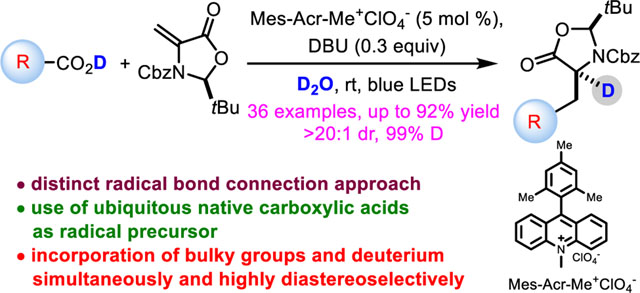
Abstract
A mild, versatile organophotoredox protocol has been developed for the preparation of diverse, enantioenriched α-deuterated α-amino acids. Distinct from the well-established two-electron transformations, this radical-based strategy offers the unrivaled capacity of the convergent unification of readily accessible feedstock carboxylic acids and a chiral methyleneoxazolidinone fragment and highly diastereo-, chemo- and regio-selective incorporation of deuterium simultaneously. Furthermore, the approach has addressed the long-standing challenge of the installation of sterically demanding side chains into α-amino acids.
Graphical Abstract
Isotopically labelled amino acids, particularly, the α-deuterated version, are broadly used in almost every sub-discipline in the life sciences for studying biosynthetic pathways,1 enzymatic mechanisms,2 and probing the secondary and tertiary structures of peptides and proteins by NMR and MS techniques.3 Furthermore, the incorporation of deuterium into α-position of amino acids can enhance metabolic stability and reduce the rate of epimerization of peptido and peptidomimetic therapeutics and thus enhance the efficacy and/or decrease the potential toxicity (see representatives in Figure 1).4 Therefore, there is a long-standing interest in the synthesis and application of enantioenriched α-deuterated amino acids.5–7
Figure 1.
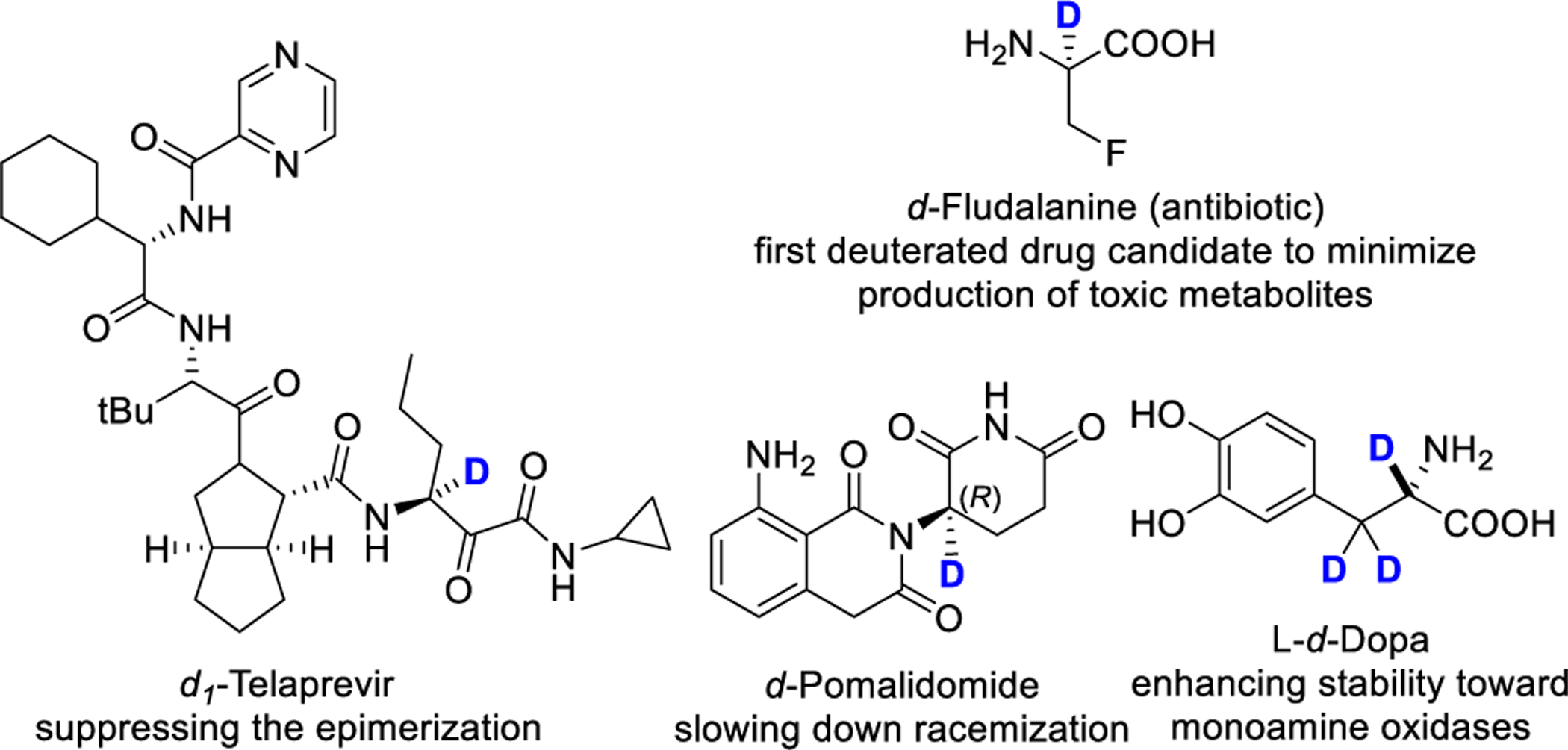
Examples of α-deuterated amino acid therapeutics.
