Abstract
Background:
In dermatology, prior authorizations (PAs) can delay treatment, decrease patient adherence, and deter providers from advocating for their patients. Patients with complex dermatological conditions, often requiring off-label treatments, may face particularly significant insurance barriers.
Objective:
Evaluate the impact of PAs in patients with complex dermatological conditions.
Methods:
This prospective cohort study assessed patients seen by a dermatologist specializing in complex dermatology over 5 months. Patients included were >18 years old, seen at V.P.W.’s rheumatology-dermatology clinic, and prescribed a medication or ordered a diagnostic procedure that elicited an insurance PA. PA outcome, administrative time, and delay to treatment were collected.
Results:
Of 51 PAs, 51% were initially denied, with systemic medications more likely denied than topical medications (p <0.0001). Total administrative time spent on 50 PAs tracked was 62.5 hours (median time per PA: 30 minutes [IQR: 17–105]). Time to access treatment was tracked for 86% of PAs; median delay was 12 days [IQR: 5.5–23].
Limitations:
Single center, single provider patient panel.
Conclusion:
Patients with complex dermatologic conditions face a significant barrier to care due to PAs. The administrative burden for provider practices to address these PAs is substantial and may warrant a streamlined system in collaboration with insurers.
Keywords: prior authorizations, complex medical dermatology, health care delivery
Capsule Summary:
• While prior authorizations serve an important clinical role, for patients with complex dermatologic conditions, prior authorizations may delay access to appropriate care, particularly for systemic medication prescriptions.
• The administrative burden for provider practices to address these PAs is substantial and warrants developing a streamlined system in collaboration with insurers.
Introduction
Prescription drug prices are one of the fastest growing healthcare expenditures. In the setting of these rising costs, insurers have begun to use prior authorization (PA) requirements to promote cost-effective prescribing practices. However, these PA requirements can increase administrative burdens and limit timely access to appropriate treatments. For instance, in 2006 the average practice devoted 1 hour of physician time, 13.1 hours of nursing time, and 6.3 hours of clerical time to the PA process per week.1
In dermatology, PAs represent a particularly heavy burden, in part due to prices for dermatologic drugs rising disproportionately in recent years.2,3 For patients, a circuitous PA process may impair access to treatment; roughly 20% of patients cite PAs as a reason for primary non-adherence to acne medications.4 Indeed, dermatologists cite PAs as one of the greatest barriers to their patients receiving necessary medications.5 Physicians may respond by no longer even prescribing some medications or refusing to address PAs to avoid the administrative costs of completing the PA process.5 While potentially reducing total health care costs by limiting unnecessary prescriptions or diverting prescriptions to lower-cost alternatives, PAs might also lead to additional costs for patients, due to increased out-of-pocket spending and insurance premiums, as well as for provider practices, who need to hire administrative staff to address PAs.6,7,8
For patients with complex or uncommon dermatological conditions such as dermatomyositis or lupus erythematosus (SLE), PAs are particularly common given frequent off-label prescriptions for these conditions; for example, roughly 60% of patients with SLE receive at least one off-label prescription.9 In addition, patients with these conditions may become acutely ill and delays in care due to the PA process could result in worse clinical outcomes. Particularly if these PAs are often ultimately approved, the administrative burden and delays in accessing therapy may be inefficient for the health system. The purpose of this study was to evaluate the outcomes of PAs for patients with complex dermatological conditions and to quantify the administrative burden and clinical impact of these PAs.
Methods
Study Population:
The study was approved by the University of Pennsylvania Health System Institutional Review Board. To be included in the study, patients had to be over 18 years old, diagnosed with a skin condition, and prescribed a medication or ordered a diagnostic procedure when seen by a dermatologist (VPW) at a clinic focusing on patients with complex rheumatologic/dermatologic conditions at the University of Pennsylvania Health System from October 2018 to April 2019.
