood glucose and plasma insulin were assessed during fasting or after stimulation with glucose + l-arginine (C–F). Glucose tolerance test (GTT) was assessed after the washout period (G). Proliferation of α-cells and β-cells was assessed by coexpression of Ki67 with glucagon or insulin, respectively (H). n = 4 (saline + IgG); n = 5 (saline + GcgR mAb); n = 6 per each DT group; data are representative from three cadaveric donors. *P < 0.05 for GcgR mAb effect; †P < 0.05 for diptheria toxin effect. Standard error shown.
Following the withdrawal of mAb treatments, the mice previously treated with GcgR mAb (but not IgG control) maintained fed (Fig. 5B), fasting (Fig. 5D), and glucose/arginine-stimulated blood glucose (Fig. 5E). Secretion of human insulin showed a mild trend toward an enhanced stimulation in response to glucose/arginine as compared with IgG control-treated mice (Fig. 5F). Twenty-seven days after the last treatment, glucose tolerance was still drastically better in mice that had been treated with GcgR mAb rather than control IgG (Fig. 5G). As expected based on our previous work, immunostaining of the human islet graft after 4 wk of treatment revealed an enhancement in α-cell proliferation in the nondiabetic mice as indicated by the Ki67 proliferation marker (Fig. 5H), while there was a nonsignificant trend toward increased β-cell proliferation in nondiabetic animals. Neither α-cell proliferation nor β-cell proliferation appeared to be strongly enhanced by GcgR mAb treatment in DT-induced diabetic mice (Fig. 5H). In the mouse pancreas, insulin content and pancreas mass remained unchanged after GcgR mAb treatment (SI Appendix, Fig. S4 A and B). Insulin content within the grafted human islet was not significantly different between treatments (SI Appendix, Fig. S4C). Circulating amino acids linked to α-cell hyperplasia were elevated (SI Appendix, Fig. S4D).
Discussion
Collectively, these results suggest that inhibiting glucagon receptor action can promote recovery of functional β-cell mass in murine models of type 1 diabetes. Moreover, lineage-tracing studies suggest that in the PANIC-ATTAC model, GcgR mAb treatment stimulates α-cell→β-cell conversion. What is most remarkable, is that this allows for sustained glycemic improvements, via enhancements in functional β-cell mass. Essentially, diabetes was cured in this artificial mouse model of type 1 diabetes. In type 2 diabetes, GcgR antagonism may be limited by α-cell hyperplasia. However, in the context of type 1 diabetes, this α-cell hyperplasia may be of benefit by providing a unique source to develop new insulin-producing cells.
Amino acid sensing within the α-cell is important for the hyperplasia that occurs with GcgR antagonism or ablation (20, 21). GcgR antagonism prompts changes in hepatic amino acid (AA) catabolism and increases circulating AAs. Media mimicking these AA levels stimulate α-cell proliferation and high levels of l-glutamine are required. Expression of the sodium-coupled neutral amino acid transporter Slc38a5 is markedly increased in α-cells of mice, with interrupted glucagon signaling, and plays an essential role in α-cell proliferation. These results indicate a liver–α-cell axis where glucagon regulates serum AA availability and, conversely, AAs regulate α-cell proliferation. Consistent with our reports, GcgR mAb treatment of nondiabetic NSG-DTR mice led to elevated levels of more than a dozen AAs in serum.
The discovery that the α-cell can serve as a precursor to form new insulin-producing cells has fueled a number of studies (22). Most recently, data suggest that primary human α-cells can also begin to secrete insulin after transplantation into diabetic rodents (23). Several studies have identified factors that aid α-cell-to-β-cell conversion (24–26). Here, our data suggest that fueling new α-cell mass can provide a potential new source for insulin-producing cells under insulinopenic conditions. Our data do not rule out contributions from β-cell replication, and further studies will be needed to assess contributions from multiple cell types.
Grafts from human islets are highly heterogeneous in size and proportion of each cell type. Thus, determining changes in β-cell mass or content is difficult to assess with accuracy, and remains an unmet need in the field. In our studies, we clearly see increases in human insulin secretion and the capacity to maintain glucose homeostasis in mice harboring human islet xenografts. Further investment in this field is required to determine if we generate a greater abundance of human β-cells as a result of glucagon receptor antagonism and if human α-cells can take on insulin-secretory functions under these conditions. These studies provide hope that a once-weekly injection of a human antibody against the glucagon receptor can enhance functional β-cell mass. Changing small amounts of residual β-cells can have a huge improvement in the quality of life for millions of patients with type 1 diabetes.
