Key Points
Question
What is the extent of neurologic involvement in US hospitalized children and adolescents with coronavirus disease 2019 (COVID-19)?
Findings
In this study of 1695 patients 21 years or younger hospitalized for acute COVID-19 or multisystem inflammatory syndrome, 365 (22%) had neurologic involvement. Forty-three patients (12%) developed COVID-19–related life-threatening neurologic disorders, 11 (26%) died, and 17 (40%) survived with new neurologic sequelae.
Meaning
In this study, COVID-19–related neurologic involvement was common in hospitalized children and adolescents and mostly transient.
Abstract
Importance
Coronavirus disease 2019 (COVID-19) affects the nervous system in adult patients. The spectrum of neurologic involvement in children and adolescents is unclear.
Objective
To understand the range and severity of neurologic involvement among children and adolescents associated with COVID-19.
Setting, Design, and Participants
Case series of patients (age <21 years) hospitalized between March 15, 2020, and December 15, 2020, with positive severe acute respiratory syndrome coronavirus 2 test result (reverse transcriptase-polymerase chain reaction and/or antibody) at 61 US hospitals in the Overcoming COVID-19 public health registry, including 616 (36%) meeting criteria for multisystem inflammatory syndrome in children. Patients with neurologic involvement had acute neurologic signs, symptoms, or diseases on presentation or during hospitalization. Life-threatening involvement was adjudicated by experts based on clinical and/or neuroradiologic features.
Exposures
Severe acute respiratory syndrome coronavirus 2.
Main Outcomes and Measures
Type and severity of neurologic involvement, laboratory and imaging data, and outcomes (death or survival with new neurologic deficits) at hospital discharge.
Results
Of 1695 patients (909 [54%] male; median [interquartile range] age, 9.1 [2.4-15.3] years), 365 (22%) from 52 sites had documented neurologic involvement. Patients with neurologic involvement were more likely to have underlying neurologic disorders (81 of 365 [22%]) compared with those without (113 of 1330 [8%]), but a similar number were previously healthy (195 [53%] vs 723 [54%]) and met criteria for multisystem inflammatory syndrome in children (126 [35%] vs 490 [37%]). Among those with neurologic involvement, 322 (88%) had transient symptoms and survived, and 43 (12%) developed life-threatening conditions clinically adjudicated to be associated with COVID-19, including severe encephalopathy (n = 15; 5 with splenial lesions), stroke (n = 12), central nervous system infection/demyelination (n = 8), Guillain-Barré syndrome/variants (n = 4), and acute fulminant cerebral edema (n = 4). Compared with those without life-threatening conditions (n = 322), those with life-threatening neurologic conditions had higher neutrophil-to-lymphocyte ratios (median, 12.2 vs 4.4) and higher reported frequency of D-dimer greater than 3 μg/mL fibrinogen equivalent units (21 [49%] vs 72 [22%]). Of 43 patients who developed COVID-19–related life-threatening neurologic involvement, 17 survivors (40%) had new neurologic deficits at hospital discharge, and 11 patients (26%) died.
Conclusions and Relevance
In this study, many children and adolescents hospitalized for COVID-19 or multisystem inflammatory syndrome in children had neurologic involvement, mostly transient symptoms. A range of life-threatening and fatal neurologic conditions associated with COVID-19 infrequently occurred. Effects on long-term neurodevelopmental outcomes are unknown.
This study evaluates the range and severity of neurologic involvement among children and adolescents associated with COVID-19.
Introduction
Coronaviruses primarily cause respiratory disease; however, severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus, and newly identified SARS-CoV-2 have been associated with a range of disorders of the peripheral and central nervous system (CNS).1,2,3,4,5,6,7,8,9 Early reports from Wuhan, China, described a spectrum of neurologic conditions associated with SARS-CoV-2 infection in 36% of 214 adults hospitalized with coronavirus disease 2019 (COVID-19).10 Reported neurologic and psychiatric symptoms in adult patients with COVID-19 include anosmia/ageusia,11,12 headaches,13 dizziness/ataxia,10 psychosis, dementia, depression, anxiety, and mania.14 Reported severe neurologic involvement in adult patients with COVID-19 includes acute encephalopathy or encephalitis,15,16,17,18 acute necrotizing encephalopathy,19,20 epilepsy/seizures,21,22 acute transverse myelitis,23,24,25 Guillain-Barré syndrome (GBS),26,27 posterior reversible encephalopathy syndrome,28 and acute ischemic or hemorrhagic stroke.14,29,30,31,32,33,34
Although most children and adolescents are spared from severe COVID-19, there have been reports of life-threatening neurologic involvement in patients developing multisystem inflammatory syndrome in children (MIS-C), a relatively rare, hyperinflammatory, severe illness temporally associated with SARS-CoV-2 infection, presumably postinfectious.35,36 Across case series published between March and August 14, 2020, between 6% and 58% of children and adolescents hospitalized with MIS-C developed central and/or peripheral nervous system involvement.36,37,38,39,40 The frequency of neurologic involvement in children hospitalized with acute COVID-19 is unclear with 150 of 4190 patients reported across 9 international case series.41,42,43,44,45,46 Using the Overcoming COVID-19 US public health surveillance registry of children and adolescents hospitalized with COVID-19–related complications,36 we aimed to describe the type and severity of neurologic involvement and documented hospital outcomes.
Methods
Study Design and Participants
Active surveillance was performed at 61 hospitals in 31 states in the Overcoming COVID-19 network to identify children and adolescents (age <21 years) with SARS-CoV-2–related illness hospitalized from March 15, 2020, to December 15, 2020. The study was approved by the central institutional review board at Boston Children’s Hospital and determined to meet the requirement of public health surveillance as defined in 45 CFR 46.102(I)(2) at Boston Children’s Hospital and the US Centers for Disease Control and Prevention under a waiver of consent.
Patients were included if they were hospitalized for acute illness at a participating site, were younger than 21 years, had a positive SARS-CoV-2 test result (reverse transcriptase–polymerase chain reaction and/or antibody) and symptoms associated with acute COVID-19, or met US Centers for Disease Control and Prevention criteria for MIS-C (eTable 1 in Supplement 1).47 Patients were excluded if they had asymptomatic SARS-CoV-2 infection or a non–COVID-19–related cause for hospitalization or death. Race and ethnicity were extracted from the patient’s medical record and included to evaluate risk of neurologic involvement.
Classification of Neurologic Involvement
Patients were stratified by the presence of neurologic involvement, defined as (1) suspected acute neurologic disease (eg, CNS infection/demyelination or stroke) on presentation or that developed during hospitalization (eMethods in Supplement 1) or (2) acute neurologic signs or symptoms on presentation.
