Abstract
Acute Hepatopancreatic Necrosis Disease (AHPND) is a newly emerging shrimp disease with mortality up to 100 percent caused by Vibrio parahaemolyticus which carries a plasmid encoding for two toxins, ToxA and ToxB. In 2013, the Global Aquaculture Alliance (GAA) estimated shrimp farming decline in Asia accounted for 1-billion US dollar lost. Currently, diagnosis using PCR method does not meet the demand of in situ detection, which is based on antigen-antibody interaction, has not been developed yet. In this present study, we proceeded to create the toxin and its antibody for lateral flow development. First, recombinant toxin ToxA was generated by gene manipulation. After that, purified ToxA was used to immunize rabbits. Finally, antisera from rabbits and protein-A purified antibodies were evaluated for titer, specificity, and detection threshold. Results showed that recombinant ToxA was overexpressed in soluble fraction at 37oC with 1mM IPTG. Purification by affinity chromatography was able to isolate recombinant ToxA with the purity up to 94.49%. In ELISA experiment, the immunized antisera reached a titer of up to 1/5,210,000 with 1µg/ml of antigen, and detection threshold was 100ng recombinant toxin. After purification, the detection threshold of purified polyclonal antibodies was 25ng toxin per dot. These results laid a groundwork for the development of AHPND detection kit based on antigen - antibody interactions.
Key Words: AHPND, Vibrio parahaemolyticus, Polyclonal antibodies, Recombinant toxin, ToxA; Immuno-interactions
INTRODUCTION
In 2018, shrimp production was estimated at more than 4 million tons (FAO) [1], with an export value of more than 19 billion US dollar, hence shrimp farming has a great potential. However, shrimp farming faces many diseases [2, 3], such as white spot disease [4], yellow head disease [5, 6], and a newly emerging disease named acute hepatopancreatic necrosis (AHPND). AHPND was first discovered in China in 2009 [7], spread to Vietnam in 2010, and continued to spread to Malaysia and Thailand in 2011 and 2012 [8, 9], Mexico in 2013 [10]. After that, AHPND also spread to the Philippines in 2015 and South America in 2016 [11], and first reported in the United State of America in 2017 [12] . The disease spreads quickly and causes death with mortality rate up to 100% within 30-35 days of pond-stocking [13-15]. Therefore, AHPND has caused huge damage to shrimp farming, from 2009 to 2016 shrimp production losses due to AHPND has been reported is estimated to be about 23 billion US dollars [16] and AHPND caused a sharp decline in shrimp production. In Vietnam, AHPND is the leading cause of damage to shrimp farming. The disease appeared in many provinces, affected about 39,000 ha of shrimp farming, causing damage of about 100 million US dollars [13]. The way to effectively prevent AHPND spreading is to develop a fast and early detection kit.
In 2013, the cause of AHPND was confirmed as Vibrio parahaemolyticus [17]. Then, studies in 2014 and 2015 indicated that only strains carrying plasmid coding for two toxins ToxA (13kDa) and ToxB (50kDa) could cause disease [18-20]. Base on this discovery, many PCR kits were developed to detect AHPND. Primers such as AP1, AP2 [21], AP3 [22] for one step PCR or AP4 for nested PCR [23], and multiplex PCR using primers VpPirA-284 and VpPirB-392 [24] opened the door for AHPND detection because of their high sensitivity and accuracy. However, PCR method takes time to get a result. It also requires equipment and laboratory for processing PCR test, thus farmers have to send their samples to diagnosis centers. This in turn wastes critical time for preventing the spread of AHPND. Therefore, a fast, simple in situ test kit is a meet need for the farmers in this situation which lateral flow assay (LFA) kit comes to the place [25]. The assay was successfully developed for diagnosis of white spot syndrome virus [26] and yellow head virus [27]. LFA bases on specific interactions between antigen and antibody, and in this case ToxA and ToxB were the antigens of focus in detection AHPND by LFA. In 2017, monoclonal antibodies against two toxins were generated. Threshold of these antibodies was 3ng for recombinant ToxA, and 0.78ng for ToxB, respectively [28]. Moreover, the cost of monoclonal antibody is quite high, and the process of production is quite complicated, which leads to increase the total cost for LFA mass production. Thus, polyclonal antibody is an alternative in this condition, because of low cost in production and easier in generation.
Recent study predicts that the function of ToxA is to bind to shrimp cell(s) and initiate pathogenesis process [29, 30]. This makes ToxA is the first target for AHPND detection. Therefore, in this present study, polyclonal antibodies specific for ToxA toxin of Vibrio parahaemolyticus causing AHPND in shrimp were generated and evaluated. These antibodies will be the source for lateral flow assay kit development based on immuno-interactions.
