Significance
Despite the importance of the activation of the cAMP/PKA signaling pathway for presynaptic modulation, its cellular and molecular mechanisms remain unclear. In this study, we tackled this issue at hippocampal mossy fiber-CA3 synapses by combining paired electrophysiological recordings, Ca2+ uncaging, total internal reflection fluorescence (TIRF) microscopy for direct measurements of presynaptic active zone Ca2+ influx, and superresolution time-gated STED microscopy for visualization of Ca2+ channel clusters within active zones. Our results suggest that Ca2+ channels near release sites might accumulate upon activation of the cAMP/PKA pathway in the time scale of only several minutes. Our findings may thus change the focus from release machinery to Ca2+ channel clusters and their dynamics as a cell biological mechanism of cAMP-dependent synaptic modulation.
Keywords: synapse, synaptic transmission, synaptic plasticity, calcium channels, hippocampal mossy fiber
Abstract
The cyclic adenosine monophosphate (cAMP)-dependent potentiation of neurotransmitter release is important for higher brain functions such as learning and memory. To reveal the underlying mechanisms, we applied paired pre- and postsynaptic recordings from hippocampal mossy fiber-CA3 synapses. Ca2+ uncaging experiments did not reveal changes in the intracellular Ca2+ sensitivity for transmitter release by cAMP, but suggested an increase in the local Ca2+ concentration at the release site, which was much lower than that of other synapses before potentiation. Total internal reflection fluorescence (TIRF) microscopy indicated a clear increase in the local Ca2+ concentration at the release site within 5 to 10 min, suggesting that the increase in local Ca2+ is explained by the simple mechanism of rapid Ca2+ channel accumulation. Consistently, two-dimensional time-gated stimulated emission depletion microscopy (gSTED) microscopy showed an increase in the P/Q-type Ca2+ channel cluster size near the release sites. Taken together, this study suggests a potential mechanism for the cAMP-dependent increase in transmission at hippocampal mossy fiber-CA3 synapses, namely an accumulation of active zone Ca2+ channels.
Communication between neurons is largely mediated by chemical synapses. Synaptic strengths are not fixed, but change dynamically in the short and longer term in an activity-dependent manner (short- and long-term plasticity, 1–3). Moreover, neuromodulators act on presynaptic terminals to modulate synaptic strength. Such activity-dependent or modulatory changes are often mediated by the activation of second messengers, such as protein kinase A and C (2). Second messenger systems, particularly the cyclic adenosine monophosphate (cAMP)/PKA-dependent system, are important for higher brain functions, including learning and memory in Aplysia (3), flies (4, 5), and the mammalian brain (6). Despite its functional importance, the cellular and molecular mechanisms of cAMP-dependent modulation are still poorly understood regardless of whether Aplysia synapses and Drosophila neuromuscular junctions have been investigated (2, 7). Mammalian central synapses are no exception here, also reflecting technical difficulties due to the generally small size of the presynaptic terminals in the mammalian brain.
Hippocampal mossy fiber-CA3 (MF-CA3) synapses are characterized by exceptionally large presynaptic terminals (hippocampal mossy fiber bouton, hMFB), which allow for the direct analysis of the cellular mechanisms of synaptic transmission and plasticity by using patch-clamp recordings (8–10). Thus, hMFBs provide a suitable model of cortical synapses in the mammalian brain. Moreover, these synapses are functionally important for brain function such as pattern separation (11). Mossy fiber synapses are known to exhibit unique presynaptic forms of short- and long-term synaptic potentiation and depression, which share the cAMP/PKA-dependent induction mechanism (12–15). In addition, the cAMP-dependent plasticity pathway is important for presynaptic modulation by dopamine and noradrenaline (16–18), which modulates hippocampal network activity and behavior. However, its underlying cellular mechanisms remain largely unclear. Enhancement of the molecular priming and docking of synaptic vesicles at mossy fiber synapses has been suggested by previous studies using genetics and electron microscopy (19–21). In particular, RIM1, an active zone scaffold protein, is crucial for cAMP-dependent long-term potentiation (LTP) (19) and is phosphorylated by PKA, although a corresponding phosphorylation mutant of RIM1 was found to have no effect on long-term potentiation (22, but see ref. 23). Other studies on hMFBs have implicated a role in positional priming, i.e., changes in the spatial coupling between Ca2+ channels and the release machinery (24). However, there is a lack of the direct visualization or manipulation of this regulation.
In order to measure the intracellular Ca2+ sensitivity of transmitter release directly and examine the mechanisms of cAMP-dependent modulation quantitatively, we here carried out Ca2+ uncaging experiments at hippocampal mossy fiber synapses. Unexpectedly, our results failed to show changes in Ca2+ sensitivity, but instead uncovered an increase in local Ca2+ concentrations at the release sites. Furthermore, by live imaging of local Ca2+ using total internal reflection fluorescence (TIRF) microscopy as well as superresolution time gated STED (gSTED) microscopy, we provided evidence that rather rapid Ca2+ channel accumulation may underlie cAMP-induced potentiation instead of release machinery modulations. This study thus provides a potential mechanism of presynaptic modulation at central synapses.
Results
Effects of cAMP on the Intracellular Ca2+ Sensitivity of Transmitter Release at Mossy Fiber Terminals.
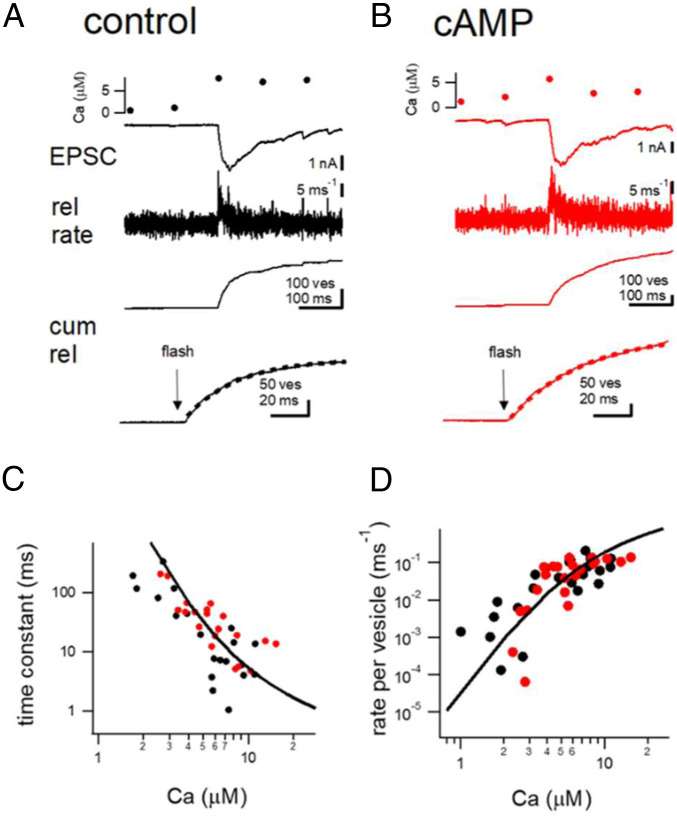
Previously, we showed that cAMP-dependent potentiation at hippocampal mossy fiber synapses involves an increase in the release probability (Pr) of synaptic vesicles (24). Pr depends on the intracellular Ca2+ sensitivity of transmitter release (25–28). However, the intracellular Ca2+ sensitivity of transmitter release had not been measured at mossy fiber terminals. Thus, we used Ca2+ uncaging to uniformly elevate the intracellular Ca2+ concentration in the terminal and measured the relationship between [Ca2+]i and transmitter release. Acute slice preparations were used to apply simultaneous voltage-clamp recordings of hMFBs and CA3 pyramidal cells. In order to perform Ca2+ uncaging experiments, DM-nitrophen (2.5 mM), and Ca2+ indicator Fura2-FF (0.2 mM) were introduced into the hMFB via a patch pipette. The AMPA receptor-mediated EPSCs were then used to track the time course of transmitter release. The extracellular solution contained 100 μM cyclothiazide (CTZ) and 50 μM D-2-Amino-5-phosphonovaleric acid (D-AP5), to block the desensitization of AMPA receptors and N-methyl-D-aspartate (NMDA) receptors, respectively. When flash photolysis was applied, [Ca2+]i was elevated to 7 μM (Fig. 1A), and large EPSCs were evoked upon the elevation of Ca2+. The release time course could be estimated by the deconvolution of the EPSCs (29, 30). Cumulative release could be estimated by the integration of the release rates (Fig. 1) and could be fitted by a single exponential of tens of milliseconds (25 ms and 40 ms in Fig. 1 A and B, respectively, dotted lines). By varying the flash intensity, we were able to systematically change the [Ca2+]i, and measure the intracellular Ca2+ dependence (see SI Appendix Fig. S1 A–C for the data from a single cell pair). As shown in Fig. 1 C and D, the release time constant estimated by an exponential fit of the cumulative release and the peak release rate per vesicle (the peak release rate divided by the readily releasable pool (RRP) size for each cell) was plotted against the [Ca2+]i (black circles). The data showed a nonlinear dependence on [Ca2+]i, with the overall dose–response curve being very similar to that of other synapse types (25–28). The solid line shows the fit expected from a five-step release model (25). Interestingly, the data could be fitted with a similar set of parameters as at the calyx of Held (25, 26), suggesting that the Ca2+ sensitivity at the hMFB synapses is per se similarly organized as at other central synapses.