Severity of neurologic involvement was adjudicated by neurology and critical care experts on the central study team (K.L.L., B.J.R., T.Y.P., and A.G.R.; eMethods in Supplement 1). Cases were classified as life-threatening based on clinical and/or neuroradiologic features associated with more severe outcomes and included the following diagnoses: acute CNS infection (aseptic meningitis, encephalitis by International Encephalitis Consortium definition,48 and Brighton criteria49), central demyelinating disorder (acute disseminated encephalomyelitis [ADEM]), acute ischemic or hemorrhagic stroke, GBS and variants, or severe encephalopathy with or without COVID-19–related neuroimaging abnormalities (eg, virus-associated necrotizing disseminated acute leukoencephalopathy50 and/or cytotoxic splenial lesions44,51,52). Cases with neurologic involvement that did not meet any of these criteria and had cerebrospinal fluid and/or neuroimaging results that were normal or not performed were categorized as non–life-threatening neurologic involvement.
Neurologic Outcome Classification
Neurology and critical care experts (K.L.L., B.J.R., and A.G.R.) determined through case review and consensus whether life-threatening neurologic conditions were directly associated with COVID-19 or secondary to exacerbation of primary neurologic disease or complication of critical illness associated with COVID-19. Sites with abnormal neuroimaging studies sent deidentified brain magnetic resonance imaging (MRI) and computed tomography studies for central review. Images were reviewed by a pediatric neuroradiologist (T.Y.P.) and discussed with a pediatric neurologist (K.L.L.) reaching consensus opinion about whether the clinicoradiologic link was directly associated with COVID-19 or secondary to an alternate etiology (eg, extracorporeal membrane oxygenation [ECMO] or preexisting neurologic condition).
Outcomes were determined at hospital discharge. Neurologic deficits were defined as gross impairment in motor, cognitive, or speech and language functions. Psychiatric sequelae (eg, anxiety, depression, and/or suicidal ideation) were not included. New neurologic deficits were determined by medical record review at each site and adjudicated by the experts for all patients with and without neurologic involvement (eMethods in Supplement 1).
Statistical Analyses
We report the frequency of clinical characteristics, underlying conditions, type of neurologic involvement on admission or during hospitalization, and hospital outcomes. Continuous variables were expressed as medians and interquartile range. Categorical variables were expressed as counts and percentages. Between-group differences were analyzed using a χ2 test, Fisher exact test, or Kruskal-Wallis test where appropriate. Two-sided P values less than .05 were considered statistically significant. We did not impute missing data. We analyzed all data using R software, version 3.6.1 (R Project for Statistical Computing).
Results
Demographics and Clinical Characteristics Among All Patients
From March 15, 2020, to December 15, 2020, a total of 1784 hospitalized children and adolescents with COVID-19–related illness were reported to the registry. Of these, 89 patients were excluded on the basis of being 21 years or older (n = 27), epidemiologic link to SARS-CoV-2 without a positive test result (n = 54), and non–COVID-19–related cause for hospitalization or death (n = 8) (eFigure in Supplement 1). We describe 1695 patients (909 male [54%]; median [interquartile range] age, 9.1 [2.4-15.3] years) from 61 sites in 31 states (Table 1). Most patients were either Hispanic or Latino (638 of 1695 [38%]) or non-Hispanic Black (442 of 1695 [26%]).
Table 1. Characteristics and Outcomes of 1695 Patients (Age <21 Years) Hospitalized for COVID-19–Related Illness by Reported Neurologic Involvement.
| Clinical characteristics | No. (%) | P value | ||
|---|---|---|---|---|
| All patients (N = 1695) | Neurological involvement | |||
| Yes (n = 365) | No (n = 1330) | |||
| Male | 909 (54) | 204 (56) | 705 (53) | .36 |
| Female | 786 (46) | 161 (44) | 625 (47) | |
| Age, median (IQR), y | 9.1 (2.4-15.3) | 9.2 (2.5-15.6) | 9.0 (2.4-15.1) | .94 |
| Race/ethnicitya | ||||
| Non-Hispanic | ||||
| White | 311 (18) | 83 (23) | 228 (17) | .004 |
| Black | 442 (26) | 108 (30) | 334 (25) | |
| Hispanic or Latino | 638 (38) | 125 (34) | 513 (39) | |
| Other race, non-Hispanic | 111 (7) | 21 (6) | 90 (7) | |
| Unknown | 216 (13) | 31 (8) | 185 (14) | |
| SARS-CoV-2 testing | ||||
| RT-PCR performed | 1589 (94) | 359 (98) | 1230 (92) | <.001 |
| Positive RT-PCR result | 1248 (74) | 298 (82) | 950 (71) | <.001 |
| Antibody test performed | 672 (40) | 140 (38) | 532 (40) | .61 |
| Positive antibody test result | 589 (35) | 121 (33) | 468 (35) | .51 |
| Underlying conditionb | ||||
| Previously healthyc | 918 (54) | 195 (53) | 723 (54) | .80 |
| ≥1 Comorbidity, excluding obesity | 714 (42) | 156 (43) | 558 (42) | .83 |
| Neurological, any condition | 194 (11) | 81 (22) | 113 (8) | <.001 |
| Seizure disorder | 100 (6) | 57 (16) | 43 (3) | <.001 |
| Neuromuscular disordersd | 59 (3) | 25 (7) | 34 (3) | <.001 |
| Autism or developmental delay | 42 (2) | 18 (5) | 24 (2) | .001 |
| Static encephalopathy | 40 (2) | 18 (5) | 22 (2) | <.001 |
| Congenital neurologic disorderse | 35 (2) | 16 (4) | 19 (1) | <.001 |
| Prior stroke/HIE | 15 (1) | 6 (2) | 9 (1) | .11 |
| Respiratory | 321 (19) | 75 (21) | 246 (18) | .42 |
| Cardiac | 110 (6) | 25 (7) | 85 (6) | .85 |
| Gastrointestinal | 173 (10) | 44 (12) | 129 (10) | .22 |
| Oncologic or immune compromised | 122 (7) | 17 (5) | 105 (8) | .04 |
| Hematological | 88 (5) | 17 (5) | 71 (5) | .70 |
| Kidney | 72 (4) | 13 (4) | 59 (4) | .56 |
| Endocrine | 129 (8) | 25 (7) | 104 (8) | .61 |
| Genetic or metabolic (not obesity) | 69 (4) | 23 (6) | 46 (3) | .02 |
| Clinically diagnosed obesityf | 184 (11) | 39 (14) | 145 (14) | >.99 |
| Non-CNS organ system involvement | ||||
| Met MIS-C criteria | 616 (36) | 126 (35) | 490 (37) | .45 |
| Other organ systems involved | ||||
| None | 182 (11) | 36 (10) | 146 (11) | .61 |
| 1 | 341 (20) | 74 (20) | 267 (20) | .99 |
| 2 | 319 (19) | 64 (18) | 255 (19) | .53 |
| 3 | 286 (17) | 56 (15) | 230 (17) | .42 |
| 4 | 567 (34) | 135 (37) | 432 (33) | .12 |
| Outcomes | ||||
| ICU | 836 (49) | 227 (62) | 609 (46) | <.001 |
| ECMO | 32 (2) | 16 (4) | 18 (1) | <.001 |
| Mechanical ventilation | 225 (13) | 103 (28) | 122 (9) | <.001 |
| Length of stay, median (IQR), d | ||||
| ICU | 4 (2-7) | 4 (2-9) | 4 (2-6) | .02 |
| Hospital | 5 (2-9) | 5 (2-11) | 5 (2-8) | .004 |
| Died | 22 (1) | 14 (4) | 8 (1) | <.001 |
| Survived, new neurological deficit | 22 (1) | 20 (5) | 2 (0.2) | .02 |
| Discharged to rehabilitation | 25 (1) | 13 (4) | 12 (1) | <.001 |
Abbreviations: CNS, central nervous system; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; HIE, hypoxic ischemic encephalopathy; ICU, intensive care unit; IQR, interquartile range; MIS-C, multisystem inflammatory syndrome in children; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Race and ethnic group were reported by the patient or by the patient’s parent or guardian. Race/ethnicity categories are not mutually exclusive.