MATERIALS AND METHODS
Strains of bacteria, and plasmids: Vibrio parahaemolyticus strains causing AHPND was provided by the Research Sub-Institute for Aquaculture in Nam Song Hau, Vietnam. Vibrio parahaemolyticus AHPND strain XN89 (VPAHPND), Vibrio parahaemolyticus non-AHPND strain XN8 (VPnon-AHPND) served as positive and negative controls, respectively was kindly provided by Dr. S. Senapin, National Center for Genetic Engineering and Biotechnology (BIOTEC), Thailand. Vibrio cholerae, Vibrio vulnificus, Vibrio alginolyticus, and White Spot Syndrome virus was kindly provided by Dr.Tuan V. Vo from University of Agriculture and Forestry, Ho Chi Minh City, Vietnam. Escherichia coli DH5α and Escherichia coli BL21(DE3) were used as strains to replicate recombinant vectors and to express recombinant protein, respectively; pET22b plasmid was used for cloning as well as target gene expression driven by T7 promoter through IPTG inducer.
Generation of E. coli BL21(DE3) strain carrying recombinant vector pET22b-toxA: The gene coding for ToxA toxin (toxA) was obtained from V. parahaemolyticus AHPND strain XN89 by PCR method with specific primers, toxA-F and toxA-R, negative control was a PCR without DNA template from V. parahaemolyticus. Next, toxA gene and plasmid pET22b were digested with two restriction enzymes, NdeI and XhoI and ligating using T4 ligase. The ligated product was transformed into E.coli DH5 and cultured on Luria broth (LB) agar containing 100µgmL-1 ampicillin and incubated overnight at 37oC. After incubated, colonies on LB agar were screened by PCR with toxA-F and T7ter primers (pairing at the T7 terminator region on the plasmid pET22b), recombinant vector was collected from a positive colony and sequenced. After that, recombinant vector pET22b-toxA was transformed into E. coli BL21(DE3) and cultured on LB agar medium with 100µgmL-1 ampicillin and screened with the same procedure as E.coli DH5. All results of PCR method were analyzed by electrophoresis on agarose 1.5% with 1kb ladder maker (Bioline, UK).
Expression of recombinant ToxA: Strain of E.coli BL21(DE3)/pET22b-toxA was cultured in 5mL LB medium containing ampicillin (100µgmL-1), at 37°C, for 16 hours. Then, sub-cultured bacteria at 1:20 (v/v) dilution continued at 37°C until OD600 reached 0.6 to 0.8. IPTG was added into the culture medium with a final concentration of 1 mM and cultured at 37°C, for 4 hours. Next, 1.5mL bacterial broth was pelleted, mixed with 300µL solution A (20 mM phosphate, 0.5M NaCl, pH 7,4), sonicated and subsequently centrifuged to collect total protein, soluble protein, and insoluble protein fractions. Expression of the recombinant protein was confirmed by SDS-PAGE with Coomassie Blue staining and Western blot probed with 6xHis antibody. Control samples were total fraction of E. coli BL21(DE3) with IPTG induction, E. coli BL21 (DE3)/pET22b with IPTG induction and E. coli BL21(DE3)/pET22b-toxA non-IPTG induction.
Purification of recombinant ToxA by affinity chromatography: The expression of E.coli BL21 (DE3)/pET22b-toxA strain was made with the scale of 100mL LB medium. Soluble protein fraction was purified by affinity chromatography with HistrapTM column HP (GE Healthcare). The column was equilibrated with solution A, then was loaded with total protein fraction. Next, the column was re-equilibrated with solution A and washed with solution B (20 mM phosphate 0.5M NaCl, 90 mM imidazole, pH 7.4). Finally, the target protein was eluted with solution C (20 mM phosphate, 0.5M NaCl, 108 mM imidazole, pH 7.4). After purification, protein ToxA was concentrated and changed to PBS buffer using 10 kDa MWCO concentrator. Result of purification ToxA was verified by SDS-PAGE with silver staining, and analyzed by Gel Analyzer software.
Production of polyclonal antibody against ToxA: All rabbits were maintained in the experimental animal facility, and experiments were performed in accordance with the guidelines provided by the Animal Care and Use Committee of Pasteur Institute in Ho Chi Minh City. Briefly, one hundred μg of recombinant ToxA in 1mL of NaCl 0.9% and 1mL of Complete Freund's Adjuvant (CFA) were emulsified for primary immunization. Each 2mL emulsion was immunized into 3 male rabbits. Before immunization, rabbit serums were collected as negative controls for antibody titer evaluation. Every 4 weeks, the rabbits were given immunization with Incomplete Freund's Adjuvant (IFA) and the amount of ToxA was reduced to 75µg for the first repetition and 50µg for the next three repetitions. Rabbit antiserum were obtained two weeks after each injection.