In the routes available for the synthesis of chiral α-deuterated amino acids, enzyme-catalyzed approaches including enzyme mediated deuteration of α-amino acids8 and enzymatic reductive amination of pyruvates,9 are largely limited by narrow substrate scope. The commonly used methods with the capacity of access to unnatural α-amino acids rely on asymmetric alkylation of deuterated glycine derived imines10 or H/D exchange of amino acids derived imines11 using chiral auxiliary (e.g., Schöllkopf’s bis-lactam ether) or chiral promoter catalyzed enolization (Scheme 1A1). Transitional metal-catalyzed C-H activation followed by H/D exchange12 or 1,3-deuteride transfer13 provides an alternative to incorporate the isotope into α-position of amino acids (Scheme 1A2). Although these techniques represent the state-of-the-art strategies for the synthesis of α-deuterated amino acids, they all rely on a polar bond connection, and therefore carrying inherent limitations such as poor chemo-, regio- and/or enantio-selectivity, and in many cases, moderate level of deuteration. Furthermore, an intrinsic limitation of these ionic strategies is difficult to synthesize highly sterically demanding amino acids, a class of structures widely used in the field of peptides and peptidomimetics to constrain their conformations, and thus improve their potency and/or selectivity, lipophilicity, and metabolic stability.14 To overcome these issues, it is clear that a new design paradigm is needed.
Scheme 1.
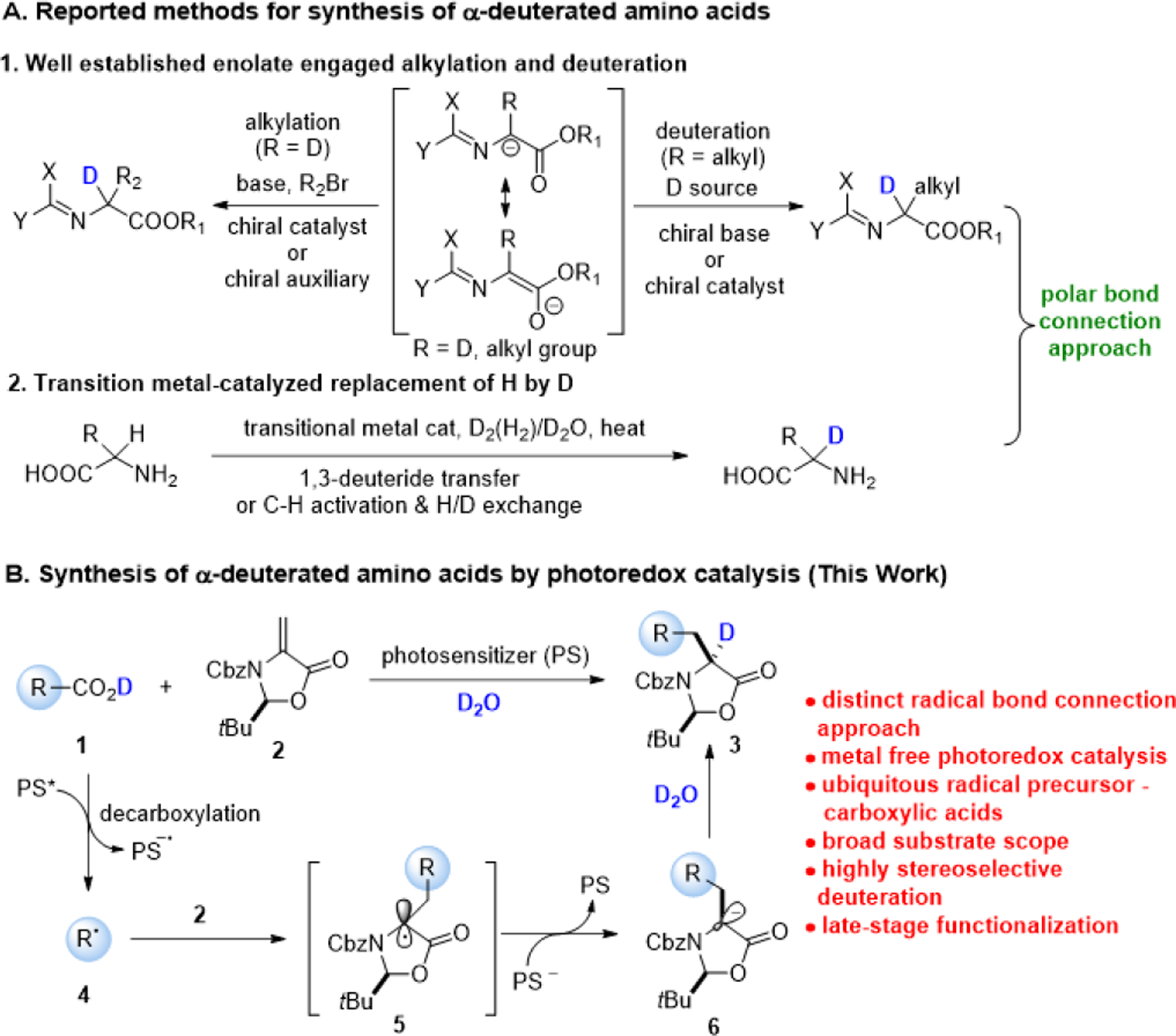
Synthesis of enantioenriched α-deuterated amino acids.
An open shell radical process would offer a distinct and pragmatic approach for introducing the bulky groups into amino acids by virtue of favorable formation of 3° radicals.15 The radical addition to dehydroalanine (Dha) derivatives has been demonstrated as a viable approach for the synthesis of α-amino acids.16 In recent efforts, notably, an efficient Giese-type reaction of tertiary amines or halogenated pyridine with Dha derivatives is realized with photoredox catalysis by Jui and coworkers.17 Molander and colleagues elegantly introduced fluorine at the α-position of amino acids by regioselective carbofluorination of Dha compounds using alkyl trifluoroborate reagents as radical precursors.18 We envisioned that direct addition of a decarboxylative radical 4 to Dha derivatives such as (S)-methyleneoxazolidinone 219 as a chiral inducer could lead to enantioenriched amino acids 3 by the employment of ubiquitous, readily accessible alkyl carboxylic acids 1 as radical progenitors (Scheme 1B).20 The ready accessibility of feedstock alkyl carboxylic acids 1 make possible for the synthesis of more structurally diverse amino acids. Furthermore, drawing from the mechanistic evidence amassed in these and our studies,17,18,21 we conceived that Re-face selective deuteration of the chiral anion intermediate 6 would potentially provide a novel approach to enantioenriched α-deuterated amino acids 3. It is expected that the power of the strategy is fueled by the chemo-, regio- and diastereo-selective incorporation of bulky side chains and deuterium into α-amino acids simultaneously. To our knowledge, a strategy of this type has not been documented previously.
