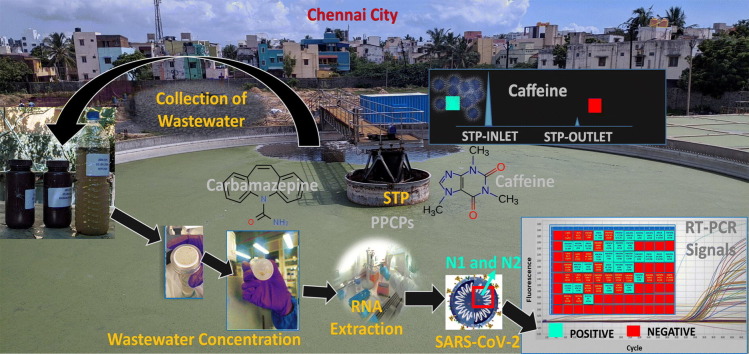
Abstract
Surveillance of SARS-CoV-2 and organic tracers (OTs) were conducted in the community wastewater of Chennai city and the suburbs, South India, during partial and post lockdown phases (August–September 2020) as a response to the coronavirus disease 2019 (COVID-19) pandemic. Wastewater samples were collected from four sewage treatment plants (STPs), five sewage pumping stations (SPSs) and at different time intervals from a suburban hospital wastewater (HWW). Four different methods of wastewater concentrations viz., composite (COM), supernatant (SUP), sediment (SED), and syringe filtration (SYR) were subjected to quantitative real time-polymerase chain reaction (qRT-PCR). Unlike HWW, STP inlet, sludge and SPS samples were found with higher loading of SARS-CoV-2 by SED followed by SUP method. Given the higher levels of dissolved and suspended solids in STPs and SPSs over HWW, we suspect that this enveloped virus might exhibit the tendency of higher partitioning in solid phase. Cycle threshold (Ct) values were < 30 in 50% of the HWW samples indicating higher viral load from the COVID-19 infected patients. In the STP outlets, a strict decline of biochemical oxygen demand, >95% removal of caffeine, and absence of viral copies reflect the efficiency of the treatment plants in Chennai city. Among the detected OTs, a combination of maximum dynamic range and high concurrence percentage was observed for caffeine and N1 gene of SARS-CoV-2. Hence, we suggest that caffeine can be used as an indicator for the removal of SARS-CoV-2 by STPs. Our predicted estimated number of cases are in line with the available clinical data from the catchments. Densely distributed population of the Koyambedu catchment could be partly responsible for the high proportion of estimated infected individuals during the study period.
Keywords: SARS-CoV-2, Wastewater, Chemical marker, RNA copies, Wastewater based epidemiology
Graphical abstract
1. Introduction
Since early 2020, the worldwide spread of coronavirus disease 2019 (COVID-19) has been a serious public health concern (Cucinotta and Vanelli, 2020). COVID-19 patients have symptoms of cough, fever, dyspnoea, diarrhoea, and anosmia. Both symptomatic and asymptomatic patients shed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) viral ribonucleic acid (RNA) in feces (Chen et al., 2020; Collivignarelli et al., 2020b; La Rosa et al., 2020a; Mallapaty, 2020). Transmission of SARS-CoV-2 via sewage is not reported to be an exposure pathway (Kitajima et al., 2020), but an increasing level of pathogens in the community dwellers will eventually increase the viral load in the sewer systems (Mallapaty, 2020). Cahill and Morris, 2020, highlighted the concerns regarding the transmission and risks of SARS-CoV-2 in recreational waters, especially those which receive inadequately treated wastewater. Wastewater-based epidemiology (WBE) aims in monitoring the prevalence of viruses in a given catchment, much before the spread of the viral load in the community dwellers (Lodder and de Roda Husman, 2020; Randazzo et al., 2020; Wu et al., 2020). WBE has been found to be a cost-effective tool to handle the coronavirus pandemic, and it is stated that worldwide about 2.1 billion people could be monitored from wastewater samples in 105,600 sewage treatment plants (STPs) (Hart and Halden, 2020).
Detection of SARS-CoV-2 in wastewater has been so far reported from developed nations such as Italy (Collivignarelli et al., 2020a; La Rosa et al., 2020b; Race et al., 2020), Cyprus (Michael-Kordatou et al., 2020), Ireland (Cahill and Morris, 2020), Japan (Haramoto et al., 2020), the Netherlands (Medema et al., 2020a, Medema et al., 2020b), Australia (Ahmed et al., 2020), France (Wurtzer et al., 2020), Spain (Randazzo et al., 2020), Switzerland (Stringhini et al., 2020) and the USA (Peccia et al., 2020). From India, SARS-CoV-2 have been reported earlier in samples from a wastewater treatment plant (WWTP) at Ahmedabad in Gujarat (Kumar et al., 2020), and municipal WWTPs and hospital wastewater at Jaipur in Rajasthan (Arora et al., 2020).
Despite the mammoth increase in the number of COVID-19 cases in a vast country like India, there is a dearth of information on the possibility of community spread from the STPs or WWTPs, especially after the end of lockdown phase. During March–May 2020, with 10,471 COVID-19 positive cases, Tamil Nadu was listed as the second-most affected state in India. Chennai is the most populated city of Tamil Nadu and the only district that was identified of having more than half of the state's COVID-19 positive cases.
Given the uncertainties of an effective vaccine and the continuing pandemic situation, it is imperative to predict and prevent the second and subsequent waves in the catchment areas. Detection of SARS-CoV-2 in STPs can be used as an important tool for public health experts to decide on the containment and relaxation measures in the catchment community.
