Significance
Treating the transthyretin (TTR) amyloidoses early maximizes clinical response. Soluble nonnative protein oligomers are cytotoxic in cultured cell lines, in primary cells, and in animal models. Here we report an immunoassay for quantifying nonnative, oligomeric TTR (NNTTR) levels in human V30M TTR polyneuropathy plasma (n = 81). High NNTTR pretreatment levels are significant for predicting which patients are less likely to respond to tafamidis (P = 0.0025). The extent of NNTTR decrease does not differ between the tafamidis responders and nonresponders, suggesting that very high pretreatment NNTTR levels could reflect pathogenic processes that might not be ameliorated by solely reducing the amount of amyloidogenic substrate. Liver transplantation, as well as tafamidis and patisiran treatment, all lower NNTTR levels substantially.
Keywords: NNTTR, biomarker, early diagnosis, response to therapy
Abstract
The transthyretin (TTR) amyloidoses (ATTR) are progressive, degenerative diseases resulting from dissociation of the TTR tetramer to monomers, which subsequently misfold and aggregate, forming a spectrum of aggregate structures including oligomers and amyloid fibrils. To determine whether circulating nonnative TTR (NNTTR) levels correlate with the clinical status of patients with V30M TTR familial amyloid polyneuropathy (FAP), we quantified plasma NNTTR using a newly developed sandwich enzyme-linked immunosorbent assay. The assay detected significant plasma levels of NNTTR in most presymptomatic V30M TTR carriers and in all FAP patients. NNTTR was not detected in age-matched control plasmas or in subjects with other peripheral neuropathies, suggesting NNTTR can be useful in diagnosing FAP. NNTTR levels were substantially reduced in patients receiving approved FAP disease-modifying therapies (e.g., the TTR stabilizer tafamidis, 20 mg once daily). This NNTTR decrease was seen in both the responders (average reduction 56.4 ± 4.2%; n = 49) and nonresponders (average reduction of 63.3 ± 4.8%; n = 32) at 12 mo posttreatment. Notably, high pretreatment NNTTR levels were associated with a significantly lower likelihood of clinical response to tafamidis. Our data suggest that NNTTR is a disease driver whose reduction is sufficient to ameliorate FAP so long as pretreatment NNTTR levels are below a critical clinical threshold.
The systemic amyloidoses are progressive human degenerative diseases, diagnosed in part by the presence of insoluble cross–β-sheet amyloid fibrils in various tissues (1). Besides the infiltrative amyloid pathology, it is now appreciated that numerous other aggregates form in vivo (2), and that some of these aggregates are likely to be cytotoxic (3).
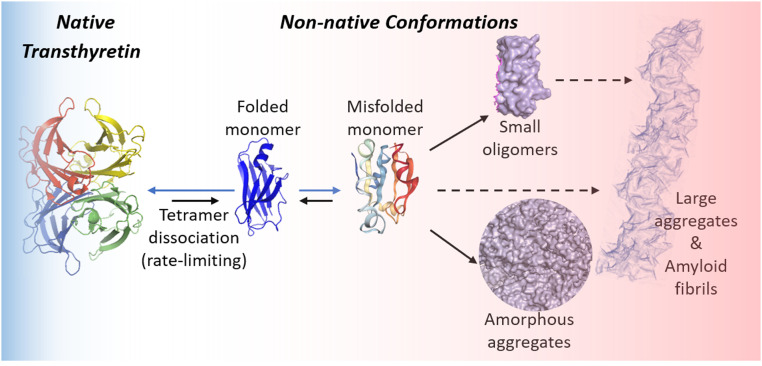
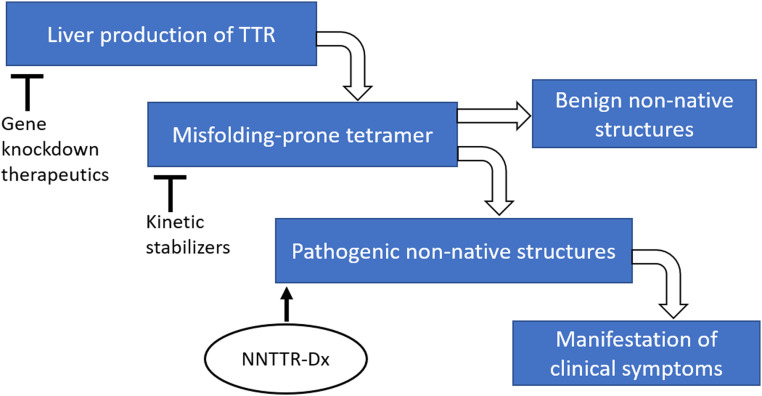
Transthyretin (TTR), a transporter of thyroxine and holo-retinol-binding protein, is synthesized primarily by the liver and secreted into the blood (4, 5). TTR is also produced locally by the choroid plexus and by retinal pigment epithelial cells secreting TTR into the cerebrospinal fluid and eye, respectively (6, 7). The TTR amyloidoses (ATTRs) are caused by rate-limiting dissociation of tetramers comprising wild-type (WT) and/or mutant TTR subunits (Fig. 1) (8, 9), followed by monomer misfolding (10) that enables aggregation into several TTR structures, including soluble nonnative TTR (NNTTR) oligomers and amyloid fibrils (2, 3). In the TTR amyloidoses, amyloid fibrils occupy the extracellular space of clinically affected organs (1), including the heart and autonomic and peripheral nerves. The vast majority of hereditary TTR amyloidosis patients are heterozygotes; thus, the majority of tetramers comprise mutant and WT TTR subunits. The 132 TTR autosomal dominant disease-associated mutations (http://amyloidosismutations.com/) destabilize the TTR heterotetramers, increasing the concentration of the misfolded aggregation-prone monomers and accelerating aggregation (10, 11).
Fig. 1.
Rate-limiting native tetrameric TTR dissociation affording monomers and subsequent monomer misfolding and aggregation produces a large number of NNTTR assemblies.