To investigate the feasibility of this proposal, in the initial attempt, we probed a reaction of deuterated methyl 2,3-O-(1-methylethylidene)-β-D-ribofuranosiduronic acid (1a, 1.5 equiv) as the glycosyl radical precursor and (S)-methyleneoxazolidinone 2 (1.0 equiv) as the amino acid surrogate, and D2O (80 equiv) as the deuterium source in the presence of a photosensitizer (PS) irradiated by a 40 W Kessil blue LED (Table 1). Commonly used Cs2CO3 (1.5 equiv) as base in photoredox decarboxylation was tested in anhydrous dichloroethane (DCE) as solvent for 24 h. It should be noted that the use of deuterated acid and anhydrous solvent (eliminating H2O) was necessary for achieving higher deuteration level. It was found that the reaction efficiency was PS dependent (entries 1–3). Among the PS probed, mesityl acridinium salt (Mes-Acr-Me+•ClO4−) delivered the desired product 3a in encouraging 59% yield (entry 1), while PS with low reduction potential Ir[dF(CF3)ppy]2(dtbpy)PF6 (entry 2) and (4CzIPN (entry 3) failed to produce the product. In addition, 85% D-incorporation with excellent diastereometric ratio (dr) >20:1 was achieved. Further optimization reaction conditions including solvent (entry 4), the amount of D2O (entry 4), 1a and base and amount (entries 5 and 6and 3), and (7revealed the optimized reaction conditions (entry 7): 0.3 equiv of DBU, 80 equiv of D2O and anhydrous MeCN. The control experiments confirmed that base, light and photocatalyst were prerequisites for this transformation (entries 8–10).
Table 1.
Exploration and optimization[a]
 | ||
|---|---|---|
| entry | derivation from standard conditions | yield (%),[b] D-content (%)[c] |
| 1 | Cs2CO3 (1.5 equiv) as base, anhydrous DCE as solvent for 24 h | 59, 92 |
| 2 | Ir[dF(CF3)ppy]2(dtbpy)PF6 as PS, Cs2CO3 (1.5 equiv) as base, and D2O (40 equiv) used in anhydrous DCE for 24 h | <5, nd[e] |
| 3 | 4CzIPN as PS, Cs2CO3 (1.5 equiv) as base, and D2O (40 equiv) used in anhydrous DCE for 24 h | <5, nd[e] |
| 4 | Cs2CO3 (1.5 equiv.) as base, D2O (40 equiv) used, anhydrous DCE as solvent for 24 h | 52, 85 |
| 5 | 0.6 equiv of DBU used | 69, 96 |
| 6 | 1.2 equiv of 1a used | 63, 96 |
| 7 | none | 70 (68),[d] 95 |
| 8 | no base | <5, nd[e] |
| 9 | no PS | <5, nd[e] |
| 10 | no light | <5, nd[e] |
Reaction conditions: unless specified, a mixture of 1a (0.3 mmol), 2 (0.2 mmol) and catalyst (0.01 mmol) in anhydrous MeCN (2.0 mL) was irradiated with 40W Kessil blue LEDs in N2 atmosphere at rt for 36 h.
Yield based on 1H NMR.
Determined by 1H NMR.
Yield of isolated products.
not determined.
With optimized reaction conditions in hand, we first evaluated the coupling reactions utilizing glycosyl carboxylic acids 1 with 2 by providing an alternative for the synthesis of β-glycosyl α-deuterated amino acids. The protocol worked well for the tested pentose and hexose to give the desired products 3a-c in moderate yield and with high level of deuterium incorporation at the desired α-position (Scheme 2A). It should be noted that the anomeric effect of the glycosyl radicals delivers highly stereoselective anomeric products, consistent with our previous works.22,23 Furthermore, the chiral (S)-oxazolidinone controlled the deuteration very well with >20:1 dr by only forming one diastereomer.
Scheme 2.
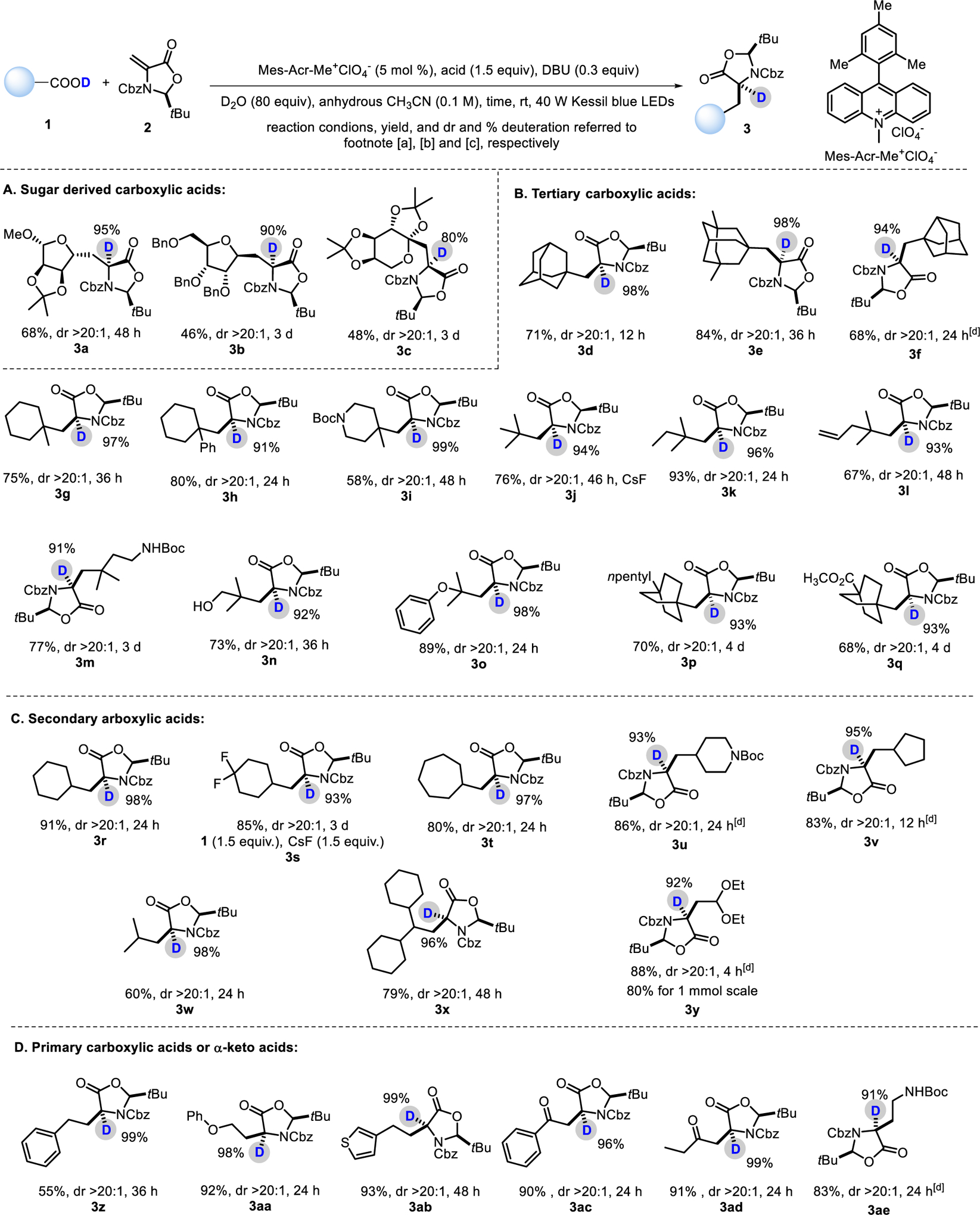
Scope of the organophotoredox-mediated asymmetric α-deuterated α-amino Acids synthesis with simple carboxylic acids[a]
[a] Reaction conditions: unless specified, a mixture of 1 (0.3 mmol), 2 (0.2 mmol) and Mes-Acr-Me+•ClO4− (0.01 mmol) in anhydrous MeCN (2.0 mL) was irradiated with 40 W Kessil blue LEDs in N2 atmosphere at rt for specified time. [b] Yield of isolated products. [c] Deuteration and dr determined by 1H NMR. [d] No desired product was obtained under the standard reaction conditions. The reaction was carried out, as follows: a mixture of 1 (0.24 mmol), 2 (0.2 mmol) Cs2CO3 (0.24 mmol) and 4CzIPN (0.01 mmol) in anhydrous DMF (2.0 mL) was irradiated with 40 W Kessil blue LEDs in N2 atmosphere at rt for specified time.