Transcripts of nucleocapsid, N gene (N1&N2) are the most abundant transcripts of the SARS-CoV-2 and are therefore, good targets for the detection of the virus in the samples (Babiker et al., 2020; Petrillo et al., 2020). According to the Centre for Public Health and Environmental Engineering Organization (CPHEEO), Ministry of Housing and Urban Affairs (MoHUA), Government of India, 70–80% of the total amount of domestic water supplied is generated as wastewater (Kaur et al., 2012). STPs or WWTPs receive raw sewage or wastewater from domestic households. Quality of treated water from STPs and WWTPs, especially during the pandemic, is a matter of public health concern. Both caffeine and carbamazepine were used as potential chemical markers to evaluate the proper functioning of STPs and WWTPs in India (Chakraborty et al., 2019; Chakraborty et al., 2021). Hence, during partial and post lockdown phases, we took the first attempt to conduct an intensive wastewater surveillance to detect the presence of SARS-CoV-2 in the four major STPs and five sewage pumping stations (SPSs) of Chennai city. Simultaneously, we conducted weekly monitoring of wastewater at different time intervals of the day in a community hospital located about 30 km away from the city. The major objectives of the study were to: (i) compare four different wastewater concentration methods for RNA extraction and subsequent detection of N1 and N2 genes of SARS-CoV-2 by quantitative real-time polymerase chain reaction (qRT-PCR) (ii) quantitatively enumerate the number of RNA copies (iii) quantitate the level of organic tracers including wastewater markers (caffeine, carbamazepine) and selected pharmaceuticals and personal care products (PPCPs) and track the concurrence of these contaminants with SARS-CoV-2 (iv) elucidate the functional efficacy of STPs, and (v) estimate the number of infected persons in each catchment area.
2. Materials and methods
2.1. Sampling site selection and sample collection
2.1.1. STPs and SPSs in Chennai city
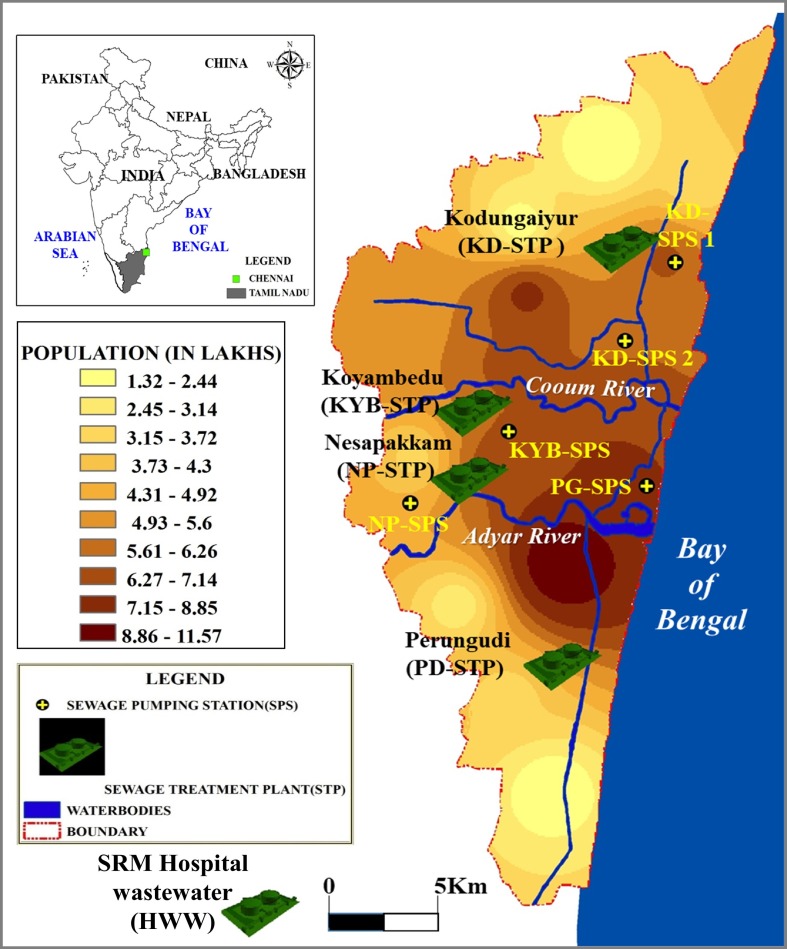
Locations of the sampling sites are given in Fig. 1 and Table S1 of the supporting information (SI). Fig. 1 was prepared using Arc GIS 9 (Version: Arc Map 9.3). Data on population distribution were obtained from elsewhere (GreaterChennnaiCorporation, 2018). During 5th–11th September 2020, composite grab samples from inlet, primary sludge and outlet were collected from each of the four STPs and five associated SPSs of Chennai city viz., Perungudi (PG-STP, PG-SPS), Nesapakkam (NP-STP, NP-SPS) Kodungaiyur (KD-STP, KD-SPS-1, KD-SPS-2) and Koyambedu (KYB-STP, KYB-SPS). The sampling locations in STPs viz., inlet, sludge and outlet have been given in Fig. S1. In total, 12 samples from STPs and 5 samples from SPSs were collected in sterile plastic containers. Strict safety protocols were followed throughout the sample collection and processing stages. Samples were transported to the laboratory in ice boxes and processed for virus concentration procedures on the same day of sampling.
Fig. 1.
Sampling map with the location of the sewage treatment plants (STPs), sewage pumping stations (SPSs) in Chennai city and SRM hospital wastewater (HWW) collection site in the suburbs.
2.1.2. Hospital wastewater
Similarly, hospital wastewater (HWW) samples were collected at different intervals of the day (7 AM, 10 AM, 1 PM, 4 PM and 7 PM) for three weeks during partial lockdown (n = 15) (August, 2020) and one week during post lockdown (September, 2020) (n = 5) from SRM hospital.
2.2. Physico-chemical water quality
Water quality parameters for the wastewater samples such as dissolved oxygen (DO) in mg/L, conductivity (COND) in mS/cm, pH, total dissolved solids (TDS) in (mg/L), salinity in parts per million (ppm), and biochemical oxygen demand (BOD) in mg/L were analysed using portable digital probes. Each device was rinsed with millipore water three times and allowed to dry before each analysis. Further details on analysis and the data on water quality parameters for STPs, SPSs, and HWW are presented in Tables S2 & S3.