Fig. 1.
The intracellular Ca2+ sensitivity of transmitter release at the hMFBs. (A and B) Example traces of flash-evoked EPSCs recorded under control conditions (A) and in the presence of cAMP (0.5 mM) in the patch pipette (B). From Top, the intracellular Ca2+ concentration measured from Fura2-FF (0.2 mM), EPSCs, release (rel) rates estimated from the deconvolution method and cumulative (cum) release are shown. At Bottom, cumulative release is shown over an expanded time scale. Dotted lines denote exponential fits. (C) Release time constants estimated from the exponential fitting to cumulative release are fitted against the Ca2+ concentration. Black and red points represent the data under control conditions and in the presence of cAMP in the patch pipette, respectively. The line shows the data expected from the five-step model proposed by ref. 25. (D) Similar to C, but the peak release rates per vesicle are shown. The peak release rates were divided by the RRP size.
In order to examine how cAMP changes the Ca2+ dependence of transmitter release, we additionally included cAMP (0.5 mM) in the presynaptic patch pipette, and performed flash photolysis experiments. Fig. 1B shows example traces, when flash photolysis was applied 5 to 10 min after the establishment of whole-cell recordings and cAMP was infused sufficiently into the terminal. The [Ca2+]i was elevated to 7 μM (Fig. 1B). The evoked EPSC showed a similar time course as that of Fig. 1A, and the cumulative release could be fitted by a single exponential with a time constant of tens of milliseconds. In Fig. 1 C and D, the data points recorded under cAMP were superimposed on the control data (red circles), indicating that cAMP did not change the intracellular Ca2+ sensitivity significantly.
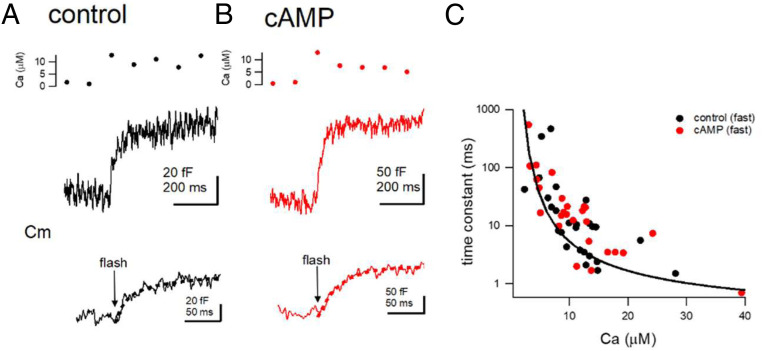
In order to estimate the Ca2+ dependence of synaptic vesicle (SV) exocytosis independently, we also performed presynaptic capacitance measurements (9), combined with flash photolysis of caged Ca2+. Capacitance measurements estimate the membrane surface area allowing to monitor synaptic vesicle exo- and endocytosis. Fig. 2 A and B shows the example capacitance traces in response to an elevation of [Ca2+]i to 10 μM. The capacitance increase could be fitted by a single exponential with time constants of 20 to 30 ms in these examples. Fig. 2C shows the intracellular Ca2+ dependence of the time constants (black: control, red: cAMP). In 4 out of 26 traces in control and 3 out of 26 traces in cAMP conditions we observed a biexponential time course of capacitance increase when [Ca2+]i was elevated much higher than 10 μM (SI Appendix, Fig. S1 D and E), similar to what was observed at the calyx of Held (31). The fast time constants were <10 ms, whereas the slow time constants were 30 to 100 ms. In contrast to the fast component which showed a steep dependence on [Ca2+]i, the slow component only shallowly depended on [Ca2+]i. The fast component displayed a capacitance jump of ∼50 fF (corresponding to 500 vesicles, 45.6 ± 6.1 fF, and 59.6 ± 7.1 fF in control and cAMP conditions, n = 10 and 7 cells, P > 0.05, SI Appendix, Fig. S1E). A solid line in Fig. 2C and SI Appendix, Fig. S1D indicates the expectation based on the calyx of Held release model (25). These data suggest that the Ca2+-sensing mechanism of the fast release is similar to that at the calyx synapses and that cAMP does not significantly change the intracellular Ca2+ sensitivity of fast release. We should note, however, that whole-cell dialysis might per se change the intrinsic Ca2+ sensitivity and its modulation by cAMP.
Fig. 2.
The intracellular Ca2+ dependence of transmitter release estimated from presynaptic capacitance measurements. (A and B) Example capacitance traces from control condition (A) and in the presence of cAMP (B). From Top, the intracellular Ca2+, capacitance traces and the expanded capacitance traces are shown. Dotted lines indicate the exponential fits. (C) The relationship between the release time constants (estimated from exponential fitting) and the Ca2+ concentration. Black and red dots indicate individual data under control condition and in the presence of cAMP, respectively. The line shows the data expected from the five-step model.
Local Ca2+ Concentrations at the Release Site Are Increased by cAMP.
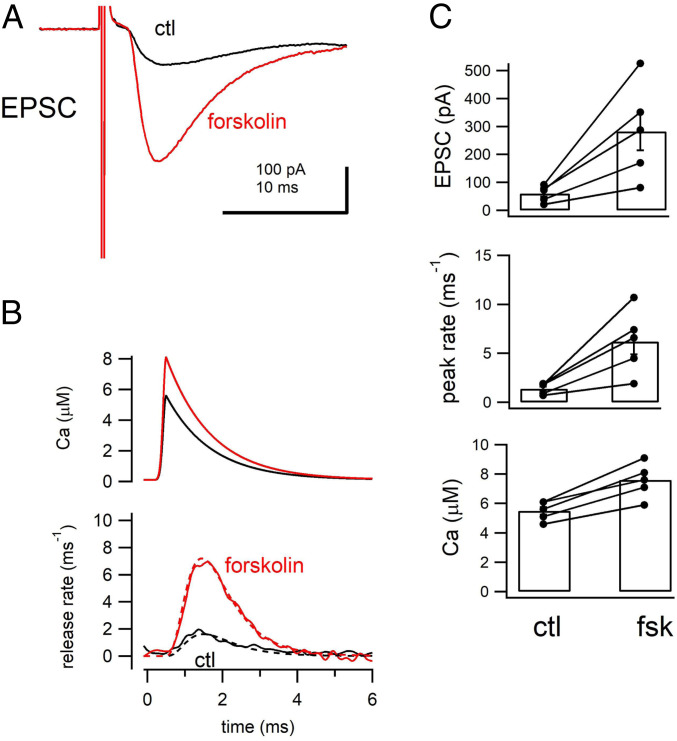
To estimate the local [Ca2+]i at the release site during an action potential, we back calculated the [Ca2+]i from the release rates using a five-step release model (25). Minimum stimulation was used to stimulate a single fiber and evoke EPSCs (Fig. 3A). Minimal stimulation was confirmed by detecting all-or-none responses after increasing the stimulation intensity. The EPSC size here was 60 ± 12 pA (n = 5 cells), corresponding to the release of a few quanta. The size was similar to that of the previous studies using the same method (32) and paired recordings (24). The release time course was estimated by deconvolution, and the peak release rate was calculated as ∼1 vesicle per millisecond in Fig. 3B (1.4 ± 0.2 ms−1, n = 5, Fig. 3C). We used the release model to estimate the local Ca2+ concentrations at the release site and the release parameters determined in Figs. 1 and 2. The input Ca2+ waveform was changed so that the release model could fit the release time course fully (dotted line in the Lower panel of Fig. 3B). A peak Ca2+ level of 5 μM (5.5 ± 0.3 μM, n = 5, Fig. 3C) was calculated, which represents only 25 to 50% of the values of other types of synapses (25, 27). The decay time constant of the local Ca2+ waveform was 1 ms, which is slower than that at most other synapses (25, 27, 33).
Fig. 3.
The estimated release site Ca2+ during an action potential. (A) The example EPSCs before (ctl, black) and after (forskolin, red) the application of forskolin. Data represent an average of 40 to 50 traces from the same cell. (B, Top) The estimated local Ca2+ concentration at the transmitter release sites. (B, Bottom) Release rates from the EPSC deconvolution method. The local Ca2+ concentration at the release site was estimated using the release model of ref. 25. The release parameters were fixed in Figs. 1 and 2, and only free parameter is the time course of the local Ca2+ at the release site. The Ca2+ waveform was set so that the entire time course of release was simulated fully (dotted lines). (C) From Top, the average EPSC amplitudes, peak release rates, and the estimated peak local Ca2+ concentration under control and in the presence of forskolin are plotted. Individual and average data are plotted. The differences are statistically significant (P < 0.05, 0.05, 0.01, respectively; paired t test).
