Abstract
Background and Aim
Ustekinumab is approved in Europe for the treatment of moderate to severe Crohn's disease (CD). Italian real‐life data are scarce, so the aim of this study was to assess the effectiveness and safety of ustekinumab in an Italian cohort of CD patients.
Methods
Data of patients with CD who started using ustekinumab were extracted from the cohort of the Sicilian Network for Inflammatory Bowel Disease. Primary end‐points were steroid‐free clinical remission at 8, 24, and 52 weeks of therapy and reduction of C‐reactive protein. Secondary end‐points were treatment response, treatment persistence at 12 months, and safety.
Results
A total of 131 patients (males 56%; mean age 46 years ±15) were included. All patients were biologics experienced except for one. At 24 and 52 weeks, 40% and 43% of patients achieved steroid‐free clinical remission, and 64% and 62% had clinical response, respectively. At the end of follow‐up, there was a significant reduction of steroid use (P = 0.012) and of the Harvey‐Bradshaw Index (P = 0.001). The probability of persistence in therapy with ustekinumab after 12 months of treatment was 89%. The only factor associated with discontinuation was older age.
Conclusions
Data from our real‐life cohort of treatment‐refractory CD patients suggest the satisfactory effectiveness and safety profile of ustekinumab.
Keywords: anti‐interleukin‐12/23, efficacy, persistence, safety

We assessed one‐year effectiveness and safety of ustekinumab in an real‐life cohort of treatment‐refractory CD patients. At 52 weeks 43% of patients achieved steroid‐free clinical remission and 62% had clinical response with a persistence in therapy of 98% and a significant reduction of steroid use (p = 0.012) and of HBI (p = 0.001). Data from our cohort suggest satisfactory effectiveness and safety profile of ustekinumab.
Introduction
Biological therapies have become the mainstay in the treatment of inflammatory bowel diseases (IBDs) representing an advance in the management of these chronic conditions. According to different mechanisms of action, biologics approved for Crohn's disease (CD) include anti‐tumor necrosis factor (TNF)α (infliximab and adalimumab) and anti‐integrin (vedolizumab). 1 , 2 These agents have proven efficacy for inducing and maintaining remission, but a consistent percentage of patients does not respond (primary failure) and experiences loss of efficacy (secondary failure) or adverse events, so new drugs with different mechanisms of action are needed.
Ustekinumab (Stelara, Janssen Biotech Inc., Horsham, PA, USA) is a monoclonal antibody targeting the p40 subunit of interleukin (IL)‐12 and IL‐23. It has recently been approved and used to treat moderate to severe active CD in adult patients unresponsive or intolerant to anti‐TNFα agents or patients in whom these biologic agents are contraindicated. Ustekinumab has been available since 2016 in the United States, since 2017 in Europe, and since January 2019 in Italy. The phase IIb trial Crohn’s Evaluation of Response to Ustekinumab anti‐IL12/23 for Induction (CERTIFI) showed short‐term clinical response in a cohort of anti‐TNF experienced patients. The following three phase III trials (UNITI ‐1, UNITI‐2, and IM‐UNITI) showed the efficacy of ustekinumab in the induction and maintenance of remission in anti‐TNF‐refractory and anti‐TNF‐naïve CD patients. 3 , 4 , 5 , 6 However, patients enrolled in clinical trials are not entirely representative of those treated in real life. In recent years, several observational real‐life studies from Europe have confirmed the external validity of randomized controlled trials with good effectiveness and safety of ustekinumab in a refractory CD population. 7 In Italy, ustekinumab is available for patients with moderate to severe CD and for those refractory or intolerant to conventional therapy or to at least one anti‐TNF agent. To our knowledge, there are no studies of real‐life effectiveness published on Italian populations. The aim of the present study was to assess the effectiveness, safety, and persistence of treatment with ustekinumab in a cohort of patients from Sicily in the first year of drug use.
Methods
Patients
In this multicenter study, prospectively collected data of patients with a confirmed clinical, endoscopic, and/or histological diagnosis of CD registered in this regional electronic database, ≥18 years of age, who started ustekinumab from January 2019 to August 2019 were extracted from the cohort of the Sicilian Network for Inflammatory Bowel Diseases (SN‐IBD). 8 There were no exclusion criteria except for incomplete uploaded data. Ustekinumab was prescribed to patients according to the national approval guidelines, that is, patients with moderately to severely active CD who had an inadequate response or loss of response or were intolerant to either conventional therapy or other biological agents (i.e. anti‐TNFα and vedolizumab). According to the recommended treatment schedule, the initial intravenous (i.v.) infusion at week 0 was weight‐based (260 mg for <55 kg, 390 mg for between 55 kg and 85 kg, and 520 mg for >85 kg) followed by a subcutaneous (s.c.) induction dose at week 8 with 90 mg and, subsequently, by a maintenance treatment with 90 mg s.c. every 8 or 12 weeks, at the discretion of the treating physician.