2.3. SARS-CoV-2
2.3.1. Concentration of SARS-CoV-2 from wastewater samples
In several studies, SARS-CoV-2 was concentrated from wastewater by different procedures. The supernatant concentration method was found to be very effective in isolating SARS-CoV-2 (Ahmed et al., 2020; Medema et al., 2020a, Medema et al., 2020b). Prior to RNA extraction from each sample, we have used four methods of wastewater concentration with some modifications: composite (COM), supernatant (SUP), sediment (SED) and syringe filtration (SYR): a) COM: 1 mL of composite wastewater sample was taken from 250 mL of wastewater from each site and treated with 20 mg/mL of proteinase K for 10 min in 65 °C dry bath. b) SED: One loop-full of the sediment was suspended in 1 mL of 1× phosphate buffer saline (PBS) which was then treated with 20 mg/mL of proteinase K for 10 min in 65 °C dry bath. c) SUP: 250 mL of the sample was centrifuged at 8100 rpm for 30 min in a cooling centrifuge (model: REMI, CPR 24 Plus) (Fig. S2). The supernatant was treated with 5 mg of aluminium hydroxide and then incubated in a Rocker Orbitek® laboratory refrigerated shaker for 12 h at 4 °C. At the end of 12 h, the supernatant was further centrifuged at 14000 rpm for 45 min and then filtered through a 0.45 μm pore size, 47 mm cellulose nitrate membrane filter (Thermo-Scientific, Cat.no.LSMCN4547NST). The filtrate was then transferred to a sterile tube and 4 mL of 1× PBS was added to it and the filtrate was incubated at 4 °C for 50 oscillations per minute for 20 ± 5 min. Again 4 mL of 1× PBS was added to the tube and the sample was concentrated using the Amicon®ultra-15ultra-centrifugal filters (Cat.no. #UFC910096) at 7000 rpm at 4 °C for 15 min. d) SYR: 10 mL of the sample was passed through a 0.2 μ syringe filter.
2.3.2. RNA extraction
RNA extraction was carried out in Bio-Safety Level II cabinet (Thermo-scientific, Model: 1300 series A2), with proper safety measures in the laboratory. RNA was extracted by using the QIAamp Viral RNA mini kit (Cat.no#52906), Qiagen, Germany, according to the manufacturer's instructions. Briefly, 140 μL of the sample was added to 560 μL of AVL buffer™ and incubated at room temperature for 10 mins and then the sample was centrifuged. Further, 560 μL of 100% ethanol was added and 630 μL of the sample was added to the mini spin column and then centrifuged at 8000 rpm for 1 min. To the column, 500 μL of AW1 wash buffer was added and centrifuged at 8000 rpm for 1 min. Then 500 μL of AW2 wash buffer was added and centrifuged at 14000 rpm for 3 mins. Further, the column was placed in a sterile 2 mL Eppendorf tube and then 60 μL of AVE elution buffer was added and then` incubated for 1 min followed by centrifugation at 8000 rpm for 1 min. The eluted RNA was stored at −80 °C until further analysis.
2.3.3. RNA analysis
RNA analysis was performed in qRT-PCR in Light cycler 96, ROCHE PCR machine. SARS-CoV-2 RNA was detected by using Prime script III qRT-PCR mix (Takara Bio, USA, Cat no#RR600A). IDT 2019-nCoV CDC-EUA kit (Integrated DNA Technologies, Coralville, IA, USA, Cat no#10006770) was used for the detection of N1, N2 of SARS-CoV-2, and RNaseP. Human RNaseP primer-probe mix serves as the internal control for monitoring the sample quality and extraction protocol. The reaction mixture consisted of 10 μL of prime script master mix, 0.5 μL of Taqman primer-probe mix of N1/N2/RNaseP of FAM-labelled primer-probe mix, 3.5 μL of nuclease-free water, and 6.0 μL of the RNA extracted from the sample (Table S4). The thermal conditions were reverse transcription at 45 °C for 15 min followed by initial denaturation at 95 °C for 2 min. Then the reaction was followed with 45 cycles of 95 °C for 3 s and 55 °C for 30 s.
QA/QC: EURM-019 obtained from the European Commission Joint Research Centre (JRC), served as an internal positive control as nucleocapsid gene CDC-N1 and CDC-N2 containing the universal synthetic ssRNA of 880 nucleotides. N1 and N2 genes were amplified by qRT-PCR with the ssRNA and served as the positive control for the assay validation. Similarly, RNA was extracted from buccal swab and amplified with RNaseP gene which served as an internal control. Basically, with positive control, N1 and N2 amplifications were seen; however, ssRNA did not show amplification with RNaseP. Furthermore, with nuclease free water (Molecular Biology grade), no amplification curve was observed. The internal positive control had Ct values of 17.02 and 16.13 for N1 and N2, respectively. RNA isolated from human buccal swab showed amplification with Ct value of 29, whereas negative control did not show any amplification with the nuclease free water.
2.3.4. Estimation of RNA copies
After the PCR, 96 well plates were taken for analysis of RNA copies. The completed cDNA of N1 and N2 together with the corresponding RNA copies present in the samples were analysed by Thermo Scientific Nano Drop 2000 UV-VIS Spectrophotometer. RNA copies were calculated according to the formula given elsewhere (IDT, 2013; Pfaffl, 2004)
where ‘X’ refers to the difference between DNA concentration after qRT-PCR and RNA concentration before qRT-PCR; N refers to the length of dsDNA amplicon and 660 g/mol is the average mass of 1 bp dsDNA. RNA copies were predicted by using the IDT online software.
2.3.5. Estimation of infected persons in the particular catchment area
We estimated the infected persons by using the formula given elsewhere (Ahmed et al., 2020).
where, RNA copies/L of wastewater = 560 multiplied with RNA copies/μL, Litres of wastewater/day in million litres/day (MLD), g feces per person/day = 243 g in low income countries (Rose, 2003), RNA copies/g feces =107 (Foladori et al., 2020).