Encouraged by the above studies, we extended the strategy for the synthesis of highly valued, structurally diverse and unique unnatural α-deuterated amino acids, which are difficult to be accessed by the established polar bond connection methods (Scheme 2B–D). The results from the studies show that the protocol serves as a general approach to various unnatural α-deuterated amino acids. In the view of biological importance of the bulky side chains of amino acids in peptido- and peptidomimetic relevant drug discovery and biological studies, the difficulty in accessing them using prior methods made them an ideal starting point. We first paied our attention on the sterically demanding tertiary alkyl carboxylic acids (Scheme 2B). To our delight, despite their high steric hindrance, the tested tertiary carboxylic acids including adamantyl group and analogue (3d-f), cyclohexyl derivatives (3g-l), tert-butyl group bearing various functional groups (3k-o) gave good to excellent yield with uniformly high diastereoselectivity (dr >20:1) and high deuteration level (91–99%). Moreover, the bridged structures (3p- q) could also be incorporated with high efficiency. Next, cyclic secondary alkyl radicals (3r-v) bearing five-, six-, and seven-membered rings were probed (Scheme 2C). The less hindered structures gave rise to higher yield (80–91%) without sacrificing deuteration level (93–98%) and diastereoselectivity (>20:1 dr). The same trend was observed for acyclic secondary carboxylic acid (3w-y), including the natural amino acid d-leucine and aldehyde precursor-acetal. This study was further expanded to primary carboxylic acids (3z-ae, Scheme 2D) as alkyl radical precursors, which are generally difficult to generate. As shown, the protocol worked smoothly for the cases of 3z-3ae in terms of reaction yield, dr and deuteration. It should also be aware that under the mild reaction conditions, this radical-based method exhibits broad functional group tolerance, as demonstrated for protected amines (3m, 3u and 3ae), free hydroxyl (3n), alkene (3l), ester (3q), ether (3a-c, 3o and 3aa), acetal (3y), carbonyl (3ac, ad), and heteroaromatic (3ab). No desired products were obtained under the standard reaction conditions for 3f, 3u, 3v, 3y, and 3ae. However, the reaction could proceed smoothly with a mixture of 1 (0.24 mmol), 2 (0.2 mmol) and 4CzIPN (0.01 mmol) in anhydrous DMF (2.0 mL) irradiated with 40 W Kessil blue LEDs in N2 atmosphere at rt.
To further demonstrate the utility of this mild decarboxylative deuteration methodology, we performed a series of late-stage modifications on medicinal agents and natural products. As shown in Scheme 3A, the standard protocol (for detailed experiments, see Scheme 2, footnote [a] and SI) was successfully applied to natively and selectively modify bezafibrate and drug gemfibrozil, clinically used lipid lowering agents, to give amino acid derivatives 7 and 8 in 79 and 68% yield, and 96 and 97% D-incorporation, respectively and with >20:1 dr. Moreover, an anti-inflammatory agent 3-indolacetic acid, indomethacin was efficiently transformed into corresponding isotopically labelled amino acid (9) in good yield (85%), high deuteration (97%) and excellent dr (>20:1). Finally, enoxolone (10) containing a secondary alcohol and an α, β-unsaturated ketone, was tolerated. Of note, a modified protocol using 0.6 equiv. of DBU with a 0.05M concentration was used to improve the reaction efficiency.
Scheme 3.