We then added forskolin (50 μM) to increase the intracellular cAMP concentrations (Fig. 3A). Forskolin does not change mEPSC amplitudes (34) or the postsynaptic sensitivity to glutamate at mossy fiber synapses (12), but potentiates EPSCs through presynaptic effects. Consistently, EPSC amplitudes were highly potentiated (fourfold) 5 to 10 min after forskolin application (283 ± 68 pA, n = 5, P < 0.05 from t test) and the release rates were elevated (6.2 ± 1.3 ms−1, n = 5, P < 0.05 from t test, Fig. 3C). Because the intracellular Ca2+ sensitivity was not changed significantly, we could use the same release model as the one used in the control condition for estimating the time course of local Ca2+ concentrations. The estimated peak Ca2+ level at the release site after forskolin application was 7.6 ± 0.5 μM (n = 5, Fig. 3C). The peak Ca2+ level was thus 40% larger than under control conditions (∼5 μM in control; P < 0.01). Compared with the increase in the EPSCs, this increase was much smaller in extent, presumably explained by the dependence of release on Ca2+ in a highly nonlinear relationship. These results suggest that the local Ca2+ concentration at the release site increases by 40% under elevated cAMP levels.
The underlying mechanisms of the increased local Ca2+ concentration effective at the release machinery may include movements of release sites toward Ca2+ channel clusters within the active zone (“positional priming”), as has been implicated in our previous studies (24, 35). On the other hand, cAMP might facilitate the accumulation of Ca2+ channels around the release sites, resulting in an increase in the local Ca2+ concentration. If so, we would expect a 40% increase in the local Ca2+ influx at the active zone. The following set of experiments was designed to directly examine these two possibilities.
cAMP/PKA Activation Amplifies Local Ca2+ Influx upon Depolarization.
Thus far, our results suggest that the potentiation of transmitter release by the cAMP-dependent presynaptic mechanism can be attributed to higher local [Ca2+]i at the release sites rather than a higher Ca2+ sensitivity of SV release (Figs. 1–3). The functional properties of the Ca2+ channels in the whole hMFBs such as Ca2+ current amplitude, voltage dependence, and kinetics were not changed by forskolin (SI Appendix, Fig. S2 A–H). Therefore, we hypothesized that the Ca2+ influx was augmented by cAMP/PKA activation in a local, active zone-bound fashion, thereby potentiating glutamate release.
Midorikawa and Sakaba (24) recently developed dissociated hMFB preparations and applied TIRF microscopy to the dissociated terminal to visualize the SV dynamics near the plasma membrane. These preparations combined with TIRF microscopy allowed us to visualize local Ca2+ influx at the release sites using a Ca2+-sensitive dye. With an internal solution containing a low-affinity Ca2+-sensitive dye together with a relatively higher concentration of ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA) to block the bulk Ca2+ increase and restrict the spread of Ca2+, one can visualize local Ca2+ influx at the limited area around the Ca2+ channel cluster (24, 36).
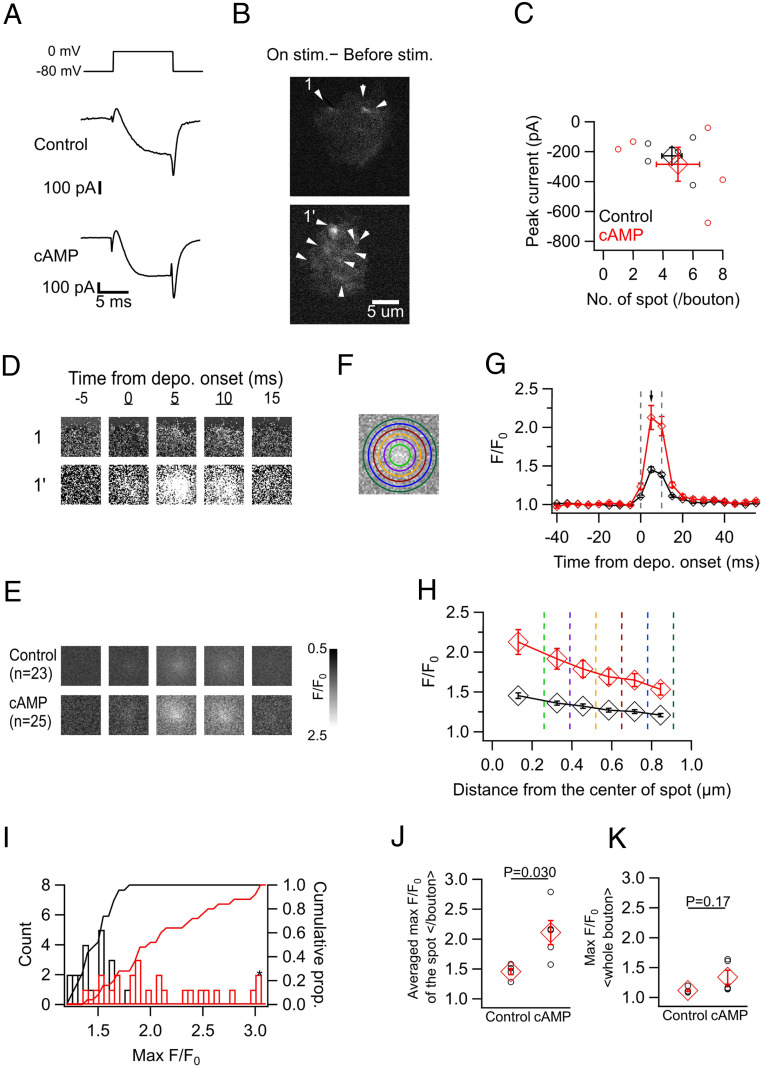
We performed whole-cell patch-clamp recording from dissociated hMFBs using an internal solution containing 0.2 mM Oregon Green BAPTA 6F (OGB6F) and 5 mM EGTA, while measuring the fluorescence of OGB6F at 200 Hz using TIRF microscopy. Depolarization to 0 mV for 10 ms induced inward Ca2+ currents and spot-like elevations of the fluorescence (Fig. 4 A and B). The local elevation was locked to the stimulus and showed an intensity peak at the midmost of the region while the intensities were attenuated toward the peripheral regions (Fig. 4 B and D–H). The hotspot was presumably the center of the active zone. Then, we directly examined the effects of the cAMP pathway by loading cAMP (0.5 mM) into the bouton via a patch pipette. After whole-cell dialysis for 5 to 10 min, we found that the spot-like elevation of the fluorescence was remarkably larger under cAMP than under control conditions (Fig. 4 B, D, and E). cAMP increased the peak F/F0 (1.47 ± 0.03, n = 23 spots, in control; 2.19 ± 0.15, n = 25 spots, with cAMP; P = 0.00011, unpaired t test), and the cumulative histogram of the peak F/F0 was strongly shifted rightwards under cAMP application (Fig. 4 G–I). The amplitudes of the whole-terminal Ca2+ currents and the number of the spots, presumably representing the active zone Ca2+ channel clusters within a terminal were not significantly different between the two conditions (Fig. 4C). We averaged the peak F/F0 of the spots for each terminal, and compared them between the conditions (comparison based on the number of terminals), confirming that cAMP significantly increased the amplitude of the Ca2+ spot (Fig. 4J). The F/F0 values averaged over the whole bouton are indicative of the bulk Ca2+ (Fig. 4K), considering that the majority of the signal derives from outside the hotspots. The difference in the depolarization-induced maximal bulk F/F0 (averaged over the whole bouton) was insignificant between the two conditions, suggesting that the bulk Ca2+ was not sensitive to cAMP. This result is consistent with the constant whole-cell Ca2+ current amplitudes (SI Appendix, Fig. S2), suggesting that the incorporation of additional Ca2+ channels from internal stores to the plasma membrane is unlikely. Furthermore, the effect of cAMP on the Ca2+ spot was significantly suppressed in the presence of KT5720, an inhibitor of PKA (SI Appendix, Fig. S3). These results support the idea that the local Ca2+ influx at the release site, rather than a global Ca2+ elevation, is augmented by cAMP/PKA activation.
Fig. 4.
cAMP amplifies the spot-like Ca2+ transients in the acute dissociated hMFBs. (A) Ca2+ current was elicited by the depolarization (Depo.) (0 mV, 10 ms) in control (Top) and with cAMP (0.5 mM) introduced via a patch pipette (Bottom). (B) Fluorescence of Ca2+-sensitive dye (OGB6F, 0.2 mM) was loaded via a patch pipette. Images represent the differences between the average intensity during the stimulation (three frames) and the one before stimulation (eight frames), for control (Top) and cAMP conditions (Bottom). Arrowheads indicate the spots where the fluorescence intensity locally increased in response to depolarization. (C) The number of Ca2+ spots per bouton against the amplitude of the inward current. These values were not significantly different between control and cAMP conditions (P = 0.81 for the number of spots; 0.67 for the current amplitude; unpaired t test). In C and G–I, control (black) and cAMP (red) data are shown. (D and E) Sequential F/F0 images at the spot 1 and 1′ in B (D) and of the averaged spots for control and cAMP conditions (E). Five consecutive frames across the depolarization (from 0 to 10 ms, underlined numbers) were depicted. In D–F, the Ca2+ spots were centered in a 2 μm × 2 μm cropped square. (F) Schematic view of the measurement of the Ca2+ spot. The mean F/F0s inside the most inner circle (light green; “midmost”) and at the filled areas between the adjacent circles (“annuli”) were measured. The circles have a radius of 0.26, 0.39, 0.52, 0.65, 0.78, and 0.91 μm, from inner to outer, respectively. (G) Sequential F/F0 changes at the midmost in control and cAMP. Gray dashed lines indicate the onset and the end of depolarization. In G and H, the values are the averages of 23 and 25 spots from five animals for control and cAMP, respectively. (H) F/F0s are plotted against the distance from the spot center at 5 ms after the onset of depolarization (arrow in G) in control and cAMP. The vertical dotted lines correspond to the radius of the circles in F, and each diamond indicates the mean F/F0 at the area. (I) Histograms and cumulative graphs of maximum F/F0 at the midmost for control and cAMP. Values over 3.0 were pooled on the rightest (asterisk). Statistical significance was evaluated by two-sample Kolmogorov–Smirnov test (P = 2.7 × 10−6). (J) Maximum F/F0 at the midmost were averaged per bouton for control and cAMP. (K) Maximum F/F0 in the whole bouton for control and cAMP.