Study Design:
We evaluated patients’ prior authorization requests using real-time documentation of full-time work-hours by administrative staff as well as our existing electronic medical record (EMR) and ordering system (Epic Systems Corporation, Verona, Wisconsin). Both quantitative review of study outcomes and qualitative review of specific patient cases (see “Narrative Summary” section) were planned.
We used a prospective time-motion study to evaluate the administrative time spent on addressing these PAs. Start- and stop-time for administrative activities associated with PAs such as form completion, telephone calls, and peer-to-peer dialogue was tracked. Start- and stop-times were self-recorded in real-time on a standardized data collection form by a licensed practical nurse (LPN) and certified registered nurse practitioner (CRNP) as they executed each of these tasks. Faxes containing third-party payer decisions and the EMR were evaluated to determine PA outcome of approval versus denial. In cases that were initially denied, administrative time spent on any ensuing appeals process continued to be measured until the conclusion of the study period. In cases that were either initially or ultimately approved following an appeal, the length of time from initial prescription or diagnostic procedure order to date of approval was measured.
Estimation of cost to the hospital system per PA was calculated based on the mean hourly wage for a LPN and CRNP in the Philadelphia metropolitan area.10 To compare the administrative cost of addressing a PA to the revenue of an outpatient specialist appointment, a sensitivity analysis using multiple benchmarks for reimbursement rates was conducted. Reimbursement rates for outpatient visits vary considerably across geographic markets and depend on negotiated rates between individual insurers and health systems.11,12 One approach to estimate reimbursement per outpatient visit was based on the hospital system’s standard charges, prices each hospital publishes as required by the Centers for Medicare and Medicaid Services (CMS). However, these published prices undergo substantial negotiation in contracts between insurer and hospital system and thus are significantly inflated compared to what insurers ultimately pay.12 Another approach was to base reimbursement estimates off of the Medicare fee schedule. Since Medicare fees are standardized across the country and accurately reflect reimbursement rates for patients covered by Medicare, this proxy was selected to provide increased generalizability to the analysis. Cost of an outpatient specialist appointment was conservatively estimated based on the national payment amount in the Medicare fee schedule using Current Procedural Terminology (CPT) codes 99213 (office/outpatient visit with an established patient) and 99214 (office/outpatient visit with an established patient, with medical decision making of moderate complexity). CPT codes were searched using the Physician Fee Schedule available from CMS, selecting the National Payment Amount at the Non-Facility Price, given that all appointments took place in the office setting.13
Statistics:
Demographic characteristics were summarized by standard descriptive summaries: means and standard deviations for continuous variables such as age, percentages for categorical variables such as gender, medians and interquartile ranges [IQR] for administrative time spent and delay to treatment. Fisher’s exact testing was used to analyze the relationship between drug class and PA outcome and insurance coverage type and PA outcome. All statistical analyses were performed using Prism GraphPad.
Results
Demographics:
During the study period, 450 unique patients were seen in VPW’s dermatology clinic (Perelman Center for Advanced Medicine, Philadelphia, Pennsylvania) between October 2018 and April 2019, and of these, 51 PAs resulted for 48 patients. The demographic characteristics of patients included are outlined in Table 1. Of note, our sample represented a specialized population as a result of the clinic’s emphasis on autoimmune dermatological conditions, with approximately half of patients with a primary diagnosis of dermatomyositis and approximately one quarter with a primary diagnosis of systemic lupus erythematosus. Table 2 summarizes the primary associated diagnosis for each patient and the medications and diagnostic procedures that resulted in a PA. Of the 42 PAs for medications prescribed, all medication prescriptions were considered “off-label” based on FDA labels.