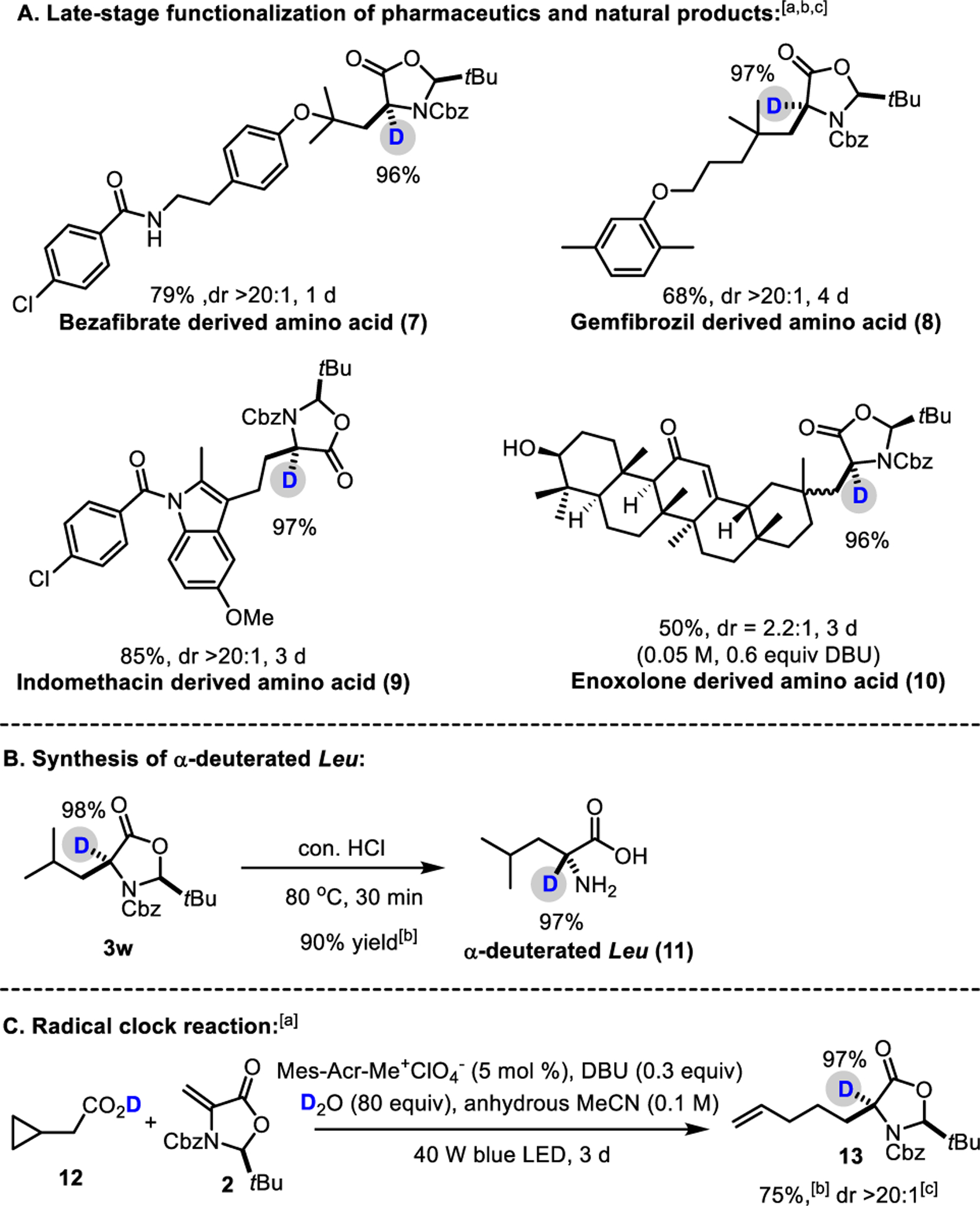
Late-stage functionalization of pharmaceutics and natural products, conversion to amino acids and radical clock reaction.
[a] Reaction conditions: unless specified, see footnote [a] in Scheme 2 and see SI. [b] Yield of isolated products. [c] Deuteration and dr determined by 1H NMR.
The synthesized products 3 could be conveniently transformed into α-deuterated α-amino acids, as showcased in the synthesis of α-deuterated Leu (11) by reacting with con. HCl for 30 min without the erosion of deuteration level (Scheme 3B). Intrigued by the apparent breadth of scope, a preliminary mechanistic inquiry was conducted (Scheme 3C). Radical clock experiments–cyclopropyl ring-opening (12) by forming alkenyl derived amino acid 13 suggest the presence of alkyl radicals. This observation is consistent with previously reported decarboxylative coupling studies.20,22,23
In summary, a mild, versatile organophotoredox protocol has been developed for the preparation of diverse, enantioenriched, α-deuterated α-amino acids. The distinct radical approach represents a significant departure from the two-electron transformations so often prescribed in the literature. This radical-based strategy offers the unrivaled capacity of the convergent unification of readily accessible feedstock carboxylic acids and a chiral methyleneoxazolidinone fragment and highly diastereo-,chemo- and regio-selective incorporation of deuterium simultaneously, which could vastly expand the domain of highly biologically and medicinally valued α-deuterated amino acids. Furthermore, the approach has addressed the long-standing challenge of the installation of sterically bulky side chains into α-amino acids. Customizable by design, the simplicity and efficiency of this procedure should resonate with medicinal chemists requiring rapid access to these highly sought building blocks.
