Abstract
Purpose
Certain patient demographics and biomarkers have been suggested to predict survival in patients infected with COVID-19. However, predictors of outcome in patients who are critically ill are unclear.
Materials and Methods
We performed a multicentre analysis of 171 consecutive patients with confirmed COVID-19 who were admitted to the intensive care unit (ICU) between 1 March 2020 and 30 April 2020 and were followed until 23 May 2020. Demographic data, past medical history, laboratory values, echocardiographic and telemetry data were analysed. Patient status was classified as either alive or deceased at hospital discharge or the end of follow-up period.
Results
Mean patient age was 66±13 and 57% were male. Mortality rate of this ICU cohort at the end of follow-up was 46.2%. A multivariable logistic regression analysis identified the presence or history of atrial fibrillation (Odds Ratio 4.8, p=0.004) as a significant cardiovascular attribute that contributed to increased mortality.
Conclusion
Mortality of critically ill COVID-19 patients is high. This study suggests a relationship between atrial fibrillation and increased mortality from COVID-19. Early aggressive treatment patients with high risk characteristics, such as atrial fibrillation could improve clinical outcome.
Keywords: COVID-19, Coronavirus, Mortality, Atrial fibrillation
Introduction
COVID-19 is a global pandemic caused by the SARS-CoV-2 virus, first identified in Wuhan, China in December 2019. COVID-19 starts as a local upper respiratory infection but can spread to affect multiple organ systems. Several cardiovascular signs of involvement have been reported, however the degree, the severity and the utility of predicting poor outcomes is unknown [1]. Of patients infected with COVID-19, it is estimated that 26–32% of these patients will need admission to the intensive care unit (ICU) [2,3]. There is some evidence that shows dexamethasone and remdesivir could be of some benefit to critically ill patients [4,5]. Certain patient demographics and biomarkers have been shown to predict survival in patients infected with COVID-19. However, predictors of outcome in patients who are critically ill are unclear. We sought to study 171 consecutive COVID-19 patients who were admitted to the intensive care unit to identify criteria which can assist in predicting mortality.
Methods
A multicentre retrospective cohort study was performed at Ascension Macomb-Oakland Hospital in Warren, MI and Sparrow Hospital in Lansing, MI and the study protocol was approved by the local Institutional Review Boards prior to data collection. A total of 171 patients with COVID-19 admitted to the ICU between 15 March 2020 and 30 April 2020 were considered for enrolment in the study. Chart review and data collection were performed at their respective institutions and were obtained through electronic medical record review, after which data was combined into one database. Patients were reviewed through hospital discharge or 23 May 2020, whichever occurred first. Variables collected include: patient demographics (dates of admission and discharge, age, gender, body mass index, race), prior medical conditions (hypertension, diabetes mellitus, [COPD] obstructive pulmonary disease, history of heart failure, history of coronary stent or bypass) and cardiac and inflammatory biomarkers (troponin, ferritin, d-dimer, lactate dehydrogenase [LDH]). Laboratory values were performed using the usual hospital laboratory assays for the respective tests. Values were considered to be elevated if above the range specified by our laboratories. For both hospitals the reference for elevated troponins were >0.05 ɳg/mg, D-Dimer>500 ɳg/mL, ferritin>120 ɳg/mL and LDH was >240 IU/L. Cardiac parameters including documented arrhythmia on telemetry, echocardiogram values (left ventricular ejection fraction [LVEF], valvular heart disease, right ventricular systolic pressure [RVSP]) were recorded. Echocardiography data was collected only within 3 months prior to admission. Specific therapies related to the COVID-19 infection including the use of hydroxychloroquine, azithromycin, anticoagulation, and intravenous vasopressors were noted. Outcome data was discharge condition (alive, deceased).
Inclusion criteria of the study includes 18 years or older, admission to the ICU at any point during their hospitalisation and a positive SARS-CoV-19 PCR test. Maximum values for troponin, ferritin and LDH were logged. Valvular heart disease was considered positive if any valvular lesion (regurgitation or stenosis) was graded moderate or worse on any echocardiogram done within 3 months of hospital admission. RVSP data was collected only if the echocardiogram was performed during the current hospital admission. LVEF was reported using the highest percentage of the range noted on the echocardiogram report. Arrhythmias were defined as ventricular tachycardia, ventricular fibrillation, atrial fibrillation or atrial flutter, sinus pause, atrioventricular block, or asystole greater than 3 seconds. Cardiac arrest was defined as having a primarily cardiac aetiology for the arrest and was excluded if the arrest was likely due to noncardiac causes such as hypoxaemia or electrolyte abnormalities. All laboratory values and telemetry data were collected through hospital discharge or 23 May 2020 whichever occurred first. At the end of the data collection period patients were either classified as either alive or deceased. Patients who were either transferred to another facility (another hospital or long term acute care facility) or who were still hospitalised at the end of the data collection period were classified as alive.