Evaluating antiserum titer: Antiserum titer was evaluated by ELISA[31]. Recombinant ToxA was mixed with 0.1M carbonate buffer, pH 9.6 with final concentration of 1µgmL-1 and incubated 100µL each well at room temperature overnight. After that, the ELISA plate was washed four times with PBS-T (PBS with 0.05% Tween). Next, wells were blocked with 5% skim milk in PBS, incubated at 37°C for 1 hour and washed 4 times with PBS-T. Then, diluted concentrations from 1/5,000 to 1/5,210,000 of antiserum in PBS with 1% skim milk were added and incubated for 1 hour at 37°C. Diluted pre-bleed at 1/5,000 was used at negative control. After washing four times with PBST, HRP conjugated goat anti-rabbit IgG secondary antibody was added and incubated at 37°C for 1 hour before washing step with PBS-T. Finally, TMB substrate was added to all wells, incubated for 5 mins before adding 100µL stop solution (H2SO4 2N). Results were read by ELISA (Multikan Ascent) plate reader at 450nm.
Specificity evaluation of antiserum: Proteins from strains VPAHPND, VPnon-AHPND, total protein E.coli BL21(DE3)/pET22b-toxA and purified recombinant ToxA protein were used for specificity evaluation of antiserum by Western blot. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 3% skim milk, antiserum was diluted to 1/10,000 and incubated for 1 hour. Then HRP conjugated goat anti-rabbit IgG was added and incubated for 1 hour. Finally, TMB substrate was added and incubated for 15 minutes. Results on membrane was digitalized documented.
In addition, the specificity of antiserum was evaluated by Dot blot method with strains V. cholerae, V. vulnificus, V. alginolyticus, and White Spot Syndrome virus. Cell lysis of bacteria were fixed onto nitrocellulose membrane and next steps were conducted as the same as Western blot method. The color dots on membrane were digitalized documented.
Purification of polyclonal antibodies and sensitivity evaluation: Polyclonal antibodies were collected from antisera by combining two methods, precipitation with 50% ammonium sulphate and chromatography with protein A column. The purity was analyzed by SDS- PAGE and silver staining. Antiserum and polyclonal antibodies were evaluated for sensitivity by Dot blot method with three difference samples including recombinant ToxA protein, protein from VPAHPND and VPnon-AHPND. The samples were diluted in two-fold dilution manner, starting from 800ng, and fixed onto nitrocellulose membrane. Next steps were performed as the same as Western blot method. The color dots on membrane were digitalized documented.
RESULTS
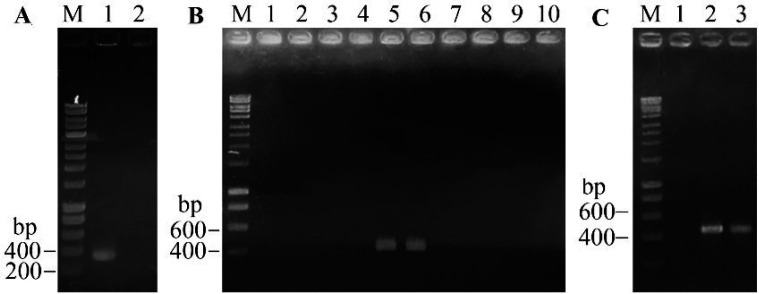
Gene encoding for toxA was obtained from the AHPND using PCR method and checked by electrophoresis on 1.5% agarose gel. The results of electrophoresis showed a single band (lane 1, Fig. 1A), and there was no band in a negative control on gel. After ligation and transformation, colonies were screened by PCR with toxA-F and T7ter primers. As expected, the toxA gene would be inserted into a region between T7 promoter and T7 terminator of plasmid pET22b, thus an amplification sequence length was 446bp. The electrophoresis results showed positive colonies with predicted band (lanes 5, 6, Fig. 1B). In addition, the negative control did not show any band, indicating that no contamination occurred during PCR (lane 1, Fig. 1B).
Figure 1.
Results of electrophoresis on agarose gel. (A) Isolation of gene toxA. M, ladder. Lane 1, gene toxA. Lane 2, negative control. (B) Screening colonies from pET22b-toxA candidates. M, ladder. 1, negative control. 2-10, candidate colonies. (C) Screening BL21(DE3)/pET22b-toxA strains. M, ladder. 1, negative control. 2-3 candidate colonies
The result was confirmed again by sequencing plasmids obtained from positive colonies. Sequencing result showed that a typical recombinant clone obtained was 100% homologous to 3HP pathogenic strain published in the GenBank [18, 32] (data not shown).