Supplementary Material
ACKNOWLEDGMENT
Financial support was provided by the NIH (5R01GM125920-03).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experiment details and spectroscopic data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Furuta T; Takahashi H; Kasuya Y Evidence for a carbanion intermediate in the elimination of ammonia from L-histidine catalyzed by histidine ammonia-lyase. J. Am Chem. Soc 1990, 112, 3633; [PubMed] [Google Scholar]; (b) Baldwin JE; Adlington RM; Marquess DG; Pitt AR; Porter MJ; Russell AT Evidence for an insertion-homolysis mechanism for carbon-sulphur bond formation in penicillin biosynthesis; 1. Synthesis of tripeptide probes. Tetrahedron, 1996, 52, 2515; [Google Scholar]; (c) Church NJ; Young DW Synthesis of the suicide substrate D-propargylglycine stereospecifically labelled with deuterium and investigation of its oxidation by D-amino acid oxidase. J. Chem. Soc. Perkin Trans. 1 1998, 52, 1475. [Google Scholar]
- (2).Rose JE; Leeson PD; Gani D J Mechanisms and stereochemistry of the activation of (2S)- and (2R)-serine O-sulfate as suicide inhibitors for Escherichia coli glutamic acid decarboxylase. J. Chem. Soc. Chem. Commun 1992, 1784. [Google Scholar]
- (3).(a) Lian L-Y Middleton DA Labelling approaches for protein structural studies by solution-state and solid-state NMR. Prog. Nucl. Msgn. Reson. Spectrosc 2001, 39, 171; [Google Scholar]; (b) Sack I; Balazs YS; Rahimipour S; Vega S Solid-State NMR Determination of Peptide Torsion Angles: Applications of 2H-Dephased REDOR. J. Am. Chem. Soc 2000, 122, 12263; [Google Scholar]; (c) Gardner KH; Kay LE Production and Incorporation of 15N, 13C, 2H (1H-δ1 Methyl) Isoleucine into Proteins for Multidimensional NMR Studies. J. Am. Chem. Soc 1997, 119, 7599; [Google Scholar]; (d) Lastra E; Hegedus LS Synthesis of compounds containing two adjacent carbon-13 labels by photolytic reactions of chromium carbene complexes. J. Am. Chem. Soc 1993, 115, 87. [Google Scholar]
- (4).For reviews of deuterium application in biomedical sciences and drug discovery, see:; (a) Atzrodt J; Derdau V; Kerr WJ; Reid M Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. Int. Ed 2018, 57, 1758; [DOI] [PubMed] [Google Scholar]; (b) Yang J in Deuterium: Discovery and Applications in Organic Chemistry, Elsevier, Amsterdam, 2016, pp. 1; [Google Scholar]; c) Gant TG Using Deuterium in Drug Discovery: Leaving the Label in the Drug. J. Med. Chem 2014, 57, 3595; [DOI] [PubMed] [Google Scholar]; d) Pirali T; Serafini M; Cargnin S; Genazzani AA Applications of Deuterium in Medicinal Chemistry. J. Med. Chem 2019, 62, 5276; [DOI] [PubMed] [Google Scholar]; (e) Simmons EM; Hartwig JF On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem., Int. Ed 2012, 51, 3066; [DOI] [PubMed] [Google Scholar]; and selected examples of deuterated therapeutics:; (f) Pirali T; Serafini M; Cargnin S; Genazzani AA Applications of Deuterium in Medicinal Chemistry. J. Med. Chem 2019, 62, 5276; [DOI] [PubMed] [Google Scholar]; (g) Maltais F; Jung YC; Chen M; Tanoury J; Perni RB; Mani N; Laitinen L; Huang H; Liao S; Gao H; Tsao H; Block E; Ma C; Shawgo RS; Town C; Brummel CL; Howe D; Pazhanisamy S; Raybuck S; Namchuk M; Bennani YL In Vitro and In Vivo Isotope Effects with Hepatitis C Protease Inhibitors: Enhanced Plasma Exposure of Deuterated Telaprevir versus Telaprevir in Rats. J. Med. Chem 2009, 52, 7993; [DOI] [PubMed] [Google Scholar]; (h) Yamamoto T; Tokunaga E; Nakamura S; Shibata N; Toru T Synthesis and configurational stability of (S)- and (R)-deuteriothalidomides. Chem. Pharm. Bull 2010, 58, 110; [DOI] [PubMed] [Google Scholar]; (i) Jacques V; Czarnik AW; Judge TM; Van der Ploeg LH; DeWitt SH Differentiation of antiinflammatory and antitumorigenic properties of stabilized enantiomers of thalidomide analogs. Proc. Natl. Acad. Sci. U.S.A 2015, 112, E1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).For reviews of synthesis deuterated building blocks, see: 4e and; (a) Atzrodt J; Derdau V; Kerr WJ; Reid M C-H Functionalisation for Hydrogen Isotope Exchange. Angew. Chem., Int. Ed 2018, 57, 3022; [DOI] [PubMed] [Google Scholar]; (b) Sattler A Hydrogen/Deuterium (H/D) Exchange Catalysis in Alkanes. ACS Catal 2018, 8, 2296. [Google Scholar]
- (6).Selected examples:; (a) Yu RP; Hesk D; Rivera N; Pelczer I; Chirik PJ Iron-catalysed tritiation of pharmaceuticals. Nature 2016, 529, 195; [DOI] [PubMed] [Google Scholar]; (b) Loh YY; Nagao KA; Hoover J; Hesk D; Rivera NR; Colletti SL; Davies IW; MacMillan DWC Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 2017, 358, 1182; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Koniarczyk J; Hesk D; Overgard A; Davies IW; McNally A A General Strategy for Site-Selective Incorporation of Deuterium and Tritium into Pyridines, Diazines, and Pharmaceuticals. J. Am. Chem. Soc 2018, 140, 1990; [DOI] [PubMed] [Google Scholar]; (d) Hale LVA; Szymczak NK Stereoretentive Deuteration of α-Chiral Amines with D2O. J. Am. Chem. Soc 2016, 138, 13489; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Valero M; Weck R; Ggssregen S; Atzrodt J; Derdau V Highly Selective Directed Iridium-Catalyzed Hydrogen Isotope Exchange Reactions of Aliphatic Amides. Angew. Chem., Int. Ed 2018, 57, 8159; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wang X; Zhu M-H; Schuman DP; Zhong D; Wang W-Y; Wu L-Y; Liu W; Stoltz BM; Liu W-B General and