Familial amyloidotic polyneuropathy (FAP), affecting 15,000 to 50,000 people worldwide (12), is a debilitating hereditary neuropathy involving sensory, motor, and autonomic nervous system dysfunction (13). Patients may also display central nervous system abnormalities, eye pathology, and involvement of other organs (14–17). The average life expectancy in FAP without treatment is 10 to 12 y (18). V30M TTR is a prominent FAP-associated mutant in many countries. Underdiagnosis or delayed diagnosis of FAP is particularly common in genetically nonendemic areas (such as the U.S.), where a positive family history may not exist or may not have been solicited (19). Currently, FAP clinical diagnosis is driven by clinical suspicion based on patient and family history and physical examination, followed by biopsy of abdominal fat, labial epithelium, or a clinically involved tissue, with recognition of congophilic fibrils and identification of TTR as the amyloid precursor by either immunohistochemistry or mass spectrometry (19, 20). Subsequent genetic analysis establishes the presence of a mutation in the TTR gene, the identification of which should be consistent with the amyloid mass spectrometry results. Positive results from both an amyloid biopsy and a genetic analysis are used by most centers to establish the clinical diagnosis. It is sometimes necessary to conduct repeat biopsies at multiple sites to detect amyloid fibrils, as tissue deposition of amyloid is not uniform.
Recently completed randomized, placebo-controlled trials have demonstrated the therapeutic efficacy of four drugs for FAP (21–24). The small molecules diflunisal and tafamidis kinetically stabilize the native tetrameric structure, slowing its dissociation and aggregation (25–27), whereas the TTR mRNA-lowering oligonucleotides patisiran and inotersen suppress the production of mutant and WT TTR by the liver. Reported rates of pharmacologic response in the randomized controlled trials have ranged from 30% to 70%, with a fraction of patients showing functional improvement (28–30).
Prior to the development of kinetic stabilizers and the oligonucleotide therapeutics, liver transplantation, a surgical form of TTR gene replacement, was the standard of care for FAP (18, 31). The liver synthesizing WT and mutant TTR was replaced by a liver carrying two WT TTR genes (32). Long-term observational studies have shown that liver transplantation extends survival and arrests progression of neuropathy in most patients with FAP (18, 31), although the threat of eye pathology and later central nervous system pathology is unchanged. There is compelling evidence that the earlier liver transplantation is initiated for FAP, the better the outcome with respect to survival, arrest of disease progression, and quality of life enhancement (31). The benefit of early treatment with the kinetic stabilizer tafamidis has also been demonstrated in the context of both polyneuropathy and cardiomyopathy (21, 27, 33, 34).
There is no plasma or urine test that is diagnostic for FAP. One useful, but not definitive, metric in FAP patients is a reduced serum TTR concentration relative to that seen in age-, sex-, and ethnicity-matched controls (30, 35, 36). However, there are other conditions, including acute and chronic inflammation, infectious diseases, and malnutrition, that result in low blood TTR concentrations (37, 38). Furthermore, in the TTR oligonucleotide trials, the extent of serum TTR reduction did not directly correlate with clinical neurologic response as measured by the NIS+7 or its variant, mNIS+7 (i.e., Pearson’s r = 0.57 for patisiran and 0.36 for inotersen) (23, 24, 28, 29). This finding suggests that a subset of FAP patients exhibited pathogenetic mechanisms unresponsive to correction of the TTR proteinopathy alone.
Numerous publications have hypothesized that soluble nonnative protein oligomers are the pathogenic drivers of amyloid diseases, based largely on evidence obtained in studies of nonnative oligomer cytotoxicity in cancer cell lines, in cultured primary neurons, or in animal models (39–44). Here we report a sandwich enzyme-linked immunosorbent assay (ELISA) for quantifying nonnative TTR (NNTTR) levels in human V30M TTR FAP patient plasma (n = 81), revealing that high pretreatment NNTTR levels are significant for predicting patients who are less likely to respond to tafamidis (P = 0.0025). The extent of NNTTR lowering was not different between the tafamidis responders and the nonresponders. Liver transplant and patisiran treatment also substantially lowered NNTTR levels. Collectively, our data suggest that high NNTTR levels are indicative of disease progression not likely to be arrested by simply correcting the proteinopathy component of V30M ATTR polyneuropathy. In contrast, in patients with V30M TTR FAP with low pretreatment NNTTR level, tafamidis treatment is generally effective in slowing or stopping disease progression. NNTTR was absent in healthy individuals but was detected in the plasma of many presymptomatic V30M carriers, as well as in all symptomatic V30M TTR FAP patients at generally higher levels, indicating its potential utility as a pathology-associated biomarker. Many presymptomatic V30M carriers are followed yearly in amyloidosis centers worldwide to anticipate the onset of symptoms and initiate treatment as early as possible. Our results suggest that treatment should commence when plasma NNTTR concentration increases above the threshold identified in this paper, that is, before NNTTR concentration increases to a level at which patients are less likely to respond to proteinopathy-based therapies.
Results
NNTTR Antibodies Specifically Recognize NNTTR Conformations.
We used an engineered monomeric version of TTR (M-TTR) as an immunogen, a construct harboring both F87M and L110M mutations that prevents normal dimer and tetramer formation (10). M-TTR is prone to misfolding and slow aggregation when incubated under physiological conditions and to fast aggregation if subjected to mild denaturation stresses, such as elevated temperature or acidic conditions (10). M-TTR displays epitopes that are buried in the native WT TTR tetrameric structure; therefore, M-TTR was used as a recombinant NNTTR, as natively folded M-TTR is always in equilibrium with NNTTR oligomers.
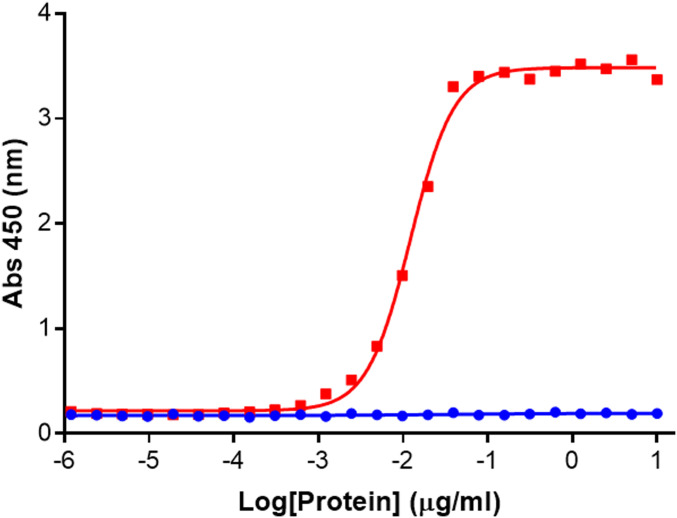
Mouse and rabbit immunizations yielded three antibodies directed against epitopes exposed only on recombinant NNTTR. All three antibodies bind to unique epitopes on NNTTR with subnanomolar affinity (SI Appendix, Fig. S1) and do not bind to natively folded tetrameric TTR (tTTR), as shown in a representative ELISA in Fig. 2. The antibodies specifically recognize a partially thermally denatured M-TTR sample (resulting in misfolded/aggregated TTR) with affinities >1,000-fold greater than those with which they bind freshly purified recombinant native WT tTTR.