Data collection and outcomes
For each patient gender, age at diagnosis and age at start of therapy, smoking status (never smoked, ex, or active smoker), family history for IBD, duration of disease, previous surgery for IBD, concomitant therapies, and comorbidities were recorded. Concerning disease characteristics, in accordance with the Montreal classification, 9 extent and behavior of CD were assessed. Data on type of previous biological agents and reason for discontinuation were also collected. Concomitant diseases were assessed for each patient and expressed as the Charlson comorbidity index (CCI). 10 All adverse events (AEs) were recorded.
We evaluated, as primary outcome, steroid‐free remission at 8, 24, and 52 weeks (defined as Harvey‐Bradshaw Index (HBI) ≤4 points without any kind of steroids) and the reduction of patients with elevated C‐reactive protein (CRP) at baseline and during the follow‐up. Secondary outcomes included the following: clinical response (defined as a reduction of HBI ≥3 compared with baseline), reduction of concomitant steroid use, AEs, and possible causes of withdrawal, together with persistence in therapy (at 12 months) over the first year of treatment.
Primary failure was defined as persistence of patient symptoms together with serologic evidence of inflammation at the end of the induction period. Loss of response (LOR) was defined as worsening of patient symptoms together with serologic evidence of inflammation after an initial response, that is, an increase in CRP above the normal limit. Regarding CRP values, due to the heterogeneity in laboratory methods at the contributing centers, we used “CRP positive” instead of mean or median CRP values. Data concerning surgery during follow‐up were also collected.
The study was approved by the Ethics Committee of the coordinating center (Messina) with protocol n. 77/19 as of 30.07.2019.
Statistical analysis
Statistical analysis was carried out using SSPS version 22.0 software for Windows. Descriptive statistics included the calculation of mean values with standard deviation (SD) or standard error (SE) for all continuous variables. Categorical variables were summarized using absolute frequencies and percentages. Wilcoxon test and McNemar test were used to compare continuous and categorical variables, respectively, at different time points of follow‐up. For multiple comparisons, Bonferroni's correction was applied, and the significance level was set at P < 0.017.
Proportional Cox risk models were estimated to assess independent predictors for treatment discontinuation. Hazard ratios (HRs) were jointly expressed with 95% confidence intervals (CI) and relative P‐values. Univariate and stepwise multivariate analyses were performed to assess independent predictors of steroid‐free remission at weeks 24 and 52. The following factors were selected and included in the analysis: age, gender, family history, smoking habit, CCI, disease duration, disease localization and behavior, perianal disease, previous resections, baseline HBI, number and type of previous biologics, and concomitant therapy with systemic steroids at baseline.
Survival analysis according to Kaplan–Meier was carried out considering the time since start of treatment up to 12 months or to the last follow‐up, discontinuation of therapy, or loss of follow‐up.
Analyses were performed on an intention‐to‐treat basis, and any treatment stop with ustekinumab for any reason, including AEs, LOR, or loss to follow‐up, was considered treatment failure from that time onward. Patients who started later and therefore did not reach a specific time point (24 or 52 weeks) were considered censored cases and were not included in the analysis of that given time point. A detailed flowchart of the included patients at every follow‐up is shown in Figure 1.
Figure 1.