Patients may have more than 1 underlying condition.
Previously healthy was defined as an absence of reported underlying conditions and taking no prescription medications.
Neuromuscular disorders include spastic quadriplegia, muscular dystrophy, neuromuscular weakness, and neuromuscular scoliosis.
Congenital neurologic disorders include hydrocephalus, neurogenetic, and neurometabolic disorders.
The determination of clinically diagnosed obesity was based on reporting by clinicians among patients who were aged at least 2 years (n = 278 for patients with neurological involvements and n = 1017 for patients without neurological involvement).
Neurologic vs Nonneurologic Involvement
There were 365 patients (22%) with neurologic involvement reported from 52 sites in 29 states. The characteristics of the patients with and without neurologic involvement are shown in Table 1. The frequencies of previously healthy patients (195 [53%] vs 723 [54%]) and patients meeting MIS-C criteria (126 [35%] vs 490 [37%]) were similar. Patients with neurologic involvement were more likely to have underlying neurologic disorders (81 [22%]) compared with those without (113 [8%]), including seizure disorders, neuromuscular disorders, and autism or developmental delay. Presenting neurologic signs and symptoms differed by age with seizures or status epilepticus most common in children younger than 5 years and anosmia and/or ageusia most common in patients between ages 13 and 20 years (Figure 1A).
Figure 1. Presenting Neurologic Symptoms and Most Abnormal Laboratory Values in Patients (Age <21 Years) Hospitalized for Coronavirus Disease 2019 (COVID-19).
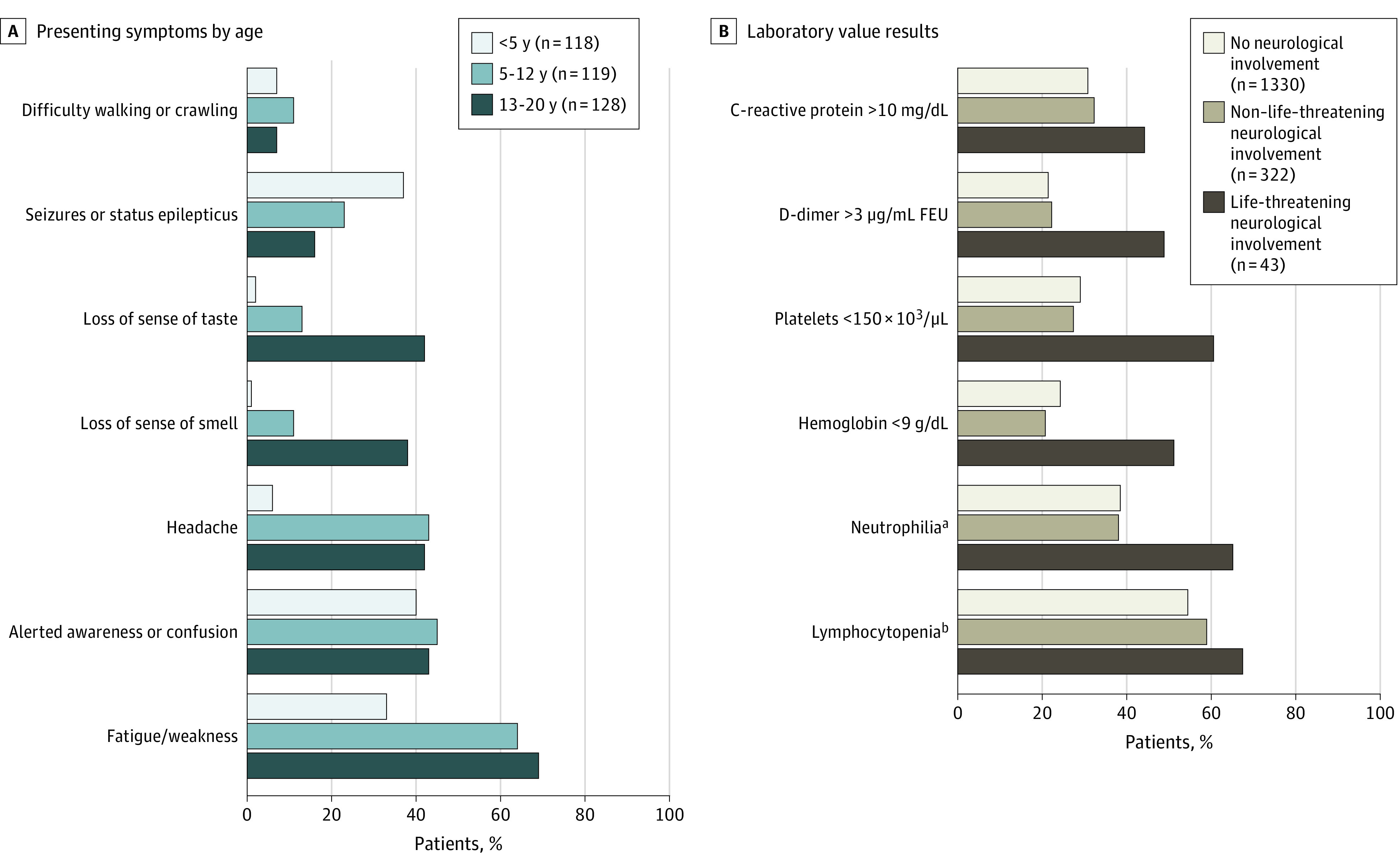
A, Presenting neurologic symptoms by age in 365 patients (age <21 years) with COVID-19–related neurologic involvement. B, Most abnormal laboratory results in 1695 patients (age <21 years) with COVID-19 by severity of neurologic involvement. Denominators varied and are provided in eTable 6 in Supplement 1. FEU indicates fibrinogen equivalent units.
SI conversion factors: To convert C-reactive protein to mg/L, multiply by 10; D-dimer to nmol/L, multiply by 5.476; hemoglobin to d/L, multiply by 10; platelet count to ×109/L, multiply by 1.
aNeutrophilia was defined as a maximum absolute neutrophil count higher than 7700/μL.
bLymphocytopenia was defined as an absolute lymphocyte count of less than 1500/μL in patients 8 months or older and of less than 4500/μL in patients younger than 8 months.
Most patients with and without neurologic involvement were discharged alive (351 [96%] and 1322 [99%], respectively). Children with neurologic involvement had a higher rate of survival with new neurologic deficits (20 of 365 [5%]) compared with those without COVID-19–associated neurologic involvement (2 of 1330 [0.2%]) (Table 1). Neurologic deficits in those without neurologic involvement included cognitive and motor impairments as a result of sequelae of critical illness and intensive care therapies.