2.4. Organic tracers
Based on frequent usage, twenty-one OTs were quantified in the wastewater samples. OTs were categorised as: a) wastewater markers (caffeine, carbamazepine) b) pharmaceuticals and personal care products (PPCPs). The list of OTs included fluoroquinolones (norfloxacin, ofloxacin, ciprofloxacin), antibiotics (erythromycin, roxithromycin, azithromycin, clarithromycin, trimethoprim, tetracycline, oxytetracycline, doxycycline hyclate, chloramphenicol), sulphonamides (sulfadiazine, sulfamethoxazole), parabens (ethyl paraben, methyl paraben, propyl paraben, butyl paraben) and antimicrobial agent (triclosan).
2.4.1. Wastewater extraction for organic tracers
100 mL of wastewater samples were filtered on the same day of sample collection using glass fibre filter (GF/F) (Whatman), acidified to pH 2 immediately after sampling and stored at −20 °C. Each Oasis hydrophilic lipophilic balanced (HLB) cartridge (500 mg 6 mL, Waters) was preconditioned with 20 mL methanol, 6 mL milli-Q water, and 6 mL milli-Q water with pH 2.0. Filtered aliquots of the water samples were added with 250 mg Na2EDTA and spiked with surrogate standards of each group of OTs. The water samples were loaded into the cartridges at a flow rate around 5 mL/min. After the cartridges were dried under an airstream for 10 min, analytes were eluted with 12 mL of methanol. The eluates were collected, evaporated to dryness with nitrogen stream and reconstituted to 1.0 mL with methanol:water (1:1, v: v).
2.4.2. Instrumental analysis
Chromatographic separation of analytes was performed using an Atlantis T3 column (100 mm × 2.1 mm, 3 μm, Waters, MA, US) by a Waters ACQUITY Ultra Performance Liquid Chromatography (UPLC) H class coupled with Xevo TQ-S micro–Triple Quadrupole Mass Spectrometry. For the positive electrospray ionization mode (ESI), two methods were used. For sulfonamides, fluoroquinolones, and tetracycline, the mobile phase A contained 0.1% formic acid in water and mobile phase B was methanol. For macrolides, carbamazepine and trimethoprim, mobile phase A contained 2 mM ammonium formate with 0.1% formic acid in water and mobile phase B contained 0.1% formic acid in acetonitrile. For parabens, chloramphenicol and triclosan, negative ESI mode was used with the mobile phase A containing water and mobile phase B containing methanol. 13C12 Caffeine used as surrogate standard was quantified in all the three methods. The sample injection volume was 2 μL. The monitored target and reference ions are given in Table S5. Nine organic tracers detected in the wastewater samples were: caffeine (CAF), carbamazepine (CBZ), azithromycin (AZT), erythromycin (ERY), methyl paraben (MP), sulfamethoxazole (SMX), ofloxacin (OFL), norfloxacin (NOR), ciprofloxacin (CIP), and triclosan (TCS).
QA/QC: Identification of each OT was performed by UPLC-MS with multiple reaction monitoring (MRM) mode, using the two highest characteristic precursor ions. Instrument detection limit (IDL) was calculated at S/N ratios ≥10. The method detection limits (MDLs) were estimated by combining instrumental quantification limits, recoveries and concentration factors. Recovery for 13C caffeine varied between 86%–104%. Recovery percentage for fluroquinolones, sulphonamides, antibiotics and parabens varied between 89 and 127% (103 ± 13), 96–113% (101 ± 7), 88–112% (99 ± 8), 93–130% (105 ± 5), respectively. Further, instrumental blank, procedural blank, blank spike, and matrix spike were applied for each batch of five samples to keep a track of the contamination level during the sample preparation procedure. All instrumental and procedural blanks were below MDL (Table S5a and 5b).
2.5. Statistical analysis
Statistical analysis was performed using SPSS version 22. Regression analysis, t-tests and one-way ANOVA was done at 95% confidence interval.
3. Results and discussion
3.1. General discussion
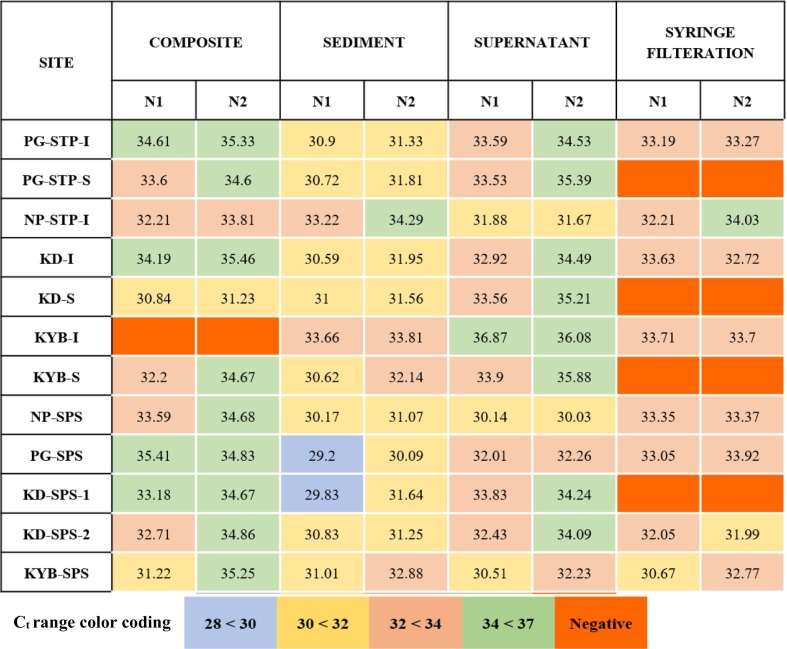
Physico-chemical parameters of wastewater are given in Tables S2 and S3. Water quality parameters such as TDS, DO and BOD drastically improved at the outlets of STPs. Based on the Ct values using qRT-PCR, positive results for SARS-CoV-2 from four different wastewater concentration methods mentioned in the earlier section are given in Table 1 . Ct values greater than 23 and less than 40 for nucleocapsid genes-N1 and N2 were considered positive. Increasing Ct values correspond to lesser number of RNA copies per litre of wastewater. Further, the semi-quantitatively detected SARS-CoV-2 RNA copies are given in Figs. 2 and S3. The number of RNA copies of N1 was significantly higher than N2 (p < 0.05) for most of the samples. Higher number of RNA copies of N1 is in agreement with the WBE study, wherein the authors identified higher sensitivity of N1 primer-probe over N2 (Medema et al., 2020a, Medema et al., 2020b). Similarly, US-FDA has also reported that sensitivity of N1 is greater than N2 in clinical samples (Hallo et al., 2020).
