Significance
Antibodies recognizing the neuronal gamma-aminobutyric acid A receptor (GABAA-R) cause severe encephalitis by triggering internalization of the antibody–receptor complexes in inhibitory synapses, which leads to hyperexcitability and dysfunction of neuronal networks. From the cerebrospinal fluid of a patient with GABAA-R encephalitis we cloned a highly expressed antibody and showed that it binds the GABAA-R and influences signal transduction in neurons, explaining clinical symptoms. Using several experimental techniques, we confirmed that the antibody cross-reacts to an oncoprotein which is known to be involved in several malignancies. We showed that cross-reactivity to this oncoprotein may also be detected in two other GABAA-R patients, suggesting that such cross-reactivity is presumably a key event in the pathogenesis of GABAA-R encephalitis.
Keywords: autoimmune encephalitis, GABA-A-receptor encephalitis, autoantibody, paraneoplastic encephalitis, epilepsy
Abstract
Encephalitis associated with antibodies against the neuronal gamma-aminobutyric acid A receptor (GABAA-R) is a rare form of autoimmune encephalitis. The pathogenesis is still unknown but autoimmune mechanisms were surmised. Here we identified a strongly expanded B cell clone in the cerebrospinal fluid of a patient with GABAA-R encephalitis. We expressed the antibody produced by it and showed by enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry that it recognizes the GABAA-R. Patch-clamp recordings revealed that it tones down inhibitory synaptic transmission and causes increased excitability of hippocampal CA1 pyramidal neurons. Thus, the antibody likely contributed to clinical disease symptoms. Hybridization to a protein array revealed the cross-reactive protein LIM-domain-only protein 5 (LMO5), which is related to cell-cycle regulation and tumor growth. We confirmed LMO5 recognition by immunoprecipitation and ELISA and showed that cerebrospinal fluid samples from two other patients with GABAA-R encephalitis also recognized LMO5. This suggests that cross-reactivity between GABAA-R and LMO5 is frequent in GABAA-R encephalitis and supports the hypothesis of a paraneoplastic etiology.
A shared feature of autoimmune encephalitis syndromes are autoantibodies recognizing either intracellular antigens or extracellular epitopes of cell-surface antigens (1–5). Intracellular antigens are primarily released by cellular immune responses and later bound by autoantibodies, whereas cell surface antigens are assumed to be targets of direct humoral immune responses. For both processes, paraneoplastic mechanisms are surmised, and tumors are indeed often detected in conjunction with both types of autoimmunity albeit at different frequencies. However, in some patients a tumor is identified neither at the onset nor during the course of the neurological disease. Here, the immune reaction may have already erased the malignancy or have kept it below the diagnostic detection limit (6, 7).
Encephalitis associated with autoantibodies against the gamma-aminobutyric acid A receptor (GABAA-R) has recently been described (8–11). Anti–GABAA-R antibodies cause GABAA-R cross-linking and internalization of the antibody–receptor complex with a selective reduction of postsynaptic GABAA-R clusters at inhibitory GABAergic synapses (9, 12). This is supposed to cause hyperexcitability and dysfunction of neuronal networks, and thus clinical disease symptoms (1). The immunopathogenesis is heterogeneous and may include viral as well as tumoral triggers of GABAA-R encephalitis: Viral infections of the central nervous system (CNS) may represent triggers of the disease in some patients (13, 14), whereas peripheral tumors are clinically detectable in about one-third of all patients at the time of GABAA-R encephalitis diagnosis (14). The malignancies comprise thymoma, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, multiple myeloma, small cell lung cancer, and rectal cancer (8–10, 13, 15, 16).
Here we describe cloning and functional analysis of a pathogenic antibody from the cerebrospinal fluid (CSF) B cells of a patient with “idiopathic” GABAA-R encephalitis, who has been described earlier and was termed “index patient 2” (IP2) (9). In the CSF and the hippocampus of this patient we have recently detected a strongly expanded CD8+ T cell clone (17). We expressed the dominant antibody from the CSF B cells of patient IP2 (termed Ab-IP2) recombinantly and showed that it binds recombinant α1-subunits of the GABAA-R, recognizes hippocampal neuropil structures, and dampens phasic GABAergic inhibitory synaptic activity and increases excitability in hippocampal CA1 pyramidal neurons. Hybridization to a protein array and confirmation by enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation revealed that Ab-IP2 also recognized LIM-domain-only protein 5 (LMO5, synonym: “Cysteine and glycine-rich protein 2,” CSRP2). We detected cross-reactivity between GABAA-R and LMO5 also in CSF samples from two other patients with GABAA-R encephalitis. Strikingly, all LMO proteins are related to tumor growth and spread (18), and this has also been demonstrated specifically for LMO5 (19–24). This may hint toward a paraneoplastic pathogenesis (induced by detectable or occult tumors) and linked T and B cell responses as a major principle in the immunopathogenesis of GABAA-R encephalitis.
Results
Repertoire Analysis Reveals an Expanded B Cell Clone in the CSF of Patient IP2.
B cell receptor repertoire analysis by next-generation sequencing revealed an almost monoclonal expansion of an IGHV3-21*01 heavy chain (H chain) with an immunoglobulin G1 Fc region in addition to several nonexpanded H chains. Some of them had closely related sequences that differed only in one or few amino acids, suggesting antigen-driven affinity maturation by somatic hypermutation. For the light chains, we found a strongly expanded IGKV3-20*01 κ chain with a short CDR3 region, and again a background of weakly or nonexpanded, diverse L chains. The very strong expansions of a single H chain and a single κ chain suggest that these chains were pairing to yield antibody Ab-IP2 (SI Appendix, Fig. S1). An essentially monoclonal expansion of a cognate B cell clone suggests a highly focused immune response to dominant antigen(s).
Ab-IP2 Recognizes GABAA-R.