Table 1:
Demographic Characteristics of patients with prior authorizations
| Demographic Characteristic | Patients (n = 48) n (%) |
|---|---|
| Mean age, years (range) | 53 (25–87) |
| Female patients | 42 (88) |
| Race | |
| Black/Non-Hispanic/Non-Latino | 10 (21) |
| White/Non-Hispanic/Non-Latino | 30 (63) |
| White/Hispanic/Latino | 2 (4) |
| Not specified | 6 (13) |
| Healthcare Coverage | |
| Medicare | 12 (25) |
| Medicaid | 5 (10) |
| Commercial | |
| Blue Cross/Blue Shield | 14 (29) |
| Aetna | 10 (21) |
| United Healthcare | 4 (8) |
| Horizon | 3 (6) |
Table 2:
Clinical characteristics of patients with prior authorizations
| Primary associated diagnosis | Patients (n = 48) n (%) |
|---|---|
| Dermatomyositis/suspected dermatomyositis | 25 (52) |
| Lupus | 13 (27) |
| Overlapping (lupus vs. dermatomyositis, lupus/rheumatoid arthritis overlap, | 4 (8) |
| dermatomyositis and morphea) | |
| Bullous pemphigoid | 2 (4) |
| Psoriasis | 1 (2) |
| Granuloma annulare | 1 (2) |
| Rosacea | 1 (2) |
| Vitiligo | 1 (2) |
| Medication | Prescriptions or Orders Placed (n = 51) n (%) |
| Topicals (tacrolimus ointment, pimecrolimus, clobetasol, fluocinolone triamcinolone) | 21 (41) |
| Mychophenolate/Mycophenolic acid | 12 (24) |
| Biologics (tofacitinib, omalizumab, secukinumab) | 4 (8) |
| Lenalidomide | 2 (4) |
| Methotrexate | 2 (4) |
| Azathioprine | 1 (2) |
| Imaging (MRI/CT/echocardiogram) | 9 (18) |
PA Outcomes:
Figure 1 summarizes the process for addressing PAs and the outcomes (approval, initial denial, continued denial) of the 51 PAs evaluated. The rate of initial approval was 49% and initial denial was 51%; some pursued an appeals process, and of those, 56% eventually received approval, culminating in a total ultimate approval rate of 