Table 1.
Cycle threshold (Ct) values for detected SARS-Cov-2 in wastewater samples from STPs and SPSs of Chennai city concentrated by four different wastewater concentration methods.
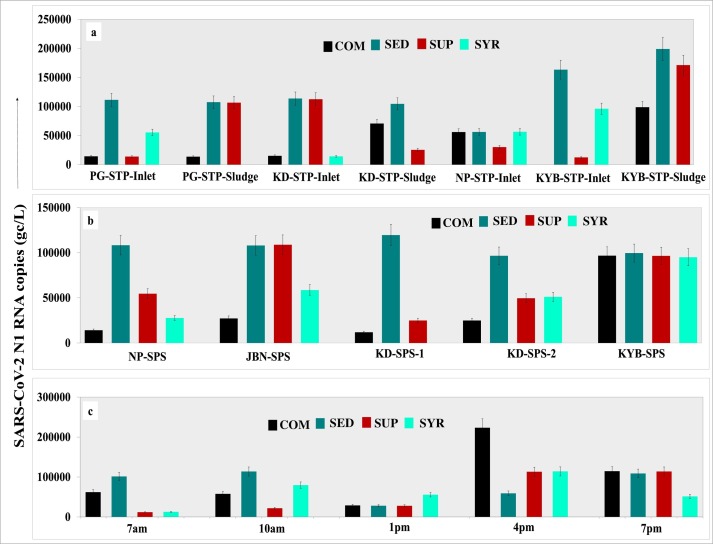
Fig. 2.
Error plots showing the four wastewater concentration techniques and corresponding SARS-CoV-2 RNA copies of N1 gene during post lock down for a). STP inlets (STP-I) and STP-sludge (STP-S) b). SPSs c). Hospital wastewater samples at different time intervals.
3.2. Detection of SARS-CoV-2
3.2.1. STPs and SPSs
Of all the 17 wastewater samples, SARS-CoV-2 was detected in all SPSs (n = 5), STP inlets (n = 4) and sludge samples excluding NP-STP (n=3). However, none of the outlet samples were positive for SARS-CoV-2. Longer survival rate of corona viruses in primary wastewater over secondary wastewater has been observed in the past due to the less inactivation by increased suspended particles (Gundy et al., 2009). With SED method, all the positive samples were found with the lowest range of Ct values (N1, 29.2 to 33.22 and N2, 30.09 to 33.81) for SARS-CoV-2 excluding one inlet sample (NP-STP-I, N1, 33.22; N2, 34.29) (Table 1). Similar positive response was seen for SUP method with slightly higher Ct values (N1, 32–38: N2, 30–36).
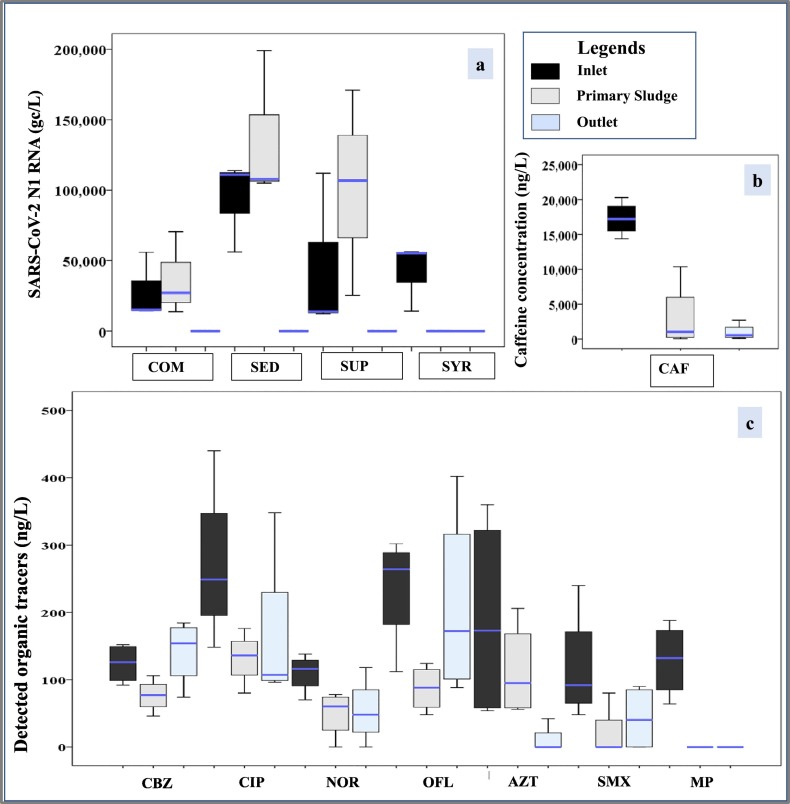
Correspondingly, maximum number of RNA copies for N1 gene obtained by the SED method in all the positive samples from STPs (range 9.66×104–1.99×105 gc/L) were higher over the SPSs (range 1.41×104–9.96×104 gc/L). In STPs and SPSs, SED followed by SUP concentration methods showed higher number of SARS-CoV-2 in the wastewater samples. It is to be noted that N1 numbers obtained from SED method were significantly higher than other methods (p < 0.01 to 0.05). Given the possibility of SARS-CoV-2 being hydrophobic, such particles in wastewater stream can have higher tendency to get associated with solids (Balboa et al., 2020). Enveloped viruses such as Murine hepatitis virus and Pseudomonas phage Φ6, exhibited higher partitioning (26%) to solid component in wastewater over non-enveloped viruses (6%) (Ye et al., 2014). Hence, higher loading of SARS-CoV-2 by SED concentration method might be due to the adsorption of the virus particles to solids in sewage and/or sludge particularly in the STP inlets. Most of the viral particles in raw sewage can settle down, thereby, reducing the viral load in the SYR method. Highest number of RNA copies were observed in Koyambedu sludge sample (KYB-STP-S, N1, 1.99×105 gc/L) followed by the Koyambedu inlet (KYB-STP-I, N1, 1.63×105 gc/L) by SED method (Fig. 2). Excluding Nesapakkam inlet (NP-STP-I, N1, 5.6×104 gc/L), N1 copies obtained from the SED method in rest of the STPs and SPSs ranged between 1.00×105–1.99×105 gc/L. Similarly, results from SUP and COM methods were found with higher number RNA copies for KYB-STP and KYB-SPS samples (Fig. 2).