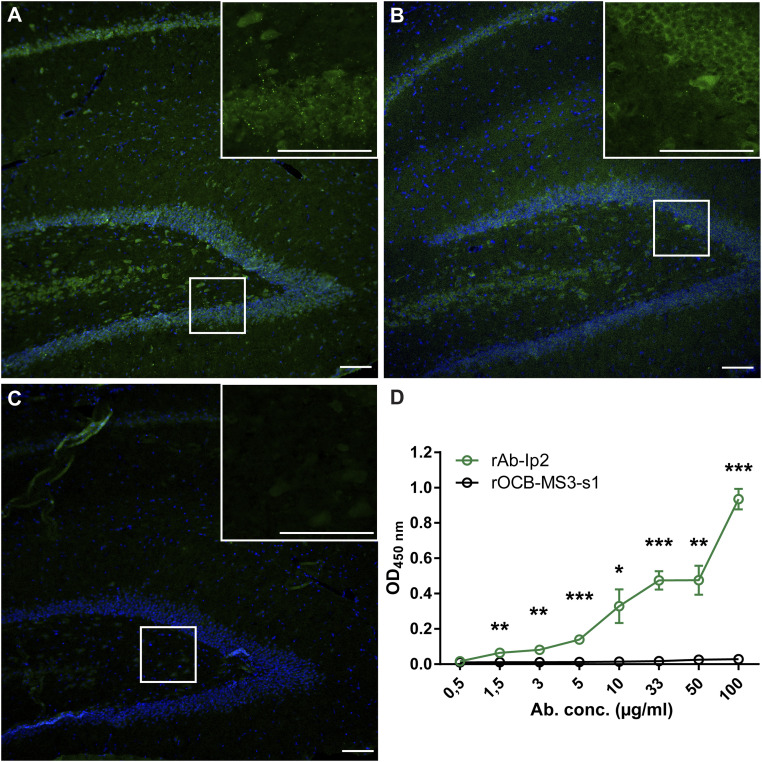
To study the function of Ab-IP2, we cloned, expressed, and purified the recombinant Ab-IP2 (rAb-IP2). Since the amino acid sequences of the extracellular domain of the α1-subunit (amino acids 28 to 251) of the human GABAA-R (GABAA-R-α1ex) in humans, mice, and rats are identical (except for Leu4), we stained serial sections from rat hippocampus with rAb-IP2 (Fig. 1A), a commercial anti–GABAA-R antibody 62-3G1 (Fig. 1B), and with the negative control antibody rOCB-MS3-s1 (25) (Fig. 1C). The staining patterns of rAb-IP2 and 62-3G1 were identical, indicating that rAb-IP2 indeed might recognize GABA-AR in the hippocampal neuropil. We validated this assumption by an ELISA, in which recombinant GABAA-R-α1ex was specifically recognized by rAb-IP2 in a dose-dependent manner (Fig. 1D). GABAA-R-α1ex was not recognized by the control antibody rOCB-MS3-s1 (25). As a further validation, we showed by flow cytometry of HEK293Expi cells that were transfected with the α1- and β3-subunits of GABAA-R that rAb-IP2 recognizes these GABAA-Rs (SI Appendix, Fig. S1).
Fig. 1.
rAb-Ip2 recognizes the extracellular domain of GABAA-R-α1. Immunohistochemical staining of formalin-fixed paraffin-embedded rat hippocampus. (A) Staining with rAb-IP2 (green). Nuclei are stained with DAPI. Isotype control staining was negative. (Scale bar: 100 µm.) (B) Staining with the commercial antibody 62-3G1 to the GABAA-R-α1 subunit (green). (C) Staining with the negative control antibody rOCB-MS3-s1. (D) The ELISA shows that rAb-IP2 recognizes recombinant GABAA-R-α1ex produced in E. coli in a concentration-dependent manner (green). Control antibody rOCB-MS3-s1 does not show any reactivity to GABAA-R-α1ex (black). Error bars indicate SEM, n = 4. Statistical significance was calculated with GraphPad Prism 6 by unpaired t test. *P < 0.05,**P < 0.01,***P < 0.001.
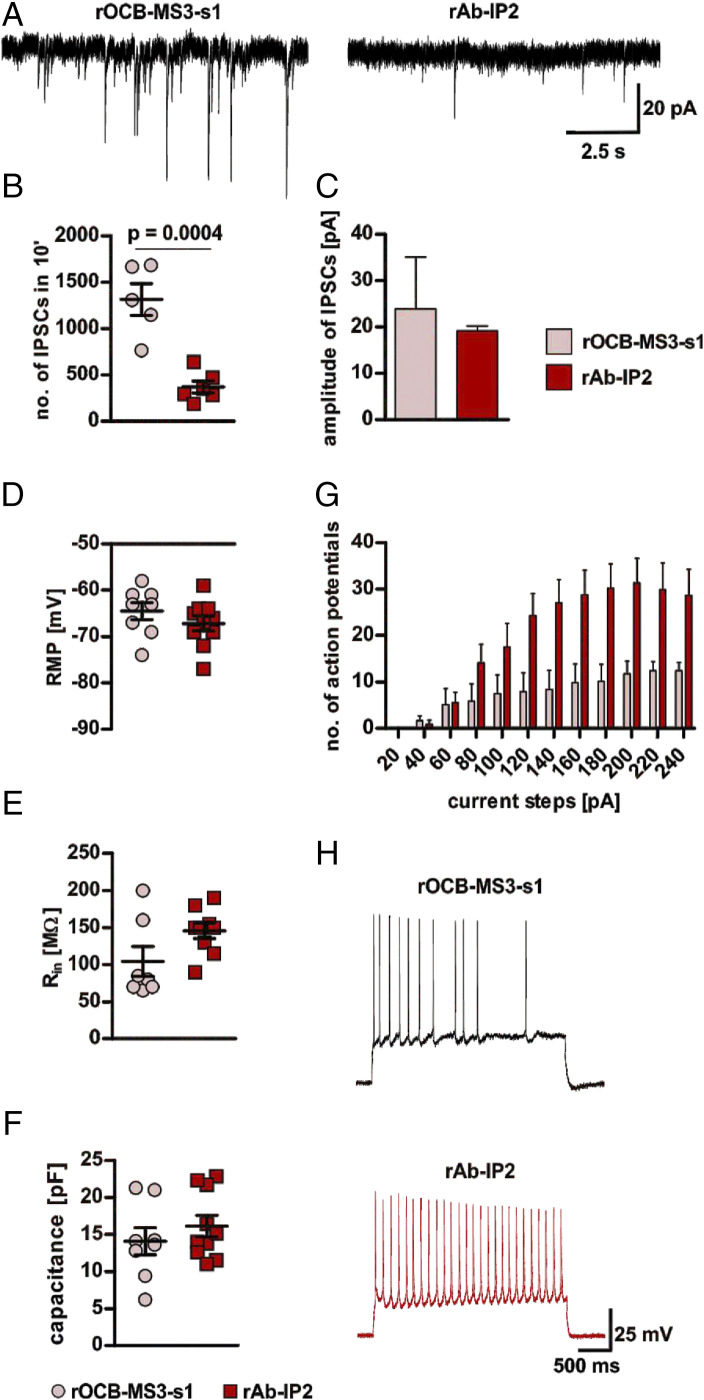
To study effects of rAb-IP2 on neuronal synaptic signaling and excitability we performed electrophysiological recordings in hippocampal CA1 pyramidal neurons using acute mouse brain slices (Fig. 2), characterized by high expression levels of the α1-subunit of GABAA-R (26). Preincubation of slices with rAb-IP2 for 2 h significantly decreased the number of spontaneous inhibitory postsynaptic currents (sIPSCs) occurring in 10 min compared to slices incubated with the negative control antibody rOCB-MS3-s1 (25) (Fig. 2 A and B). Incubation with rAb-IP2 also reduced the amplitude of sIPSC compared to slices incubated with rOCB-MS3-s1, although this change did not reach significance threshold (Fig. 2 A and C). Next, hippocampal CA1 pyramidal neurons were challenged with a series of depolarizing current steps at the individual resting membrane potentials (RMPs) and the number of elicited action potentials (APs) was recorded. Notably, no differences in RMPs were found between the rAb-IP2– and rOCB-MS3-s1–incubated slices (Fig. 2D). Moreover, analysis of the passive electrical properties of CA1 pyramidal neurons, namely the input resistance (Rin) and the whole-cell capacitance, as major determinants of intrinsic neuronal excitability, did not differ between rAb-IP2– and rOCB-MS3-s1–incubated slices (Fig. 2 E and F). However, preincubation of slices with rAb-IP2 reduced the AP firing threshold in CA1 pyramidal neurons compared to rOCB-MS3-s1–incubated slices. Moreover, at a given depolarizing current step, the number of APs in CA1 pyramidal neurons was higher in rAb-IP2–incubated compared to rOCB-MS3-s1–incubated slices (Fig. 2 G and H).