Life-threatening Neurologic Involvement
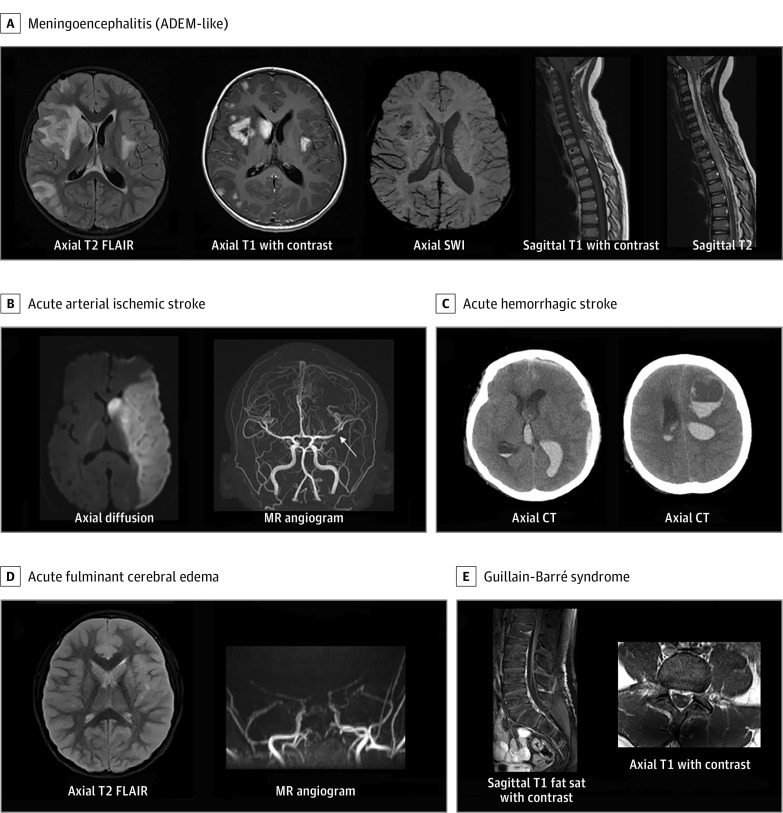
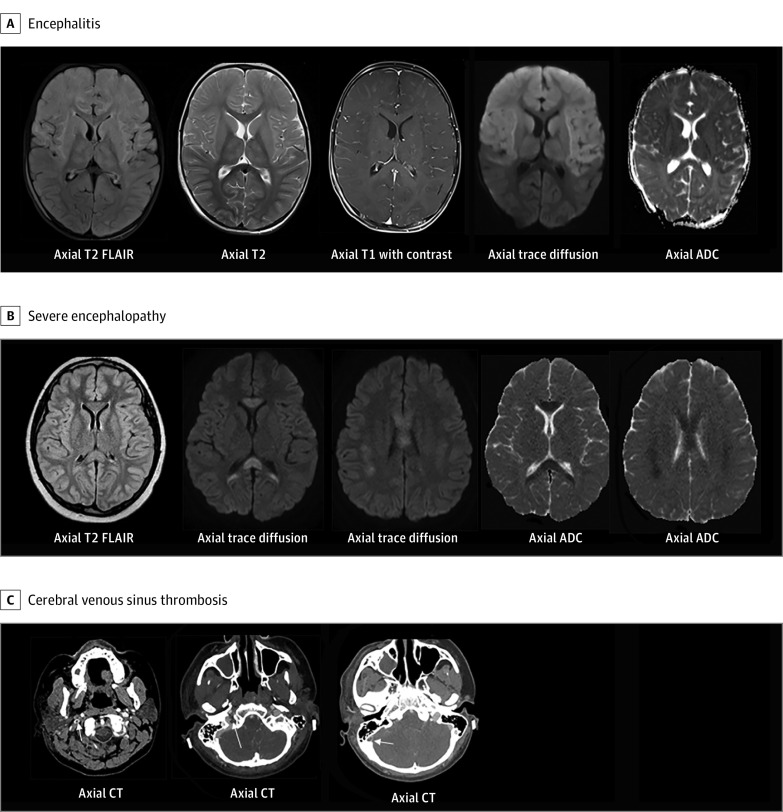
Among 365 patients with neurologic involvement, 43 (12%) had life-threatening neurologic involvement associated with COVID-19 (Table 2). Among these, 34 of 43 (79%) had no major underlying conditions, 20 (47%) met criteria for MIS-C, and 3 (7%) had a preexisting neurologic disorder. Life-threatening neurologic conditions included severe encephalopathy (n = 15; 5 with white-matter hyperintensities and splenial lesions), acute ischemic or hemorrhagic stroke (n = 12), acute CNS infection/ADEM (n = 8), acute fulminant cerebral edema (n = 4), and GBS (n = 4) (Table 2; eTable 2 in Supplement 1). Eight patients with stroke had underlying risk factors (5 experienced stroke during ECMO [eTable 3 in Supplement 1]; 2 were attributed to possible COVID-19–related exacerbation of an underlying primary neurologic disorder [eg, arteriovenous malformation rupture and ischemic stroke in a patient with history of moyamoya syndrome]; and a previously healthy patient presented with a new diagnosis of acute myelogenous leukemia). Four patients were previously healthy and did not have stroke risk factors (eTable 4 in Supplement 1). Five children with severe encephalopathy had brain MRI findings of diffuse white-matter hyperintensities on T2-weighted images and restricted diffusion in the periventricular white matter, deep white matter, and/or corpus callosum (60% with MIS-C; 3 of 5 with unfavorable neurologic outcomes). Representative CNS images from patients with life-threatening neurologic involvement associated with COVID-19 are shown in Figure 2 and Figure 3.
Table 2. Life-threatening COVID-19–Related Neurologic Conditions and Deaths in 43 Patients (Age <21 Years) Hospitalized for COVID-19.