Fig. 2.
NNTTR antibodies are specific for misfolded conformations of TTR. Heat-treated M-TTR (50 °C for 120 min) (red) that had been freshly purified (within 24 h) and freshly prepared WT TTR (blue) were titrated using the NNTTR-Dx ELISA. 2504A and 4C1 were used as the capture and detection antibodies, respectively.
NNTTR-Dx ELISA Accurately and Specifically Identifies ATTR FAP Patients Using Plasma.
Using the three antibodies specific for nonnative TTR, we tested multiple configurations of capture and detection antibodies to optimize the NNTTR ELISA. The NNTTR-Dx sandwich assay uses a rabbit polyclonal NNTTR antibody specific for the C terminus of TTR (2504A) as the capture antibody and uses 4C1 as the detection monoclonal antibody. This NNTTR-Dx ELISA configuration exhibited the best clinical correlation (as demonstrated below) and assay performance and thus was used in all ELISA experiments for analysis of the clinical samples. Freshly purified and then thermally denatured M-TTR (50 °C, 120 min) displaying all three NNTTR epitopes recognized by our antibodies was aliquoted, lyophilized, and used as the reference standard to generate a standard curve on which the relative amount of NNTTR of each test sample was calculated.
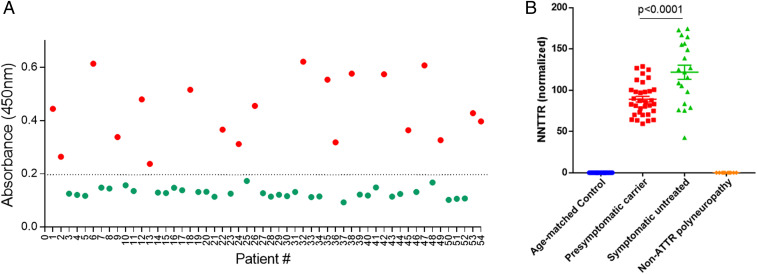
Fig. 3A depicts the results obtained when the NNTTR-Dx assay was performed on blinded plasma samples from either symptomatic Portuguese V30M TTR FAP patients prior to any treatment (n = 20, red circles) or age-matched healthy Portuguese controls (n = 34, green circles). All the patient samples had clearly detectable levels of NNTTR, whereas the signal in the control samples was uniformly below the lower limit of detection (P < 0.001).
Fig. 3.
NNTTR-Dx ELISA accurately and specifically identifies TTR FAP patients and presymptomatic V30M carriers using plasma samples. (A) The assay accurately distinguished 20 V30M TTR FAP patients (red circles) from 34 age-matched controls (green circles) in a set of blinded plasma samples. (B) NNTTR detection in plasma samples from groups of age-matched controls (n = 34), presymptomatic V30M TTR mutation carriers (n = 33), symptomatic pretreatment V30M TTR FAP patients (n = 20), and non-TTR polyneuropathy patients (n = 10, including 3 with diabetic neuropathy, 4 with chronic inflammatory demyelinating polyneuropathy, and 4 with idiopathic neuropathy). NNTTR values are presented as relative values normalized to a pooled pretreatment FAP sample (Materials and Methods). Error bars represent SEM. P values were calculated using one-way ANOVA with Tukey’s multiple comparisons test. All plasma samples used in the experiment are from the Amyloidosis Reference Center (Porto, Portugal), except for the non-TTR polyneuropathy samples, which are from the United States.
To further determine the specificity of the NNTTR-Dx assay, we examined plasma samples from patients with non-TTR polyneuropathies, including diabetic neuropathy, chronic inflammatory demyelinating polyneuropathy, and idiopathic neuropathy (graciously provided by Thomas Brannagan III, Neurological Institute, Columbia University College of Physicians and Surgeons). No signal was detected in the 10 non-ATTR polyneuropathy patient plasma samples (Fig. 3B). Thus, the assay accurately and specifically identified V30M TTR FAP patients using a 5- to 10-μL plasma sample.
Using the NNTTR-Dx assay, we studied plasma from five patients who received livers from symptomatic V30M TTR FAP patients in the course of domino liver transplantation for liver failure due to causes other than TTR amyloidosis (SI Appendix, Fig. S2; iatrogenic liver transplantation). It has been shown that such ATTR liver recipients may develop iatrogenic TTR polyneuropathy or cardiomyopathy due to deposition of mutant TTR produced by the transplanted liver at 3 to 6 y posttransplantation (45, 46). NNTTR was detected in most patient plasma samples tested, albeit at reduced levels compared to untreated FAP patient samples (mean of 35.8 vs. 100 after normalization; difference not statistically significant) (SI Appendix, Fig. S2).
NNTTR Is Detected in Presymptomatic TTR-V30M Carriers.
On the NNTTR-Dx assay, NNTTR was also present but at lower levels in presymptomatic V30M carriers, consisting of 15 females and 18 males (Fig. 3B). Based on clinical evaluation and family history of age of onset, these individuals were estimated to be within a few years of developing symptoms. NNTTR levels are presented as relative values normalized to a pooled pretreatment FAP sample (Materials and Methods).
NNTTR Levels Dramatically Decrease during Treatment.
We performed the NNTTR-Dx ELISA on plasma samples from a group of V30M TTR FAP patients prior to the initiation of tafamidis treatment (20 mg once daily) and after 12 and 24 mo of treatment, using the “tafamidis treatment response” cohort sample set recently studied by Monteiro et al. (30). All samples were assayed blinded with respect to treatment response and treatment status. The patients were followed for at least 1.5 y and up to 5.5 y on treatment, a period sufficient to establish whether they were clinically responsive to the drug. To reduce uncertainty and simplify data interpretation, we only analyzed samples from “tafamidis treatment response” patients (n = 81; Table 1) who were classified clinically as either “responders” (R) or “nonresponders” (NR) based on a comprehensive clinical evaluation and an expert opinion from the attending physicians (30).
Table 1.
“Tafamidis treatment response” cohort demographics (n = 81)
| Characteristic | Value |
| Sex, n (%) | |
| Male | 44 (54.3) |
| Female | 37 (45.7) |
| Age at disease onset, % | |
| 18–49 y | 63.0 |
| 50–69 y | 16.0 |
| 70+ y | 3.7 |
| Baseline NIS, % | |
| 0–9 | 58.0 |
| 10–20 | 23.5 |
| ≥21 | 14.8 |
| Unknown | 3.7 |
| Disease duration, % | |
| 0–2 y | 69.1 |
| ≥3 y | 30.9 |
Patient plasma samples from clinically defined responders and nonresponders from the Centro Hospitalar do Porto (Porto, Portugal) were correlated with baseline NNTTR levels.