Fig. 2.
Electrophysiological effects of rAb-IP2 on murine hippocampal CA1 pyramidal neurons. (A) Exemplary traces showing sIPSCs recorded from rOCB-MS3-s1– (control; Left) and rAb-IP2–incubated (Right) pyramidal neurons in the hippocampal region CA1. (B) Scatter plot showing that incubation with rAb-IP2 leads to a significant decrease of the number of sIPSCs recorded in an overall period of 10 min in comparison to control (rAb-IP2: 371.8 ± 65.8, n = 6; rOCB-MS3-s1: 1,316.0 ± 171.8, n = 5; unpaired Student’s t test: t = 5.511, degrees of freedom [df] = 9, P = 0.0004). (C) Bar graphs show a tendency to reduced amplitudes of sIPSCs in rAb-IP2–incubated pyramidal neurons in comparison to controls (rAb-IP2: 19.19 ± 0.60 pA, n = 3; rOCB-MS3-s1: 23.9 ± 5.6 pA, n = 4; Mann–Whitney U test: P = 0.4). (D) Scatter plot showing the RMP of rAb-IP2–incubated neurons and controls (rAbIP2: −67.20 ± 1.56, n = 10; rOCB-MS3-s1: −64.5 ± 1.83 mV, n = 8; unpaired Student’s t test: t = 1.128, df = 16, P = 0.2764). (E) Scatter plot showing the Rin of rAb-IP2–incubated neurons and controls (rAb-IP2: 145.6 ± 10.3 MΩ, n = 9; rOCB-MS3-s1: 104.3 ± 20.2 MΩ, n = 7, respectively; Mann–Whitney U test: P = 0.11). (F) Scatter plot showing the whole-cell capacitance of rAb-IP2–incubated neurons and controls (rAb-IP2: 16.12 ± 1.44 pF, n = 10; rOCB-MS3-s1: 14.07 ± 1.82 pF, n = 8, respectively; unpaired Student’s t test: t = 0.8988, df = 16, P = 0.382). (G) Bar graphs showing quantification of APs generated in response to depolarizing current steps of increasing intensity (from +20 to +240 pA, 20-pA increments, duration of 2.5 ms) in rAb-IP2–incubated pyramidal neurons and controls [mixed design two-way ANOVA, main effect of rAb-IP2- vs. rOCB-MS3-s1-incubation: F(1,165) = 6.538, P = 0.02, Sidak’s multiple comparisons test for rAb-IP2- vs. rOCB-MS3-s1: at 120 pA, P = 0.0459; at 140 pA, P = 0.0127; at 160 pA, P = 0.0115; at 180 pA, P = 0.0055; at 200 pA, P = 0.0076 and at 220 pA, P = 0.0257]. Of note, for both groups the input/output relation reflected by the proportional increase of the number of APs in response to the increasing current intensity did not differ, indicating a lower threshold for AP firing in rAb-IP2–incubated cells rather than a defective AP generation in rOCB-MS3-s1–incubated cells (main effect of input/output relation: F(11, 165) = 24,90, P < 0,0001, Sidak’s multiple comparisons test: no significance between groups). (H) Exemplary traces showing APs generated in response to a +140-pA current step at resting membrane potential in rAb-IP2–incubated (red) and rOCB-MS3-s1–incubated (control; black) pyramidal neurons.
Cross-Reactivity of rAb-IP2 to LMO5.