In order to confirm the cAMP/PKA-dependent increases in the local Ca2+ influx we compared the Ca2+ signals before and after the extracellular application of forskolin in a given cell (SI Appendix, Fig. S2 I–L). Forskolin did not amplify the inward Ca2+ current (SI Appendix, Figs. S2 E, F, and I). In five out of eight boutons, we observed a higher peak F/F0 at the Ca2+ hotspot after forskolin application for 5 to 10 min (SI Appendix, Fig. S2 J and K). SI Appendix, Fig. S2L plots the line profile of the signal intensity, showing the emergence of the hotspots. On the other hand, we did not observe a bulk Ca2+ increase after forskolin application, but rather a slight decrease (peak F/F0: 1.13 ± 0.03 vs. 1.09 ± 0.02; P = 0.006, paired t test). These data are consistent with the accumulation of Ca2+ channels at release sites in a cAMP/PKA-dependent manner, resulting in the observed potentiation of glutamate release.
cAMP Increases Pr but Does Not Significantly Alter the RRP Size.
If cAMP/PKA activation increases the local Ca2+ influx, we should observe a faster time course of SV exocytosis. A previous study indeed demonstrated that cAMP induced acceleration of the exocytosis time course, which was blocked by the application of KT5720, an inhibitor of PKA (24). To quantitatively examine the time course of exocytosis, we measured cell capacitances from dissociated hMFBs and assessed the effects of cAMP by adding cAMP via a patch pipette (Fig. 5 and SI Appendix, Fig. S4). We measured cell capacitances before and after depolarization (Fig. 5A). The depolarization induced Ca2+ currents and provoked an increase of cell capacitance (ΔCm; Fig. 5B). While the amplitudes of the evoked Ca2+ currents remained relatively constant throughout the experiments (SI Appendix, Fig. S4C), the ΔCms got larger with increasing pulse duration. The time course could be fitted by a single time constant of 11.0 ms (in control, Fig. 5 D, Left and black traces in SI Appendix, Fig. S4 E and F) and two time constants of 0.083 and 37.8 ms (with cAMP), revealing the emergence of a fast release component specifically under cAMP conditions (Fig. 5 D, Right and red traces in SI Appendix, Fig. S4 E and F). In particular, we observed a larger ΔCm at 2-ms depolarization (4.2 ± 0.9 fF in control, n = 13; 9.7 ± 1.4 fF with cAMP, n = 13; P = 0.0034, unpaired t test), confirming the emergence of a fast, cAMP-specific component. The ΔCms became saturated with increasing pulse duration and reached 16.2 ± 2.9 fF in control (n = 13) and 21.7 ± 3.1 fF with cAMP (n = 14) at 100-ms pulses (P = 0.20, unpaired t test), suggesting that the size of the RRP is similar between the two conditions as the 100-ms pulse depletes the RRP (24, 37). Furthermore, the ratio of ΔCm at the 2-ms pulse to that at the 100 ms was 1.8 times higher with cAMP than that in control (P = 0.00017, unpaired t test; SI Appendix, Fig. S4G), suggesting a higher Pr with elevated cAMP levels. The higher Pr under conditions of elevated cAMP, which was measured by capacitance measurements, is consistent with previous studies (12, 24) and with the larger Ca2+ influx at the release sites measured optically (Fig. 4).
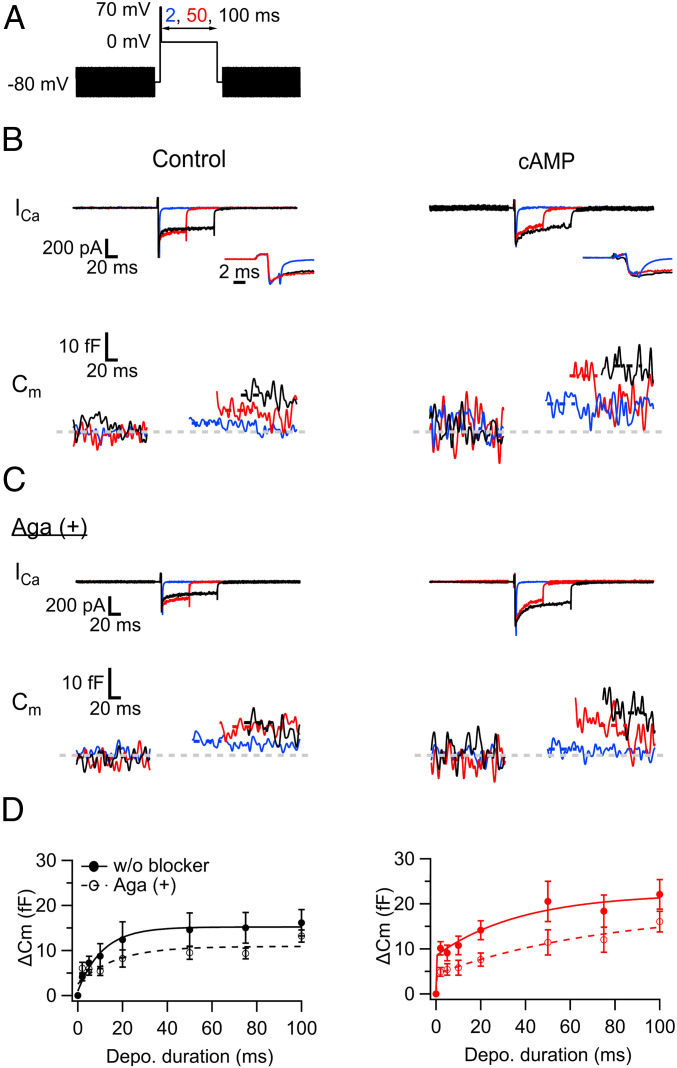
Fig. 5.
cAMP accelerates exocytosis in hMFBs via increasing P/Q-type Ca2+ channel-sensitive release component. (A) Scheme of the pulse. The duration of 0-mV depolarization was varied between 2 and 100 ms, which followed short and strong depolarization (2 ms, 70 mV). The sinusoidal voltage (30 mV, 1,000 Hz) was applied for membrane capacitance (Cm) measurements before and after the depolarization. (B) Ca2+ current and Cm were measured without (control, Left) and with cAMP (0.5 mM) in the patch pipette (Right). The depolarization induced the Ca2+ currents (Top) and elevated the Cm (Bottom). The examples at the 0-mV depolarization for 2 ms (blue), 50 ms (red), and 100 ms (black) are shown. Note that the increase of the Cm caused by the 2-ms depolarization was prominent with cAMP. Insets are the magnified views of the onsets of Ca2+ currents. (C) The same as B, but with data obtained in the presence of ω-agatoxin IVA (Aga, 0.5 μM), P/Q-type Ca2+ channel blocker in the bath solution. Note that the capacitance jump (ΔCm) in response to a 2-ms pulse was inhibited in the presence of cAMP. In B and C, the traces at the 2-ms pulses are the averages of three trials, and the capacitance after the depolarizing pulse was measured at the periods indicated by the dotted lines. Baseline is indicated as a gray dotted line. (D) The ΔCms are plotted against the pulse duration. The panels show the blockage of ΔCms by Aga under the conditions without (Left) and with cAMP in the patch pipette (Right). The filled and open circles represent the ΔCms without and with Aga, and they were fitted by a single (for control) and a double exponential function (for cAMP), whose results are indicated as solid (without Aga) and dashed lines in the graphs (with Aga): 9 to 13 (control) and 10 to 14 (cAMP) without Aga; 8 to 15 (control) and 8 to 13 terminals (cAMP) with Aga.
P/Q-Type Ca2+ Channels Play a Role in cAMP-Induced Potentiation.
The release of glutamate at the hMFB-CA3 synapses is mainly mediated by P/Q- and N-type Ca2+ channels (38–40). We examined which subtype would play a major role in synaptic transmission and cAMP/PKA-dependent potentiation. For this purpose, we used capacitance measurements to assess the effect of cAMP on ΔCm in the presence of either ω-conotoxin GVIA (Cono; 1 μM), a selective N-type Ca2+ channel blocker, or ω-agatoxin IVA (Aga; 1 μM), a selective P/Q-type Ca2+ channel blocker. Compared with ΔCm without blockers, Cono seemingly suppressed ΔCm almost throughout all pulse durations tested, irrespective of the presence of cAMP (SI Appendix, Fig. S5 G and H). Interestingly, the suppression of ΔCm by Aga was most prominent at short depolarizing pulses in the presence of cAMP (Fig. 5C and SI Appendix, Fig. S5 A–F and H); the ΔCm at the 2-ms pulse was reduced to 51% of that without Aga (P = 0.024, Dunn’s post hoc multiple comparisons test following Kruskal–Wallis test; SI Appendix, Fig. S5H). In strong contrast, ΔCm seemed relatively insensitive to Aga in the control conditions (Fig. 5D and SI Appendix, Fig. S5H). These data imply that P/Q-type Ca2+ channels boost fast glutamate release upon cAMP/PKA activation.