Practical Potassium Methoxide/Disilane-Mediated Dehalogenative Deuteration of (Hetero)Arylhalides. J. Am. Chem. Soc 2018, 140, 10970; [DOI] [PubMed] [Google Scholar]; (g) Puleo TR; Strong AJ; Bandar JS Catalytic α-Selective Deuteration of Styrene Derivatives. J. Am. Chem. Soc 2019, 141, 1467; [DOI] [PubMed] [Google Scholar]; (h) Liang X; Duttwyler S Efficient Brønsted-Acid-Catalyzed Deuteration of Arenes and Their Transformation to Functionalized Deuterated Products. Asian J. Org. Chem 2017, 6, 1063; [Google Scholar]; (i) Zhou R; Li J; Cheo HW; Chua R; Zhan G; Hou Z; Wu J Visible-light-mediated deuteration of silanes with deuterium oxide. Chem. Sci 2019, 10, 7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).The studies from our labs:; (a) Geng H; Chen X; Gui J; Zhang Y; Shen Z; Qian P; Chen J; Zhang S; Wang W Practical synthesis of C1 deuterated aldehydes enabled by NHC catalysis. Nature Catal 2019, 2, 1071; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shen Z; Zhang S; Geng H; Wang J; Zhang X; Zhou A; Yao C; Chen X; Wang W Trideuteromethylation Enabled by a Sulfoxonium Metathesis Reaction. Org. Lett 2019, 21, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Stevenson DE; Akhtar M; Gani D Structural and stereochemical studies of methiomine decarboxylase from dryopteris felix-mas. Tetrahedron Lett 1986, 27, 5661; [Google Scholar]; (b) Milne JJ; Malthouse JPG Enzymatic synthesis of α-deuterated amino acids. Biochem. Soc. Trans 1996, 24, 133S; [DOI] [PubMed] [Google Scholar]; (c) Faleev NG; Ruvinov SB; Saporovskaya MB; Belikov VM; Zakomyrdina LN; Sakharova IS; Torchinsky YM Preparation of α-deuterated L-amino acids using E.coli B/It7-A cells containing tryptophanase. Tetrahedron Lett 1990, 31, 7051. [Google Scholar]
- (9).(a) Raap J; Nieuwenhuis S; Creeners A; Hexspoor S; Kragl U; Lugtenburg J Synthesis of Isotopically Labelled L-Phenylalanine and L-Tyrosine. Eur. J. Org. Chem 1999, 10, 2609; [Google Scholar]; (b) Wong CH; Whitesides GM Enzyme-catalyzed organic synthesis: regeneration of deuterated nicotinamide cofactors for use in large-scale enzymatic synthesis of deuterated substances. J. Am. Chem. Soc 1983, 105, 5012. [Google Scholar]
- (10).(a) Rose JE; Leeson PD; Gani D Stereospecific synthesis of α-deuteriated α-amino acids: regiospecific deuteriation of chiral 3-isopropyl-2,5-dimethoxy-3,6-dihydropyrazines. J. Chem. Soc. Perkin Trans 1995, 157; [Google Scholar]; (b) Taylor PJM; Bull SD An improved synthesis of deuterated Schöllkopf’s bis-lactim ether and its use for the asymmetric synthesis of (R)-[α−2H]-phenylalanine methyl esters. Tetrahedron: Asymmetry 2006, 17, 1170; [Google Scholar]; (c) Lygo B; Humphreys LD Enantioselective synthesis of α-carbon deuterium-labelled L-α-amino acids. Tetrahedron Lett 2002, 43, 6677; [Google Scholar]; (d) Takeda R; Abe H; Shibata N; Moriwaki H; Izawa K; Soloshonok VA Asymmetric synthesis of α-deuterated α-amino acids. Org. Biomol. Chem 2017, 15, 6978; [DOI] [PubMed] [Google Scholar]; (e) Elemes Y; Ragnarsson U Synthesis of enantiopure α-deuteriated Boc-L-amino acids. J. Chem. Soc. Perkin Trans. 1 1996. 537. [Google Scholar]
- (11).(a) Oh J-S; Kim K; Song CE Enantioselective synthesis of α-deuterium labelled chiral α-amino acids via dynamic kinetic resolution of racemic azlactones. Org. Biomol. Chem 2011, 9, 7983; [DOI] [PubMed] [Google Scholar]; (b) Moozeh K; So SM; Chin J Catalytic Stereoinversion of L-Alanine to Deuterated D-Alanine. Angew. Chem., Int. Ed 2015, 54, 9381. [DOI] [PubMed] [Google Scholar]
- (12).(a) Taglang C; Martínez-Prieto LM; Rosal I; Maron L; Poteau R; Philippot K; Chaudret B; Perato S; Lone AS; Puente C; Dugabe C; Rousseau B; Pieters G Enantiospecific C-H Activation Using Ruthenium Nanocatalysts. Angew. Chem., Int. Ed 2015, 54, 10474; [DOI] [PubMed] [Google Scholar]; (b) Michelotti A; Rodrigues F; Roche M Development and Scale-Up of Stereoretentive α-Deuteration of Amines. Org. Process Res. Dev 2017, 21, 1741; [Google Scholar]; (c) Bhatia S; Sphlinger G; Boukhumseen N; Boll Q; Li Z; Jackson JE Stereoretentive H/D Exchange via an Electroactivated Heterogeneous Catalyst at sp3C–H Sites Bearing Amines orAlcohols. Eur. J. Org. Chem 2016, 4230; [Google Scholar]; (d) Maegawa T; Akashi A; Esaki H; Aoki F; Sajiki H; Hirota K Palladium-Catalyzed Base-Selective H-D Exchange Reaction of Nucleosides in Deuterium Oxide. Synlett 2005, 5, 845; [Google Scholar]; (e) Michelotti A; Roche M 40 Years of Hydrogen–Deuterium Exchange Adjacent to Heteroatoms: A Survey. Synthesis 2019, 51, 1319; [Google Scholar]; (f) Taglang C; Korenchan DE; Morze C; Yu J; Najac C; Wang S; Blecha JE; Subramaniam S; Bok R; VanBrocklin HF; Vigneron DB; Ronen SM; Sriram R; Kurhanewicz JD; Wilson M; Flavell RR Late-stage deuteration of 13C-enriched substrates for T1 prolongation in hyperpolarized 13C MRI. Chem. Commun 2018, 54, 5233; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Jere FT; Miller DJ; Jackson JE Stereoretentive C-H Bond Activation in the Aqueous Phase Catalytic Hydrogenation of Amino Acids to Amino Alcohols. Org. Lett 2003, 5, 527. [DOI] [PubMed] [Google Scholar]
- (13).Chatterjee B; Krishnakumar V; Gunanathan C Selective α-Deuteration of Amines and Amino Acids Using D2O. Org. Lett 2016, 18, 5892. [DOI] [PubMed] [Google Scholar]