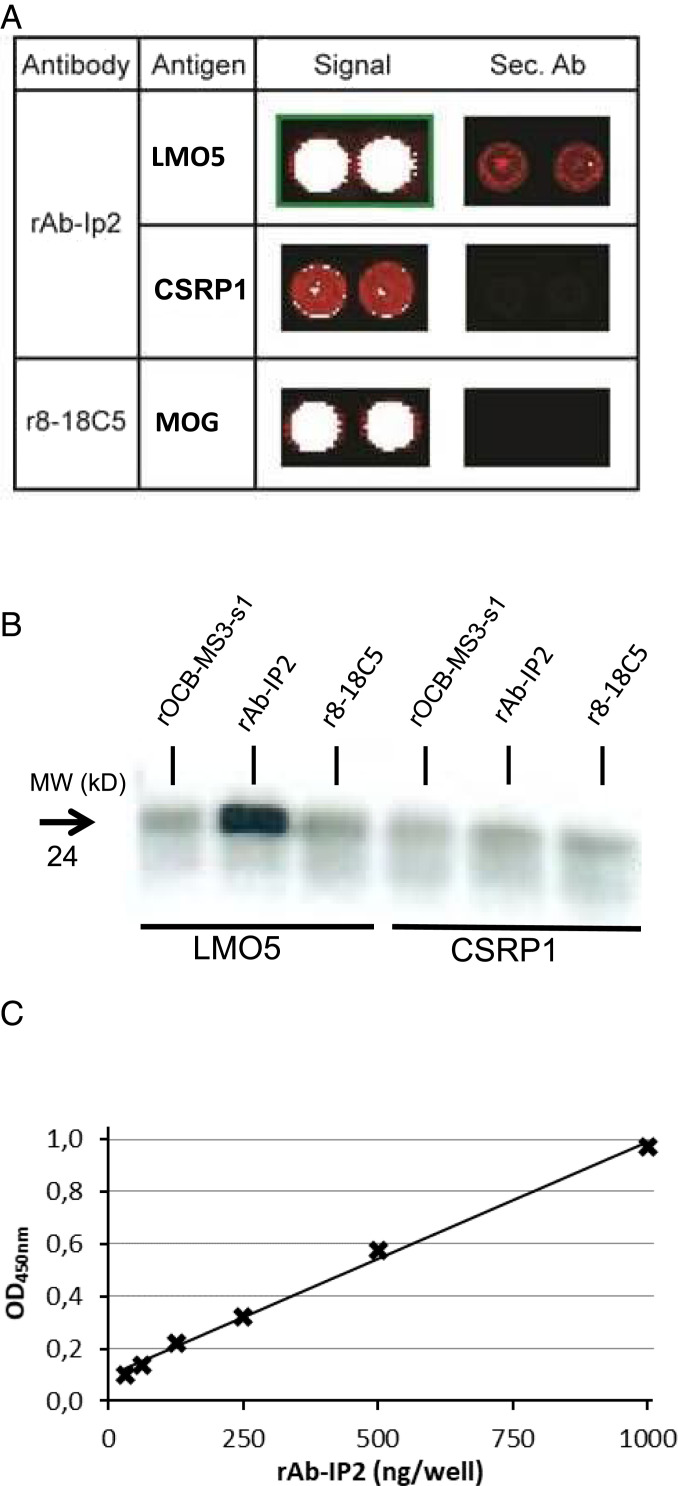
To test whether rAb-IP2 is cross-reactive to other antigens, which might eventually have initiated immune responses, we hybridized rAb-IP2 to a commercial ProtoArray microarray that contained ∼9,400 different human proteins expressed in insect cells. We observed strong signals of rAb-IP2 with several intracellular proteins, including some with LIM domains. LMO5 (synonym: CSRP2) yielded the strongest signals, while other LMO family members, such as CSRP1, were also recognized, although with lower affinity (Fig. 3A). Because all proteins spotted on the ProtoArray were produced in insect cells, we confirmed recognition of LMO5 by rAb-IP2 through immunoprecipitation using commercial recombinant LMO5 produced in HEK293 cells (Fig. 3B). CSRP1 was not immunoprecipitated, consistent with the much lower affinity of rAb-IP2 to CSRP1 as compared to LMO5 seen by array hybridization (Fig. 3A). Furthermore, rAb-IP2 recognized LMO5 produced in Escherichia coli in an ELISA in a dose-dependent manner (Fig. 3C). Taken together, in three independent experiments we showed that rAb-IP2 specifically recognized LMO5.
Fig. 3.
Identification of LMO5 as antigen of Ab-IP2. (A) Hybridization of rAb-IP2 to a protein array. Sections of the developed array are shown. The first column lists the antibodies used, the second column the detected target antigens, the third column the signals of the antigens, and the fourth column the signals of the secondary antibody alone. The color code ranges from black (no reactivity) to red (medium reactivity) and white (strong reactivity). All samples were spotted in duplicate. The upper row shows the detection of LMO5 (synonym CSRP2) by rAb-IP2. The middle row shows the detection of the homologous CSRP1 by rAb-IP2. Both proteins are recognized specifically as the secondary antibody alone yields no or a much weaker signal on the array. The lowest row shows recognition of the positive control antigen major oligodendrocyte glycoprotein (MOG) by the MOG-specific antibody r8-18C5. LMO5 and MOG were detected with high affinity, whereas CSRP1 was detected with low affinity. Reprinted with permission from ref. 32. (B) Validation of LMO5 recognition by rAb-IP2 by immunoprecipitation. Recombinant proteins LMO5 (lanes 1–3) and CSRP1 (lanes 4–6) were produced in HEK293 cells and precipitated with antibodies rOCB-MS3-s1 (lanes 1 and 4), rAb-IP2 (lanes 2 and 5), and r8-18C5 (lanes 3 and 6). Only LMO5 could be precipitated by rAb-IP2. The blot is representative for three independent experiments. (C) Validation of LMO5 recognition by rAb-IP2 by ELISA. LMO5 was coated to plates and detected by rAb-IP2 between 0 and 1,000 µg/mL rAb-IP2 recognition of recombinant LMO5 produced in E. coli occurred in a dose-dependent manner. The shown ELISA is representative for two independent experiments. The parameter for the linear equation is y = 0,0009x + 0,0942, and the correlation coefficient is R2 = 0.9954 with P < 0.001.
LMO5 Is Recognized by CSF Samples from Other Patients with Anti–GABAA-R Encephalitis.