Capacitance measurements can reliably detect the exocytosis of more than tens of synaptic vesicles per a single hMFB, but are unable to detect lower amount. We therefore recorded evoked EPSCs at MF-CA3 synapses and assessed effects of Aga and Cono on the EPSCs in the control and forskolin-treated condition (SI Appendix, Fig. S6). In the controls, Aga or Cono application reduced the EPSC to ∼40% (SI Appendix, Fig. S6 A–C and G and H), suggesting that both P/Q- and N-type channels are involved in hMFB synaptic transmission under control conditions. After forskolin application, the EPSCs were, as expected, augmented, and the subsequent application of Aga considerably suppressed the EPSC (to ∼10%), whereas Cono was less effective (to >95%; SI Appendix, Fig. S6 D–H). This indicates that the contribution of the P/Q-type Ca2+ channels to the EPSC is apparently larger than that of the N-type Ca2+ channels after cAMP-induced potentiation, supporting that P/Q-type Ca2+ channels play a more important role in cAMP-dependent potentiation, although N-type channels also play a role. In addition, we cannot entirely exclude the possibility that the inputs other than mossy fibers may participate in the EPSCs.
gSTED Microscopy Detects an Accumulation of P/Q-Type Ca2+ Channels near Release Sites upon cAMP/PKA Activation.
The data above suggest that P/Q-type Ca2+ channels play a key role in the cAMP-induced potentiation of glutamate release. Therefore, we examined the distribution of P/Q-type Ca2+ channels near the release site in dependence on cAMP elevation. A previous study combining electrophysiology and computer simulation reported that the distances between readily releasable vesicles and Ca2+ channels within an hMFB active zone can vary between 50 and 200 nm (on average ∼80 nm) (10). Such a nanoscopic arrangement is below the diffraction limit of conventional light microscopy (200 to 300 nm according to illumination wavelength), such as confocal microscopy (Fig. 6 A, Top). We therefore turned to two-dimensional (2D) gSTED (Figs. 6 and 7 and SI Appendix, Figs. S7–S10) displaying a lateral resolution of about 40 nm (SI Appendix, Supplementary Materials and Methods), to image acute hippocampal slices labeled for Cav2.1 (P/Q-type Ca2+ channels, green), the release site marker Munc13-1 (red) (41, 42), and the postsynaptic density (PSD) marker Homer1 (blue) (Fig. 6 A, Bottom; see ref. 43). Notably, the Cav2.1 signals were located within areas positive for ZnT3, an hMFB marker, allowing us to specifically target our analysis on the active zones of hMFBs (SI Appendix, Fig. S7).
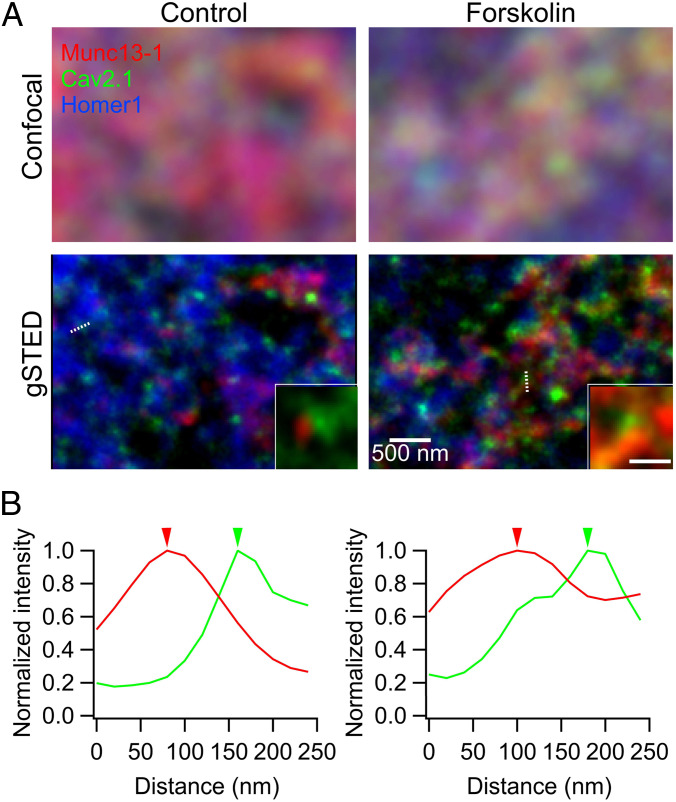
Fig. 6.
gSTED microscopy reveals Cav2.1 and Munc13-1 clusters at hMFBs. (A) Confocal (Top) and corresponding deconvolved gSTED images (Bottom) of Munc13-1 (red), Cav2.1 (green), and Homer1 (blue) labeled by the specific antibodies in the CA3 stratum lucidum, where MF-CA3 synapses are located, in control (Left) and forskolin-treated (Right) hippocampal slices (150 μm). Munc13-1 and Cav2.1 clusters at release sites were identified as ones opposed to the Homer1 signals (Bottom Insets show clusters on the white dotted lines). (Scale bar for Inset: 200 nm.) (B) Examples of intensity profiles, normalized to the intensity peak, across the line profiles drawn in A for Munc13-1 (red), Cav2.1 (green) in control (Left), and forskolin (Right). Arrowheads indicate the peak locations of Cav2.1 and Munc13-1 clusters (SI Appendix, Fig. S8).
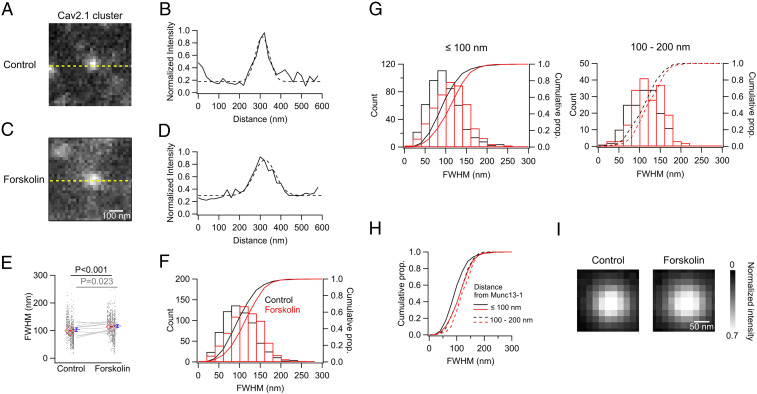
Fig. 7.
Forskolin application increases the size of Cav2.1 clusters near release sites. (A–D) Cropped raw gSTED images representing Cav2.1 clusters near a given Munc 13-1 cluster within the active zone in control (A) and forskolin-treated (C) hippocampal slices (150 µm). Intensities were measured at the yellow dotted lines in A and C, normalized to the peak intensity of the target cluster, and plotted as the solid lines in B and D. The intensity profiles across the sampling line were fitted to Gaussian function (dashed lines), and the full widths of half-maximum amplitudes (FWHMs) were estimated to be 78.8 nm (B) and 118.6 nm (D) for these examples. (E) FWHMs in control (n = 660 synapses) and forskolin (n = 655 synapses). The values for the individual profiles (black dots) were vertically aligned animal by animal (10 animals), and the mean values per animal were compared between the conditions (gray lines). The averages for these are shown as the red and blue symbols, respectively. (F) Histograms and cumulative proportions (prop.) of FWHM in a 20-nm bin in control (black) and forskolin (red). (G and H) Histograms and cumulative proportions of FWHM in a 20-nm bin in control (black) and forskolin (red) in relation to the distances to the Munc13-1 cluster. Within 100 nm (G, Left; n = 495 in control and 472 in forskolin) and 100 to 200 nm of the Munc13-1 cluster (G, Right; n = 165 in control and 183 in forskolin). Cumulative proportions are overlaid in H. Solid (within 100 nm) and dashed lines (100 to 200 nm). Statistical significance was tested with two-sample Kolmogorov–Smirnov test for F (P = 1.8 × 10−12) and G (Left, P = 1.0 × 10−11; Right, P = 0.04). (I) Averaged images of the Cav2.1 clusters located within 100 nm in control (Left) and forskolin (Right). Two-dimensional Gaussian fitting yielded the FWHMs for control (98.3 nm for x; 99.0 nm for y) and forskolin (110.0 nm for x; 107.3 nm for y).