69%. Table 3 summarizes these initial outcomes based on medication class or diagnostic procedure. PAs were significantly more likely to be initially denied if for a systemic medication compared to a topical medication (p < 0.0001). Whether patients had public (Medicare or Medicaid) or commercial insurance was not statistically significantly related to initial approval or denial (p > 0.7761).
Figure 1:
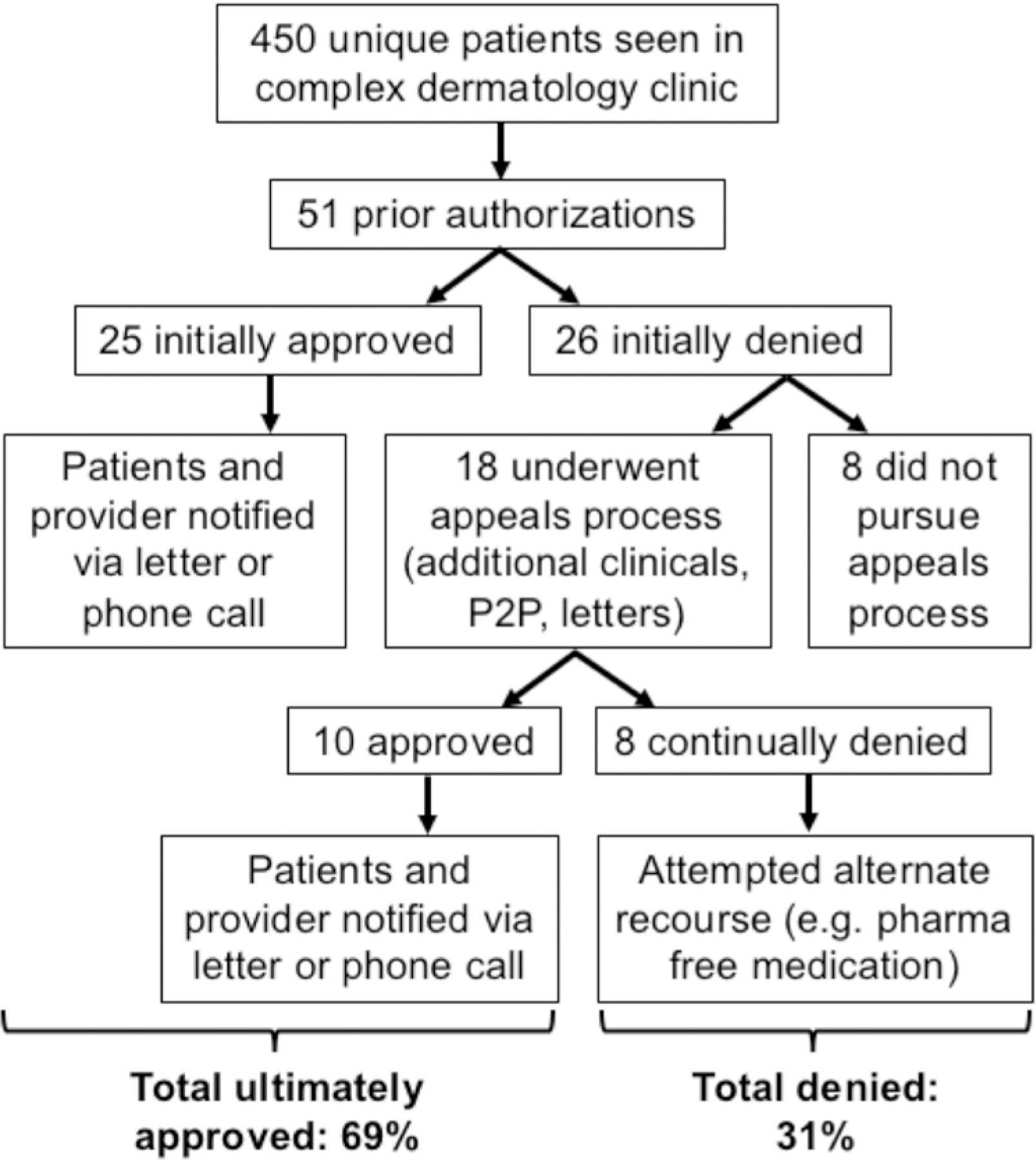
Prior authorization workflow and outcomes
Table 3:
Prior authorization outcome based on medication class, diagnostic order, and insurance type
| Medication Class or Diagnostic Order | Initial approval (n = 25) n (%) |
Initial denial (n = 26) n (%) |
|---|---|---|
| Topicals (tacrolimus ointment, pimecrolimus, clobetasol, fluocinolone, triamcinolone) | 18 (86) | 3 (14) |
| Immunosuppressives (mycophenolate/methotrexate/azathioprine) | 3 (20) | 12 (80) |
| Biologics (tofacitinib, omalizumab, secukinumab)/Lenalidomide | 0 (0) | 6 (100) |
| Imaging | 4 (44) | 5 (55) |
| Insurance Type | ||
| Public (Medicare/Medicaid) | 10 (53) | 9 (47) |
| Commercial | 15 (47) | 17 (53) |
Delay to Treatment:
Time to receive treatment was measured from the date of the medication prescribed or diagnostic procedure ordered to the date of approval or procurement of the medication via alternate methods (free medication programs or out-of-pocket payment). Of the 51 PAs tracked, an exact date of approval or denial was documented for 41 (80%), the remainder lacking a documented decision date due to variable receipt of faxed decision letters; in the cases lacking documentation, approval or denial was directly confirmed with patients. Median time to access the prescribed medication or diagnostic procedure was 12 days [IQR: 5.5–23].