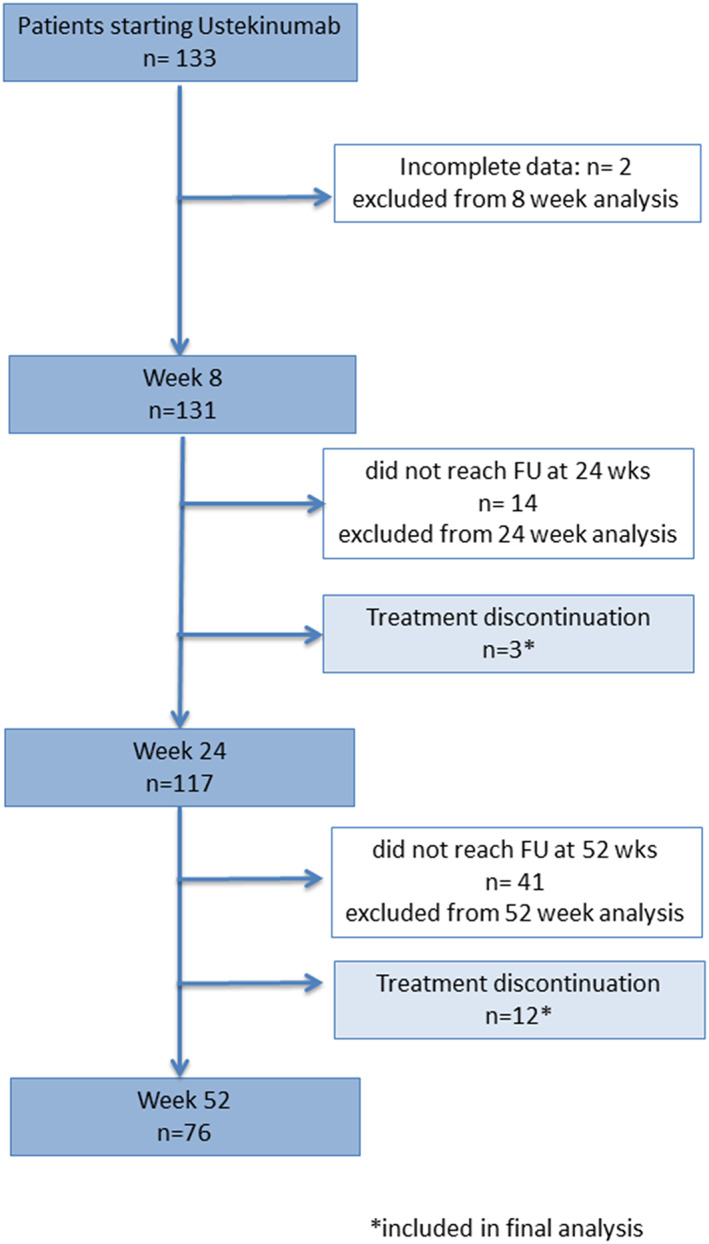
Flowchart of the present study. FU, follow‐up. *patients included in final analysis.
P‐values <0.05 were considered statistically significant with the exception for the above‐mentioned multiple comparisons.
Results
Patients and descriptive data
From the SN‐IBD database, prospectively collected data from 131 patients who started treatment with ustekinumab from January 2019 to August 2019 were identified (see Fig. 1). Patient baseline characteristics are summarized in Table 1. Patients' mean age at diagnosis and at start of ustekinumab was 33 years ±16 and 46 years ±15, respectively; 73 (56%) of them were males, 28 (26%) were current smokers, and 30 (23%) had a family history of IBD. Mean duration of disease was 12 ± 9 years. Almost all patients (99%) had previous treatments with one or more biologic agents (see Table 1) and discontinued mainly for secondary failure (70%); only one patient was naïve to biological agents (an elderly patient with several concomitant pathologies). Previous intestinal surgery was reported in 78 (60%) patients. In almost all patients (95%), an 8‐week interval regimen was followed.
Table 1.