Acute hippocampal slices (150-μm thickness) were prepared and preincubated for 10 min before fixation in control artificial cerebrospinal fluid (ACSF) or ACSF containing forskolin. With improved lateral resolution by gSTED imaging and subsequent deconvolution with the instrument-specific point spread function (Lorentzian function, full widths of half-maximum amplitude [FWHM]:40 nm), we could identify subactive zone spots enriched in Cav2.1 and Munc13-1 proteins, herewith termed clusters, similar to what was previously observed at murine hMFBs (43). We then detected the peak location of each cluster and quantified peak-to-peak distances by line-profile measurements between Cav2.1 and Munc13-1 clusters (Fig. 6 A, Bottom Insets and Fig. 6B). We measured the distances for cluster pairs opposed to a given Homer1 cluster, in order to limit our measurements to protein clusters belonging to the same active zone. Only well-defined planar or vertical synapses with Cav2.1 and Munc13-1 spots on the same focal plane, were considered for analysis. Histograms revealed that the mapped Cav2.1-Munc13-1 distances within one active zone varied substantially between 20 and 150 nm (Fig. 6B and SI Appendix, Fig. S8A) and that their distributions were similar between control and forskolin application (SI Appendix, Fig. S8A). Averaging the obtained distances per individual animal (81.4 ± 3.4 nm in control; 82.7 ± 3.4 nm in forskolin; n = 10 animals/group; P = 0.85, paired t test; SI Appendix, Fig. S8B) retrieved similar values between conditions (SI Appendix, Fig. S9 for further analysis). Notably, the distance values observed here are similar to those previously measured in mice by immunohistochemistry and gSTED (∼70 nm) (43), as well as electrophysiological estimates of the coupling distances (10). Nevertheless, as peak-to-peak distances may only be slightly affected by changes in the distribution of Cav2.1 molecules in the peripheral regions of the cluster, we cannot fully exclude the possibility that increases in cAMP could still alter the physical coupling distances between Ca2+ channels and the release sites in a subtle manner (Discussion).
The average density of Cav2.1 clusters within the entire visible ZnT3(+) area was similar between the control and forskolin conditions (SI Appendix, Fig. S7), which is compatible with the unchanged amplitude of bulk Ca2+ current after forskolin application (SI Appendix, Fig. S2 A, E, and F). The number of Cav2.1 clusters near the release sites (<200 nm to restrict sampling to a single active zone) (44) mapped by Munc13-1 was unchanged by forskolin, suggesting that forskolin application does not significantly alter the number of Ca2+ channel clusters within an active zone (SI Appendix, Fig. S9). Next, we focused on the size of the Cav2.1 clusters nearest to the release sites, which should be a measure of the local accumulation of P/Q-type Ca2+ channels, and examined the effects of forskolin (Fig. 7). Using raw gSTED images to detect subtle changes in cluster size, we measured the intensity distribution along lines centering the peak of a given Cav2.1 cluster, previously identified by line profile measurements on the deconvolved images (Figs. 6A and 7 A–D), which indicated the nearest neighbor relative to a Munc13-1 cluster. Notably, peaks across the line tended to be broader under forskolin application than in controls (Fig. 7 B and D–F). Thus, we fitted the Cav2.1 intensity profiles across the line to a Gaussian function and estimated the FWHM as a measure of cluster size. The FWHMs were mostly between 20 and 200 nm in both conditions, but the distribution for forskolin-treated samples was relatively skewed to the wider range, which was also obvious in about a 20% increase of the median (94.7 nm in controls versus 114.7 nm under forskolin; Fig. 7F). Consistently, we could see significant increases in the average FWHM values, either when pooled over the total number of clusters analyzed (99.2 ± 1.5 nm, n = 660 synapses, in control; 113.9 ± 1.49 nm, n = 655 synapses, in forskolin: P = 5.2 × 10−12, unpaired t test), as well as when averaging the values per animal (103.3 ± 6.5 nm, in control; 116.7 ± 4.8 nm, in forskolin; n = 10 animals per group; P = 0.023, paired t test; Fig. 7E). Note that an increase of 15 to 20% in the diameter may indicate an even larger area. Furthermore, we focused on the Cav clusters located within 100 nm of the Munc13-1 cluster (Fig. 7 G–I), since a previous study demonstrated that Ca2+ channels are mostly located within 100 nm of the release sites (10). In both cases, that is, clusters within 100 nm and 100 to 200 nm from the Munc13-1 clusters, the sizes of the Cav clusters were increased by forskolin (Fig. 7G). Notably, the closer Cav clusters showed a larger shift in FWHMs, with median increases of 23.4% (within 100 nm) and 7.3% (100 to 200 nm) (Fig. 7H). This enlargement of the Cav clusters was also visible in the averaged images of the closer Cav clusters (Fig. 7I). Fitting these images to 2D Gaussian function estimated the FWHMs (98.3 nm for x and 99.0 nm for y in control; 110.0 nm for x and 107.3 nm for y in forskolin), consistent with the analysis of the individual line profiles. It might be possible that the observed expansion could be due to spreading of the Ca2+ channels within a cluster. Nonetheless, given the augmentation of the local Ca2+ influx observed by TIRF microscopy (Fig. 4), our results more likely mean that cAMP/PKA activation results in an accumulation of P/Q-type Ca2+ channel boosting channel cluster size and channel number close to the release sites mapped by Munc13-1.
The same type of analyses was performed for the size of synaptic Munc13-1 clusters to see if there was any change in the release sites upon cAMP potentiation (SI Appendix, Fig. S10). Here, the FWHMs did not show a significant difference between control and forskolin conditions with an average for the total clusters of 140.4 ± 2.5 nm in control (n = 440 synapses, from nine animals) and 139.7 ± 2.2 nm in forskolin (n = 442 synapses, from nine animals; P = 0.83, unpaired t test; SI Appendix, Fig. S10E). We could not detect any significant difference in the average FWHMs per animal or in the distribution of the FWHMs either, suggesting that cAMP modulation does not affect the size and the number of the release sites, at least over the 10 min of forskolin application (SI Appendix, Fig. S10 E and F).
Taken together, these results suggest that increase of cAMP may increase Pr via the accumulation of P/Q-type Ca2+ channels.
Discussion
We studied here the mechanistic underpinnings of how cAMP application in the minutes range enhances the release of hMFB. A prime candidate was the enhanced Ca2+ sensitivity of transmitter release. Surprisingly, however, no indications for changes in the Ca2+ sensitivity of synaptic vesicle release through cAMP application could be observed. Our results instead suggest that peak local Ca2+ concentrations at the hMFB release sites were increased by cAMP. Indeed, live imaging of the Ca2+ microdomains by TIRF microscopy demonstrated that local Ca2+ influx at the release site was increased by cAMP. Because the whole cell dialysis took 5 to 10 min, the cAMP effects were quite rapid. Finally, gSTED microscopy provided evidence that the size of the P/Q type Ca2+ channel clusters near the release sites, mapped by Munc13-1, increased after forskolin application. Taken together, we favor a scenario where rapid accumulation of Ca2+ channels at the release sites might be responsible for cAMP-dependent potentiation of the release at MF-CA3 synapses. Although synaptically and chemically induced potentiations occlude each other, whether mossy fiber LTP and the cAMP-induced potentiation are mechanistically identical remains to be examined in the future (15).
Intracellular Ca2+ Sensitivity of Transmitter Release at the hMFBs.
By measuring the intracellular Ca2+ sensitivity of transmitter release at the hMFBs, we found that hMFB-CA3 synapses were strongly facilitated during repetitive stimulations. At neuromuscular junctions, facilitating synapses are less Ca2+ sensitive compared to depressing synapses (45, 46). We here found that the intracellular Ca2+ sensitivity for transmitter release is strikingly similar to that of other mammalian synapses (25–28, 33), suggesting that Ca2+-sensing mechanisms and the resulting Ca2+ sensitivity of release is rather fixed generically across mammalian central synapses. This result also implies that the low release probability, which is one of the key features of facilitating synapses, might rather be due to lower Ca2+ concentrations at the release site. Indeed, the local Ca2+ concentration (5 μM) we measured here corresponds to only 25 to 50% of the value typically measured at other synapses (25, 27). At 5 μM, the release should be highly sensitive to a small change in the local Ca2+ concentrations, providing a lot of room for plastic changes. Such a change could be caused by increases in Ca2+ influx, the accumulation of residual Ca2+, or the saturation of Ca2+ buffers (10) such as calretinin (47). In addition, low Ca2+ at the release site may shift the relative importance of residual Ca2+ (48) over local Ca2+, and therefore, the relative importance of fast Ca2+ sensor synaptotagmin 1/2 over other sensors such as synaptotagmin 7 (49, 50) may be different from other synapses.
Importantly, we found that cAMP did not change the intracellular Ca2+ sensitivity of release. This was somewhat surprising, given that second messenger activation drastically changes the intrinsic Ca2+ sensitivity of release at the calyx of Held synapses (51, 52). In hMFBs, it has been shown that cAMP changes the state of docking/priming (20, 21). Thus, enhanced docking/priming may not change Ca2+-sensitive steps but rather act on the Ca2+-insensitive steps of synaptic vesicle fusion and synaptic vesicle recruitment (21). It is equally possible that docked vesicles are not necessarily equal to the RRP determined by depolarizing pulses as used here, or else some undocked vesicles might participate in the RRP (53). Enhanced docking/priming may increase the number of superprimed synaptic vesicles (54), which have higher Pr in response to action potentials. In this scenario, the superprimed vesicles at hippocampal mossy fiber synapses would have similar intracellular Ca2+ sensitivity as other vesicles, when probed with Ca2+ uncaging, but the release probability for those vesicles close to Ca2+ channels may be increased due to the physical growth of Ca2+ channel clusters.
Increased Ca2+ Concentration at Mossy Fiber Release Sites under cAMP.