Data processing and statistical analysis were done using RStudio version Version 1.2.5042 and R version 3.6.3 (2020-02-29). Outliers were detected and included in the analysis as the values were biologically plausible. Univariate analysis for continuous variables reported p-values using the t-test for normally distributed variables and the Wilcoxon Rank Sum test otherwise, and for categorical variables the Fisher’s exact test was used for low counts and the Chi-squared test otherwise. Multivariate logistic regression models were developed using generalised linear models with variables that were significant on univariate analysis. A multivariate analysis was attempted with all significant univariate variables, however due to the number of significant factors, a single model could not be generated. Instead a multivariate model was generated focussing on cardiac specific parameters including elevated troponin, prior history of cardiac disease, and new onset atrial fibrillation. Diabetes was not positive in the univariate analysis however was included in the final multivariate as prior studies have shown that diabetes contributes to COVID-19 mortality rates [6,7]. Kaplan-Meier survival curves were generated using atrial fibrillation and Cox proportional hazards models were subsequently developed.
Results
A total of 171 patients (77 patients at Ascension Macomb-Oakland Hospital and 94 patients at Sparrow Hospital) were analysed for the study. Baseline characteristics are summarised in Table 1 . By the end of the run- through date of 23 May 2020, 92 (53.8%) patients were still alive and 79 (46.1%) patients were deceased.
Table 1.
Univariate analysis of demographics and history.
| Demographics and History | Alive at Discharge n=92 |
Died in Hospital n=79 |
P-value |
|---|---|---|---|
| Age | 0.002 | ||
| Mean±SD (median) | 63±16 (64) | 70±11 (71) | |
| Min to max | 26–96 | 46–94 | |
| Males | 53 (57.6%) | 46 (58.2%) | 0.93 |
| BMI | 0.45 | ||
| Mean±SD (median) | 30±8.2 (29) | 32±9.8 (30) | |
| Min to max | 16–65 | 18 to 68 | |
| Race | N=90 | N=78 | 0.59 |
| White | 55 (61.1%) | 40 (51.3%) | |
| Black | 26 (28.9%) | 26 (33.3%) | |
| Asian | 3 (3.3%) | 4 (5.1%) | |
| Other (declines removed) | 6 (6.7%) | 8 (10.3%) | |
| Hypertension | 61 (66.3%) | 60 (76.0%) | 0.17 |
| Diabetes | 28 (30.4%) | 35 (44.3%) | 0.061 |
| Insulin dependent | 16 | 15 | |
| CKD | 23 (25.0%) | 21 (26.6%) | 0.81 |
| COPD | 14 (15.2%) | 23 (29.1%) | 0.028 |
| Oxygen dependent | 5 (5.4%) | 12 (15.2%) | 0.034 |
| Prior history of stent | 9 (9.8%) | 20 (25.3%) | 0.007 |
| Prior ICD | 2 (2.2%) | 6 (7.6%) | 0.15 |
| Prior heart failure history | 14 (15.2%) | 20 (25.3%) | 0.1 |
| Prior AF history | 6 (6.5%) | 21 (26.6%) | 0.0003 |
| New onset AF | 9 (9.8%) | 23 (29.1%) | 0.001 |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter defibrillator; AF, atrial fibrillation; BMI, body mass index.
Bolded values indicates a statistically significant value (p-value <0.05).
A univariate analysis was performed including prior medical history (Table 1), laboratory findings (Table 2 ), medications received during the hospitalisation and telemetry and echocardiographic findings (Table 3 ). Patients who died were significantly more likely to have advanced age (p<0.002), COPD (p=0.028), prior history of stent or bypass (p=0.007), prior history of atrial fibrillation (p=0.0003) and new onset atrial fibrillation (p=0.001) compared to the alive cohort. Higher levels of troponin (p<0.0001) ferritin (p<0.0001), and LDH (p<0.0001) were also associated with higher mortality. Patients who were intubated (p<0.0001), given hydroxychloroquine (p=0.001), azithromycin (p=0.048), or intravenous vasopressor support (p<0.0001) had a statistically higher rate of death as well. A multivariate analysis of these variables showed age (OR 1.05, p=0.021), atrial fibrillation (AF) (OR 4.8, p=0.004) and intubation (OR 21.2, p<0.001) were independent predictors of mortality (Table 4 ).
Table 2.