| Variable | Life-threatening COVID-19-related neurologic conditions, No. (%) | |||||
|---|---|---|---|---|---|---|
| Overall | Severe encephalopathy | Ischemic or hemorrhagic stroke | Acute CNS infection or ADEM | Acute fulminant cerebral edema | Guillain-Barré syndrome | |
| No. | 43 | 15 | 12 | 8 | 4 | 4 |
| Age, median (IQR), ya | 12 (7-15) | 1 Infant | 1 Preschooler | 1 Infant | 1 Infant | 2 School aged |
| 1 Toddler | 5 School-aged | 1 Toddler | 1 Preschooler | 2 Adolescents | ||
| 2 Preschoolers | 5 Adolescents | 2 Preschoolers | 2 School-aged | NA | ||
| 5 School-aged | 1 Young adult | 1 School-aged | NA | NA | ||
| 6 Adolescents | NA | 3 Adolescents | NA | NA | ||
| Male | 27 (63) | 11 (73) | 6 (50) | 4 (50) | 2 (50) | 4 (100) |
| RT-PCR or antibody results | ||||||
| Positive RT-PCR result only | 19 (44) | 7 (47) | 7 (58) | 2 (25) | 3 (75) | 0 |
| Positive antibody result only | 11 (26) | 3 (20) | 3 (25) | 3 (38) | 0 | 2 (50) |
| Positive RT-PCR and antibody results | 13 (30) | 5 (33) | 2 (17) | 3 (38) | 1 (25) | 2 (50) |
| MIS-C diagnosis | 20 (47) | 8 (53) | 3 (25) | 6 (75) | 2 (50) | 1 (25) |
| No major underlying conditions | 34 (79) | 11 (73) | 8 (67) | 8 (100) | 4 (100) | 3 (75) |
| Underlying neurologic disorder | 3 (7) | 1 (7) | 2 (17) | 0 | 0 | 0 |
| Death | 11 (26) | 4 (27) | 4 (33) | 0 | 3 (75) | 0 |
| Discharged alive, new CNS deficit | 17 (40) | 2 (13) | 7 (58) | 5 (63) | 0 | 3 (75) |
Abbreviations: ADEM, acute disseminated encephalomyelitis; CNS, central nervous system; COVID-19, coronavirus disease 2019; IQR, interquartile range; MIS-C, multisystem inflammatory syndrome in children; NA, not applicable; RT-PCR, reverse transcriptase–polymerase chain reaction.
Age categories reported for privacy reasons for subcategories of complications: infant (age <1 year), toddler (age 1-2 years), preschool (age 3-5 years), school-aged (age 6-12 years), adolescent (age 13-17 years), and young adult (age 18-21 years).
Figure 2. Representative Central Nervous System Images From Patients With Life-threatening COVID-19–Related Neurologic Involvement.
A, Young boy with headache, fatigue, and weakness. Enhancing cerebral lesions with basal ganglia punctate blood products, and abnormal spinal cord signal with focal nodular enhancement. B, Male adolescent with right-sided hemiparesis, confusion, and conjunctivitis. Left middle cerebral artery infarct with middle cerebral artery bifurcation intraluminal thrombus (arrow). C, Adolescent with cerebral palsy in acute hypoxemic respiratory/kidney failure. During recovery sudden respiratory decompensation and shock requiring venovenous extracorporeal membrane oxygenation for 3 to 4 weeks. Computed tomography (CT) for mental status change and anisocoria shows intraventricular, subdural, and frontal intraparenchymal hemorrhage. D, Acute fulminant cerebral edema. Young girl with altered awareness, seizure, nausea, vomiting, acute respiratory failure, and shock requiring vasopressors. Severe cerebral edema with reduced diffusivity and magnetic resonance (MR) angiography with little flow above the level of the supraclinoid internal carotid arteries consistent with brain death. E, Adolescent presents with lethargy, paresthesia, and extremity weakness. There are enhancing cauda equina nerve roots. COVID-19 indicates coronavirus disease 2019; fat sat, fat saturation; FLAIR, fluid-attenuated inversion recovery; SWI, susceptibility weighted imaging.
Figure 3. Representative Central Nervous System Images From Patients With Life-Threatening COVID-19–Related Neurologic Involvement.
A, Previously healthy toddler with multisystem inflammatory syndrome in children (Ab+) with fever, rash, fatigue, vomiting, decreased oral intake, compensated cardiogenic shock, and generalized tonic-clonic status epilepticus. There is diffuse T2 hyperintensity, leptomeningeal enhancement, and reduced diffusivity within the bilateral frontal lobes, basal ganglia, and thalami. B, School-aged child with coronavirus disease 2019 (COVID-19) and multisystem inflammatory syndrome in children presented with fever, headache, neck pain, abdominal pain, encephalopathy, and visual hallucinations. T2 prolongation with reduced diffusivity in the genu and splenium of corpus callosum, periventricular, and parietal white matter. C, Previously healthy teenager with fever, vomiting, diarrhea, headache, and fatigue presented with altered mental status, visual hallucinations, left hemiparesis, septic shock, and respiratory distress requiring intubation. Axial computed tomography (CT) images demonstrate nonocclusive thrombus in the right internal jugular vein within the upper neck, jugular bulb, and right sigmoid sinus. ADC indicates apparent diffusion coefficient map; FLAIR, fluid-attenuated inversion recovery.
Compared with those with non–life-threatening neurologic involvement, children with life-threatening neurologic disease were more likely to undergo lumbar puncture (20 of 43 [47%] vs 72 of 322 [22%]), head computed tomography (23 of 43 [53%] vs 40 of 322 [12%]) or brain MRI (26 of 43 [60%] vs 28 of 322 [9%]). The cerebrospinal fluid results showed unremarkable findings in both groups (eTable 5 in Supplement 1). As shown in Figure 1B, patients with life-threatening neurologic conditions were more inflamed and coagulopathic than those with no or non–life-threatening neurologic involvement. Patients with life-threatening vs non–life-threatening neurologic involvement had higher neutrophil-to-lymphocyte ratios (median, 12.2 vs 4.4), and higher reported frequency of D-dimer >3 μg/mL fibrinogen equivalent units (21 [49%] vs 72 [22%]; to convert D-dimer to nanomoles per liter, multiply by 5.476; Figure 1; eTable 6 in Supplement 1).
In patients who developed life-threatening neurologic involvement, 11 (26%) died and 17 (40%) were discharged from hospital with new neurologic deficits (Table 2). Of survivors with new deficits, 16 (94%) were previously healthy, none had prior neurologic disorders, 7 (41%) met MIS-C criteria, and 14 (82%) required rehabilitative services on discharge (eTable 2 in Supplement 1).
Association of COVID-19 Neurologic Involvement With Fatality
Fourteen patients with COVID-19 neurologic involvement died in the hospital. Three deaths were associated with acute COVID-19 cardiorespiratory disease. Two patients with asthma had cardiac arrest on hospital presentation, and 1 previously healthy teenager with anosmia/ageusia died of multiorgan failure. These patients were excluded from further evaluation. The other 11 deaths were classified by expert consensus as either directly associated with COVID-19 neurologic involvement or with catastrophic neurologic events secondary to COVID-19–related critical illness (Table 2). These cases are briefly summarized below (eTable 2 in Supplement 1).
Three previously healthy children with acute fulminant cerebral edema died within 48 hours of hospital admission (eTable 7 in Supplement 1). One male infant with COVID-19 presented with fever, seizures, and gastrointestinal symptoms and within 24 hours of hospitalization developed status epilepticus and had a cardiac arrest, with subsequent imaging showing global cerebral edema. One elementary school–aged girl presented with fever and sore throat, then developed status epilepticus with subsequent imaging revealing cerebral edema with tonsillar herniation. One elementary school–aged boy met criteria for MIS-C 1 month after a positive SARS-CoV-2 respiratory test result. He developed status epilepticus shortly after hospital admission and imaging showed global cerebral edema and uncal herniation.
Four patients with stroke died. Three of these patients had strokes with malignant edema and examinations consistent with brain death on ECMO. The fourth died of multiple ischemic strokes owing to rapidly progressive large-vessel CNS vasculitis despite intensive immunotherapies.