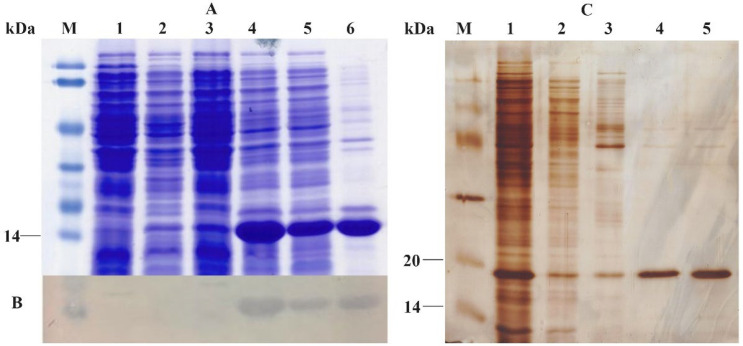
In E. coli BL21(DE3)/pET22b-toxA strain, ToxA protein was overexpressed as a recombinant protein about 14kDa (lane 5, Fig. 2A), a predicted size of ToxA, in soluble fraction. Also, there was no band of target protein in control samples. Next, the expression of ToxA was confirmed indirectly using Western blot probed with anti-6xHis antibody. Western blot result showed that the overexpressed protein in SDS-PAGE gel was ToxA protein with 6xHis fusion (Fig. 2B).
Figure 2.
Results of SDS-PAGE (A), Western blot (B) of expression and purification (C) ToxA. In (A) and (B) M, protein ladder. 1, E. coli BL21(DE3) (+1mM IPTG). 2, E. coli BL21(DE3)/pET22b (+1mM IPTG). 3, E. coli BL21(DE3)/pET22b-toxA (-IPTG); 4-6. E.coli BL21(DE3)/pET22b (+IPTG) total, soluble, insoluble proteins. (C) M, protein ladder. 1, total protein. 2, flow-through fraction. 3 washing fraction. 4-5, elution fractions
After that, ToxA protein was purified by Ni2+ chromatographic column. Then, purified fractions were analyzed using SDS-PAGE and Gel Analyzer software. As shown in Figure 2C, flow through and washing fractions (Fig. 2C, lanes 2-3) appeared many protein bands, including partial ToxA protein. Evaluation by Gel Analyzer showed that ToxA protein in elution fraction had a purity of more than 94%, and recovery efficiency of purification process was 40.29%.
Table 1.
Quantity and recovery efficiency of purification protein ToxA
| Volume (ml) |
Concentration
(µg/ml) |
Percent of protein (%) | Quantity of protein (µg) | Recovery efficiency (%) | |
|---|---|---|---|---|---|
| Total protein Purified protein |
20 | 1808 | 21.01 | 7235.62 | |
| 1.5 | 2088 | 94.49 | 2959.43 | 40.29 |
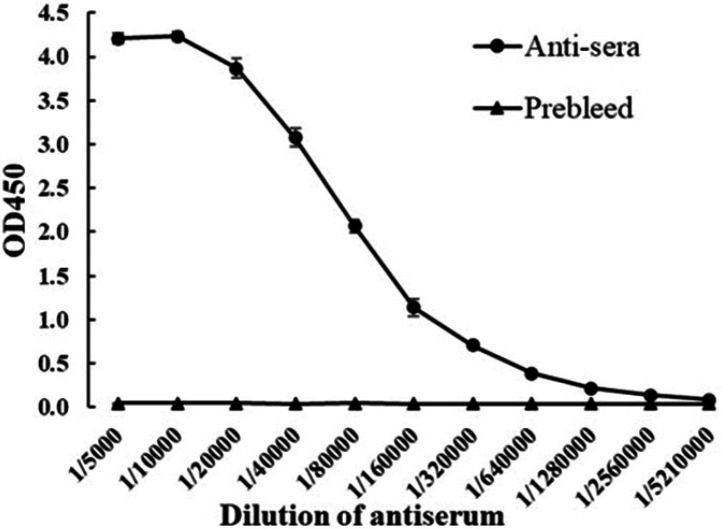
Antibody titer evaluation was performed by indirect ELISA with 1µgmL-1 antigen and antiserum diluted from 1/5,000 to 1/5,210,000. Signals were measured at 450nm and graphed in Figure 3. The antiserum titer was defined as the highest dilution that still gives a positive value. The result showed that antiserum gave a high signal from 1/5,000 and 1/10,000 dilutions. After that, the signal began gradually declined at next dilutions and reached the cut-off value (mean 3SD) [33] at 1/5,210,000 dilution. Collectively, the titer of antiserum was 5,210,000. Along with that, there was no signal in the negative control, indicating serum before immunization did not react with ToxA.