Univariate analysis of laboratory values.
| Laboratory Values | Alive at Discharge n=92 |
Died in Hospital n=79 |
P-value |
|---|---|---|---|
| Troponin | N=90 | N=74 | <0.0001 |
| Median (25th, 75th) | 0.03 (0.01, 0.06) | 0.08 (0.03, 0.36) | |
| Min to max | 0–1.9 | 0.01–4.1 | |
| D-dimer | N=58 | N=56 | 0.34 |
| Median (25th, 75th) | 2.0 (0.79, 14.9) | 4.8 (1.2, 9.4) | |
| Min to max | 0.3–30 | 0.39–30 | |
| LDH | N=72 | N=70 | <0.0001 |
| Median (25th, 75th) | 380 (265, 520) | 629 (485, 877) | |
| Min to max | 122–976 | 172–6,089 | |
| Ferritin | N=72 | N=70 | <0.0001 |
| Median (25th, 75th) | 709 (354, 1,326) | 1524 (796, 3,904) | |
| Min to max | 6–16,018 | 161–82,360 |
Abbreviation: LDH, lactate dehydrogenase.
Bolded values indicates a statistically significant value (p-value <0.05).
Table 3.
Univariate analysis of medication, telemetry and echocardiographic findings.
| Medication, Telemetry, Echocardiographic Findings | Alive at Discharge n=92 |
Died in Hospital n=79 |
P-value |
|---|---|---|---|
| Moderate-severe VHD | 0/15 | 5/23 (21.7%) | NA too few |
| Decreased RV function | 6/17 (35.3%) | 8/18 (44.4%) | NA too few |
| Hydroxychloroquine | 61 (66.3%) | 69 (87.3%) | 0.001 |
| Azithromycin | 48 (52.2%) | 53 (67.1%) | 0.048 |
| Anticoagulant | 29 (31.5%) | 36 (45.6%) | 0.059 |
| IV vasopressors | 23 (25.0%) | 55 (70.5%) | <0.0001 |
| NSVT | 1 (1.1%) | 7 (8.9%) | 0.025 |
| Sustained VT | 1 (1.1%) | 6 (7.6%) | 0.0497 |
| Sinus pause | 2 (2.2%) | 6 (7.6%) | 0.15 |
| PEA or VF arrest | 1 (1.1%) | 6 (7.6%) | 0.0497 |
| AV block | 2 (2.2%) | 1 (1.3%) | 1 |
| Intubated | 20 (21.7%) | 66 (83.5%) | <0.0001 |
| Total days intubated | <0.0001 | ||
| Median (25th, 75th) | 0 (0, 0) | 7 (3, 10) | |
| Min to max | 0–55 | 0–30 | |
| LOS | 0.12 | ||
| Median (25th, 75th) | 11 (6, 26) | 9 (6, 17) | |
| Min to max | 1–53 | 1–34 |
Abbreviations: VHD, valvular heart disease; RV, right ventricle; IV, intravenous; VT, ventricular tachycardia; PEA, pulseless electrical activity; VF, ventricular fibrillation; AV, atrioventricular; LOS, length of stay.
Bolded values indicates a statistically significant value (p-value <0.05).
Table 4.
Multivariate analysis.
| Multivariate Analysis | OR | 95% CI | P-value |
|---|---|---|---|
| Age | 1.05 | 1.01, 1.14 | 0.021 |
| Rhythm | |||
| Normal sinus rhythm | — | — | |
| Any atrial fibrillation | 4.80 | 1.70, 14.7 | 0.004 |
| Peak troponin >0.50 ng/dL | |||
| No | — | — | |
| Yes | 7.20 | 1.02, 152 | 0.092 |
| History of COPD | |||
| No | — | — | |
| Yes | 1.64 | 0.51, 5.39 | 0.40 |
| History of diabetes | |||
| No | — | — | |
| Yes | 1.07 | 0.37, 3.00 | >0.9 |
| History of stent/CABG | |||
| No | — | — | |
| Yes | 0.80 | 0.19, 3.37 | 0.80 |
| Intubation | |||
| No | — | — | |
| Yes | 21.2 | 5.99, 91.8 | <0.001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass graft.