Baseline patient's characteristics
| Baseline characteristics | n = 131 |
|---|---|
| Age at start of treatment | |
| Median (IQR) | 44 (32–57) |
| Range | 18–84 |
| Gender‐male; n (%) | 73 (56) |
| Age diagnosis | |
| Median (IQR) | 29 (19–43) |
| Range | 4–77 |
| Months of follow‐up | |
| Median (IQR) | 10 (6–12) |
| Range | 2–27 |
| Family history; n (%) | 20 (15) |
| Disease location † ; n (%) | |
| L1 | 44 (34) |
| L2 | 13 (10) |
| L3 | 66 (50) |
| L4 | 10 (8) |
| B1 | 70 (53) |
| B2 | 52 (40) |
| B3 | 9 (7) |
| P | 43 (33) |
| Duration of disease (years) | |
| Median (IQR) | 11 (6–17) |
| Range | 1–57 |
| Current smokers; n (%) | 30 (23) |
| Charlson Comorbidity index | |
| 0; n (%) | 77 (59) |
| 1; n (%) | 20 (15) |
| 2; n (%) | 14 (10) |
| 3; n (%) | 15 (11) |
| >3; n (%) | 5 (4) |
| EIMs; n (%) | |
| Joints | 35 (27) |
| Skin | 19 (15) |
| Eyes | 2 (2) |
| Previous biologics; n (%) | 130 (99) |
| One Anti‐TNFα | 37 (28) |
| Two Anti‐TNFα | 38 (29) |
| Only Vedolizumab | 9 (7) |
| Vedo + Anti‐TNF | 46 (35) |
| Reason for discontinuation of first anti‐TNFα; n (%) | n = 121 |
| Primary failure | 19 (16) |
| Secondary failure | 52 (43) |
| Adverse events | 47 (39) |
| Others | 3 (2) |
| Reason for discontinuation of second anti‐TNFα; n (%) | n = 71 |
| Primary failure | 19 (27) |
| Secondary failure | 43 (60) |
| Adverse events | 9 (13) |
| Reason for discontinuation vedolizumab; n (%) | n = 48 |
| Primary failure | 15 (31) |
| Secondary failure | 27 (56) |
| Adverse events | 6 (13) |
| Concomitant medications | |
| Steroids; n (%) | 56 (43) |
| IMM; n (%) | 14 (11) |
| Previous intestinal resections; n (%) | 78 (60) |
Disease location is expressed according to Montreal Classification.
Data are expressed as numbers (percentages) or median with range and IQR.
EIMs, extraintestinal manifestations; IMM, immunomodulators; IQR, interquartile range.
At baseline, the mean HBI was 7, with 56 (43%) patients on steroids, and CRP was elevated in 70 (53%) patients (Table 2). Fourteen patients (11%) were treated concomitantly with immunomodulators (IMM).
Table 2.
Outcomes at different time points in follow‐up
| Baseline n = 131 | 8 weeks n = 131 | 24 weeks n = 117 | 52 weeks n = 76 | |
|---|---|---|---|---|
| Steroid‐free remission; n (%) | — | 46 (35) | 47 (40) | 33 (43) |
| Response; n (%) | — | 76 (58) | 75 (64) | 45 (59) |
| CRP positive; n (%) | 70 (53) | 56 (43) | 45 (38) | 15 (25) |
| AEs overall; n (%) | — | 7 (5) | 12 (10) | 2 (3) |
| Discontinuation of treatment (overall); n (%) | — | 3 (2) | 11 (9) | 1 (1.3) |
| Primary failure | 1 (1) | 4 (3) | 0 | |
| Secondary failure | 0 | 7 (6) | 1 (1.3) | |
| AEs | 2 (1.5) | 0 | 0 | |
| Surgery; n (%)§ | — | 3 (3) | 3 (2.5) | 0 |
§Surgery for perianal disease included, analysis intention‐to‐treat.
Numbers are expressed as crude numbers and percentages.
CRP, C‐reactive protein; AEs, adverse events.
Efficacy, predictors of response, and steroid‐free remission
At week 8, clinical response and steroid‐free remission was achieved in 75 of 131 (68%) and 46 of 131 (35%) patients, respectively. Persistent positive CRP levels were observed in 56 patients (43%). Thirty‐five patients (27%) were still on treatment with steroids.
At week 24, clinical response and remission was achieved in 75 of 117 (64%) and 47 of 117 (40%) patients, respectively. A positive CRP was observed in 45 patients (38%). Twenty‐four patients (20%) were still on treatment with steroids. On multivariate analysis, factors independently associated with failure to achieve steroid‐free remission at week 24 were family history [Odds ratio (OR) 0.18; 95% CI 0.10–0.63, P = 0.036], a higher CCI (OR 0.25; 95% CI 0.10–0.63, P = 0.003), and a higher HBI at baseline (OR 0.59; 95% CI 0.48–0.73, P < 0.001).
Seventy‐six patients had sufficient follow‐up through week 52 and were analyzed, including the 15 patients who had discontinued treatment. At week 52, clinical response and remission were achieved in 45 of 76 (59%) and 33 of 76 (43%) patients, respectively. A positive CRP was observed in 15 patients (25%). Only three patients (4%) were still on treatment with steroids. On multivariate analysis, the only factor independently associated with failure to achieve steroid‐free remission at week 52 was a higher HBI at baseline (OR 0.43; 95% CI 0.25–0.74, P = 0.002).