Administrative Burden:
Administrative time was tracked for 50/51 (98%) of PAs. A dedicated LPN and CRNP spent a total of 62.5 hours addressing these PAs and a median of 30 minutes per PA [IQR: 17–105]. Activities documented were filling out PA forms, phone calls with payers clarifying patient or pharmacy information, phone calls with patients regarding PA progress, writing appeals letters, peer-to-peer calls, and faxing documents. Based on the mean hourly salaries of a LPN and CRNP of $26.12 and $49.60 per hour, respectively, total administrative cost for these 50 PAs was $1,690.76, with an average administrative cost per PA of $33.82. Using Medicare fee schedules, compared to the reimbursement rate for a typical outpatient specialist appointment during which these medications or diagnostic procedures were ordered ($75.32 to $110.28 per visit for a 99213 and 99214 encounter, respectively), this cost per PA constituted 31–45% of the visit gross revenue billed per visit.13 Based on the hospital system’s standard charge for an outpatient visit for an established patient of $384, which may be assumed to be inflated compared to the actual reimbursement received per visit, the cost per PA would account for at least 9% of the visit’s gross revenue.14
Narrative Summary:
While the majority of PAs were ultimately approved and required a median of 30 minutes of administrative time, several “outlier” patients required exceptional administrative time and experienced a greater delay to receiving appropriate medications or diagnostic procedures. We present selected vignettes of these patients below.
A patient with erosive discoid lupus erythematosus/systemic lupus erythematosus had been previously treated with multiple agents including methotrexate, hydroxychloroquine, quinacrine, and prednisone without success. The next best step was deemed to be a trial of lenalidomide, which was continually denied. Despite approximately 10 hours of administrative work, including an appeal letter and peer-to-peer conversation, this patient ultimately became septic through denudation of the patient’s skin, requiring a 39-day hospital course. Lenalidomide was finally approved 70 days after initial prescription, to which the patient responded favorably. Four months later coverage was abruptly discontinued, forcing additional written appeals in an ongoing review until the time of the study conclusion.
One patient with type 2 diabetes mellitus was newly diagnosed with urticarial bullous pemphigoid. Omalizumab was selected based on studies demonstrating efficacy in urticarial bullous pemphigoid, but since the drug was not on the patient’s insurance company’s formulary as indicated for bullous pemphigoid, the patient was continually denied coverage despite appeals. The patient was started on prednisone to manage symptoms, leading to hyperglycemia, reported worsening vision, and a subsequent 4-day hospital admission. Omalizumab was finally approved 22 days after initial prescription, after 3.5 hours of administrative work, but the patient switched providers before trialing the new medication.
The barriers to care were not only restricted to patients requiring advanced therapies. For a 54-year-old patient with dermatomyositis, a CT scan of the abdomen and pelvis was ordered given the risk for malignancy. The patient’s insurance would not cover the procedure, requiring an initial ultrasound. Provider-to-provider review and multiple phone calls between nursing staff, physician, and the patient amounted to approximately 5 hours spent addressing this insurance barrier. Ultimately, the patient received a CT scan 84 days after it was originally ordered.
Discussion
Our study describes the burden due to PAs in a patient population with complex dermatologic conditions seen at a single clinic. By quantifying the delay to treatment and associated cost of these barriers to care, we demonstrate that in this patient population, PAs represent a far-reaching burden, from the delay patients experience to the administrative time spent handling PA requirements. These patients experience significant delays to treatment, and in extreme cases may be hospitalized before receiving the appropriate medications. Compared to a reported 64% initial approval rate in a general dermatology setting and 68% in a primary care setting, only 49% of prescriptions and orders were initially approved among this patient population, with a preponderance of these being topical medications.6,15 Our estimate of the labor costs associated with each PA, solely based on salary, is similar to or higher than prior estimates; in primary care and subspecialty, non-dermatologic settings, the estimated direct labor cost including benefits in addition to salary was $12.79 to $37.50 per PA, respectively.16,15 Furthermore, our estimate is likely conservative as the effective cost burden on physician practices should include indirect costs such as employee benefits in addition to salary, the materials cost of printed pages required to address each PA, and the opportunity cost of spending time addressing PAs rather than participating in revenue-generating activities such as direct patient care. This suggests that the clinical complexity of these patients may lead to a relatively higher administrative burden, a lower likelihood of initial approval, a more frequent need to undergo an appeal process, and thus a longer delay to treatment for patients. This study was limited by its relatively narrow patient population, from a single region of the country, which may limit generalizability, and selection from a single provider’s clinic schedule during a limited timeframe, leading to smaller sample size. Future studies could be conducted in other populations to validate generalizability.