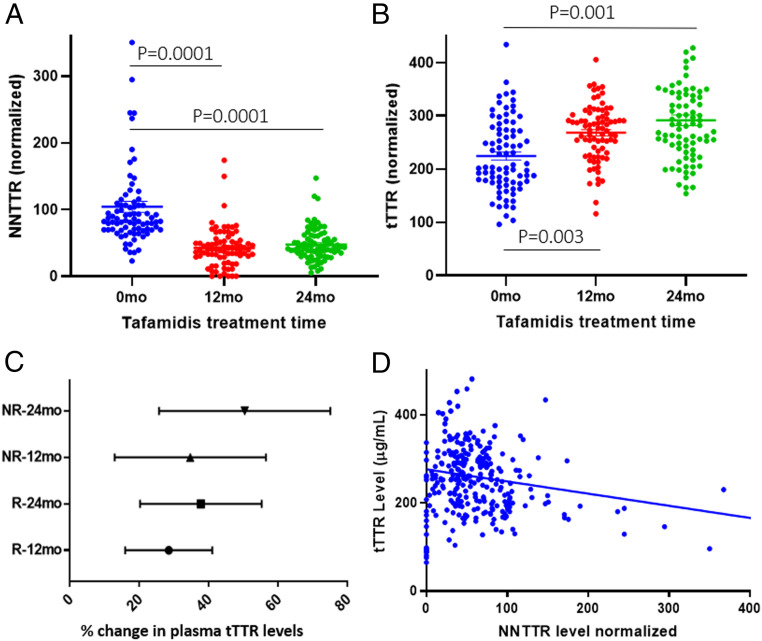
Fig. 4A shows the NNTTR levels before treatment and after 12 and 24 mo of tafamidis treatment (n = 81) in the R and NR cohorts (30). NNTTR levels are significantly reduced at 12 and 24 mo compared with the pretreatment baseline. At the individual level, 97.5% of patients have a reduction in NNTTR from their pretreatment level and >81% have a ≥30% reduction from their pretreatment level (SI Appendix, Fig. S3A; n = 81). Notably, the decrease was seen in both the R (n = 49) and NR (n = 32) groups, with an average reduction in NNTTR level of 56.4 ± 4.2% and 63.3 ± 4.8% for the R and NR groups, respectively, at 12 mo posttreatment.
Fig. 4.
Tafamidis treatment significantly reduced circulating NNTTR levels while increasing tetrameric or tTTR levels in V30M TTR FAP patients (n = 81). (A) NNTTR levels were measured using the NNTTR-Dx assay (one-way ANOVA, Dunnett test, P = 0.0001 vs. pretreatment for both 12 and 24 mo). (B) tTTR levels measured employing a sandwich ELISA using antibody 6H3 as the capture and detection pair (one-way ANOVA, Dunnet test, P = 0.003 and 0.001 for 12 and 24 mo, respectively). All assays were run with blinded plasma samples. (C) % Change in total native TTR levels after tafamidis treatment for the R and NR groups at 12 and 24 mo (range with 95% confidence interval). No statistically significant difference in total native TTR changes was seen among the groups. (D) There is a weak negative correlation (Pearson’s r coefficient is −0.3773) between NNTTR and native TTR levels in plasma samples (n = 219).
In a separate cohort of 57 V30M TTR FAP patients, a significant reduction in NNTTR levels was also seen in patients treated with the TTR kinetic stabilizer tafamidis at as early as 3 mo after initiation of therapy, the earliest time point sampled (SI Appendix, Fig. S3B). Levels remained low for the remainder of the study period in the majority of patients.
In addition to the reduction in NNTTR levels using the kinetic stabilizer, other effective therapeutic interventions yielded similar results. For example, NNTTR levels assessed by the NNTTR-Dx ELISA were significantly reduced in FAP patients after liver transplantation when their livers produced only WT TTR (SI Appendix, Fig. S2). Similarly, V30M TTR FAP patients from a set of participants selected at random from the APOLLO patisiran trial (Onpattro; Alnylam Pharmaceuticals) showed reduced NNTTR levels by the NNTTR-Dx ELISA (SI Appendix, Fig. S4; n = 18) (24). Eighty plasma samples taken from 17 healthy controls at different time points during the Phase I patisiran trial were also tested blinded, with none of the samples showing any detectable level of NNTTR (47).
We concurrently examined the level of tTTR in the plasma samples using the mouse monoclonal antibody 6H3, which selectively recognizes the native tetrameric quaternary structure of human TTR in a sandwich ELISA configuration (see SI Appendix, Fig. S5 for description of the ELISA). Fig. 4B shows that tafamidis treatment significantly increased total tTTR levels in the same patient group, most likely due to its pharmacologic chaperoning activity (48). Posttreatment the plasma concentration of tTTR was close to normal (“normalization effect”), a phenomenon also seen post-liver transplantation but opposite in direction to the reduction seen with RNA interference or antisense oligonucleotide treatment. Tetrameric TTR levels measured by the sandwich ELISA showed increases in both the R (27.6% ± 6.5%) and NR (34.7% ± 6.5%) groups at 24 mo posttreatment (increase not significantly different). The native TTR levels for the R and NR groups (30) were within error at each of the three time points (0, 12, and 24 mo after tafamidis treatment). The total native TTR concentration increases at 12 and 24 mo between the R and NR were also similar (Fig. 4C) according to the antibody 6H3-based ELISA. Notably, there is only a weak negative correlation between NNTTR and tTTR levels assessed by antibody 6H3 in the V30M TTR FAP samples (R = −0.3773) (Fig. 4D), indicating the relative independence of these two biomarkers.
High Baseline Plasma NNTTR Levels Correlate with Poor Clinical Response to Tafamidis, Especially in Males.