Four patients who developed severe encephalopathy died. One immunocompromised adolescent with leukemia and acute COVID-19 pneumonia had diffuse T2 prolongation and reduced diffusivity in the bilateral periventricular white matter, with involvement of the splenium and genu of the corpus callosum, who also developed acute motor-sensory axonal neuropathy confirmed by electromyography/nerve conduction study. Two other patients who died required intubation for severe encephalopathy, complicated by cardiovascular collapse with cannulation for venoarterial ECMO and progression to brain death. One teenager with obesity who died had preexisting hypertension and diabetes and received venoarterial ECMO for cardiorespiratory failure. A brain MRI on decannulation obtained for prolonged encephalopathy showed multifocal areas of restricted diffusion and hemorrhage throughout the posterior white matter and brainstem.
Discussion
In a large, multicenter case series of US children and adolescents hospitalized with acute COVID-19 or MIS-C, 22% of reported patients had neurologic involvement. Approximately half of patients with and without neurologic involvement were previously healthy, a similar percentage had MIS-C, but more patients with neurologic involvement had underlying neurologic disorders (22% vs 8%). Neurologic involvement in most patients was transient and resolved by hospital discharge; however, 43 patients (12%) developed a range of life-threatening neurologic conditions associated with COVID-19, and 66% of these patients had unfavorable outcomes, including death or new neurologic disability at hospital discharge.
The range of neurologic symptoms associated with COVID-19 in children and adolescents was broad and varied by age including seizures/status epilepticus in the younger patients and reports of anosmia and/or ageusia, headache, and fatigue/weakness in older patients. Approximately 1 in 4 patients with neurologic involvement across age groups presented with altered awareness or confusion. The range of severe neurologic complications including peripheral nerve disorders (GBS and variants), focal CNS disease (ischemic stroke due to large vessel occlusion, cerebral venous sinus thrombosis, and focal cerebral arteriopathy), and diffuse CNS involvement (CNS infection, ADEM, severe encephalopathy with white matter and corpus callosum lesions, and acute fulminant cerebral edema) make it likely that multiple mechanisms underlie this wide spectrum of disease. These include putative mechanisms such as neuroinvasive or neurotropic (direct viral entry and/or neuronal infection via angiotensin-converting enzyme 253,54 and/or olfactory tract55,56), neuroinflammatory (exaggerated cytokine/immune mediated response leading to blood brain barrier breakdown57,58), postinfectious immune dysregulation,59,60 and/or as secondary injury from complications of systemic inflammation or other non-CNS organ failure.61
We observed 4 cases of GBS that presented with classic neurologic signs, symptoms, and electrophysiologic features within 1 month following SARS-CoV-2 exposure, similar to reports in adults and children in association with COVID-19,26,27 and 1 case of acute motor-sensory axonal neuropathy. Animal models and clinicopathological evidence support an autoimmune mechanism and potential molecular mimicry between antibodies against myelin and gangliosides in the nervous system and recent infectious agents, now including COVID-19 infection,62,63 and suggests a potential role for antiganglioside antibodies in immunomodulatory therapies.
We report 12 cases of acute ischemic or hemorrhagic stroke, with 8 having underlying stroke risk factors. Five of these cases occurred while receiving ECMO, but were included because COVID-19 may have exacerbated an underlying pathophysiologic state (eg, hypercoagulability, hyperinflammation, increased risk of bleeding, and endothelial dysfunction) predisposing to stroke while receiving ECMO.64,65 The 4 cases without stroke risk factors were directly associated with COVID-19. In the pediatric literature consisting of case reports and 2 international case series, ischemic stroke type has been reported in 18 children with COVID-19 with stroke mechanisms similar to those observed in our study.44,66,67,68,69 Acute ischemic stroke in hospitalized adults with COVID-19 is not uncommon.8,33,70
We also describe global cerebral involvement in 15 patients (8 with MIS-C) with severe encephalopathy, 8 patients with acute CNS infection (encephalitis, aseptic meningitis) or postinfectious, central demyelination (ADEM), and 4 patients with acute fulminant cerebral edema. We identified 5 previously healthy patients who presented with severe encephalopathy, focal neurologic deficits, and visual hallucinations (4 of 5 cases) and had diffuse abnormal T2 hyperintensities and reduced diffusivity involving the white matter and genu or splenium of the corpus callosum on MRI. These imaging features have been ascribed to COVID-19 in adults50,71 and in children with MIS-C.44,51,52 Cytotoxic lesions in the corpus callosum are thought to be associated with increased numbers of glutamate and cytokine receptors in the corpus callosum, particularly the splenium.44,72,73 Similar to the range of outcomes in 1 small adult case series,50 3 patients had unfavorable outcomes (1 died and 2 were discharged with new deficits including cognitive impairment and painful neuropathy requiring gabapentin). Of those with acute CNS infections/ADEM, 7 patients in our study could be confirmed as having probable acute CNS infection using published case definitions.48,49 Case reports and small case series also support a link between meningoencephalitis and COVID-19 in adults74,75,76 and children.44,77,78,79
There were 4 cases of previously healthy children who developed acute fulminant cerebral edema directly associated with COVID-19 or MIS-C, and 3 died. Acute fulminant cerebral edema has been previously reported in a child with COVID-1980 and is a recognized phenotype with high mortality in adults81,82 and children44,83 associated with other viral causes.
Our study has several strengths. There was expert adjudication of cases with fatal and life-threatening neurological involvement and new neurologic deficits by pediatric neurology, pediatric critical care, and pediatric neuroradiology experts. The central study team also had personal communication with site clinicians contributing cases with fatal and life-threatening neurologic involvement or new neurologic deficits to confirm diagnoses and clinical course. Neuroimaging was associated with clinical information to document supportive imaging findings and confirm diagnoses. We also captured patients across most US states from a large number of pediatric centers.
Limitations
The study has certain limitations. First, cases of COVID-19–related neurologic involvement were identified only at reporting hospitals and may not accurately reflect the true range and severity of COVID-19 neurologic involvement. Second, in patients with underlying neurologic diseases, neurologic presentations may be owing to COVID-19 neurologic effects or exacerbation of underlying neurologic conditions. Third, not all patients underwent neuroimaging (possibly owing to infection control concerns or critical illness-related instability) and image acquisition was not standardized, which could result in misclassification or an underestimation of neurologic involvement. Fourth, although standardized case report forms were used, we may not have captured certain variables completely, such as the indications for procedures (eg, lumbar puncture and imaging). Fifth, some neurologic symptoms (eg, anosmia or ageusia) may be underreported in very young patients. Sixth, nonstandardized diagnostic workups performed under routine clinical conditions may have missed non–COVID-19–related causes of life-threatening neurological conditions attributed to COVID-19. Seventh, standardized and validated assessments of neurologic outcomes at or after hospital discharge were not performed, likely underestimating the nature and extent of neurologic sequelae. Eighth, this is not a prospective cohort study but a case series, and caution is warranted in interpreting these data to identify risk factors for neurologic involvement.