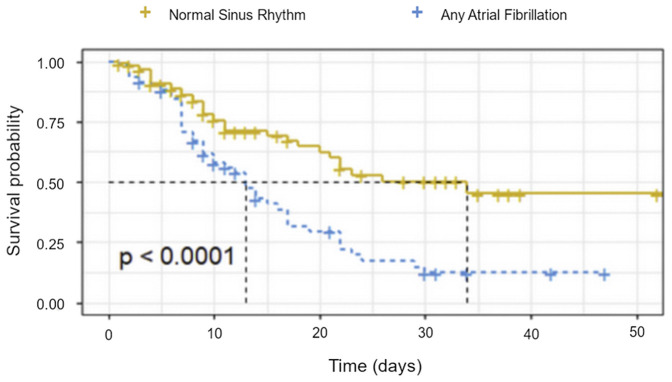
The presence of atrial fibrillation led to a 2.38 times greater risk of mortality compared to normal sinus rhythm (Table 5 ) and is represented in a Kaplan-Meier survival curve (Figure 1 ). History of COPD, history of stent or bypass were significant in the univariate analysis, however were not statistically significant in multivariate analysis.
Table 5.
Hazard ratio of atrial fibrillation vs. normal sinus rhythm.
| Atrial Fibrillation vs. Normal Sinus Rhythm | HR | 95% CI | P-value |
|---|---|---|---|
| Normal sinus rhythm | – | – | |
| Atrial fibrillation | 2.38 | 1.52, 3.71 | <0.001 |
Abbreviation: HR, hazard ratio.
Figure 1.
Kaplan-Meier survival curve of patients in atrial fibrillation vs. normal sinus rhythm.
A Kruskal-Wallis test was then performed on three specific subsets of the patients in the study, using rhythm as the primary characteristic. Patients were divided into a normal sinus rhythm group, previous history of atrial fibrillation group and a new-onset atrial fibrillation group. From the analysis (Table 6 ) it was discovered that there were statistically significant differences (p<0.001) between the groups. Patients who developed new-onset atrial fibrillation and those with a prior history of atrial fibrillation tended to be older, had a higher propensity to be intubated during their ICU stay, had a higher maximum troponin value and had a higher mortality rate compared to those who remained in sinus rhythm.
Table 6.
Kruskal-Wallis Test comparing rhythm to specific patient characteristics.
| Kruskal-Wallis Test Comparing Rhythm to Specific Patient Characteristics | N | 0. Normal Sinus Rhythm | 1. Prior History of AF | 2. New Onset AF | P-valuea |
|---|---|---|---|---|---|
| Characteristics | |||||
| Mean age (years) | 171 | 63 | 72 | 76 | <0.001 |
| Intubated | 171 | <0.001 | |||
| 1. Not intubated | 67 (60%) | 10 (38%) | 8 (24%) | ||
| 2. Intubated | 44 (40%) | 16 (62%) | 26 (76%) | ||
| Status at discharge | 171 | <0.001 | |||
| 1. Alive | 77 (69%) | 6 (23%) | 9 (26%) | ||
| 2. Deceased | 34 (31%) | 20 (77%) | 25 (74%) | ||
| Mean length of stay (days) | 171 | 9 | 8 | 13 | 0.092 |
Abbreviation: AF, atrial fibrillation.
The p-value for the continuous variable above is from the Kruskal-Wallis Test where the null hypothesis assumes that the samples (groups) are from identical populations while the alternative hypothesis assumes that at least one of the samples (groups) comes from a different population than the others.
Discussion
Recent literature has described an association with new atrial fibrillation with patients with COVID-19 [[8], [9], [10]]. Interestingly, our study suggests that the presence of atrial fibrillation contributes to an increased morbidity and mortality in COVID-19 patients. Patients who either had a prior history of atrial fibrillation (63%) or new onset atrial fibrillation (76%) both had statistically significantly higher rates of intubation rates compared to those who remained in sinus rhythm (40%). This relationship is reflected in mortality as well with the majority of patients with a prior history of atrial fibrillation (78%) and new onset (74%) passing away compared to normal sinus patients (31%). One (1) possible explanation could be that patients who develop or present with atrial fibrillation are generally sicker. Prior studies have shown that patients who have severe sepsis, also have higher levels of proinflammatory markers which triggers a cascade of biochemical reactions leading to higher rates of atrial fibrillation [11]. This is consistent with our data, as patients in the deceased cohort did exhibit higher levels of ferritin, LDH and troponin in the univariate analysis. The mechanism by which atrial fibrillation contributes to mortality in these patients is unclear, however it is known, that atrial fibrillation can create haemodynamic instability and clinical deterioration through mechanisms such as the loss of atrial kick and rapid ventricular response. Additionally, atrial fibrillation has long been known to be a source of cardioembolism which increases mortality rates [12] which may have a compounding effect as some studies have shown that COVID-19 promotes a pro-coagulable state [13]. Further studies will be required to characterise the association between COVID-19 and atrial fibrillation, and to specifically identify the mechanism by which atrial fibrillation causes increased mortality in these patients.