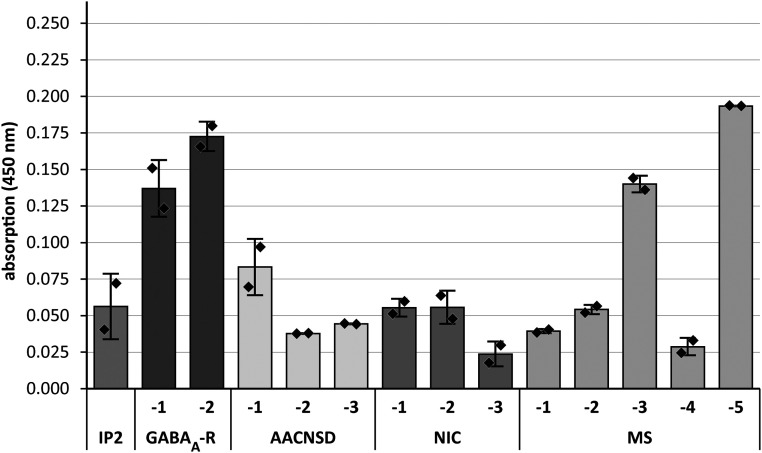
To investigate whether cross-reactivity between GABAA-R and LMO5 was unique to patient IP2, we tested CSF samples from IP2 and two other patients with “idiopathic” GABAA-R encephalitis (GABAA-R-1 and -2). Further, we included three subjects with other forms of antibody-associated CNS diseases (AACNSD-1 to -3), three with noninflammatory neurological diseases (NIC-1 to -3), and five with multiple sclerosis (MS-1 to -5). We could not establish a presumably more sensitive cell-based assay by expressing LMO5 fused to transmembrane domains at the surface of eukaryotic cell lines. We failed presumably because LMO5 is a small intracellular protein with 16 reduced cysteine residues of 193 amino acids in total (https://www.uniprot.org/uniprot/Q16527) which will form disulfide bonds at the cell surface, leading to denaturation of the protein. Furthermore, intracellular overexpression is hampered by the function of LMO5 as a cell-cycle regulator. Therefore, we produced LMO5 in E. coli, purified it to homogeneity, and tested its recognition by CSF supernatants in an ELISA (Fig. 4). In two CSF samples from other patients with GABAA-R encephalitis (GABAA-R-1 and GABAA-R-2) LMO5 was recognized with twofold higher signals than the highest signals in most other samples. This finding was reproducible in two independent experiments, each performed in duplicate. These results suggest that cross-reactivity between GABAA-R and LMO5 is not a unique feature of Ab-IP2 but a more general feature of GABAA-R encephalitis.
Fig. 4.
Detection of anti-LMO5 antibodies in other patients and controls. CSF samples from IP2, two other patients with GABAA-R encephalitis (GABAA-R-1 and -2), three with other forms of antibody-associated CNS diseases (AACNSD-1 to -3), three with NIC (NIC-1 to -3), and five with MS (MS-1 to -5) were tested in an ELISA experiment. Clinical details are given in SI Appendix, Table S1. CSF samples were diluted 1:10. We could also detect significant signals when the samples from GABAA-R-1 and GABAA-R-2 were diluted 1:20 or 1:40, respectively. Relative absorbance units were measured as optical density at 450 nm. A representative experiment of two independent assays is shown. Assays were performed in duplicate. Error bars indicate SDs of duplicates.
Surprisingly, however, CSF from patient IP2 did not recognize LMO5 in this assay—probably because the CSF sample used here was taken briefly before death, that is, it was not identical to the sample used for initial diagnosis (9). In the time between initial diagnosis and death, patient IP2 had received escalated immunotherapy including immediately antibody-depleting treatments (ref. 9 and SI Appendix, Table S1). In the CSF sample drawn at initial diagnosis, the detection limit of Ab-IP2 was 1:320 (ref. 9 and SI Appendix, Table S1). In our CSF sample, however, it was decreased to 1:32, that is, it was 10-fold lower. Furthermore, the LMO5 ELISA used here is not as sensitive as the cell-based assay that was used for detecting GABAA-R binding. MS-3 and MS-5 also recognized LMO5, which is expressed at high levels in brain tissue (https://www.proteinatlas.org/ENSG00000175183-CSRP2/tissue). CNS tissue destruction caused by long-lasting inflammation has presumably released LOM5 from the cytosol of destroyed cells, leading to antibody responses against this obviously highly immunogenic protein (19–24). Thus, high anti-LMO5 antibody titers in MS-3 and MS-5 are consistent with our earlier observation that recognition of intracellular ubiquitous proteins is often seen in MS subjects with oligoclonal bands (25). Notably, samples of GABAA-R-1 and GABAA-R-2 were obtained at the disease onset and exhibited no oligoclonal bands (SI Appendix, Table S1), indicating a more specific immune response. Except MS-3 and MS-5, all other control CSF samples from subjects with AACNSD, NID, and MS yielded considerably lower signals than GABAA-R-1 and GABAA-R-2.
Discussion
Analysis of the B cell receptor repertoire in the CSF of patient IP2 revealed a strongly expanded B cell clone that produces the dominant antibody Ab-IP2. By three independent experiments we could demonstrate that rAb-IP2 recognizes the extracellular domain of GABAA-R. First, we observed identical neuropil staining patterns in immunohistochemistry of hippocampal tissue sections as with a commercial anti–GABAA-R antibody. Although not a strict proof, this suggests that rAb-IP2 recognizes GABAA-R. Second, rAb-IP2 strongly and specifically bound to the extracellular domain of the α1-subunit of the human GABAA-R in an ELISA in a dose-dependent manner. Third, we could show that rAb-IP2 is functional because it reduced the amplitude and frequency of spontaneous postsynaptic GABAergic events in hippocampal CA1 pyramidal neurons. This is consistent with a reduction of postsynaptic GABAA-R clusters at GABAergic inhibitory synapses and a dampening net effect on the interneuron network activity. Both effects lead to a strongly reduced AP firing threshold and increased AP firing of hippocampal CA1 pyramidal neurons. These disinhibiting effects on neuronal function may form the basis of the clinical symptoms of patients with GABAA-R encephalitis (8–10).
We identified LMO5 as a major target of rAb-IP2, and in addition we found reactivity to several other proteins with lower affinities. Some, but not all, of these target proteins contained LIM domains. Although the “original” antigen that recruited Ab-IP2 in patient IP2 will remain elusive, it is tempting to speculate that LIM-domain proteins might have been released from (clinically occult) peripheral tumor cells following antitumoral immunity, most probably initiated by CD8+ T cells. Of note, we have recently described a CD8+ T cell clone that was strongly expanded in the CSF and in the hippocampus of patient IP2 but not in the operculo-insular cortex (17). Thus, a CD8+ T cell-mediated early antitumor response in the periphery might have released intracellular proteins and cryptic epitopes of oncoproteins might then have initiated production of Ab-IP2, which accidentally was cross-reactive to GABAA-R in the CNS. The parallel expansions of a B and T cell clone might indicate a functional link between these two main constituents of the adaptive immune system both in the periphery and the CNS, although the antigens recognized by the B and T cell clones do not necessarily have to be identical. However, an initiation of the adaptive immune response within the CNS eventually induced by an (occult) viral infection (27) with CD8+ T cell-mediated tissue destruction, release of intracellular LMO5, and formation of Ab-IP2, which then cross-reacts with the GABAA-R, appears less likely but cannot formally be excluded. Thus, the association of GABAA-R encephalitis with clinically detectable peripheral tumors in about one-third of all patients (14) and the possibility of an even higher portion of patients with clinically occult peripheral malignancies strongly argues in favor of the initiation of the autoimmune response by a neoplastic process in the periphery.