Figure 3.
ELISA results of evaluation antiserum titer
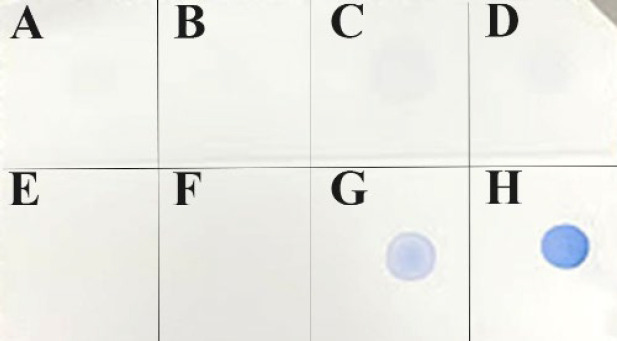
Bacteria and virus, which usually reside in shrimp were used for evaluating the specificity of antiserum by Dot blot method (Figure 4). Result showed that only two dots of recombinant ToxA and VPAHPND gave positive signals, other samples including VPnon-AHPND strain, V. cholerae, V. vulnificus, V. alginolyticus, and White Spot Syndrome virus (WSSV) showed no signal.
Figure 4.
Dot blot result of specific interaction between polyclonal antibody and V. alginolyticus (A), V. vulnificus (B), V. cholerae (C), V. parahaemolyticus XN8 (D), White Spot Syndrome virus (C), V. parahaemolyticus XN89 (G), and recombinant ToxA (H).
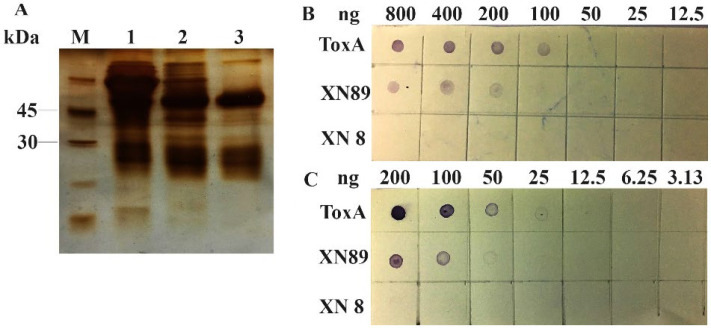
Antibodies in antiserum were precipitated by ammonium sulfate 50% and purified by affinity chromatography using protein A column, and analyzed by SDS-PAGE. Results showed that the precipitation fraction with 50% ammonium sulfate has not removed completely unwanted proteins (lane 2, Figure 5A). Therefore, we purified the precipitated fraction to eliminate these unwanted proteins by protein A column. The results in lane 3, figure 5A showed that, there was no band of unwanted protein as in precipitation fraction. In the presence of 1,4-Dithiothreitol (DTT), IgG was split into two clusters of protein, a heavy chain about 50kDa and light chain about 25-30kDa. Thus, polyclonal antibody was purified from antiserum, unwanted proteins were removed.
Figure 5.
Result of antibody purification and evaluating threshold of antibody. (A) SDS-PAGE result of purification polyclonal antibody by precipitation with ammonium sulphate 60% and protein A column. M, protein ladder. 1, antisera sample. 2, precipitated proteins. 3, purified antibody. Evaluating threshold of antisera (B) and purified polyclonal antibody (C).
Purified antibody was expected to give higher threshold; thus antiserum and purified antibody were again evaluated threshold using dot blot with ToxA and natural toxin. Antiserum with dilution 1/10,000 could detect 100ng ToxA and 200ng natural toxin (Figure 5B). Expectedly, purified antibody with the same concentration of antiserum reached a lower detection as it could detect 25ng ToxA and 50ng natural toxin (Figure 5C), and both antiserum and purified antibody showed no signal with VPnon-AHPND.
DISCUSSION
AHPND is a highly contagious disease in shrimp with mortality rate of up to 100%. To promptly prevent, methods of detecting diseases need to be fast, accurate, and convenient. However, lateral flow kit with many advantages for in situ detection has not been developed yet. A body of studies shows that the disease is caused by two toxins ToxA and ToxB, thus they are both targets for disease detection. Recently, Lin et.al predicted that ToxA is necessary for receptor binding and hepatopancreatic cytotoxicity [29, 30]. Therefore, we have firstly made antibody specific for ToxA, an important component in lateral flow kit development.