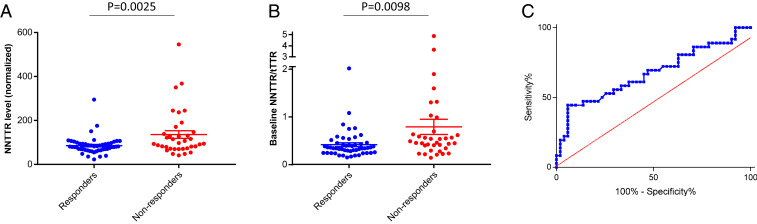
Monteiro et al. (30) proposed an equation wherein baseline native TTR concentrations (determined by the fluorogenic probe A2), 12-mo tafamidis concentrations, sex, and disease severity as measured by a neurophysiology score are the key terms for predicting tafamidis responders and nonresponders. We hypothesized that NNTTR levels before treatment should also be useful as part of an algorithm to predict clinical response. Identification of TTR misfolding and aggregation as major drivers of hereditary TTR polyneuropathy pathology is supported by the positive tafamidis and diflunisal clinical trial results (21, 22), as well as by the clinically observed interallelic trans-suppression of V30M ATTR FAP in V30M/T119M compound heterozygotes (49–51). In the open-label tafamidis study (30), the mean pretreatment NNTTR level was significantly lower in the R group compared with the NR group (85.3 ± 5.6 vs. 135.5 ± 17.5; P = 0.0025, unpaired t test) (Fig. 5A). Thus, a low NNTTR level alone may be a useful prognostic biomarker in predicting response to tafamidis therapy, suggesting either earlier or less severe disease. In fact, 84% (43 out of 51) of the tafamidis responders had a baseline NNTTR level below the mean NNTTR value for the entire R cohort. This result suggests that NNTTR levels could be very useful for indicating when treatment should commence in presymptomatic carriers, with high baseline NNTTR level indicating a lower probability of success with tafamidis (20 mg once daily), particularly when considered in combination with terms of an equation like that proposed by Monteiro et al. (30).
Fig. 5.
High baseline NNTTR levels correlated with poor response to tafamidis treatment. (A) Responders (R, blue) had significantly lower baseline NNTTR levels than nonresponders (NR, red) (P = 0.0025, unpaired t test). (B) Responders (R, blue) had significantly lower baseline NNTTR/tTTR ratio than nonresponders (NR, red) (P = 0.0098, unpaired t test). (C) ROC curve for NNTTR with AUC = 0.6833.
Sex has long been known to play a role in disease penetrance, disease progression, severity, and response to liver transplantation for TTR FAP, with males showing more aggressive disease (46). In the present cohort, female FAP patients had lower NNTTR levels than males at baseline, but the difference was not significant (average level, 97.8 vs. 112.5; P = 0.38) (SI Appendix, Fig. S6). In the responder cohort, 83% (24 of 29) of the female Rs and 86% (19 of 22) of male Rs had a baseline NNTTR level below the mean NNTTR value (106.07) for the entire cohort. Importantly, male V30M TTR FAP patients with low baseline NNTTR levels were more likely to respond to tafamidis treatment.
When baseline NNTTR levels are plotted as a function of the age of disease onset, it appears that a subset of patients with early disease onset (<50 y) have significantly higher NNTTR levels, a trend suggesting that a younger age of disease onset is associated with higher NNTTR levels (SI Appendix, Fig. S7).
In addition, based on the observation that the typical R has lower NNTTR levels and higher total native TTR (tTTR) plasma levels at baseline, we examined the NNTTR/tTTR ratio. Not surprisingly, baseline pretreatment NNTTR/tTTR ratios were significantly different between the R and NR groups (average ratios, 0.41 ± 0.04 units and 0.79 ± 0.16 units, respectively; P = 0.0098, unpaired t test) (Fig. 5B). In fact, 90% of all Rs had a baseline NNTTR/tTTR ratio <0.62, including 95% of the male Rs (21 of 22). A baseline NNTTR/tTTR ratio <0.62 does not ensure that a given patient will respond to tafamidis; however, a ratio >0.62 suggests that the patient is much less likely to respond. A receiver operating characteristic (ROC) curve is shown in Fig. 5C with an area under the curve (AUC) = 0.6833. The data suggest that the NNTTR/tTTR ratio could be very useful in guiding the clinical decision on when treatment should begin, especially as part of an equation.
Discussion
Five positive placebo-controlled clinical trials strongly support the amyloid hypothesis in the context of the TTR amyloidoses (21–24, 27), the notion that the process of TTR aggregation causes the degenerative phenotypes in TTR polyneuropathy and TTR cardiomyopathy (52). Moreover, here we provide evidence that soluble nonnative TTR conformations may directly contribute to the disease phenotypes of FAP. Liver transplantation, as well as tafamidis and patisiran treatment, all significantly lower NNTTR levels while clinically improving TTR polyneuropathy in most FAP patients, evidence consistent with NNTTR being a driver of disease. Moreover, a high NNTTR level prior to treatment makes it less likely that the patient will respond to tafamidis, also supporting the notion that NNTTR is a major contributor to TTR polyneuropathy. It will be interesting to test whether NNTTR pretreatment levels will predict response to other FAP therapies. It is also important to establish whether the NNTTR-Dx assay will be useful in genotypes other than V30M.
Numerous lines of evidence indicate that early treatment of TTR amyloidosis is key to slowing or stopping disease progression (21, 27, 33, 46). To date, early treatment has been hampered by the lack of biomarkers to aid early diagnosis (19). Preventing newly synthesized TTR from aggregating by stabilizing the tetramer (tafamidis, diflunisal) or lowering the TTR level by mRNA degradation (inotersen, patisiran) slows or stops TTR polyneuropathy progression, even though it appears that amyloid fibrils are not cleared on the timescale of the clinical response (21–24, 52). Furthermore, V30M/T119M compound heterozygotes have a milder clinical course than heterozygous V30M/WT individuals because the T119M subunit inclusion in the tetramer kinetically stabilizes it, reducing the quantity of aggregation-prone substrate (i.e., the misfolded monomer), thus inhibiting aggregation (49–51). While we lack a complete understanding of the aggregate structure-proteotoxicity relationship driving nervous system and organ damage in the TTR amyloidoses (2, 52), the reduction in NNTTR levels upon liver transplantation (31), tafamidis/diflunisal treatment (21, 22), and treatment with the TTR mRNA-lowering agent patisiran (23, 24) suggest that the nonnative TTR conformations detected by the NNTTR assay are candidate proteotoxicity drivers that merit further scrutiny (3).
Our data suggest that NNTTR is a disease driver whose reduction is sufficient to improve clinical manifestations of FAP when NNTTR levels are relatively low before treatment. High pretreatment NNTTR level seems to be associated with a form or stage of V30M TTR polyneuropathy, perhaps with sustained inflammation, immune activation, and/or organ dysfunction, that has more than the aggregation of TTR driving pathology (52, 53). For these roughly one-third of polyneuropathy patients with at least two putative disease drivers, we hypothesize that ameliorating the proteinopathy by liver transplantation or by tafamidis, diflunisal, inotersen, or patisiran treatment is necessary but not sufficient to stop polyneuropathy progression (28–30, 53). In other words, lowering NNTTR levels leads to a profound clinical response only in those patients in whom NNTTR was low to begin with and whose V30M TTR polyneuropathy is driven principally by TTR aggregation (including NNTTR formation).