Cross-reactivity between a neural cell surface and an intracellular oncoprotein points toward such a paraneoplastic pathogenesis. All LIM-domain proteins are located intracellularly, often involved in regulating cell growth, and thus related to tumor growth and metastasis (28). In particular, all LMO proteins are involved in tumor outgrowth (15). Indeed, LMO5 has recently been related as an oncoprotein to a variety of hematological malignancies (19–21, 23) and solid tumors (22) and might also be involved in other malignancies. Likewise, the association of GABAA-R encephalitis with hematological and solid tumors has been described before (8–10, 13–16). The pattern of cross-reactivity between GABAA-R and LMO5 observed for rAb-IP2 might be quite common in GABAA-R encephalitis as we could detect anti-LMO5 reactivity in two other patients with GABAA-R encephalitis. The signals were not very strong and variable in the control CSF samples, but the signals found for GABAA-R-1 and GABAA-R-2 in two independent experiments were reproducibly increased twofold and significantly higher than in controls. Although our cohort is small, and more patients need to be tested, our data suggest that cross-reactivity between GABAA-R and LIM-domain proteins is not restricted to patient IP2. Detecting such common patterns in the pathogeneses of patients with GABAA-R encephalitis might help to develop rational strategies for early diagnosis and therapy.
Materials and Methods
Clinical Samples.
Clinical data of patients with GABAA-R encephalitis and control subjects with other forms of antibody associated CNS diseases, multiple sclerosis, or noninflammatory neurological diseases are given in SI Appendix, Table S1.
To test CSF samples for antibodies against GABAA-R, we used a cell-based assay with fixed HEK293 cells expressing either α1- and β3-subunits or α1-, β3-, and γ2-subunits of the GABAA-R or a mock control. This assay was shown to be equally sensitive to a cell-based assay with living HEK293 cells (16).
Clinical data of patient IP2 are described (9). The CSF sample analyzed here was collected briefly before death. CSF samples of other patients with GABAA-R encephalitis were collected within the German Network for Research on Autoimmune Encephalitis (https://generate-net.de/). CSF samples from patients with MS, AACNSD, and NID were collected at the Ludwig Maximilians University of Munich. The study was approved by the ethics committees of the University of Valencia, Spain (PT13/0010/0026), Munich, Germany (project 163-16), Kiel, Germany (AZ 13-162), and Münster, Germany (AZ 2013-682-b-S). All participants gave written informed consent prior to study conduct, according to the principles of the Declaration of Helsinki. All mice were kept under pathogen-free conditions and had access to food and water ad libitum. All experiments were conducted according to the German law and were approved by local authorities (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen).
B Cell Receptor Repertoire Analyses by Next-Generation Sequencing.
To analyze the B cell receptor repertoire, RNA was isolated from 1.5 mL of a fresh CSF sample and transcribed into complementary DNA (cDNA). Reverse transcription and two rounds of PCR amplification were carried out as described (29) except that the reverse inner primers for the second PCR were tagged with multiplex identifiers for sample identification. Nested multiplex PCR products for immunoglobulin G (IgG) H, κ, and λ chains obtained from each sample were purified with QIAquick Gel Extraction Kit (Qiagen) and pooled in equimolar concentrations. The mixtures were further processed by the sequencing service at FISABIO, Valencia, Spain, where adaptors A and B were ligated and the samples sequenced with a Roche GS FLX sequencer and Titanium chemistry, which yields long enough reads to identify full-length Vn(D)nJ sequences of the H and L chains. Productively rearranged Ig sequences were analyzed using the program IMGT/HighV-QUEST tool.
Expression and Characterization of rAb-IP2.
The IGHV3-21*01 and IGκV3-20*01 chains were obtained by gene synthesis (Invitrogen) or amplified from cDNA, respectively. They were cloned into the expression plasmid pTT5, which adds V5- and His6-tags to the H chains, cotransfected and expressed in HEK-293E cells, purified by immobilized metal affinity chromatography (IMAC), and characterized as described (25).
Recognition of GABAA-R was confirmed by immunohistochemistry, ELISA, and flow cytometry. Immunohistochemistry was performed on hippocampal slices from Wistar rat brain. The tissue was fixed with 4% paraformaldehyde overnight in freezing compound medium, sectioned, and dried. After heat-induced epitope retrieval, slides were blocked with 2% bovine serum albumin, 10% fetal calf serum (Sigma-Aldrich), 0.1% Tween20 (Karl Roth), 1% hu/mouse/rb serum in PBS (Dako) and stained with rAb-IP2 (50 µg/mL), the mouse anti-GABAA-R antibody MAB339 (1:500; Merck) that recognizes the α-subunits of GABAA-R, and with the negative control antibody rOCB-MS3-s1 (50 µg/mL). As secondary antibodies the antibodies Alexa-Fluor-488 goat anti-mouse IgG (1:1,000; Invitrogen), and goat anti-human IgG-AF488 (1:1,000; Invitrogen), respectively, were used. Nuclei were stained with DAPI and autofluorescent background was reduced using Sudan Black B (0.1% in 70% ethanol; Sigma-Aldrich). Signal was visualized using an inverted Leica DMi8 epifluorescence microscope.
To validate recognition of GABAA-R by ELISA, the extracellular domain of the α1-subunit of the human GABAA-R (GABAA-R-α1ex) was produced in E. coli as fusion protein with a thioredoxin-His6 tag. From the soluble fraction, GABAA-R-α1ex was purified by IMAC, coated overnight to Costar ELISA plates at 15 µg/mL at 4 °C, and exposed to varying concentrations of rAb-IP2 or the control antibody rOCB-MS3-s1 (25) for 1 h at 37 °C. For detection we used a rabbit anti-human IgG H and L chain horseradish peroxidase (HRP)-conjugated secondary antibody (1:10,000; Abcam). Signals were detected using 3,3′,5,5′-tetramethylbenzidine (Invitrogen) at 450 nm.