Polyclonal antibody is a potential solution because of its low production cost and ability to scale up in a short time. As antigen purity is directly linked with antibody specificity, instead of using the total protein from VPAHPND as an antigen [28], we generated purified recombinant ToxA toxin. The more purity of antigen, the more specific of its antibody. Because ToxA is from bacteria, E. coli BL21 is the ideal host for the expression of recombinant ToxA protein. Indeed, our result showed that ToxA protein was expressed in soluble fraction at 37°C without of soluble tag [34] or decreasing culture temperature [35], which helps to produce antigen easier. High purity of antigen is necessary to improve antibody quality. Therefore, during purification by His-trap column, an amount of ToxA protein was waived in washing fraction to increase the purity up to 94.49% by only one step of purification.
With long-term aim to develop AHPND lateral flow test kit, large amount of antibodies is needed, thus rabbit was the animal of choice instead of mouse [36, 37]. After immunization, antiserum from rabbits was evaluated. It had extremely high titer, up to 5,210,000 which has never been documented before with similar researches [38, 39] . Dot blot method was used to evaluate antigen-antibody interactions which can directly observe the result, similar to lateral flow method [28]. Bacterial strains of the Vibrio family, commonly reside through shrimp lifespan [40, 41], were selected as control samples for specificity evaluation [28]. Our results show that the antibody did not give any signal to control strains indicating no false-positive case. Similar result was also obtained using WSSV. We also collected natural toxin from VPAHNPD for evaluation, following previous method [34]. Regarding of detection threshold, current monoclonal antibody can detect 6.25ng natural toxin [28], and our polyclonal antibodies could detect up to 25ng natural toxin. It’s worth noting that in lethal test, a dose of 2ug per 1g of shrimp is capable of killing shrimp [34], thus, the threshold of detecting 25ng toxin was suitable for disease detection.
Taken together, we have been successful in generating antibody specific to ToxA with high specificity and detection threshold. These results laid a premise for the development of AHPND detection kit based on antigen - antibody interactions.
Acknowledgment
This research is funded by the Mekong Delta Program Office under project No: 19/2018/HĐ-KHCN-TNB.ĐT/14-19/C31.
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Food and Agriculture Organization. An estimated 3 million tonnes of shrimp entered the international trade in 2018. GLOBEFISH. Information and Analysis on World Fish Trade; 2019. [Google Scholar]
- 2.Lightner DV. The penaeid shrimp viruses TSV, IHHNV, WSSV, and YHV: current status in the Americas, available diagnostic methods, and management strategies. J Appl Aqua. 1999;9:27–52. [Google Scholar]
- 3.Seibert CH, Pinto AR. Challenges in shrimp aquaculture due to viral diseases: distribution and biology of the five major penaeid viruses and interventions to avoid viral incidence and dispersion. Braz J Microbiol. 2012;43:857–864. doi: 10.1590/S1517-83822012000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou HY, Huang CY, Wang CH, Chiang HC, Lo CF. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis Aquat Organ. 1995;23:165–173. [Google Scholar]
- 5.Chantanachookin C, Boonyaratpalin S, Kasornchandra J, Direkbusarakom S, Ekpanithanpong U, Supamataya K, Sriurairatana S, Flegel TW. Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by yellow-head disease. Dis Aquat Organ. 1993;17:145–145. [Google Scholar]
- 6.Boonyaratpalin S, Supamattaya K, Kasornchandra J, Direkbusaracom S, Aekpanithanpong U, Chantanachooklin C. Non-occluded baculo-like virus, the causative agent of yellow head disease in the black tiger shrimp (Penaeus monodon) Fish Pathol. 1993;28:103–109. [Google Scholar]
- 7.NACA‐FAO. Quarterly Aquatic Animal Disease Report (Asia and Pacific Region), 2011/2, April-June 2011. NACA Bangkok; 2011. [Google Scholar]