Overall, from week 8 to the end of follow‐up, we observed a significant reduction of HBI (from baseline to weeks 8 and 24, P < 0.001; from baseline to week 52, P = 0.001 )(Fig. 2) and of steroid use (from baseline to weeks 8 and 24, P < 0.001; from baseline to week 52, P = 0.012). Although not statistically significant, a reduction of CRP from baseline to the end of follow‐up was observed (Table 2). Three patients at week 8 and three patients at week 12 underwent surgery, one patient for intestinal volvulus and five patients for perianal fistulas.
Figure 2.
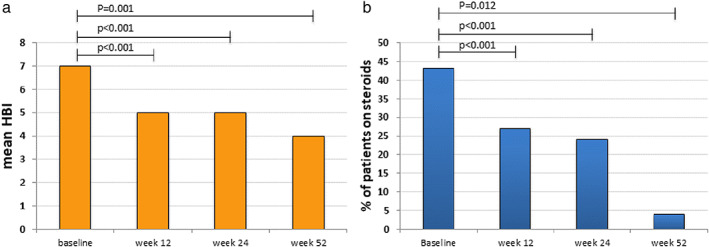
Reduction of Harvey‐Bradshaw Index (HBI) (Wilcoxon‐test) (a) and of steroid use (b) during follow‐up (McNemar test).
Persistence on therapy and predictors of discontinuation
At the end of follow‐up, only 15 patients discontinued treatment. Kaplan Meier survival analysis showed a probability to persist in therapy of 89% (95% CI 10.8–11.6) (Fig. 3). Cox model analysis showed that older age was the only factor independently associated with treatment discontinuation (HR 0.91; 95% CI 0.85–0.98, P = 0.034). Causes for treatment discontinuation are shown in Table 2. No differences were found for treatment persistence comparing groups with different lines of previous biologics (data not shown).
Figure 3.
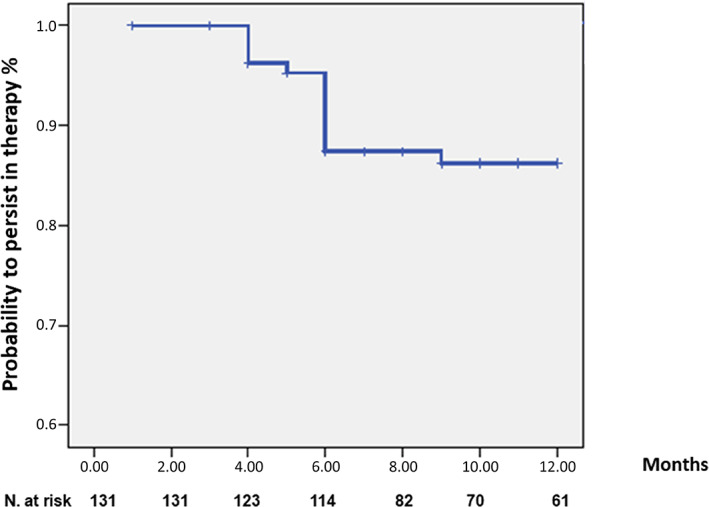
Kaplan–Meier survival analysis showed persistence at 12 months of treatment.
Safety
AEs were reported in 21 patients (16%), of which 3 led to treatment discontinuation (Table 3). Treatment failure and consequent surgery were reported in two patients, whereas one patient stopped treatment due to a reduction of visual acuity.
Table 3.