Conclusions
In this study, neurologic involvement was common in children and adolescents with COVID-19–related hospitalization and is mostly transient. A spectrum of life-threatening neurologic involvement infrequently occurred and was associated with more extreme inflammation and severe sequelae. Future immunologic studies of cell-mediated and cytokine immune responses in young individuals may provide insight into the pathogenesis of neurologic disease in COVID-19 and MIS-C.84 Patients with less severe neurologic involvement could have future sequelae. Long-term follow-up of pediatric patients with COVID-19–related neurologic involvement is needed to evaluate effects on cognition and development.
eMethods.
eFigure. Eligibility flowchart of hospitalized patients with COVID-19-related neurologic involvement, March 15–December 15, 2020
eTable 1. Case definition used in this study for multisystem inflammatory syndrome in children (MIS-C)
eTable 2. Life-threatening COVID-19-related neurologic disorders and outcomes in 43 children and adolescents (< 21 years) hospitalized for COVID-19
eTable 3. Detailed clinical descriptions for 5 patients who experienced stroke while supported by extracorporeal membrane oxygenation (ECMO)
eTable 4. Detailed clinical descriptions for 4 previously healthy patients (< 21 years) with acute ischemic stroke
eTable 5. Lumbar puncture and neurodiagnostic imaging results for children and adolescents (<21 years) hospitalized for COVID-19 by severity of neurological involvement
eTable 6. Most abnormal laboratory results for children and adolescents (<21 years of age) hospitalized for COVID-19 by severity of neurological involvement
eTable 7. Detailed clinical descriptions of 3 patients with acute fulminant cerebral edema progressing to brain death
eReferences.
Nonauthor Collaborators. Overcoming COVID-19 Investigators.
References
- 1.Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767-783. doi: 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26(5):499-501. doi: 10.1111/cns.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104-3120. doi: 10.1093/brain/awaa240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018-1027. doi: 10.1001/jamaneurol.2020.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koralnik IJ, Tyler KL. COVID-19: A global threat to the nervous system. Ann Neurol. 2020;88(1):1-11. doi: 10.1002/ana.25807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit Care. 2020. doi: 10.1007/s12028-020-01049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favas TT, Dev P, Chaurasia RN, et al. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol Sci. 2020;41(12):3437-3470. doi: 10.1007/s10072-020-04801-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stafstrom CE, Jantzie LL. COVID-19: neurological considerations in neonates and children. Children (Basel). 2020;7(9):E133. doi: 10.3390/children7090133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. doi: 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bénézit F, Le Turnier P, Declerck C, et al. ; RAN COVID Study Group . Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. 2020;20(9):1014-1015. doi: 10.1016/S1473-3099(20)30297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolay H, Gül A, Baykan B. COVID-19 is a real headache! Headache. 2020;60(7):1415-1421. doi: 10.1111/head.13856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varatharaj A, Thomas N, Ellul MA, et al. ; CoroNerve Study Group . Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882. doi: 10.1016/S2215-0366(20)30287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-58. doi: 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268-2270. doi: 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farhadian S, Glick LR, Vogels CBF, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20(1):248. doi: 10.1186/s12883-020-01812-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etemadifar M, Salari M, Murgai AA, Hajiahmadi S. Fulminant encephalitis as a sole manifestation of COVID-19. Neurol Sci. 2020;41(11):3027-3029. doi: 10.1007/s10072-020-04712-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119-E120. doi: 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delamarre L, Gollion C, Grouteau G, et al. ; NeuroICU Research Group . COVID-19-associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J Neurol Neurosurg Psychiatry. 2020;91(9):1004-1006. doi: 10.1136/jnnp-2020-323678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hepburn M, Mullaguri N, George P, et al. Acute symptomatic seizures in critically ill patients with COVID-19: is there an association? Neurocrit Care. 2020. doi: 10.1007/s12028-020-01006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohal S, Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20:e00782. doi: 10.1016/j.idcr.2020.e00782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AlKetbi R, AlNuaimi D, AlMulla M, et al. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol Case Rep. 2020;15(9):1591-1595. doi: 10.1016/j.radcr.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valiuddin H, Skwirsk B, Paz-Arabo P. Acute transverse myelitis associated with SARS-CoV-2: a case-report. Brain Behav Immun Health. 2020;5:100091. doi: 10.1016/j.bbih.2020.100091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakraborty U, Chandra A, Ray AK, Biswas P. COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep. 2020;13(8):e238668. doi: 10.1136/bcr-2020-238668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2020. doi: 10.1007/s00415-020-10124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uncini A, Vallat JM, Jacobs BC. Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry. 2020;91(10):1105-1110. doi: 10.1136/jnnp-2020-324491 [DOI] [PubMed] [Google Scholar]
- 28.Parauda SC, Gao V, Gewirtz AN, et al. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. 2020;416:117019. doi: 10.1016/j.jns.2020.117019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J Stroke Cerebrovasc Dis. 2020;29(7):104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morassi M, Bagatto D, Cobelli M, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267(8):2185-2192. doi: 10.1007/s00415-020-09885-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi: 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7(5):001691. doi: 10.12890/2020_001691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majidi S, Fifi JT, Ladner TR, et al. Emergent large vessel occlusion stroke during New York City’s COVID-19 outbreak: clinical characteristics and paraclinical findings. Stroke. 2020;51(9):2656-2663. doi: 10.1161/STROKEAHA.120.030397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team . Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347-358. doi: 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334-346. doi: 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aronoff SC, Hall A, Del Vecchio MT. The natural history of severe acute respiratory syndrome coronavirus 2-related multisystem inflammatory syndrome in children: a systematic review. J Pediatric Infect Dis Soc. 2020;9(6):746-751. doi: 10.1093/jpids/piaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaushik A, Gupta S, Sood M, Sharma S, Verma S. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J. 2020;39(11):e340-e346. doi: 10.1097/INF.0000000000002888 [DOI] [PubMed] [Google Scholar]
- 40.Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;S0022-3476(20)30985-9. doi: 10.1016/j.jpeds.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeBiasi RL, Song X, Delaney M, et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J Pediatr. 2020;223:199-203.e1. doi: 10.1016/j.jpeds.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative . Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868-873. doi: 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prata-Barbosa A, Lima-Setta F, Santos GRD, et al. ; Brazilian Research Network in Pediatric Intensive Care, (BRnet-PIC) . Pediatric patients with COVID-19 admitted to intensive care units in Brazil: a prospective multicenter study. J Pediatr (Rio J). 2020;96(5):582-592. doi: 10.1016/j.jped.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindan CE, Mankad K, Ram D, et al. ; ASPNR PECOBIG Collaborator Group . Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2020;S2352-4642(20)30362-X. doi: 10.1016/S2352-4642(20)30362-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin JE, Asfour A, Sewell TB, et al. Neurological issues in children with COVID-19. Neurosci Lett. 2021;743:135567. doi: 10.1016/j.neulet.2020.135567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panda PK, Sharawat IK, Panda P, Natarajan V, Bhakat R, Dawman L. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J Trop Pediatr. 2020;fmaa070. doi: 10.1093/tropej/fmaa070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention . Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). Accessed December 4, 2020. https://www.cdc.gov/mis-c/hcp/