This study demonstrated that forskolin application provoked an increase in P/Q-type Ca2+ channel cluster size near mossy fiber release sites, as measured by gSTED, together with an increase in the peak local Ca2+ concentration at release sites in the Ca2+ uncaging and TIRF experiments. gSTED microscopy did not reveal any signs that the spatial distance between Ca2+ channel clusters and the putative release site marker Munc13-1 (41, 42), measured by peak-to-peak analysis, change upon forskolin application (Fig. 6 and SI Appendix, Fig. S9). In addition, we failed to observe any changes in either the size or the number of Munc13-1 clusters around the Ca2+ channels within our settings.
Thus, our data favor a simple mechanism contributing to the minutes range “early” cAMP-dependent potentiation: an increased physical accumulation of Ca2+ channels at release sites. This is supported by two major pieces of evidence. First, TIRF microscopy suggests that local Ca2+ influx was enhanced by 30 to 50% at the release sites. Second, we observed a spatial spread in the geometry of individual Cav2.1 Ca2+ channel clusters nearest to the release sites after forskolin application. Although the expansion may indicate a spread of the Ca2+ channel molecules within a given cluster analyzed, it is equally consistent with an increase in channel numbers. The 30 to 40% increase in Ca2+ influx we observed per se suffices to explain the potentiation of transmitter release, which has been estimated by the model based on Ca2+-secretion coupling (Fig. 3), further confirming that the increased Ca2+ influx is a major mechanism supporting potentiation. Additionally, the pharmacological block of P/Q-type channels had more pronounced effects on SV exocytosis and EPSCs under the elevated cAMP conditions compared with control conditions (Fig. 5 and SI Appendix, Fig. S6), consistent with the idea that, during potentiation, Ca2+ influx through P/Q-type channels, compared with N-type channels, is more relevant for exocytosis at hMFBs.
The increased local Ca2+ influx we report here superficially seems inconsistent with our previous study, which suggested that cAMP changes the coupling between the Ca2+ channels and release sites (24). This statement was based on the observation that cAMP reduces the sensitivity of transmitter release to EGTA. The recent modeling study indicates that an increased local Ca2+ influx can also reduce EGTA sensitivity (55, 56). This explanation likely reconciles the present study with Midorikawa and Sakaba (24). However, we cannot categorically exclude the possibility of altered physical coupling distances in response to cAMP elevation, because a physical expansion of Cav2.1 clusters near release sites would consequently cause the nearest Cav2.1 Ca2+ channels at the periphery of the cluster to be located closer to the release sites, without such a scenario producing any detectable change in the peak-to-peak distances (SI Appendix, Figs. S8 and S9). The expansion may be related to an increase of the mEPSC frequency by the application of forskolin (34), which could well be mediated by stochastic openings of the Ca2+ channels located near the release sites. We should also note that retrieving true coupling distance using 2D-STED may be difficult as the shape of active zones of hMFBs is highly complex.
Cav2.1 Ca2+ channel accumulation could be explained by several possible mechanisms. One possibility is that new Ca2+ channels may be inserted into the active zones. The biosynthetic precursor of the active zone complexes and release sites seems to be transported by specialized vesicles also containing active zone scaffold proteins (57), and it appears possible that Ca2+ channels might be added via insertion of such active zone “building blocks” (58). Notably, however, the amplitudes of whole-cell Ca2+ currents were not influenced by cAMP. This rather suggests that Ca2+ channels might be recruited from plasma membrane pools in the time scale of minutes under conditions of elevated cAMP. Consistently, mobility of Ca2+ channels can be relatively high within presynaptic terminals (59). It has been shown that active zones recruit Ca2+ channels via active zone scaffold proteins such as RIM (60, 61) and RIM-BP (62, 63). Indeed, RIM1 is crucial for induction of long-term potentiation at hippocampal mossy fiber synapses (19). Therefore, a likely possibility to us is that cAMP/PKA enhances the recruitment of Ca2+ channels to the active zone release sites from the plasma membrane via regulations entailing specific active zone scaffold proteins. Nevertheless, this study provides no direct molecular demonstrations of rapid Ca2+ channel accumulation, and the molecular mechanism underlying the cAMP-induced rapid Ca2+ channel accumulation will need to be investigated further in future studies.
Possible Increase in the Number of Release Sites by cAMP.
This study found the RRP size was found to be unchanged by cAMP. When a 100-ms depolarization pulse was applied to the hMFB, no change in the capacitance increase between control conditions and cAMP conditions was observed, consistent with a previous study (24). At the same time, however, the time course of release got much faster, indicating that the fraction of synaptic vesicles displaying high release probability, being particularly sensitive to action potentials (64), was increasing. In other words, the RRP measured by depolarizing pulses is markedly larger than that recruited by action potentials. The subpool with high Pr (fast-releasing pool, superprimed pool) may correspond to tightly docked vesicles (54), whereas depolarization may also recruit loosely docked vesicles, which are remote from Ca2+ channels. Although we cannot entirely exclude the possibility that the addition of new release sites close to Ca2+ channels could explain the high number of high Pr SVs, it appears more likely that the subpool of high Pr vesicles may be increased by cAMP due to accumulation of Ca2+ channels at the active zone, as the increased Ca2+ concentration at the release site is sufficient to explain the EPSC potentiation (Fig. 3).
We should emphasize that our recording time frame was 30 min at most, corresponding to the early phase of long-term potentiation. Over longer time periods, other mechanisms potentially enhancing numbers of docked and primed vesicles (20, 21, 65) and increasing the number of release sites (66) likely take place. This putative temporal sequence of presynaptic plastic events and their mechanisms should be a subject warranting future investigation.
Taken together, this study provides direct evidence that changes in the membrane distribution of Ca2+ channels can occur in the range of minutes, a possibility that has received less attention previously (67). The Ca2+ channel accumulation possibly induced at hMFBs upon forskolin application is reminiscent of the redistribution of AMPA receptors at postsynaptic densities during postsynaptic plasticity (68, 69). Our results thus suggest that pre- and postsynaptic compartments may ultimately use similar mechanisms to regulate synaptic strength in the minutes time frame.
Materials and Methods
A complete description of materials and methods is available in SI Appendix.
Animals.
All animal experiments were performed in accordance with the guidelines of the Japanese Physiological Society and approved by the animal committee of Doshisha University. Wistar rats (18 to 30 d old) were used for the experiments.
Slice Physiology.
Hippocampal slices were prepared as described previously (24). The normal ACSF contained (in millimoles): NaCl 125, KCl 2.5, glucose 25, NaHCO3 25, NaH2PO4 1.25, ascorbic acid 0.4, myo-inostiol 3, and Na-pyruvate 2, CaCl2 2, MgCl2 1, oxygenated with 95% O2, and 5% CO2. Patch-clamp recordings were applied to hMFBs and CA3 pyramidal cells using an EPC10/2 amplifier (HEKA). Flash photolysis was applied by a UV flash lamp (Rapp), and the [Ca2+]i was measured by a Till imaging system (Polychrome 5, Till Photonics) by excitation wavelengths of 350 and 380 nm (25, 26).
Dissociation of hMFBs and Ca2+ Imaging.
The procedures were similar to those described in a previous study (24). For measurements, the dissociated terminals were transferred on the stage of an inverted microscope (Nikon Eclipse Ti-u) equipped with a custom-made TIRF system. An EPC10/2 amplifier (HEKA) was used for the patch-clamp capacitance measurements.
Immunohistochemistry and Time-Gated STED Microscopy.
The procedures were similar to those described previously (43). gSTED microscopy was performed using an Abberior Instruments Expert Line STED setup equipped with an inverted IX83 microscope (Olympus), two pulsed STED lasers for depletion at 775 nm (0.98-ns pulse duration, up to 80-MHz repetition rate) and at 595 nm (0.52-ns pulse duration, 40-MHz repetition rate) and pulsed excitation lasers (at 488 nm, 561 nm, and 640 nm).
Statistics.
Normality was assessed with the Shapiro–Wilk test and inspecting data distribution. For significance test, paired or unpaired t tests were performed, unless otherwise stated. Difference in variance was tested with the f test before paired or unpaired t test. Only two-tailed P values were considered.