Worldwide, numerous presymptomatic V30M and other FAP mutation carriers are followed annually in amyloidosis centers to ensure that therapy is instituted at the first appearance of symptoms. Our data indicate that treatment should commence before the NNTTR/tTTR ratio exceeds 0.62 points (or its equivalent in fully analytically and clinically validated assays), to maximize the chance of obtaining a good response to tafamidis treatment. It appears from our data that further increases in plasma NNTTR render the patient less likely to respond to tafamidis, and perhaps to TTR proteinopathy therapies in general. We suggest that NNTTR levels and/or the NNTTR/tTTR ratio be measured yearly in asymptomatic carriers, in conjunction with clinical and pathologic measures of peripheral nerve dysfunction (30), such the neurophysiology score (54); intraepidermal, sweat gland, and pilomotor nerve fiber densities (55); nerve lesions based on magnetic resonance neurography (56); and neurofilament light chain and phosphorylated neurofilament heavy chain levels (57, 58), to establish/refine changes or thresholds for initiating therapy at a stage that will maximize the chance of a good clinical response. Polyneuropathy mutation carriers are currently considered asymptomatic even though their intraepidermal, sweat gland, and pilomotor nerve fiber densities are all reduced relative to age-matched controls (55). In addition, asymptomatic polyneuropathy mutation carriers have twice as many nerve lesions relative to controls and one-half as many as patients with a positive amyloid biopsy, based on magnetic resonance neurography (56, 59).
In summary, we propose NNTTR thresholds and/or NNTTR/native TTR tetramer level ratio guidelines as a starting point for using NNTTR as a biomarker to help diagnose TTR FAP earlier using 5 to 10 μL of patient plasma and to help guide treatment initiation. Our data clearly demonstrate that the lower the NNTTR level on initiation of stabilizer therapy, the more likely disease progression will be slowed or stopped by tafamidis treatment; thus, adding a NNTTR level term into emerging equations to predict the likelihood of clinical response is appealing (30). Notably, the NNTTR levels fall in response to liver transplantation, as well as to tafamidis and patisiran treatment, and thus measuring the change in NNTTR level is a promising response-to-therapy biomarker to potentially speed drug development. Finally, changes in NNTTR have the potential to be a surrogate biomarker that is reasonably likely to predict clinical response, or at least part of an equation to do so in the subset of patients with nonnative TTR and/or NNTTR/native TTR ratio below a threshold level. While the data presented herein are based on a research study using blinded clinical samples whenever feasible, further analytical and clinical validations are necessary before the assay, or an improved version thereof, can be fully utilized in clinical decision making (Fig. 6).
Fig. 6.
NNTTR-Dx monitors NNTTR level, an apparent pathogenic driver of TTR proteinopathy.
Materials and Methods
Antibody Generation.
M-TTR, an engineered monomeric form of TTR, generated as described previously (10), and a C-terminal peptide (AALLSPYSYSTTAV) of TTR, were used to immunize mice and rabbits to generate monoclonal and polyclonal antibodies, respectively, following standard procedures. Similarly, WT TTR was used as the immunogen to generate a mouse monoclonal antibody that can recognize native tTTR. Clone 6H3 was identified as an antibody that recognizes native features on tTTR and was used in our assays to quantify native tTTR in biological fluids.
Antibody screening.
Mouse hybridoma supernatant fluids were screened to identify clones specific for nonnative TTR (NNTTR) and with little or no affinity for tTTR. A direct ELISA in which recombinant M-TTR was coated to the assay plate was used to identify lead antibodies. In the direct ELISA, Corning 96-well high-binding EIA/RIA plates were coated with 2 µg/mL M-TTR in 50 mM sodium carbonate/bicarbonate buffer (pH 9.6) and blocked with Superblock (Pierce). Conditioned media from hybridoma clones from neat to a 1:1,000 dilution in TBST were added to the wells and incubated at ambient temperature for 1 h. The wells were washed three times with TBST. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG or Fc antibody was added to detect the monoclonal antibodies bound to the antigen. Excessive HRP was removed by washing three times with TBST, 350 μL per well for each wash. TMB (3,3′,5,5′-tetramethylbenzidine) solution was then added as substrate for HRP color development. The reaction was stopped, and plates were scanned by a plate reader at 450 nm. Positive clones were subjected to additional ELISA screenings.
Positive antibody clones were further assayed in a competition assay in which each hybridoma supernatant was spiked with various conformers of TTR (native tetrameric and nonnative conformations) or bovine serum albumin (BSA) as a negative control. The mixtures were added to ELISA plates precoated with M-TTR, followed by incubation at ambient temperature for 1 h. The wells were washed, and then HRP-conjugated goat anti-mouse IgG antibody was added. TMB was used for detection following the same protocol as in the direct ELISA. Binding to immobilized M-TTR by a number of the hybridoma clones, including the clones used in the current study, was inhibited by NNTTR but not by tTTR, demonstrating the selectivity of these monoclonal antibodies for nonnative conformers.
NNTTR-Dx assay performance.
For quantification purposes, a reference standard was prepared by aliquoting and lyophilizing a batch of heat-treated M-TTR protein. A standard curve using the reference standard is included on each plate to calculate a relative level of NNTTR in each sample. In addition, a calibrator was prepared by mixing plasma samples from 16 FAP patients (symptomatic pretreatment). The calibrator was aliquoted and stored at −80 °C. The calibrator is included on each assay plate, and its value is used to normalize other plasma samples on the same plate. We optimized the assay with respect to the capture/detection antibody configuration, antibody concentration, incubation time, and incubation temperature. Using the M-TTR reference standard, the lower limit of quantification of the NNTTR-Dx assay was determined to be 2.5 to 10 ng/mL. Analytical accuracy was determined using four sample concentrations in five replicates and in three separate assays. Assay accuracy was within ±20% across the concentration range. Intra-assay and interassay precision were also established, showing a coefficient of variation (CV%) <15% (SI Appendix, Fig. S8). Sample freeze-thaw studies showed stable sample NNTTR levels after three separate freeze-thaw cycles.