- 8.Flegel TW. Historic emergence, impact and current status of shrimp pathogens in Asia. J Invertebr Pathol. 2012;110:166–173. doi: 10.1016/j.jip.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Lightner, D, Flegel, T. Diseases of Crustaceans–Acute Hepatopancreatic Necrosis Syndrome (AHPNS)(Disease card). Asia Pacific Emergency Regional Consultation on Early Mortality Syndrome (EMS)/Acute Hepatopancreatic Necrosis Syndrome (AHPNS), Workshop organized by the Australian Government Department of Agriculture, Fisheries and Forestry and the Network of Aquaculture Centres in Asia-Pacific (NACA) Bangkok, Thailand (August 9-10, 2012): 2012. [Google Scholar]
- 10.Nunan L, Lightner D, Pantoja C, Gomez-Jimenez S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis Aquat Organ. 2014;111:81–86. doi: 10.3354/dao02776. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo L, Bayot B, Betancourt I, Pinzón A. Draft genome sequence of pathogenic bacteria Vibrio parahaemolyticus strain Ba94C2, associated with acute hepatopancreatic necrosis disease isolate from South America. Genome Data. 2016;9:143–144. doi: 10.1016/j.gdata.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhar AK, Piamsomboon P, Caro LFA, Kanrar S, AdamiJr R, Juan YS , First report of acute hepatopancreatic necrosis disease (AHPND) occurring in the USA. Dis Aquat Organ. 2019;132:241–247. doi: 10.3354/dao03330. [DOI] [PubMed] [Google Scholar]
- 13.FAO. Report of the FAO/MARD Technical Workshop on Early Mortality Syndrome (EMS) or Acute Hepatopancreatic Necrosis Syndrome (AHPND) of Cultured Shrimp (under TCP/VIE/3304). Hanoi, Viet Nam, 25–27 June 2013. 2013. FAO Fisheries and Aquaculture Report No. 1053. [Google Scholar]
- 14.Hong XP, Xu D, Zhuo Y, Liu HQ, Lu LQ. Identification and pathogenicity of Vibrio parahaemolyticus isolates and immune responses of Penaeus (Litopenaeus) vannamei (Boone) J Fish Dis. 2016;39:1085–1097. doi: 10.1111/jfd.12441. [DOI] [PubMed] [Google Scholar]
- 15.Asia-Pacific, N.o.A. Ci. Report of the Asia Pacific Emergency Regional Consultation on the Emerging Shrimp Disease: Early Mortality Syndrome (Ems)/Acute Hepatopancreatic Necrosis Syndrome (AHPNSj, 9-10 Aug 2012. Network of Aquaculture Centres in Asia and the Pacific Bangkok. 2012 [Google Scholar]
- 16.Shinn AP, Pratoomyot J, Griffiths D, Trong T, Vu N, Jiravanichpaisal P, Briggs M. Asian shrimp production and the economic costs of disease. Asian Fish Sci. 2018;31S:29–58. [Google Scholar]
- 17.Tran L, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons K, Lightner DV. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Organ. 2013;105:45–55. doi: 10.3354/dao02621. [DOI] [PubMed] [Google Scholar]
- 18.Lee CT, Chen IT, Yang YT, Ko TP, Huang YT, Huang JY, Huang MF, Lin SJ, Chen CY, Lin SS, Lightner DV, Wang HC, Wang A, Wang HC, Hor LI, Lo CF. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci UAS. 2015;112:10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YT, Chen IT, Lee CT, Chen CY, Lin SS, Hor LI, Tseng TC, Huang YT, Sritunyalucksana K, Thitamadee S, Wang HC, Lo CF. Draft genome sequences of four strains of Vibrio parahaemolyticus, three of which cause early mortality syndrome/acute hepatopancreatic necrosis disease in shrimp in China and Thailand. Genome Announc. 2014;2:e00816–14. doi: 10.1128/genomeA.00816-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo H, Tinwongger S, Proespraiwong P, Mavichak R, Unajak S, Nozaki R, Hirono I. Draft genome sequences of six strains of Vibrio parahaemolyticus isolated from early mortality syndrome/acute hepatopancreatic necrosis disease shrimp in Thailand. Genome Announc. 2014;2:e00221–14. doi: 10.1128/genomeA.00221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TW F Researches Release Free Information on AHPND Detection. 2013. available at: www.shrimpnews.com/FreeReportsFolder/NewsReportsFolder/ThailandTaiwanFreeEMStests.html.
- 22.Sirikharin R, Taengchaiyaphum S, Sritunyalucksana K, Thitamadee S. A new and improved PCR method for detection of AHPND bacteria. Network of Aquaculture Centres in Asia-Pacific (NACA) 2014. available at: ( http://www.enaca.org/modules/news/article.php.