Supplementary Material
Acknowledgments
We thank Takafumi Miki and Erwin Neher for comments, Niclas Gimber for the Python script, and the BioSupraMol Optical Microscopy facility (Freie Universität Berlin) for use of the Abberior Instruments gSTED microscope (SupraFAB, Freie Universität Berlin) and for assistance. This study was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) (Grants JP18H02530, JP19H04760, and JP20KK0171 to T.S. and JP20K15905 to R.F.), JSPS Core-to-Core Program A. Advanced Research Networks (Grant JPJSCCA20170008 to T.S.), Germany´s Excellence Strategy – EXC-2049 – 390688087, Research Unit FOR 2705, and Collaborative Research Grant SFB 1315 provided by the Deutsche Forschungsgemeinschaft (DFG) (to S.J.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016754118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Zucker R. S., Regehr W. G., Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Byrne J. H., Kandel E. R., Presynaptic facilitation revisited: State and time dependence. J. Neurosci. 16, 425–435 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel E. R., The molecular biology of memory storage: A dialogue between genes and synapses. Science 294, 1030–1038 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Tempel B. L., Bonini N., Dawson D. R., Quinn W. G., Reward learning in normal and mutant Drosophila. Proc. Natl. Acad. Sci. U.S.A. 80, 1482–1486 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heisenberg M., Mushroom body memoir: From maps to models. Nat. Rev. Neurosci. 4, 266–275 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Monday H. R., Younts T. J., Castillo P. E., Long-term plasticity of neurotransmitter release: Emerging mechanisms and contributions to brain function and disease. Annu. Rev. Neurosci. 41, 299–322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh Y. H., Gramates L. S., Budnik V., Drosophila larval neuromuscular junction: Molecular components and mechanisms underlying synaptic plasticity. Microsc. Res. Tech. 49, 14–25 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Geiger J. R., Jonas P., Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron 28, 927–939 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Hallermann S., Pawlu C., Jonas P., Heckmann M., A large pool of releasable vesicles in a cortical glutamatergic synapse. Proc. Natl. Acad. Sci. U.S.A. 100, 8975–8980 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vyleta N. P., Jonas P., Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science 343, 665–670 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Rolls E. T., A quantitative theory of the functions of the hippocampal CA3 network in memory. Front. Cell. Neurosci. 7, 98 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisskopf M. G., Castillo P. E., Zalutsky R. A., Nicoll R. A., Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 265, 1878–1882 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Huang Y. Y., Li X. C., Kandel E. R., cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell 79, 69–79 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Nicoll R. A., Schmitz D., Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 6, 863–876 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Jones B. W., et al., Targeted deletion of AKAP7 in dentate granule cells impairs spatial discrimination. eLife 5, e20695 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi K., Suzuki H., Dopamine selectively potentiates hippocampal mossy fiber to CA3 synaptic transmission. Neuropharmacology 52, 552–561 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Hopkins W. F., Johnston D., Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J. Neurophysiol. 59, 667–687 (1988). [DOI] [PubMed] [Google Scholar]
- 18.Huang Y. Y., Kandel E. R., Modulation of both the early and the late phase of mossy fiber LTP by the activation of β-adrenergic receptors. Neuron 16, 611–617 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Castillo P. E., Schoch S., Schmitz F., Südhof T. C., Malenka R. C., RIM1alpha is required for presynaptic long-term potentiation. Nature 415, 327–330 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Maus L., et al., Ultrastructural correlates of presynaptic functional heterogeneity in hippocampal synapses. Cell Rep. 30, 3632–3643.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandael D., Borges-Merjane C., Zhang X., Jonas P., Short-term plasticity at hippocampal mossy fiber synapse is induced by natural activity patterns and associated with vesicle pool engram formation. Neuron 107, 509–521.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaeser P. S., et al., RIM1α phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. Proc. Natl. Acad. Sci. U.S.A. 105, 14680–14685 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaeser-Woo Y. J., et al., Synaptotagmin-12 phosphorylation by cAMP-dependent protein kinase is essential for hippocampal mossy fiber LTP. J. Neurosci. 33, 9769–9780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midorikawa M., Sakaba T., Kinetics of releasable synaptic vesicles and their plastic changes at hippocampal mossy fiber synapses. Neuron 96, 1033–1040.e3 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Schneggenburger R., Neher E., Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature 406, 889–893 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Wadel K., Neher E., Sakaba T., The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron 53, 563–575 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Bollmann J. H., Sakmann B., Borst J. G., Calcium sensitivity of glutamate release in a calyx-type terminal. Science 289, 953–957 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Sun J., et al., A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature 450, 676–682 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neher E., Sakaba T., Combining deconvolution and noise analysis for the estimation of transmitter release rates at the calyx of held. J. Neurosci. 21, 444–461 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamond J. S., Jahr C. E., Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron 15, 1097–1107 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Wölfel M., Schneggenburger R., Presynaptic capacitance measurements and Ca2+ uncaging reveal submillisecond exocytosis kinetics and characterize the Ca2+ sensitivity of vesicle pool depletion at a fast CNS synapse. J. Neurosci. 23, 7059–7068 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonas P., Major G., Sakmann B., Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J. Physiol. 472, 615–663 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaba T., Two Ca(2+)-dependent steps controlling synaptic vesicle fusion and replenishment at the cerebellar basket cell terminal. Neuron 57, 406–419 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Kamiya H., Umeda K., Ozawa S., Manabe T., Presynaptic Ca2+ entry is unchanged during hippocampal mossy fiber long-term potentiation. J. Neurosci. 22, 10524–10528 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neher E., Sakaba T., Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59, 861–872 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Zenisek D., Davila V., Wan L., Almers W., Imaging calcium entry sites and ribbon structures in two presynaptic cells. J. Neurosci. 23, 2538–2548 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyano R., Miki T., Sakaba T., Ca-dependence of synaptic vesicle exocytosis and endocytosis at the hippocampal mossy fibre terminal. J. Physiol. 597, 4373–4386 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Castillo P. E., Weisskopf M. G., Nicoll R. A., The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron 12, 261–269 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Pelkey K. A., Topolnik L., Lacaille J. C., McBain C. J., Compartmentalized Ca(2+) channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron 52, 497–510 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Chamberland S., Evstratova A., Tóth K., Short-term facilitation at a detonator synapse requires the distinct contribution of multiple types of voltage-gated calcium channels. J. Neurosci. 37, 4913–4927 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakamoto H., et al., Synaptic weight set by Munc13-1 supramolecular assemblies. Nat. Neurosci. 21, 41–49 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Rebola N., et al., Distinct nanoscale calcium channel and synaptic vesicle topographies contribute to the diversity of synaptic function. Neuron 104, 693–710.e9 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Brockmann M. M., et al., RIM-BP2 primes synaptic vesicles via recruitment of Munc13-1 at hippocampal mossy fiber synapses. eLife 8, e43243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rollenhagen A., et al., Structural determinants of transmission at large hippocampal mossy fiber synapses. J. Neurosci. 27, 10434–10444 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Millar A. G., Zucker R. S., Ellis-Davies G. C., Charlton M. P., Atwood H. L., Calcium sensitivity of neurotransmitter release differs at phasic and tonic synapses. J. Neurosci. 25, 3113–3125 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan B., Zucker R. S., A general model of synaptic transmission and short-term plasticity. Neuron 62, 539–554 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blatow M., Caputi A., Burnashev N., Monyer H., Rozov A., Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron 38, 79–88 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Regehr W. G., Tank D. W., The maintenance of LTP at hippocampal mossy fiber synapses is independent of sustained presynaptic calcium. Neuron 7, 451–459 (1991). [DOI] [PubMed] [Google Scholar]
- 49.Jackman S. L., Turecek J., Belinsky J. E., Regehr W. G., The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 529, 88–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groffen A. J., et al., Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science 327, 1614–1618 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lou X., Scheuss V., Schneggenburger R., Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature 435, 497–501 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Yao L., Sakaba T., cAMP modulates intracellular Ca2+ sensitivity of fast-releasing synaptic vesicles at the calyx of Held synapse. J. Neurophysiol. 104, 3250–3260 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Kaeser P. S., Regehr W. G., The readily releasable pool of synaptic vesicles. Curr. Opin. Neurobiol. 43, 63–70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neher E., Brose N., Dynamically primed synaptic vesicle states: Key to understand synaptic short-term plasticity. Neuron 100, 1283–1291 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Nakamura Y., Reva M., DiGregorio D. A., Variations in Ca influx can alter chelator-based estimates of Ca channel-synaptic vesicle coupling distance. J. Neurosci. 38, 3971–3987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z., Das B., Nakamura Y., DiGregorio D. A., S. M. Young, Jr, Ca2+ channel to synaptic vesicle distance accounts for the readily releasable pool kinetics at a functionally mature auditory synapse. J. Neurosci. 35, 2083–2100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vukoja A., et al., Presynaptic biogenesis requires axonal transport of lysosome-related vesicles. Neuron 99, 1216–1232.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Böhme M. A., et al., Rapid active zone remodeling consolidates presynaptic potentiation. Nat. Commun. 10, 1085 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider R., et al., Mobility of calcium channels in the presynaptic membrane. Neuron 86, 672–679 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Kaeser P. S., et al., RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144, 282–295 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han Y., Kaeser P. S., Südhof T. C., Schneggenburger R., RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron 69, 304–316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acuna C., Liu X., Gonzalez A., Südhof T. C., RIM-BPs mediate tight coupling of action potentials to Ca2+-triggered neurotransmitter release. Neuron 87, 1234–1247 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Liu K. S., et al., RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science 334, 1565–1569 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Neher E., Merits and limitations of vesicle pool models in view of heterogeneous populations of synaptic vesicles. Neuron 87, 1131–1142 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Imig C., et al., The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron 84, 416–431 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Reid C. A., Dixon D. B., Takahashi M., Bliss T. V., Fine A., Optical quantal analysis indicates that long-term potentiation at single hippocampal mossy fiber synapses is expressed through increased release probability, recruitment of new release sites, and activation of silent synapses. J. Neurosci. 24, 3618–3626 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heck J., et al., Transient confinement of Cav2.1 Ca2+-channel splice variants shapes synaptic short-term plasticity. Neuron 103, 66–79.e12 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Choquet D., Triller A., The role of receptor diffusion in the organization of the postsynaptic membrane. Nat. Rev. Neurosci. 4, 251–265 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Diering G. H., Huganir R. L., The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.