Materials and Methods
Animals.
All animal experimental protocols were approved by the Institutional Animal Care and Use Committees of the University of Texas Southwestern Medical Center at Dallas, Dallas Veterans Administration Medical Center, University of Utah, or Vanderbilt University Medical Center (VUMC). Institutional guidelines for animal care and use were followed. With the exception of NOD mice, all mice were bred in-house with constant temperature on 12-h light cycles. PANIC-ATTAC experiments were performed on male or female mice beginning at 9 to 14 wk of age (17, 27). Female NOD mice (The Jackson Laboratory) were maintained in-house beginning at 8 wk of age. Transgenic mice, with two autosomal copies of the RIP-DTR transgene (22), in which DTR expression is driven by the RIP, were previously backcrossed onto the NSG background (28) for more than 10 generations, resulting in the NSG-DTR mouse maintained at VUMC (29).
Diabetes Induction.
Homozygous PANIC-ATTAC mice were administered a single dose of AP20187 homodimerizer (0.08125 mg/kg) prepared according to the manufacturer’s instructions (17). Female NOD mice were surveyed twice-weekly by glucometer, until spontaneous diabetes was diagnosed by glucose >250 mg/dL for two consecutive measurements. DT (List Biological Laboratories; 150) was administered to NSG-DTR mice in a single, 300-μL intraperitoneal injection of 5.0 ng total DT. Control NSG-DTR mice (phosphate-buffered saline; PBS) were treated with an equal volume of 1× PBS (Sigma-Aldrich). All animals in a cohort (with human islets from the same human donor) were injected with DT or PBS on the same day (29).
Plasma Measurements.
Blood glucose was measured in conscious animals from a hand-held glucosemeter (Bayer HealthCare) on tail vein blood around 10:00 AM. Plasma insulin and C-peptide levels were measured using rat/mouse enzyme-linked immunosorbent assay kits (Crystal Chem).
Human and Mouse Islets.
Non-diabetic human islets were obtained from islet isolation centers that are part of the Integrated Islet Distribution Program, Allegany Health Network (Rita Bottino), or Alberta Diabetes Institute including a 59-year-old male donor (BMI of 31, RRID:SAMN08769061), a 19-year-old male donor (BMI 20, no RRID), and a 47-year-old male donor (BMI 27.6, no RRID), respectively. Assessment of human islet function was performed by perifusion on the day of islet arrival, as previously described (29). Sixty size-matched islets were perifused with 5.6 mM glucose, 16.7 mM glucose, and 16.7 mM glucose with 100 μM 3-isobutyl-1-methyl-xanthine (Sigma-Aldrich; I5879-1G). Insulin secretion was normalized to islet equivalents (IEQs) (30).
Islet Transplantation.
NSG or NSG-DTR male mice, between 12 and 20 wk of age, were used for transplantation as described (31). For the NSG-DTR model, each recipient mouse received 1,000 IEQs of human islets transplanted under the kidney capsule. All data with human islets from all models were normalized to 1,000 transplanted IEQs.
Immunohistochemistry of Pancreas and Human and Mouse Grafts.
Immunohistochemical studies were performed as described (29). Antibodies used in this study were as follows: guinea pig anti-human insulin (0564; Dako), rabbit anti-human Ki67 (ab15580; Abcam), and rabbit anti-glucagon (ab92517; Abcam). Slides of tissue sections were examined under a BZ-X700 fluorescence microscope (Keyence) and areas of insulin- and glucagon-positive cells were quantified using Keyence’s BZ-X Analyzer software.
Statistical Analysis.
Results are presented as mean ± SD and were evaluated by using Student’s t test for the comparison of two groups.