The NNTTR-Dx ELISA can be performed in 96-well plate format. Using three NNTTR antibodies that recognize different cryptic epitopes, multiple configurations of the sandwich ELISA were evaluated. As expected, different configurations of the sandwich assay detect related but nonidentical NNTTR species. For example, when the same antibody is used as both the capture and detection antibody, only large oligomers and aggregates with multiple copies of the same epitope on the protein complex are detected. We chose an assay configuration that was most consistent in terms of detection sensitivity and specificity when tested against clinical samples.
Biological samples.
Before shipment, all plasma samples were curated and assigned an arbitrary code by the clinician. The samples remained deidentified to the researchers running and analyzing the assays. Plasma samples were obtained from Portuguese asymptomatic V30M TTR carriers and V30M TTR FAP patients participating in a long-term observational study of the therapy of V30M TTR FAP approved by the Ethical and Institutional Review Boards at the Centro Hospitalar do Porto, from Japanese liver transplant recipients with approval by the Ethical Committee of Shinshu University School of Medicine, or from non-ATTR polyneuropathy patients with Institutional Review Board approval from the Columbia University College of Physicians and Surgeons and with approval from The Scripps Research Institute Review Board before subject enrollment. Each subject provided written informed consent. Only patients who were treated with commercially available, physician-prescribed tafamidis were included in the study.
Plasma was prepared from whole blood obtained by venipuncture into BD Vacutainer cell preparation tubes (CPT or PPT) containing sodium citrate or EDTA. Tubes were allowed to stand upright at room temperature for 30 to 45 min, mixed by inversion, and then centrifuged for 20 min at 1,500 RCF at ambient temperature. Plasma was carefully removed to avoid disturbing the mononuclear cell and platelet layer, flash-frozen, and stored at −80 °C. Plasma samples were aliquoted and used with no more than five freeze-thaw cycles. Samples were analyzed by sandwich ELISA performed by an individual blinded to the treatment or response status.
Sandwich assay.
NNTTR-specific antibodies were used to configure sandwich ELISAs following standard procedures. An NNTTR standard was pretreated by repeated heating and cooling of recombinant M-TTR to induce misfolding of the protein. An aliquot of that preparation was used to generate the standard curve on each assay plate. It is important to point out that due to the heterogenous nature of the NNTTR conformations within the standard, the NNTTR value calculated based on the standard curve is likely a proportional representation of the true NNTTR level in the samples. To address this, a calibrator plasma sample prepared by mixing equal volumes of plasma samples from 16 untreated symptomatic FAP patients was included on all assay plates. Its value was arbitrarily set as 100 and used to normalize the NNTTR level of the biological test samples on the same assay plates. All biological fluids used in the sandwich ELISA were diluted 1:10 to 1:200 or until the majority of the samples were in the linear detection range. Data fitting was done by four-parameter variable slope nonlinear regression using GraphPad Prism.
Statistical Analysis.
Assays were performed with stock samples in 96-well plates and their identities blinded to the person performing the experiment whenever possible. After NNTTR levels were calculated, the results were unblinded by a different observer. All statistical analysis were performed using GraphPad Prism 7. For multiple groups or treatment comparisons, 1-way ANOVA was performed using either the Tukey or Dunnett multiple comparisons test. These analyses assume a Gaussian distribution of each sample group and take into account the scatter of all the groups when calculating the P value. When comparing two sets of data containing different groups of patients, an unpaired t test was used, and a one-tailed P value was presented. The exact test used for each study, as well as the sample sizes, are indicated on the individual figure or in the figure legend. All samples assayed are included in the analysis unless outliers are specified and excluded. Outliers were identified in GraphPad Prism using the ROUT method, setting the maximum desired false discovery rate at Q = 0.1%. Such outliers are likely to be associated with damaged samples caused by repeated freeze/thaw cycles.
Supplementary Material
Acknowledgments
We thank Dr. Yoshi Sekijima for providing the valuable plasma samples. The research reported in this paper was supported by awards from the National Center for Advancing Translational Sciences (R43TR002436) and the National Institute of Neurological Disorders and Stroke (U44NS114151), a research grant from Alnylam Pharmaceuticals, NIH Grant DK46335 (to J.W.K. and T.C.), and the Skaggs Institute for Chemical Biology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016072118/-/DCSupplemental.
Data Availability
All study data are included in the main text and SI Appendix.
References
- 1.Schmidt M., et al., Cryo-EM structure of a transthyretin-derived amyloid fibril from a patient with hereditary ATTR amyloidosis. Nat. Commun. 10, 5008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisele Y. S., et al., Targeting protein aggregation for the treatment of degenerative diseases. Nat. Rev. Drug Discov. 14, 759–780 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schonhoft J. D., et al., Peptide probes detect misfolded transthyretin oligomers in plasma of hereditary amyloidosis patients. Sci. Transl. Med. 9, eaam7621 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monaco H. L., Rizzi M., Coda A., Structure of a complex of two plasma proteins: Transthyretin and retinol-binding protein. Science 268, 1039–1041 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Wojtczak A., Cody V., Luft J. R., Pangborn W., Structures of human transthyretin complexed with thyroxine at 2.0 A resolution and 3′,5′-dinitro-N-acetyl-L-thyronine at 2.2 A resolution. Acta Crystallogr. D Biol. Crystallogr. 52, 758–765 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Herbert J., et al., Transthyretin: A choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award. Neurology 36, 900–911 (1986). [DOI] [PubMed] [Google Scholar]
- 7.Cavallaro T., Martone R. L., Dwork A. J., Schon E. A., Herbert J., The retinal pigment epithelium is the unique site of transthyretin synthesis in the rat eye. Invest. Ophthalmol. Vis. Sci. 31, 497–501 (1990). [PubMed] [Google Scholar]
- 8.Colon W., Kelly J. W., Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry 31, 8654–8660 (1992). [DOI] [PubMed] [Google Scholar]
- 9.Lai Z., Colón W., Kelly J. W., The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry 35, 6470–6482 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Jiang X., et al., An engineered transthyretin monomer that is nonamyloidogenic, unless it is partially denatured. Biochemistry 40, 11442–11452 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Hammarström P., Jiang X., Hurshman A. R., Powers E. T., Kelly J. W., Sequence-dependent denaturation energetics: A major determinant in amyloid disease diversity. Proc. Natl. Acad. Sci. U.S.A. 99 (suppl. 4), 16427–16432 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt H. H., et al., Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve 57, 829–837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade C., A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 75, 408–427 (1952). [DOI] [PubMed] [Google Scholar]