For flow cytometry, HEK293Expi cells (Thermo Fisher) were transiently transfected with different molar ratios of the plasmids pTriEx-1-GABARA1 and pTriEx-1-GABARB3p, which contain either cDNA of the GABAA-R α1- or β3-subunit. The cells were grown in Expi293TM Expression Medium (Thermo Fisher) for 48 h, washed three times with phosphate-buffered saline (PBS) containing 5 µM ZnCl2, and incubated with 5 µg/mL of either rAb-IP2, MAB339, or rOCB-MS3-s1 for 30 min on ice. After three washing steps, the cells were incubated with either goat anti-human IgG-AF-488 (1:500; Invitrogen) or goat anti-mouse IgG-AF-488 (1:500; Invitrogen) for 30 min on ice, resuspended in FACS buffer containing TO-PROTM-1 iodide (1:6,000; Thermo Fisher), and analyzed using a BD FACSVerse (BD Biosciences) and the software FlowJo (Tree Star).
Functional Recognition of GABAA Receptor by rAb-IP2.
Functional recognition of GABAA-R was validated by electrophysiology in acute coronal brain slices from C57BL/6 mice. The mice were anesthetized (4% isoflurane in O2) and the brain was quickly removed and acute coronal brain slices were obtained by cutting 300-µm-thick slices using a vibratome (Leica). Recordings were performed on visually identified pyramidal neurons of the CA1 hippocampal region. Slices were continuously perfused with an extracellular solution (artificial cerebrospinal fluid) containing 120 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 22 mM NaHCO3, 20 mM C6H12O6, 2 mM CaCl2, and 2 mM MgSO4, set to pH of 7.35 with carbogen. Next, in order to test its effect on neuronal function, brain slices were incubated for 2 h with 4.84 µg/mL rAb-IP2 or rOCB-MS3-s1 as control. Control slices were kept under the same conditions in the absence of rAb-IP2. Recordings were performed using glass-pipette electrodes pulled from borosilicate glass (GC150TF-10; Clark Electromedical Instruments), connected to an EPC-10 amplifier (HEKA Elektronik). Typical electrode resistance was 4 to 5 MΩ, with a series resistance in the range of 5 to 15 MΩ (compensation ≥25%). All recordings were governed by Patchmaster software (HEKA Elektronik) and corrected for the liquid junction potential.
Spontaneous GABAergic synaptic activity was recorded in whole-cell configuration in the voltage clamp mode using pipettes filled with a KCl-based, high-chloride intracellular solution containing 10 mM NaCl, 110 mM KCl, 11 mM EDTA, 10 mM Hepes, 1 mM MgCl2, 0.5 mM CaCl2, 15 mM phosphocreatin, 3 mM Mg-ATP, and 0.5 mM Na-GTP, set to pH 7.25 with KOH and osmolality of 295 mOsmol/kg. Spontaneous GABAergic activity was analyzed by recording for 10 min and then calculating number and amplitude of sIPSCs obtained by clamping the cells at a potential of −70 mV in the presence of the glutamate receptor blockers DNQX (10 µM), an AMPA/kainate receptor blocker, and AP-5 (10 µM), a NMDA-receptor blocker. Analyses were performed using Mini Analysis (Statsoft) and Fitmaster.
RMP and APs were recorded in whole-cell configuration in the current clamp mode using pipettes filled with a K gluconate-based intracellular solution containing 10 mM NaCl, 88 mM K gluconate, 20 mM K3 citrate, 10 mM Hepes, 3 mM BAPTA, 15 mM phosphocreatine, 1 mM MgCl2, 0.5 mM CaCl2, 3 mM Mg-ATP, and 0.5 mM Na-GTP, set to pH 7.25 with KOH and osmolality of 295 mOsmol/kg. The RMP was registered in current clamp mode by adjusting the injected direct current (DC) to 0 pA. For experiments evaluating the AP firing pattern of hippocampal neurons, a series of depolarizing current steps (from +20 to +160 pA, increments of 20 pA and duration of 2.5 s) was performed at RMP by adjusting the DC current. Number and amplitude of elicited APs were analyzed as described before (30). The Rin and the whole-cell capacitance of CA1 pyramidal neurons were obtained as described before (31).
Recognition of LMO5 by rAb-IP2 and by CSF Samples from Other Patients and Controls.
rAb-IP2 and the control antibody r8-18C5 were hybridized to ProtoArrays (Invitrogen) as described (25).
Recognition of LMO5 was validated by immunoprecipitation and ELISA. For immunoprecipitation, rAb-IP2 or the control antibodies r8-18-C5 and OCB-MS3-s1 (both ref. 20) were bound to magnetic Protein G beads (Thermo Fisher), incubated with 2 µg LMO5 or CSRP1 produced in HEK293 cells (both Origene), and washed six times with PBS. Antigens were eluted with sodium dodecyl sulfate loading buffer. Eluates were detected by Western blotting as described (25).
For ELISA, cDNA of human LMO5, extended for a 3C-proteinase cleavage site, a V5 tag and a His6 tag at its C terminus, was cloned into pET19b and expressed in E. coli BL21 at 30 °C in the presence of 10 µM ZnCl2. After lysis by ultrasound and ultracentrifugation, LMO5 was purified from the supernatant in the presence of 20 mM Hepes (pH 7.5), 350 mM NaCl, 20 µM ZnCl2, and 0.1% β-mercaptoethanol ± 250 mM imidazole by IMAC, dialyzed against 20 mM Hepes (pH 7.5), 150 mM NaCl, 5 µM ZnCl2, and 0.1% β-mercaptoethanol and digested by His6-tagged HRV-3C protease (Sigma). The digestion products were applied to another IMAC column, untagged LMO5 eluted at the above buffer with 75 mM imidazole, and dialyzed as above. For control samples, exactly the same purification was performed from E. coli BL21 transfected with pET19b that lacked LMO5 but carried all tags described above. For ELISA, high-binding 96-well plates (Costar) were coated with 10 µg/mL LMO5 or corresponding fractions from the mock-plasmid purification. After blocking by 50% Blocker Casein in Tris-buffered saline (TBS) (Thermo Fisher) in dialysis buffer for 2.5 h, either 0 to 1,000 µg/mL rAb-IP2 or 1:10 diluted CSF samples from patients, controls, or no CSF (all diluted in TBS + 5% Blocker Casein in TBS) were added for 2 h. Plates were washed with TBS-T (TBS + 0.01% Triton X-100), followed by incubation in goat-anti-human IgG HRP antibody (Dianova; 1:5,000 diluted TBS + 5% Blocker Casein in TBS) and washing steps. The ELISA was developed by TMB Substrate Solution (Invitrogen) and absorbance measured at 450 nm. For analysis, data of the samples without CSF were subtracted from the data of patients and controls. The sensitivity limit of this assay was 0.5 µg/mL as determined by titrating purified rAb-IP2.