Adverse events during ustekinumab treatment
| Adverse event | Patients (n) | Discontinued treatment (n of patients) |
|---|---|---|
| Infections | ||
| Fever | 2 | No |
| Herpes simplex infection | 1 | No |
| Pneumonia | 1 | No |
| Crohn's‐related | ||
| Subocclusion | 3 | Yes (2) |
| Perianal disease | 2 | No |
| Other | ||
| Anemia | 1 | No |
| Asthma | 1 | No |
| Reduction of visual acuity | 1 | Yes (1) |
| Skin reactions | 2 | No |
| Abdominal pain and weight loss | 1 | No |
| Arthralgia | 1 | No |
| Alopecia | 1 | No |
| Lymphadenopathy | 1 | No |
| Nephrolithiasis | 1 | No |
| Edema of the legs | 1 | No |
| Abortion | 1 | No |
Discussion
In the present study, we assessed the real‐world efficacy and safety of ustekinumab treatment in a cohort of refractory CD patients. Corticosteroid‐free remission, the primary outcome, was achieved in 40% and 43% after 24 and 52 weeks of treatment, respectively, while clinical response was achieved in 64% and 67% of patients at 24 and 52 weeks, respectively. We showed good persistence on treatment together with a very good safety profile. Only three patients discontinued treatment because of AEs. Although the biochemical response represented by a reduction of CRP during follow‐up did not reach statistical significance, we observed a significant reduction of HBI and an almost total discontinuation of steroids at the end of follow‐up. Good effectiveness of ustekinumab was seen also in other real‐world studies, but only five of these reached 1 year of treatment. A recent study by Biemans et al. showed rates of steroid‐free remission at week 52 of 37.1% in the Dutch Initiative on Crohn and Colitis (ICC) Registry cohort in 221 refractory CD patients (98.6% anti‐TNF and 46.6% vedolizumab exposed). 11 This is a slightly lower percentage compared to our result (43%) despite similar baseline characteristic regarding the severity and duration of disease, exposure to anti‐TNFs and vedolizumab, and HBI. Differences in the ICC Registry cohort were lower steroid use at baseline with approximately 25% versus 43% in the present paper. Other reports indicated 44.7%, 12 26.9%, 13 24.3%, 14 and 14% 15 at 48–52 weeks, differences that may be explained by higher HBI scores at the start, 14 more patients exposed to more than one anti‐TNF, 14 or low numbers of included patients. 15
A very recent Finnish study assessed ustekinumab effectiveness in 155 patients in terms of clinical benefit (defined as the sum of patients in clinical response and clinical remission) and biomarker response (reduction of fecal calprotectin and CRP) together with endoscopic evaluation. A significant reduction of biomarkers and Simple Endoscopic Score for Crohn’s disease (SES‐CD) score from baseline up to 1 year was observed, together with a decrease of clinical active disease. However, their data are difficult to compare with our findings as clinical activity was assessed with a modified HBI (mHBI) without indication of mean or median mHBI values. Moreover, data on biomarkers were available only from half of the patients at 1 year. Similar to our population, 41.4% of patients were on steroids at baseline, with a significant reduction to 13% at 1 year. 16
In our cohort, HBI at baseline was low (mean = 7 ± 0.4 SE), but 56 (43%) patients were administered steroids with or without concomitant IMM, and CRP was elevated in 70 (53%) patients at the start of treatment. Another explanation for this apparently low HBI score may be represented by the fact that secondary failures and AEs to previous treatments were observed in 70% of patients, that is, in the former case, progressive clinical worsening despite interval shortening was bought under control with ustekinumab in a timely manner, and in the latter case, disease worsening after an AE was prevented by the therapeutic swap. Other above‐mentioned studies also included patients with a mild disease at baseline, for example, the work by Alric et al. (median HBI = 6) or by Biemans et al. (HBI = 7), but the percentage of patients on steroids was lower compared to our cohort (28% and 15.8%, respectively). 11 , 12
Concerning treatment persistence, our patients showed a very high persistence, with 89% at 52 weeks, but similar high rates when reported were achieved with 83.6%, 17 80%, 15 and 71.5%. 12 In all the aforementioned studies, including the present one, an 8‐week interval regimen was followed in the majority of patients. Persistence in the Dutch study 11 was much lower at 62.9%, but this difference may be explained by the fact that approximately 25% of their population was on maintenance therapy with a 12‐week interval, resulting in a higher discontinuation rate (42.6% vs 20% P = 0.01) compared to the 8‐week regimen.
With regard to the data obtained at 24 weeks with obviously more consistent patient's numbers in all studies, 11 , 18 , 19 , 20 percentages of steroid‐free remission are more homogenous, with 40% in the present study and 38.3%, 11 38.4%, 19 35.1% in other studies, 19 with only one exception of 15%. 18
In the present study, disease severity at baseline in terms of HBI score was the only factor independently associated with failure to achieve steroid‐free remission, both at weeks 24 and 52. Family history and a higher CCI at baseline were associated with failure to achieve steroid‐free remission at week 24. Several other predictors of failure or success have been identified in other studies, like body mass index, 11 , 14 male gender and penetrating disease, 13 colonic disease, 14 age, and tobacco use 21 , together with other indicators of earlier clinical success (e.g. CRP drop, clinical response, or remission at 24 weeks 15 ).