- 23.Dangtip S, Sirikharin R, Sanguanrut P, Thitamadee S, Sritunyalucksana K, Taengcha-iyaphum S, Mavichak R, Proespraiwong P, Flegel TW. AP4 method for two-tube nested PCR detection of AHPND isolates of Vibrio parahaemolyticus. Aquaculture Reports. 2015;2:158–162. [Google Scholar]
- 24.Devadas S, Banerjee S, Yusoff F, Bhassu S, Shariff M. Experimental methodologies and diagnostic procedures for acute hepatopancreatic necrosis disease (AHPND) Aquaculture. 2019:389–400. [Google Scholar]
- 25.Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60:111–120. doi: 10.1042/EBC20150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sithigorngul W, Rukpratanporn S, Pecharaburanin N, Longyant S, Chaivisuthangkura P, Sithigorngul P. A simple and rapid immunochromatographic test strip for detection of white spot syndrome virus (WSSV) of shrimp. Dis Aquat Organ. 2006;72:101–106. doi: 10.3354/dao072101. [DOI] [PubMed] [Google Scholar]
- 27.Sithigorngul W, Rukpratanporn S, Sittidilokratna N, Pecharaburanin N, Longyant S, Chaivisuthangkura P, Sithigorngul PA. convenient immunochromatographic test strip for rapid diagnosis of yellow head virus infection in shrimp. J Virol Methods. 2007;140:193–199. doi: 10.1016/j.jviromet.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 28.Wangman P, Chaivisuthangkura P, Sritunyalucksana K, Taengchaiyaphum S, Senapin S, Pengsuk C, Sithigorngul P, Longyant S. Development of monoclonal antibodies specific to ToxA and ToxB of Vibrio parahaemolyticus that cause acute hepatopancreatic necrosis disease (AHPND) Aquaculture. 2017;474:75–81. [Google Scholar]
- 29.Lin SJ, Hsu KC, Wang HC. Structural insights into the cytotoxic mechanism of Vibrio parahaemolyticus PirAvp and PirBvp Toxins. Mar Drugs. 2017;15:373. doi: 10.3390/md15120373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin SJ, Chen YF, Hsu KC, Chen YL, Ko TP, Lo CF, Wang HC, Wang HC. Structural insights to the heterotetrameric interaction between the vibrio parahaemolyticus pirAvp and pirBvp toxins and activation of the cry-like pore-forming domain. Toxins. 2019;11:233. doi: 10.3390/toxins11040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran Van Hieu NVD, Tran Linh Thuoc. Developing a sanwich ELISA for rapid detection of Listeria monocytogenes. J Biotechnol Vietnam Acad Sci Technol. 2007;5:411–417. [Google Scholar]
- 32.Joshi J, Srisala J, Truong VH, Chen IT, Nuangsaeng B, Suthienkul O, Lo CF, Flegel TW, Sritunyalucksana K, Thitamadee S. Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND) Aquaculture. 2014;428:297–302. [Google Scholar]
- 33.Barajas-Rojas J, Riemann H, Franti C. Notes about determining the cut-off value in enzyme linked immunosorbent assay (ELISA) Prev Vet Med. 1993;15:231–233. [Google Scholar]
- 34.Sirikharin R, Taengchaiyaphum S, Sanguanrut P, Chi TD, Mavichak R, Proespraiwong P, Nuangsaeng B, Thitamadee S, Flegel TW, Sritunyalucksana K. Characterization and PCR detection of binary, Pir-like toxins from Vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in shrimp. PLoS One. 2015;10:e0126987. doi: 10.1371/journal.pone.0126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han JE, Tang KF, Tran LH, Lightner DV. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis Aquat Organ. 2015;113:33–40. doi: 10.3354/dao02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stills HF. Polyclonal antibody production. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Elsevier; 2012. pp. 259–274. [Google Scholar]
- 37.Delahaut P. Immunisation–Choice of host, adjuvants and boosting schedules with emphasis on polyclonal antibody production. Methods. 2017;116:4–11. doi: 10.1016/j.ymeth.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Aref NEM, Saeed AM. Generation of high-titer of neutralizing polyclonal antibodies against heat-stable enterotoxin (STa) of enterotoxigenic Escherichia coli. Vaccine. 2012;30:6341–6346. doi: 10.1016/j.vaccine.2012.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miao W, Wei B, Yang R, Wu C, Lou D, Jiang W, Zhou Z. Highly specific and sensitive detection of bisphenol A in water samples using an enzyme-linked immunosorbent assay employing a novel synthetic antigen. New J Chem. 2014;38:669–675. [Google Scholar]
- 40.Jayasree L, Janakiram P, Madhavi R. Characterization of Vibrio spp associated with diseased shrimp from culture ponds of Andhra Pradesh (India) J World Aquaculture Society. 2006;37:523–532. [Google Scholar]
- 41.Sung HH, Hsu SF, Chen CK, Ting YY, Chao WL. Relationships between disease outbreak in cultured tiger shrimp (Penaeus monodon) and the composition of Vibrio communities in pond water and shrimp hepatopancreas during cultivation. Aquaculture. 2001;192:101–110. [Google Scholar]