3.2.2. Hospital wastewater (HWW)
Unlike STPs, maximum positive response (85%) was seen in samples concentrated by SUP method with Ct values for N1 and N2 ranging from 28.02 to 35.51 and 26.38 to 35.83, respectively. About 40% of the total HWW samples concentrated by SED method showed positive response, with Ct values for N1 ranging from 28.68 to 38.24 and N2 ranging from 25.85 to 32.07. Out of the four sampling dates for HWW samples, we observed relatively lesser suspended solids in HWW over STPs particularly during partial lockdown period (Table S3). SYR concentration method gave either non-detects or much less viral load in the samples. With COM method, we found lesser RNA copies/L (1.19 ×104–9.89×104 gc/L) compared to the other three methods (Fig. S3) and is in agreement with a study conducted in Bozeman, Montana (USA) (Nemudryi et al., 2020). Thus, SUP concentration method was found to be most effective in concentrating SARS-CoV-2 from HWW samples and is in line with other studies (Ahmed et al., 2020). RNA copies by SUP method increased from 4.25 ×105 gc/L (on 10/08/20) to 1.62×106 gc/L (on 17/08/20) (Fig. S3). Even though the RNA copies were less, all complete post lockdown HWW samples were positive for SARS-CoV-2 in all the methods. In HWW, RNA copies for N1 were significantly different (p < 0.01) between partial and post lockdown phases. Higher viral loading in HWW samples during partial lock-down (1.10×105–1.62×105 gc/L) over post-lockdown (1.20×104–1.15×105 gc/L) can be related with the lower number of active COVID-19 patients admitted in SRM hospital during that period.
3.3. Organic tracers and concurrence with SARS-CoV-2
3.3.1. Organic tracers (OTs)
This study encompasses a broad range of OTs with log KOW values from very hydrophilic compound (log KOW < 1, caffeine) to hydrophobic compound (log KOW > 4, triclosan). Eight detected OTs viz., CAF, CBZ, CIP, NOR, OFL, AZT, SMX and MP showed 100% detection frequency in STP inlets and sludge samples. In outlets and SPSs, the detection frequency varied between 20 and 100% (Table 2 ). The concentration of eight frequently detected OTs in the outlet samples were significantly lower (p < 0.05) than the inlet samples with the removal efficiencies varying between 13% (ERY)–98% (CAF) (Table 2). CAF concentration is over hundred-fold higher than the other detected OTs. Such dominance of CAF in STPs and WWTPs has been reported from previous studies in China (Zhou et al., 2010) and India (Anumol et al., 2016). AZT concentrations and removal efficiencies in WWTP (88–100%) were comparable with other studies from Japan (Kobayashi, 2006) and Egypt (Younes et al., 2019). Dominance of AZT and FQs reflect the consumption of these commonly prescribed drugs for ailments among SARS-CoV-2 patients having cold, fever and respiratory infections (Kotwani and Holloway, 2011).
Table 2.
Detection and concentration range of chemical markers and organic tracers in wastewater samples from STP, SPS and hospital (ND: not detected).
| Chemical markers | Detection frequency |
Concentration in ng/L |
||||||
|---|---|---|---|---|---|---|---|---|
| Inlet |
Sludge |
Outlet |
SPSs |
|||||
| STP-Inlet | STP-Sludge | STP-Outlet | SPS | Range (Avg ± STDEV) | ||||
| Caffeine (CAF) | 100% | 100% | 100% | 100% | 14,376–20,280 (17,272 ± 2459) | 40–10,366 (3114 ± 4881) | 94–2700 (966 ± 1180) | 12,096–32,794 (17,710 ± 8657) |
| Carbamazepine (CBZ) | 100% | 100% | 100% | 80% | 92–152 (124 ± 30) | 46–106 (77 ± 25) | 74–184 (142 ± 49) | ND-128 (76 ± 51) |
| Ciprofloxacin (CIP) | 100% | 100% | 100% | 100% | 148–440 (272 ± 122) | 80–176 (132 ± 40) | 96–348 (165 ± 123) | 122–656 (282 ± 218) |
| Norfloxacin (NOR) | 100% | 80% | 80% | 100% | 70–138 (110 ± 29) | ND-78 (50 ± 35) | ND-118 (54 ± 49) | 46–190 (88 ± 61) |
| Ofloxacin (OFL) | 100% | 100% | 100% | 100% | 112–302 (236 ± 85) | 48–124 (87 ± 34) | 88–402 (209 ± 143) | 132–1156 (365 ± 443) |
| Azithromycin (AZT) | 100% | 100% | 20% | 100% | 54–360 (190 ± 156) | 56–206 (113 ± 71) | ND-42 (11 ± 21) | 46–250 (125 ± 94) |
| Sulfamethoxazole (SMX) | 100% | 25% | 50% | 50% | 48–240 (118 ± 84) | ND-80 (20 ± 40) | ND-90 (43 ± 49) | ND-144 (56 ± 61) |
| Methyl Paraben (MP) | 100% | – | – | 100% | 64–188 (129 ± 55) | ND | ND | 40–192 (105 ± 56) |
3.3.2. Wastewater markers
CAF has been used as a source marker for human wastewater associated with domestic sewage (Buerge et al., 2006; Chakraborty et al., 2021). Concentration of CAF in the SPSs and STP inlets varied between 12 and 33 μg/L. About 97% of CAF was observed in the inlets and only 3% in primary sludge due to it's hydrophilic nature (Nam et al., 2014). Highest concentration of CAF was found in inlet of KD-STP (20.2 μg/L) followed by inlets of KYB-STP (17.8 μg/L), PG-STP (16.6 μg/L) and NP-STP (14.3 μg/L). Among SPSs, highest CAF concentration was observed in PG-SPS (32.7 μg/L) followed by NP-SPS (17 μg/L), KD-SPS 2 (14.1 μg/L), KYB-SPS (12.4 μg/L) and KD-SPS1 (12 μg/L). Removal efficiency of caffeine in the STPs varied between 84 and 98% (Fig. 3). Such high removal efficiency of CAF is in line with a previous study from Chennai (Anumol et al., 2016). Maximum removal efficiency of CAF (98%) was observed at NP-STP. It is noteworthy that we observed negative qRT-PCR results for all the STP outlet samples. Furthermore, >80% removal of CAF at the STP outlets suggest proper functioning of STPs in Chennai city (Fig. 3 and Table S6).