- 48.Venkatesan A, Tunkel AR, Bloch KC, et al. ; International Encephalitis Consortium . Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114-1128. doi: 10.1093/cid/cit458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sejvar JJ, Kohl KS, Bilynsky R, et al. ; Brighton Collaboration Encephalitis Working Group . Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5771-5792. doi: 10.1016/j.vaccine.2007.04.060 [DOI] [PubMed] [Google Scholar]
- 50.Agarwal S, Conway J, Nguyen V, et al. Serial Imaging of virus-associated necrotizing disseminated acute leukoencephalopathy (VANDAL) in COVID-19. AJNR Am J Neuroradiol. 2021;42(2):279-284. doi: 10.3174/ajnr.A6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel-Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020. doi: 10.1001/jamaneurol.2020.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin J, Lawson EC, Verma S, Peterson RB, Sidhu R. Cytotoxic lesion of the corpus callosum in an adolescent with multisystem inflammatory syndrome and SARS-CoV-2 infection. AJNR Am J Neuroradiol. 2020;41(11):2017-2019. doi: 10.3174/ajnr.A6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 54.Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133(1):4-12. doi: 10.1016/j.virusres.2007.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235(2):277-287. doi: 10.1002/path.4461 [DOI] [PubMed] [Google Scholar]
- 56.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264-7275. doi: 10.1128/JVI.00737-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919-929. doi: 10.1016/S1474-4422(20)30308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in vitro models of the human blood-brain barrier. bioRxiv. Posted June 15, 2020.doi: 10.1101/2020.06.15.150912 [DOI] [PMC free article] [PubMed]
- 59.Alberti P, Beretta S, Piatti M, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e741. doi: 10.1212/NXI.0000000000000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalakas MC. Guillain-Barré syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e781. doi: 10.1212/NXI.0000000000000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183(1):16-27.e1. doi: 10.1016/j.cell.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366(24):2294-2304. doi: 10.1056/NEJMra1114525 [DOI] [PubMed] [Google Scholar]
- 63.Kreye J, Reincke SM, Prüss H. Do cross-reactive antibodies cause neuropathology in COVID-19? Nat Rev Immunol. 2020;20(11):645-646. doi: 10.1038/s41577-020-00458-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kowalewski M, Fina D, Słomka A, et al. COVID-19 and ECMO: the interplay between coagulation and inflammation-a narrative review. Crit Care. 2020;24(1):205. doi: 10.1186/s13054-020-02925-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350-361. doi: 10.7326/M20-2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beslow LA, Linds AB, Fox CK, et al. ; International Pediatric Stroke Study Group . Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol. 2020. doi: 10.1002/ana.25991 [DOI] [PubMed] [Google Scholar]
- 67.Appavu B, Deng D, Dowling MM, et al. Arteritis and large vessel occlusive strokes in children following COVID-19 infection. Pediatrics. 2020;e2020023440. doi: 10.1542/peds.2020-023440 [DOI] [PubMed] [Google Scholar]
- 68.Gulko E, Overby P, Ali S, Mehta H, Al-Mufti F, Gomes W. Vessel wall enhancement and focal cerebral arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(12):2348-2350. doi: 10.3174/ajnr.A6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirzaee SMM, Gonçalves FG, Mohammadifard M, Tavakoli SM, Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297(2):E274-E275. doi: 10.1148/radiol.2020202197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majmundar N, Ducruet A, Prakash T, Nanda A, Khandelwal P. Incidence, pathophysiology, and impact of coronavirus disease 2019 (COVID-19) on acute ischemic stroke. World Neurosurg. 2020;142:523-525. doi: 10.1016/j.wneu.2020.07.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radmanesh A, Derman A, Lui YW, et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297(1):E223-E227. doi: 10.1148/radiol.2020202040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rasmussen C, Niculescu I, Patel S, Krishnan A. COVID-19 and involvement of the corpus callosum: potential effect of the cytokine storm? AJNR Am J Neuroradiol. 2020;41(9):1625-1628. doi: 10.3174/ajnr.A6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moonis G, Filippi CG, Kirsch CFE, et al. The spectrum of neuroimaging findings on CT and MRI in adults with coronavirus disease (COVID-19). AJR Am J Roentgenol. 2020. doi: 10.2214/AJR.20.24839 [DOI] [PubMed] [Google Scholar]
- 74.Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. 2020;87:33. doi: 10.1016/j.bbi.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernard-Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020;27(9):e43-e44. doi: 10.1111/ene.14298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi: 10.1007/s00415-020-09990-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375(5):497-498. doi: 10.1056/NEJMc1509458 [DOI] [PubMed] [Google Scholar]
- 78.Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1 pt 1):e73-e76. doi: 10.1542/peds.113.1.e73 [DOI] [PubMed] [Google Scholar]
- 79.Shenker J, Trogen B, Schroeder L, Ratner AJ, Kahn P. Multisystem inflammatory syndrome in children associated with status epilepticus. J Pediatr. 2020;227(Jul):300-301. doi: 10.1016/j.jpeds.2020.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim MG, Stein AA, Overby P, et al. Fatal cerebral edema in a child with COVID-19. Pediatr Neurol. 2021;114:77-78. doi: 10.1016/j.pediatrneurol.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piliero PJ, Brody J, Zamani A, Deresiewicz RL. Eastern equine encephalitis presenting as focal neuroradiographic abnormalities: case report and review. Clin Infect Dis. 1994;18(6):985-988. doi: 10.1093/clinids/18.6.985 [DOI] [PubMed] [Google Scholar]
- 82.Wendell LC, Potter NS, Roth JL, Salloway SP, Thompson BB. Successful management of severe neuroinvasive eastern equine encephalitis. Neurocrit Care. 2013;19(1):111-115. doi: 10.1007/s12028-013-9822-5 [DOI] [PubMed] [Google Scholar]
- 83.Krishnan P, Glenn OA, Samuel MC, et al. Acute fulminant cerebral edema: a newly recognized phenotype in children with suspected encephalitis. J Pediatric Infect Dis Soc. 2020;piaa063. doi: 10.1093/jpids/piaa063 [DOI] [PubMed] [Google Scholar]
- 84.Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26(11):1701-1707. doi: 10.1038/s41591-020-1054-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure. Eligibility flowchart of hospitalized patients with COVID-19-related neurologic involvement, March 15–December 15, 2020
eTable 1. Case definition used in this study for multisystem inflammatory syndrome in children (MIS-C)
eTable 2. Life-threatening COVID-19-related neurologic disorders and outcomes in 43 children and adolescents (< 21 years) hospitalized for COVID-19
eTable 3. Detailed clinical descriptions for 5 patients who experienced stroke while supported by extracorporeal membrane oxygenation (ECMO)
eTable 4. Detailed clinical descriptions for 4 previously healthy patients (< 21 years) with acute ischemic stroke
eTable 5. Lumbar puncture and neurodiagnostic imaging results for children and adolescents (<21 years) hospitalized for COVID-19 by severity of neurological involvement
eTable 6. Most abnormal laboratory results for children and adolescents (<21 years of age) hospitalized for COVID-19 by severity of neurological involvement
eTable 7. Detailed clinical descriptions of 3 patients with acute fulminant cerebral edema progressing to brain death
eReferences.
Nonauthor Collaborators. Overcoming COVID-19 Investigators.