Safety is an important issue for evaluating new therapies. In the present study, 15 patients discontinued treatment mainly because of primary or secondary failure, and only 2 patients, excluding temporary stopping due to surgery, terminated treatment because of AEs. Similarly, in previous studies, the overall discontinuation rate was very low. 11 , 12 , 13 , 14 , 15
The main strength of our multicenter real‐life study was that we reported the high effectiveness of ustekinumab using prospectively collected data with a strong steroid‐sparing effect in a population of “difficult‐to‐treat” patients based on a high rate of previous surgeries and previous treatment attempts. Another strength was the use of clinical scores for outcomes evaluation and the homogeneity of patient management as a result of the presence of a consolidated network. Surely more studies on larger populations are also needed in order to allocate the use of this drug to a more correct position in the treatment algorithm for moderate to severe CD, not least for its excellent safety profile.
There are several limitations to our study, such as the small number of patients reaching 52 weeks. Another important limitation is the lack of information regarding endoscopy and/or radiological assessment of treatment efficacy. In addition, data regarding fecal calprotectin were scarce and were therefore excluded from analysis. The costs for calprotectin measurements are not covered by the National Health System in Italy, and consequently, assessment is not required routinely.
Conclusions
The present results obtained from the database of the SN‐IBD show a high rate of treatment persistence together with a very low number of patients on steroids at the end of follow‐up, suggesting excellent treatment effectiveness of ustekinumab.
Disease severity at baseline was the only factor independently associated with failure to achieve steroid‐free remission at both weeks 24 and 52. Ustekinumab seems to be a safe, effective treatment for biological‐experienced CD. Further studies are needed to determine the most appropriate place of ustekinumab in the treatment algorithm for CD.
Acknowledgment
The authors would like to thank the additional SN‐IBD investigators: A.O.U. Policlinico “G. Martino”, Messina: C. Romano, MD, S. Pellegrino, MD, A. Costa, MD. A.O.O.R. “Papardo Piemonte”, Messina: C. Bertolami, MD. P.O. S. Vincenzo, Taormina (ME): R. Bellerone, MD; F. D'Amore, MD. A.O.O.R. “Villa Sofia‐Cervello”, Palermo: S. Renna, MD, A. Casà, MD, B. Scrivo, MD, R. Orlando, MD, E. Orlando, MD. A.R.N.A.S.‘Civico Di Cristina Benfratelli’, Palermo: R. Di Mitri, MD, F. Cavataio, MD; A.O.U. Policlinico “P. Giaccone”, Palermo: S. Accomando, MD. A.O.O.R. “S. Elia‐ M. Raimondi”, Caltanissetta: S. Camilleri, MD. A.O. Buccheri La Ferla, Palermo: R. Vassallo, MD; M. G. Minissale, MD. A.O. “Guzzardi”, Vittoria (RG): E. Giangreco, MD, N. Belluardo, MD, G. Mogavero, MD. A.O.U. Policlinico “V.E. Rodolico”, Catania: G. Inserra, MD. A.O. Garibaldi, Catania. C. Virgilio, MD; S. Siringo, MD. P.O. S. Venera, Acireale (CT): C. Cavallaro, MD. P.O. Umberto I, Siracusa: A Trovatello, MD. P.O. Giovanni Paolo II, Sciacca (AG): T. Catalano, MD. P.O. S. Antonio Abate, Trapani: S. Genova.
Declaration of Conflict of Interest: Fabio Salvatore Macaluso is an advisory board member and/or received lecture grants from AbbVie, Biogen, MSD, Pfizer, and Takeda Pharmaceuticals. Maria Cappello received lecture fees and/or is an advisory board member for AbbVie, Ferring, Janssen, MSD, Takeda, and Shire. Filippo Mocciaro is an advisory board member for AbbVie and MSD Pharmaceuticals and received lecture grants from AbbVie, MSD, and Takeda Pharmaceuticals. Sara Renna served as an advisory board member for AbbVie and MSD Pharmaceuticals and received lecture grants from AbbVie, MSD, and Takeda Pharmaceuticals. Ambrogio Orlando is an advisory board member for AbbVie, MSD, Janssen, Pfizer, and Takeda Pharmaceuticals and received lecture grants from AbbVie, MSD, Sofar, Chiesi, Janssen, Pfizer, and Takeda Pharmaceuticals. Walter Fries is an advisory board member and/or received speaker fees from Takeda Pharmaceuticals, Janssen, Zambon, Pfizer Biogen, Mundipharma, Abbvie, Ferring, Brystol Myers Squibb, and Shire. Antonino Carlo Privitera served as consultant to Mundipharma, Abbvie, MSD, Takeda, and Janssen. He received lecture fees from Abbvie, Aboca, Recordati. Giuseppe Costantino served as consultant/advisory board member for Abbvie, Janssen, and Takeda. All the other authors have no conflict to interest to declare.