Supplementary Material
Acknowledgments
We thank our friend and colleague R.H.U. for 62 y of scientific inspiration. We thank Pat Kilian and the staff at the Juvenile Diabetes Research Foundation (JDRF) for their expert guidance and support. Human pancreatic islets were provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-funded Integrated Islet Distribution Program at City of Hope (NIH Grant 2UC4DK098085). This research was supported by JDRF SRA-2016-149-Q-R (to W.L.H., P.E.S., R.H.U., A.C.P., and E.D.D.), R01DK112866 (to W.L.H.), and K01DK117969 (to E.D.D.). This research was performed using resources and/or funding provided by the NIDDK-supported Human Islet Research Network (RRID:SCR_014393; https://hirnetwork.org; UC4 DK104211 and DK112232) and by DK106755 (to A.C.P.), DK20593 (to A.C.P.), DK117147 (to A.C.P.), and the Department of Veterans Affairs (BX000666).
Footnotes
Competing interest statement: R.H.U. is a founding scientist of SynAlpha Therapeutics, LLC. K.W.S., A.M.E., and R.E.G. are employees and shareholders of Eli Lilly and Company.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022142118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Pearson M. J., Unger R. H., Holland W. L., Clinical trials, triumphs, and tribulations of glucagon receptor antagonists. Diabetes Care 39, 1075–1077 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamoto H., et al., Glucagon receptor blockade with a human antibody normalizes blood glucose in diabetic mice and monkeys. Endocrinology 156, 2781–2794 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Gu W., et al., Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J. Pharmacol. Exp. Ther. 331, 871–881 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Yan H., et al., Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J. Pharmacol. Exp. Ther. 329, 102–111 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Gelling R. W., et al., Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 100, 1438–1443 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longuet C., et al., Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: Evidence for a circulating α-cell growth factor. Diabetes 62, 1196–1205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M. Y., et al., Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc. Natl. Acad. Sci. U.S.A. 112, 2503–2508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damond N., et al., Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist. eLife 5, e13828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst J. J., et al., Insulin and glucagon: Partners for life. Endocrinology 158, 696–701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettus J., et al., REMD-477, a human glucagon receptor antibody, reduces daily insulin requirements and improves glycemic control in people with type 1 diabetes. Diabetes 66 (suppl. 1), A100 (2017). [Google Scholar]
- 11.Kazda C. M., et al., A euglycaemic clamp pilot study assessing the effects of the glucagon receptor antagonist LY2409021 on 24-hour insulin requirement in patients with type 1 diabetes mellitus. Diabetes 62, LB64 (2013). [Google Scholar]
- 12.Okamoto H., et al., Glucagon receptor inhibition normalizes blood glucose in severe insulin-resistant mice. Proc. Natl. Acad. Sci. U.S.A. 114, 2753–2758 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jun L. S., et al., Absence of glucagon and insulin action reveals a role for the GLP-1 receptor in endogenous glucose production. Diabetes 64, 819–827 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Maruyama H., Hisatomi A., Orci L., Grodsky G. M., Unger R. H., Insulin within islets is a physiologic glucagon release inhibitor. J. Clin. Invest. 74, 2296–2299 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y., et al., Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc. Natl. Acad. Sci. U.S.A. 109, 14972–14976 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conarello S. L., et al., Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50, 142–150 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Wang Z. V., et al., PANIC-ATTAC: A mouse model for inducible and reversible beta-cell ablation. Diabetes 57, 2137–2148 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye R., et al., Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. eLife 3, e03851 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye R., et al., Autonomous interconversion between adult pancreatic α-cells and β-cells after differential metabolic challenges. Mol. Metab. 5, 437–448 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean E. D., et al., Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab. 25, 1362–1373.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J., et al., Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab. 25, 1348–1361.e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorel F., et al., Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464, 1149–1154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuyama K., et al., Diabetes relief in mice by glucose-sensing insulin-secreting human α-cells. Nature 567, 43–48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Othman N., et al., Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 168, 73–85.e11 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Li J., et al., Artemisinins target GABAA receptor signaling and impair α cell identity. Cell 168, 86–100.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z., et al., A new way for beta cell neogenesis: Transdifferentiation from alpha cells induced by glucagon-like peptide 1. J. Diabetes Res. 2019, 2583047 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland W. L., et al., Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shultz L. D., Ishikawa F., Greiner D. L., Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Dai C., et al., Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J. Clin. Invest. 126, 1857–1870 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayton N. S., et al., Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am. J. Physiol. Endocrinol. Metab. 308, E592–E602 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brissova M., et al., Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 19, 498–511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.