While PAs may serve an important role in monitoring appropriate care and curbing overall healthcare spending, this study suggests that the PA process may incur unintended costs, both direct and indirect. In two instances, patients faced continued denial of their prescribed medications such that they were ultimately hospitalized, incurring additional, perhaps unnecessary hospital expenditures during their in-patient stays as well as causing distress for patients and their families. Intangible yet important additional costs include the time burden on patients and their families spent calling the provider’s office or insurance company, the distress experienced when facing repeated denials, the downstream costs associated with any progression of disease while awaiting insurance coverage, and the opportunity cost of time spent by providers on these insurance claims rather than direct patient care.
The PA burden is currently borne by provider practices and patients. Provider practices do not currently receive reimbursement for the time spent on addressing PAs, which in our study population was found to absorb at least one tenth, but more likely a third to a half of the hospital system’s gross revenue from the clinic visit. Future directions based on these findings include investigating potential ways to streamline this process, particularly for patients with complex dermatologic conditions, whether through reconsidering the PA process for the individual provider practice or instituting “checks and balances” to realign incentives between provider, payer, and patient. For example, as described, delays to care for patients who are very sick can lead to increased risk of serious harm and possible hospitalization; an expedited PA process that takes into account the clinical urgency of individual patient cases would be in the financial interest of payers. Additional process improvement strategies could include stratifying patients based on complexity of dermatologic diagnosis such that they are triaged to an immediate peer- to-peer process; stratifying providers such that those with frequent approval following appeals are granted a status that does not require PAs for certain medications; improving the “electronic PA” that can be completed at the time of prescribing or placing the diagnostic test order; expanding drug formularies to more thoroughly address dermatologic diagnoses; retroactively increasing reimbursement for visits involving medication prescriptions or diagnostic orders that later result in a prior authorization; or, as an incentive to expedite decisions and reduce treatment delays, requiring payers to retrospectively reimburse patients for out-of-pocket medication costs accrued during the appeals process if a PA is eventually approved.17 Although administratively challenging, these latter solutions would provide negative feedback on the broad use of PAs, for which there is currently minimal downside for payers. Ultimately, understanding the impact and cost of PAs on patients, providers, and health care systems will help to inform decisions surrounding health policy, reimbursement, and health care administration.
Funding/Support:
This work was supported by the United States Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development) and NIH R01AR071653 (VPW) and National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number T32-AR-007465 and receives partial salary support through a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania (JSB).
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- PA
prior authorization
- EMR
electronic medical record
- LPN
licensed practical nurse
- CRNP
certified registered nurse practitioner
- P2P
peer-to-peer dialogue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Notes: All authors have no conflicts of interest to report. The authors consent to publication. The authors confirm that all the research meets ethical guidelines, including adherence to the legal requirements of the study country. All information and materials in the manuscript are original.