- 14.Maia L. F., et al., CNS involvement in V30M transthyretin amyloidosis: Clinical, neuropathological and biochemical findings. J. Neurol. Neurosurg. Psychiatry 86, 159–167 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Sekijima Y., et al., Cerebral amyloid angiopathy in posttransplant patients with hereditary ATTR amyloidosis. Neurology 87, 773–781 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Freitas Castro V., Nascimento Alves P., Franco A. C., Martins I. P., Conceição I., Cognitive impairment in liver transplanted patients with transthyretin-related hereditary amyloid polyneuropathy. Amyloid 24, 110–114 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Martins da Silva A., et al., Age-dependent cognitive dysfunction in untreated hereditary transthyretin amyloidosis. J. Neurol. 265, 299–307 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Coelho T., et al., Natural history and survival in stage 1 Val30Met transthyretin familial amyloid polyneuropathy. Neurology 91, e1999–e2009 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Adams D.; European Network for TTR-FAP (ATTReuNET) , Optimizing the management of transthyretin familial amyloid polyneuropathy in Europe: Early diagnosis and effective care. Curr. Opin. Neurol. 29 (suppl. 1), S1–S2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortese A., et al., Diagnostic challenges in hereditary transthyretin amyloidosis with polyneuropathy: Avoiding misdiagnosis of a treatable hereditary neuropathy. J. Neurol. Neurosurg. Psychiatry 88, 457–458 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coelho T., et al., Tafamidis for transthyretin familial amyloid polyneuropathy: A randomized, controlled trial. Neurology 79, 785–792 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berk J. L.et al.; Diflunisal Trial Consortium , Repurposing diflunisal for familial amyloid polyneuropathy: A randomized clinical trial. JAMA 310, 2658–2667 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson M. D., et al., Inotersen treatment for patients with hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 22–31 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Adams D., et al., Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Bulawa C. E., et al., Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc. Natl. Acad. Sci. U.S.A. 109, 9629–9634 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekijima Y., Dendle M. A., Kelly J. W., Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid 13, 236–249 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Maurer M. S.et al.; ATTR-ACT Study Investigators , Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N. Engl. J. Med. 379, 1007–1016 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Buxbaum J. N., Oligonucleotide drugs for transthyretin amyloidosis. N. Engl. J. Med. 379, 82–85 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Buxbaum J. N., Treatment of hereditary and acquired forms of transthyretin amyloidosis in the era of personalized medicine: The role of randomized controlled trials. Amyloid 26, 55–65 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Monteiro C., et al., Predictive model of response to tafamidis in hereditary ATTR polyneuropathy. JCI Insight 4, e126526 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ericzon B. G., et al., Liver transplantation for hereditary transthyretin amyloidosis: After 20 Years still the best therapeutic alternative? Transplantation 99, 1847–1854 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Holmgren G., et al., Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 341, 1113–1116 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Coelho T., et al., Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J. Neurol. 260, 2802–2814 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waddington Cruz M., et al., Early intervention with tafamidis provides long-term (5.5-year) delay of neurologic progression in transthyretin hereditary amyloid polyneuropathy. Amyloid 23, 178–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson J. L. S., et al., Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circ. Heart Fail. 11, e004000 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinner M., et al., Lowered prealbumin levels in patients with familial amyloid polyneuropathy (FAP) and their non-affected but at-risk relatives. Am. J. Med. Sci. 289, 17–21 (1985). [DOI] [PubMed] [Google Scholar]
- 37.Ingenbleek Y., Bernstein L. H., Plasma transthyretin as a biomarker of lean body mass and catabolic states. Adv. Nutr. 6, 572–580 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sreedhara R., et al., Prealbumin is the best nutritional predictor of survival in hemodialysis and peritoneal dialysis. Am. J. Kidney Dis. 28, 937–942 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Lambert M. P., et al., Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caughey B., Lansbury P. T., Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 26, 267–298 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Hou X., et al., Transthyretin oligomers induce calcium influx via voltage-gated calcium channels. J. Neurochem. 100, 446–457 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Reixach N., Deechongkit S., Jiang X., Kelly J. W., Buxbaum J. N., Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc. Natl. Acad. Sci. U.S.A. 101, 2817–2822 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laganowsky A., et al., Atomic view of a toxic amyloid small oligomer. Science 335, 1228–1231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh D. M., et al., Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Carvalho A., Rocha A., Lobato L., Liver transplantation in transthyretin amyloidosis: Issues and challenges. Liver Transpl. 21, 282–292 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Okamoto S., et al., Liver transplantation for familial amyloidotic polyneuropathy: Impact on Swedish patients’ survival. Liver Transpl. 15, 1229–1235 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Coelho T., et al., Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 369, 819–829 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Sekijima Y., et al., The biological and chemical basis for tissue-selective amyloid disease. Cell 121, 73–85 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Coelho T., et al., A strikingly benign evolution of FAP in an individual found to be a compound heterozygote for two TTR mutations: TTR MET 30 and TTR MET 119. J. Rheumatol. 20, 179 (1993). [Google Scholar]
- 50.Coelho T., et al., Compound heterozygotes of transthyretin Met30 and transthyretin Met119 are protected from the devastating effects of familial amyloid polyneuropathy. Neuromuscul. Disord. 6, 27 (1996).8845715 [Google Scholar]
- 51.Hammarström P., Schneider F., Kelly J. W., Trans-suppression of misfolding in an amyloid disease. Science 293, 2459–2462 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Kelly J. W., Does protein aggregation drive postmitotic tissue degeneration? Sci. Transl. Med. 13, eaax0914 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurian S. M., et al., Peripheral blood cell gene expression diagnostic for identifying symptomatic transthyretin amyloidosis patients: Male- and female- specific signatures. Theranostics 6, 1792–1809 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams D., et al., Familial amyloid polyneuropathy: When does it stop to be asymptomatic and need a treatment? Rev. Neurol. (Paris) 172, 645–652 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Ebenezer G. J., et al., Cutaneous nerve biomarkers in transthyretin familial amyloid polyneuropathy. Ann. Neurol. 82, 44–56 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Kollmer J., et al., Sural nerve injury in familial amyloid polyneuropathy: MR neurography vs clinicopathologic tools. Neurology 89, 475–484 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Kapoor M., et al., Plasma neurofilament light chain concentration is increased and correlates with the severity of neuropathy in hereditary transthyretin amyloidosis. J. Peripher. Nerv. Syst. 24, 314–319 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Ticau S., et al., Neurofilament light chain (NfL) as a biomarker of hereditary transthyretin-mediated amyloidosis. Neurology, 96, e412–e422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kollmer J., et al., In vivo detection of nerve injury in familial amyloid polyneuropathy by magnetic resonance neurography. Brain 138, 549–562 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.