Fig. 3.
Box and whisker plots showing the range of a) SARS-CoV-2 N1 RNA copies obtained from four wastewater concentration methods b) caffeine c) other majorly detected organic tracers in STP inlets, primary sludge and outlets.
Up to 30% CBZ was observed in the sludge samples from the STPs (Fig. 3 ). Owing to its moderately hydrophobic nature, nearly 5–20% CBZ gets absorbed onto activated sludge (Wijekoon et al., 2013; Zhang et al., 2008). Furthermore, unlike STPs and SPSs, HWW did not show any CBZ peak (Table 2). CBZ has increased adsorption tendency towards solid matrix in the environment (Chakraborty et al., 2019). Hence, lesser solid particles can be reasoned for low CBZ in HWW when compared with STPs and SPSs samples. Among SPSs, PG-SPS (116 ng/L) had the highest level, followed by KYB-SPS (128 ng/L), KD-SPS 2 (76 ng/L) and KD-SPS 1 (62 ng/L). The range of CBZ (74–184 ng/L) in STP outlets were much lower than Canada, Germany, South Korea and Taiwan, but was slightly higher than USA and Japan (Hai et al., 2018). Excluding NP-STP, negative removal efficiency was seen for CBZ in the other three STPs. Maximum CBZ was observed in the outlet samples of KD-STP (184 ng/L) followed by PG-STP (170 ng/L), KYB-STP (138 ng/L) and inlet sample of NP-STP (92 ng/L). Similar to this study, higher concentrations of CBZ in outlets or effluents have been observed earlier in Chennai due to deconjugation of CBZ precursors during conventional activated sludge process (Anumol et al., 2016). Complex adaptive system (CAS) followed during biological treatment in STPs can lead to higher concentrations of CBZ in the outlets of STPs (Kwon and Rodriguez, 2014; Spongberg and Witter, 2008).
3.3.3. PPCPs
Prevalence of FQs in wastewater can be reasoned with higher consumption rate of these compounds in India (Arun et al., 2020). Among macrolides, only AZT and ERY were observed with a detection frequency of 100% and 75%, respectively. More than three-fourth of total AZT concentration was from STP sludge samples, most likely due to partitioning into bio-solids (Jones-Lepp and Stevens, 2007). In treatment plants, protonation of the basic dimethyl amino group can be reasoned for AZT loading in bio-solids (Younes et al., 2019). The range of AZT in STPs of Chennai city was comparable with earlier studies from Switzerland (Göbel et al., 2005), India (Mohapatra et al., 2016), Japan (Yasojima et al., 2006) and USA (Loganathan et al., 2009).
SMX and MP showed a detection frequency of 100%. Major contribution of SMX stemmed from the primary sludge samples and MP was mostly seen in STP inlet samples. Elevated usage of TCS as an anti-microbial agent in sanitizers might have resulted in high TCS in hospital wastewater (11.2 μg/L).
3.3.4. Concurrence and dynamic range
High prevalence of pepper mild mottle virus (PMMoV) and PPCPs have been reported earlier by using the dynamic range of the detection method and persistence of PMMoV (Kuroda, 2015). We have used a similar approach of concurrent percentage and dynamic range in this study to enumerate the co-existence of OTs and SARS-CoV-2 viral load. CAF was detected in all the samples. The concurrence percentage between CAF and viral load (gc/L) for N1and N2 was highest by SED and SUP (92%) followed by COM (75%) method (Table S7). The concurrence percentages of CBZ for SED, SUP and COM were found to be between 83 and 100%. The maximum concurrences of N1 and N2 from SED and SUP concentration methods were observed for AZT and NOR. This might be due to increased use of these drugs to combat the common symptoms caused by SARS-CoV-2. For OTs and SARS-CoV-2 RNA copies, dynamic range has been estimated as the ratio of their arithmetic mean concentration in the influent samples with the corresponding limit of quantification. Dynamic range for OTs varied between 62 and 8636 with the highest value for CAF and lowest for CBZ, indicating ubiquitous presence of CAF in wastewater (Table S7). Dynamic range for SARS-CoV-2 by all the wastewater concentration methods varied between 1008-2891 and 469-1920 for N1 and N2, respectively. The dynamic ranges for CIP, OFL, AZT were higher than CBZ and varied between 95 and 136. A hundred-fold higher dynamic range of CAF over the other frequently detected OTs, coupled with the concurrence of N1 and N2 genes of SARS-CoV-2 indicate that CAF can be used as a potential marker even for less polluted aquatic environment.