References:
- 1.Casalino LP, Nicholson S, Gans DN, Hammons T, Morra D, Karrison T, et al. What Does It Cost Physician Practices To Interact With Health Insurance Plans. Health Aff (Millwood). 2009. Jul-Aug;28(4):w533–43. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht J, Lebwohl M, Asgari MM, Bennett DD, Cook A, Evans CC, et al. The state and consequences of dermatology drug prices in the United States. J Am Acad Dermatol. 2016. September;75(3):603–605. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg ME, Rosenberg SP. Changes in Retail Prices of Prescription Dermatologic Drugs From 2009 to 2015. JAMA Dermatol. 2016. February;152(2):158–63. [DOI] [PubMed] [Google Scholar]
- 4.Ryskina KL, Goldberg E, Lott B, Hermann D, Barbieri JS, Lipoff JB. The Role of the Physician in Patient Perceptions of Barriers to Primary Adherence With Acne Medications. JAMA Dermatol. 2018. April 1;154(4):456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Secrest AM, Asgari MM, Kourosh AS, Barbieri JS, Albrecht J; American Academy of Dermatology Drug Pricing and Transparency Task Force. Prior authorizations for dermatologic medications: An American Academy of Dermatology survey of US dermatology providers and staff. J Am Acad Dermatol. 2017. October; 77(4):784–786. [DOI] [PubMed] [Google Scholar]
- 6.Popatia S, Flood KS, Golbari NM, Patel PV, Olbricht SM, Kimball AB, Porter ML. Examining the prior authorization process, patient outcomes, and the impact of a pharmacy intervention: A single-center review. J Am Acad Dermatol. 2019. May 16. [DOI] [PubMed]
- 7.Balkrishnan R, Bhosle MJ, Fleischer AB Jr, Feldman SR. Prior authorization for topical psoriasis treatments: is it cost-beneficial for managed care? J Dermatolog Treat (2010; 21(3):178–84. [DOI] [PubMed] [Google Scholar]
- 8.Feldman SR, Fleischer AB Jr, Chen GJ. Is prior authorization of topical tretinoin for acne cost effective? Am J Managed Care (1999); 5(4):457–63. [PubMed] [Google Scholar]
- 9.Chu B, Fleischer A Jr, Barbieri JS. The frequency of off-label prescribing in the treatment of dermatologic disease: 2006–2015. J Am Acad Dermatol. 2019. July 18. [DOI] [PMC free article] [PubMed]
- 10.Nurse Salary in Pennsylvania. https://nursesalaryguide.net/nurse-salary-in-pennsylvania/#Licensed_Practical_Nurse_LPN_LVN_Salary_in_Pennsylvania.
- 11.Ginsburg PB. Wide Variation in Hospital and Physician Payment Rates Evidence of Provider Market Power. Res Brief. 2010. November;(16):1–11. [PubMed] [Google Scholar]
- 12.Baker L, Bundorf MK, Royalty A. Private insurers’ payments for routine physician office visits vary substantially across the United States. Health Aff (Millwood). 2013. September;32(9):1583–90. [DOI] [PubMed] [Google Scholar]
- 13.Center for Medicare & Medicaid Services. Physician Fee Schedule Search. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
- 14.Hospital of the University of Pennsylvania Charge Description Master, Updated January 17, 2020, accessed February 20, 2020. https://www.pennmedicine.org/for-patients-and-visitors/patient-information/insurance-and-billing/financial-transparency/financial-transparency-for-the-hospital-of-the-university-of-pennsylvania.
- 15.Cutler T, She Y, Barca J, Lester S, Xing G, Patel J, Melnikow J. Impact of Pharmacy Intervention on Prior Authorization Success and Efficiency at a University Medical Center. J Manag Care Spec Pharm. 2016. October;22(10):1167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raper JL, Willig JH, Lin HY, Allison JJ, Broner MB, Mugavero MJ, Saag MS. Uncompensated Medical Provider Costs Associated with Prior Authorization for Prescription Medications in an HIV Clinic. Clin Infect Dis. 2010. September 15;51(6):718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbieri JS, St Claire K, Mostaghimi A, Albrecht J. Evaluation of Clinical Compendia Used for Medicare Part D Coverage Determinations for Off-label Prescribing in Dermatology. JAMA Dermatol. 2019. January 23. [DOI] [PMC free article] [PubMed]