Statistics.
For the electrophysiological experiments, statistical significance was analyzed using Prism6 (GraphPad) two-way factorial ANOVA in case of multiple comparisons, followed by Bonferroni post hoc test, while comparisons of two groups was performed using Student’s t test. For ELISA experiments, SDs of the mean are given. Statistical significance was calculated with GraphPad Prism 6 by unpaired t test and values of *P < 0.05, **P < 0.01, and ***P < 0.001 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Simone Mader and Naoto Kawakami for comments on the manuscript; Joachim Malotka, Reinhard Mentele, and Monika Wart for expert technical assistance; Zoe Hunter for editing the manuscript; and the Core Facility Bioimaging of the Biomedical Center of the Ludwig Maximilians University of Munich. The study was supported by the Else Kröner Fresenius Foundation (grant 2016_A115), the Wilhelm Sander Foundation (grant 2011,113.1,2), and the German Research Council through grants CRC128-A5 and the Munich Cluster for Systems Neurology (SyNergy, grant EXC 2145, projekt ID 390857198), the German Federal Ministry of Science and Education (grant CONNECT GENERATE; 01GM1908), and the Münster Cells-in-Motion Cluster of Excellence (grant FF2014-05). Part of the electrophysiological data are included in the medical thesis of A.F.M.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916337118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Dalmau J., Graus F., Antibody-mediated encephalitis. N. Engl. J. Med. 378, 840–851 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Melzer N., Meuth S. G., Wiendl H., Paraneoplastic and non-paraneoplastic autoimmunity to neurons in the central nervous system. J. Neurol. 260, 1215–1233 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bost C., Pascual O., Honnorat J., Autoimmune encephalitis in psychiatric institutions: Current perspectives. Neuropsychiatr. Dis. Treat. 12, 2775–2787 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent A., Bien C. G., Irani S. R., Waters P., Autoantibodies associated with diseases of the CNS: New developments and future challenges. Lancet Neurol. 10, 759–772 (2011). [DOI] [PubMed] [Google Scholar]
- 5.van Coevorden-Hameete M. H., de Graaff E., Titulaer M. J., Hoogenraad C. C., Sillevis Smitt P. A., Molecular and cellular mechanisms underlying anti-neuronal antibody mediated disorders of the central nervous system. Autoimmun. Rev. 13, 299–312 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Titulaer M. J.et al.; European Federation of Neurological Societies , Screening for tumours in paraneoplastic syndromes: Report of an EFNS task force. Eur. J. Neurol. 18, 19-e3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalmau J., Rosenfeld M. R., Paraneoplastic syndromes of the CNS. Lancet Neurol. 7, 327–340 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohkawa T., et al., Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J. Neurosci. 34, 8151–8163 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petit-Pedrol M., et al., Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: A case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 13, 276–286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettingill P., et al., Antibodies to GABAA receptor α1 and γ2 subunits: Clinical and serologic characterization. Neurology 84, 1233–1241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor K., et al., GABAA receptor autoimmunity: A multicenter experience. Neurol. Neuroimmunol. Neuroinflamm. 6, e552 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou C., et al., Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome. J. Biol. Chem. 288, 21458–21472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spatola M., et al., Investigations in GABAA receptor antibody-associated encephalitis. Neurology 88, 1012–1020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo C. Y., Gelfand J. M., Geschwind M. D., Anti-gamma-aminobutyric acid receptor type A encephalitis: A review. Curr. Opin. Neurol. 33, 372–380 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Simabukuro M. M., et al., GABAA receptor and LGI1 antibody encephalitis in a patient with thymoma. Neurol. Neuroimmunol. Neuroinflamm. 2, e73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lancaster E., Encephalitis, severe seizures, and multifocal brain lesions: Recognizing autoimmunity to the GABAA receptor. Neurol. Neuroimmunol. Neuroinflamm. 6, e554 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bracher A., et al., An expanded parenchymal CD8+ T cell clone in GABAA receptor encephalitis. Ann. Clin. Transl. Neurol. 7, 239–244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews J. M., Lester K., Joseph S., Curtis D. J., LIM-domain-only proteins in cancer. Nat. Rev. Cancer 13, 111–122 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Midorikawa Y., et al., Identification of genes associated with dedifferentiation of hepatocellular carcinoma with expression profiling analysis. Jpn. J. Cancer Res. 93, 636–643 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Z., et al., The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics 7, 96 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann C., et al., CRP2, a new invadopodia actin bundling factor critically promotes breast cancer cell invasion and metastasis. Oncotarget 7, 13688–13705 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlick B., et al., Serum autoantibodies in chronic prostate inflammation in prostate cancer patients. PLoS One 11, e0147739 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann C., et al., Hypoxia promotes breast cancer cell invasion through HIF-1α-mediated up-regulation of the invadopodial actin bundling protein CSRP2. Sci. Rep. 8, 10191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S. J., et al., Cysteine and glycine-rich protein 2 (CSRP2) transcript levels correlate with leukemia relapse and leukemia-free survival in adults with B-cell acute lymphoblastic leukemia and normal cytogenetics. Oncotarget 8, 35984–36000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brändle S. M., et al., Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc. Natl. Acad. Sci. U.S.A. 113, 7864–7869 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelson H. B., Wong R. K., Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science 253, 1420–1423 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Schuster S., et al., Fatal PCR-negative herpes simplex virus-1 encephalitis with GABAA receptor antibodies. Neurol. Neuroimmunol. Neuroinflamm. 6, e624 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sala S., Ampe C., An emerging link between LIM domain proteins and nuclear receptors. Cell. Mol. Life Sci. 75, 1959–1971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermeier B., et al., Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 14, 688–693 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Cerina M., et al., Thalamic Kv 7 channels: Pharmacological properties and activity control during noxious signal processing. Br. J. Pharmacol. 172, 3126–3140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaesse P., et al., μ-Opioid receptor-mediated inhibition of Intercalated neurons and effect on synaptic transmission to the central amygdala. J. Neurosci. 35, 7317–7325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brändle S. M., “Analysis of oligoclonal band antibodies from patients with neurological diseases,” PhD thesis, Ludwig Maximilians University of Munich, Munich, Germany (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.