References
- 1. Hanauer SB, Feagan BG, Lichtenstein GR et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359: 1541–9. [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Feagan BG, Rutgeerts P et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 2013; 369: 711–21. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Gasink C, Gao LL et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N. Engl. J. Med. 2012; 367: 1519–28. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Feagan BG, Fedorak RN et al. A randomized trial of Ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with moderate‐to‐severe Crohn's disease. Gastroenterology. 2008; 135: 1130–41. [DOI] [PubMed] [Google Scholar]
- 5. Simon EG, Ghosh S, Iacucci M, Moran GW. Ustekinumab for the treatment of Crohn's disease: can it find its niche? Therap. Adv. Gastroenterol. 2016; 9: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feagan BG, Sandborn WJ, Gasink C et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 2016; 375: 1946–60. [DOI] [PubMed] [Google Scholar]
- 7. Macaluso FS, Maida M, Ventimiglia M, Cottone M, Orlando A. Effectiveness and safety of Ustekinumab for the treatment of Crohn's disease in real‐life experiences: a meta‐analysis of observational studies. Expert Opin. Biol. Ther. 2020; 20: 193–203. [DOI] [PubMed] [Google Scholar]
- 8. Porcari S, Viola A, Orlando A et al. Persistence on anti‐tumour necrosis factor therapy in older patients with inflammatory bowel disease compared with younger patients: data from the Sicilian network for inflammatory bowel diseases (SN‐IBD). Drugs Aging. 2020; 37: 383–92. [DOI] [PubMed] [Google Scholar]
- 9. Silverberg MS, Satsangi J, Ahmad T et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005; 19: 5A–36A. [DOI] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 11. Biemans VBC, van der Meulen‐de Jong AE, van der Woude CJ et al. Ustekinumab for Crohn's disease: results of the ICC registry, a Nationwide Prospective Observational Cohort Study. J. Crohns Colitis. 2020; 14: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alric H, Amiot A, Kirchgesner J et al. The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn's disease refractory to anti‐tumour necrosis factor. Aliment. Pharmacol. Ther. 2020; 51: 948–57. [DOI] [PubMed] [Google Scholar]
- 13. Kubesch A, Rueter L, Farrag K et al. Short and long‐term effectiveness of ustekinumab in patients with Crohn's disease: real‐world data from a German IBD cohort. J. Clin. Med. 2019; 8: 2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liefferinckx C, Verstockt B, Gils A et al. Long‐term clinical effectiveness of ustekinumab in patients with Crohn's disease who failed biologic therapies: a National Cohort Study. J. Crohns Colitis. 2019; 13: 1401–9. [DOI] [PubMed] [Google Scholar]
- 15. Harris RJ, McDonnell M, Young D et al. Early real‐world effectiveness of ustekinumab for Crohn's disease. Frontline Gastroenterol. 2019; 11: 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Af Björkesten CG, Ilus T, Hallinen T et al. Objectively assessed disease activity and drug persistence during ustekinumab treatment in a nationwide real‐world Crohn's disease cohort. Eur J Gastroenterol Hepatol. 2020; 32: 1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Obando C, Ding Z, Muser E et al. Persistence, dose titration, and health care resource utilization among Crohn's disease patients treated with ustekinumab: a real‐world analysis in the United States. Adv. Ther. 2020; 37: 2127–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bar‐Gil Shitrit A, Ben‐Ya'acov A, Siterman M et al. Safety and effectiveness of ustekinumab for induction of remission in patients with Crohn's disease: a multicenter Israeli study. United Eur Gastroenterol J. 2020; 8: 418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffmann P, Krisam J, Wehling C et al. Ustekinumab: "Real‐world" outcomes and potential predictors of nonresponse in treatment‐refractory Crohn's disease. World J. Gastroenterol. 2019; 25: 4481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verstockt B, Dreesen E, Noman M et al. Ustekinumab exposure‐outcome analysis in Crohn's disease only in part explains limited endoscopic remission rates. J. Crohns Colitis. 2019; 13: 864–72. [DOI] [PubMed] [Google Scholar]
- 21. Casas Deza D, García López S, Lafuente Blasco M et al. Efficacy and safety of ustekinumab in real clinical practice. Retrospective multicentre study. ARAINF cohort. Gastroenterol Hepatol. 2020; 43: 126–32. [DOI] [PubMed] [Google Scholar]