Advanced age has been shown in multiple studies to play a significant role in predicting mortality which is consistent with our findings in the univariate analysis [3,6,7,14]. Though studies have shown age as being an independent risk factor, none of the studies assessed atrial fibrillation as a possible confounding variable. Advanced age may appear to contribute to mortality because of its association with developing other medical problems as one gets older, however without controlling for the effect of these underlying conditions, age may carry more significance than it independently has. Given how impactful atrial fibrillation is on our mortality data, this is an association that should be explored further. A prior observational study from Italy did not find an effect on mortality from new onset or recurrent atrial fibrillation [15]. However, our study investigated the US population, and the variation in findings may result from underlying differences between US and Italian populations. For example, rates of obesity, diabetes, heart failure were higher in our study population compared to the Italian population. Intubation status was also identified as an independent predictor of mortality, though this is likely a generalised surrogate for the overall severity of the patient’s disease.
This was a multi-centred study which allowed for the collection of data from different institutions and different patient populations. Early in the onset of the pandemic, Michigan saw a large number of COVID-19 cases which allowed for a large sample population for analysis. There were some limitations in this study. As the COVID-19 pandemic was still in its infancy, the lack of standardisation and recommended testing led to incomplete data for some patients. Patients who were alive and still hospitalised or who were transferred out of the hospital system were assumed to be alive at the end date of data collection, however only 13 of the 171 patients fell into this category which limits its impact.
Conclusions
Mortality of critically ill COVID-19 patients is high. Early identification and aggressive treatment of high-risk patients identified (age, presence of atrial fibrillation, intubation) in this study could improve clinical outcome. The predictive value of the presence of atrial fibrillation on survival highlights the importance of cardiac involvement in COVID-19 infections. While atrial fibrillation may not be the sole driver, findings from this study suggest that it can be considered a risk factor for increased mortality. The findings from this study are consistent with similar studies that have reported a significant mortality risk in COVID-19 patients who develop or have a history of atrial fibrillation [16]. This could help guide treatment with vigilant monitoring for arrhythmias in COVID-19 patients, as well as identifying at-risk patients early in their hospital course to provide optimal medical care. Future directions could include assessing each patient’s comorbid factors, including atrial fibrillation, to provide aggressive treatment in those deemed high risk including providing intravenous (IV) steroids and antibodies, however further studies are needed. Although the exact mechanism by which atrial fibrillation increases mortality is unknown and likely multifactorial, since this study was observational in design, this association must be investigated further to provide optimal patient care.
Funding Sources
We report no relevant funding sources associated with this manuscript.
Conflicts of Interest
There are no conflicts of interest to disclose.
References
- 1.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Qu C.Q., He J.Z., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., RECOVERY Collaborative Group, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. 2020:1. [Google Scholar]
- 5.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 6.Lai P.H., Lancet E.A., Weiden M.D., Webber M.P., Zeig-Owens R., Hall C.B., et al. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020;5:1154–1163. doi: 10.1001/jamacardio.2020.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L., Yu J., He W., Chen L., Yuan G., Dong F., et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34:2173–2183. doi: 10.1038/s41375-020-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seecheran R., Narayansingh R., Giddings S., Rampaul M., Furlonge K., Abdool K., et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taha M.E., Alsafi W., Taha M., Eljack A., Ibrahim H. Coronavirus disease and new-onset atrial fibrillation: two cases. Cureus. 2020;12(5):e8066. doi: 10.7759/cureus.8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuipers S., Peter M.C., Klouwenberg K., Olaf L., Cremer Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Critical Care. 2014;18(6):688. doi: 10.1186/s13054-014-0688-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walkey A.J., Hammill B.G., Curtis L.H., Benjamin E.J. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llau J.V., Ferrandis R., Sierra P., Hidalgo F., Cassinello C., Gómez-Luque A., et al. SEDAR-SEMICYUC consensus recommendations on the management of haemostasis disorders in severely ill patients with COVID-19 infection. Rev Esp Anestesiol Reanim. 2020;67:391–399. doi: 10.1016/j.redar.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toraih E.A., Elshazli R.M., Hussein M.H., Elgaml A., Amin M., El-Mowafy M., et al. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID-19 patients: a meta-regression and decision tree analysis. J Med Virol. 2020;92:2473–2488. doi: 10.1002/jmv.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo V., Di Maio M., Mottola F.F., Pagnano G., Attena E., Verde N., et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: a multicenter observational study. Eur J Clin Invest. 2020;50(12):e13387. doi: 10.1111/eci.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peltzer B., Manocha K.K., Ying X., Kirzner J., Ip J.E., Thomas G., et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol. 2020;31:3077–3085. doi: 10.1111/jce.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]