- (14).Reviews of conformationally constrained amino acids and their applications in peptido- and peptidomimetics:; (a) Cowell SM; Lee YS; Cain JP; Hruby VJ Exploring Ramachandran and chi space: conformationally constrained amino acids and peptides in the design of bioactive polypeptide ligands. Curr. Med. Chem 2004, 11, 2785; [DOI] [PubMed] [Google Scholar]; (b) Halab L; Gosselin F; Lubell WD Design, synthesis, and conformational analysis of azacycloalkane amino acids as conformationally constrained probes for mimicry of peptide secondary structures. Peptide Sci 2000, 55, 101. [DOI] [PubMed] [Google Scholar]
- (15).For selected reviews of photoredox catalysis:; (a) Narayanam JMR; Stephenson CRJ Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev 2011, 40, 102; [DOI] [PubMed] [Google Scholar]; (b) Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev 2013, 113, 5322; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hopkinson MN; Sahoo B; Li J-L; Glorius F Dual Catalysis Sees the Light: Combining Photoredox with Organo-, Acid, and Transition-Metal Catalysis. Chem. Eur. J 2014, 20, 3874; [DOI] [PubMed] [Google Scholar]; d) Koike T; Akita M Trifluoromethylation by Visible-Light-Driven Photoredox Catalysis. Top. Catal 2014, 57, 967; [Google Scholar]; (e) Meggers E Asymmetric catalysis activated by visible light. Chem. Commun 2015, 51, 3290; [DOI] [PubMed] [Google Scholar]; (f) Jamison CR; Overman LE Fragment Coupling with Tertiary Radicals Generated by Visible-Light Photocatalysis. Acc. Chem. Res 2016, 49, 1578; [DOI] [PubMed] [Google Scholar]; (g) Yan M; Lo JC; Edwards JT; Baran PS Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc 2016, 138, 12692; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Skubi KL; Blum TR; Yoon TP Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev 2016, 116, 10035; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Tellis JC; Kelly CB; Primer DN; Jouffroy M; Patel NR; Molander GA Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3-sp2 Cross-Coupling. Acc. Chem. Res 2016, 49, 1429; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Romero NA; Nicewicz DA Organic Photoredox Catalysis. Chem. Rev 2016, 116, 10075; [DOI] [PubMed] [Google Scholar]; (k) Chen J-R; Hu X-Q; Lu L-Q; Xiao W-J Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev 2016, 45, 2044; [DOI] [PubMed] [Google Scholar]; (l) Liu Q; Wu L-Z Recent advances in visible-light-driven organic reactions. Natl. Sci. Rev 2017, 4, 359; [Google Scholar]; m) Wang C-S; Dixneuf PH; Soulé J-F Photoredox Catalysis for Building C-C Bonds from C(sp2)-H Bonds. Chem. Rev 2018, 118, 7532. [DOI] [PubMed] [Google Scholar]
- (16).For reviews, see:; (a) Easton CJ Free-Radical Reactions in the Synthesis of α-Amino Acids and Derivatives. Chem. Rev 1997, 97, 53; [DOI] [PubMed] [Google Scholar]; (b) deGruyter JN; Malins LR; Baran PS Residue-Specific Peptide Modification: A Chemist’s Guide. Biochemistry 2017, 56, 3863; [DOI] [PMC free article] [PubMed] [Google Scholar]; for a leading reference, see:; (c) Sibi MP; Asano Y; Sausker JB Enantioselective Hydrogen Atom Transfer Reactions: Synthesis of N-Acyl-alpha-Amino Acid Esters This work was supported by the National Institutes of Health (NIH-GM-54656). Angew. Chem., Int. Ed 2001, 40, 1293. [DOI] [PubMed] [Google Scholar]
- (17).(a) Aycock RA; Vogt DB; Jui NT A practical and scalable system for heteroaryl amino acid synthesis. Chem. Sci 2017, 8, 7998; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Aycock RA; Pratt CJ; Jui NT Aminoalkyl Radicals as Powerful Intermediates for the Synthesis of Unnatural Amino Acids and Peptides. ACS Catal 2018, 8, 9115. [Google Scholar]
- (18).Sim J; Campbell MW; Molander GA Synthesis of α-Fluoro-α-amino Acid Derivatives via Photoredox-Catalyzed Carbofluorination. ACS. Catal 2019, 9, 1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Axon JR; Beckwith ALJ Diastereoselective radical addition to methyleneoxazolidinones: an enantioselective route to α-amino acids. J. Chem. Soc. Chem. Commun 1995, 549. [Google Scholar]
- (20).For recent reviews of photoredox decarboxylative reactions,; (a) Xuan J; Zhang Z-G; Xiao W-J Visible-Light-Induced Decarboxylative Functionalization of Carboxylic Acids and Their Derivatives. Angew. Chem., Int. Ed 2015, 54, 15632; [DOI] [PubMed] [Google Scholar]; (b) Huang H; Jia K; Chen Y Radical Decarboxylative Functionalizations Enabled by Dual Photoredox Catalysis. ACS Catal 2016, 6, 4983; [Google Scholar]; (c) Jin Y; Fu H Visible-Light Photoredox Decarboxylative Couplings. Asian J. Org. Chem 2017, 6, 368; [DOI] [PubMed] [Google Scholar]; (d) Liu Q; Wu L-Z Recent advances in visible-light-driven organic reactions. Natl. Sci. Rev 2017, 4, 359. [Google Scholar]
- (21).Huang H; Yu C-G; Zhang Y-T; Zhang Y-Q; Mariano PS; Wang W Chemo- and Regioselective Organo-Photoredox Catalyzed Hydroformylation of Styrenes via a Radical Pathway. J. Am. Chem. Soc 2017, 139, 9799. [DOI] [PubMed] [Google Scholar]
- (22).Ji P; Zhang Y; Wei Y; Huang H; Hu W; Mariano PS; Wang W Visible-Light-Mediated, Chemo- and Stereoselective Radical Process for the Synthesis of C-Glycoamino Acids. Org. Lett 2019, 21, 3086. [DOI] [PubMed] [Google Scholar]
- (23).Nagatomo M; Kamimura D; Matsui Y; Masuda K; Inoue M Et3B-mediated two- and three-component coupling reactions via radical decarbonylation of α-alkoxyacyl tellurides: single-step construction of densely oxygenated carboskeletons. Chem. Sci 2015, 6, 2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