3.4. Estimated number of infected persons in different catchments
With the estimated RNA (in gc/L) in wastewater samples, we have predicted the number of infected individuals from the population of a given catchment area (Fig. S4). It was noted that during post lockdown, the estimated number of persons infected obtained by SED method for PG inlet (3983) was in line with the number of actual active COVID-19 cases in the PG catchment (3418). Our estimates obtained from average of all methods showed that the maximum number of infected individuals was found in KYB catchment (5523). It is noteworthy that KYB-STP caters to a relatively lesser population (1.4 million) and receives less than half the amount of wastewater (90 MLD) in comparison with KD catchment (3 million, 202 MLD) (Table S1). Yet, the numbers of predicted infected cases were maximum in KYB catchment. High population density in KYB can be an important factor for the spread of infection. Similar spread of SARS-CoV-2 with increasing population density was observed in Algeria (Kadi and Khelfaoui, 2020). Maximum number of RNA copies and very high level of CAF indicate higher wastewater discharge from this catchment. The number of COVID-19 cases in Tamil Nadu with a massive number of active cases (1,07,109) within four months of the first detected case in Chennai city (on 18th March 2020) seems to be attributed mainly to the delay in shutting the Koyambedu market, one of the largest perishable goods market in Asia (Rakkini and Vincent, 2018). The community spread was evident as people from diverse backgrounds contracted the infection. Unlike KYB, PG catchment houses a population of 3.1 million and generates almost similar amount of wastewater as KYB (95 MLD). PG covers a relatively posh locality with the possibility of better sanitation and hygiene conditions. Hence, percentage of predicted infected individuals was four-fold lesser than KYB catchment. Such detection of SARS-CoV-2 in community wastewater is a cost-effective mode of obtaining an early sign for the spread of COVID-19 infection in Chennai after the complete lockdown phase.
4. Conclusion and way forward
This study has gathered evidence from analysis of a total of 4 STPs, 5 SPSs and HWW for detection of SARS-CoV-2 viral load during partial lock-down and post-lockdown periods in Chennai city. Of the four wastewater concentration methods, SED, and SUP wastewater concentration methods gave comparable values for predicted and real infected cases.
The study was further strengthened by incorporating the quantitative presence of organic tracers to justify the efficacy of STPs in removing the viral load in treated wastewater. Removal of >80% caffeine, 100% of SARS-CoV-2 viral load and a steady decline of BOD in the treated wastewater reflect the efficiency of the treatment plants in Chennai city. It further gives a valuable information that CAF can be used as a potential indicator for removal of SARS-CoV-2 by STPs. The wastewater surveillance showed the presence of higher number of infected people in communities with high population density (as in the case of Koyambedu catchment).
Such wastewater monitoring of SARS-CoV-2 viral loading in STPs can help the local government, water boards and public health experts to estimate the number of infected persons in a given catchment and predict the severity of the infection spread even before an outbreak of an epidemic/pandemic in the near future. This study suggests the need for regular monitoring of community wastewater as a cost-effective tool to obtain an early signal for the future waves of COVID-19 pandemic in both urban and rural areas of India. This can further support policy makers in taking decisions on intensifying testing and imposing/lifting of restrictions.
CRediT authorship contribution statement
Paromita Chakraborty: Funding acquisition, Formal analysis, Conceptualization, Methodology, Software, Supervision, Writing – review & editing. Mukesh Pasupuleti: Investigation. M.R. Jai Shankar: Methodology. Girija K. Bharat: Writing – review & editing. Sundar Krishnasamy: Formal analysis. Sakshi Chadha Dasgupta: Writing – review & editing. Shyamal Kumar Sarkar: Writing – review & editing. Kevin C. Jones: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The study was supported by the Swiss Agency for Development and Cooperation (SDC), Swiss Cooperation Office India, Embassy of Switzerland, New Delhi for funding the project 7F-09271.04.01. The authors would like to thank the engineers and staff members from the Chennai Metropolitan Water Supply and Sewerage Board (CMWSSB), Chennai, Tamil Nadu and the Environmental Science and Technology Research scholars from SRM Research Institute, SRMIST, for their extended support during sampling and data collection.
Editor: Jay Gan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.146252.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;138764 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumol T., Vijayanandan A., Park M., Philip L., Snyder S.A. Occurrence and fate of emerging trace organic chemicals in wastewater plants in Chennai, India. Environ. Int. 2016;92:33–42. doi: 10.1016/j.envint.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Arora S., Nag A., Sethi J., Rajvanshi J., Saxena S., Shrivastava S.K., Gupta A.B. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. MedRxiv. 2020;82(12):2823–2836. doi: 10.2166/wst.2020.540. [DOI] [PubMed] [Google Scholar]
- Arun S., Kumar R.M., Raj J., Mukhopadhyay M., Ilango K., Chakraborty P. Occurrence, source and risk assessment of fluoroquinolones in dumpsite soil and sewage sludge from Chennai, India. Environ. Toxicol. Pharmacol. 2020;103410 doi: 10.1016/j.etap.2020.103410. [DOI] [PubMed] [Google Scholar]
- Babiker A., Myers C.W., Hill C.E., Guarner J. Oxford University Press US; 2020. SARS-CoV-2 Testing: Trials and Tribulations. [Google Scholar]
- Balboa S., Mauricio-Iglesias M., Rodríguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B., Lema J.M. The fate of SARS-CoV-2 in wastewater treatment plants points out the sludge line as a suitable spot for incidence monitoring. MedRxiv. 2020 doi: 10.1101/2020.05.25.20112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerge I.J., Buser H.-R., Poiger T., Müller M.D. Occurrence and fate of the cytostatic drugs cyclophosphamide and ifosfamide in wastewater and surface waters. Environ. Sci. Technol. 2006;40:7242–7250. doi: 10.1021/es0609405. [DOI] [PubMed] [Google Scholar]
- Cahill N., Morris D. Recreational waters–a potential transmission route for SARS-CoV-2 to humans? Sci. Total Environ. 2020;140122 doi: 10.1016/j.scitotenv.2020.140122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Mukhopadhyay M., Sampath S., Ramaswamy B.R., Katsoyiannis A., Cincinelli A., Snow D. Organic micropollutants in the surface riverine sediment along the lower stretch of the transboundary river Ganga: Occurrences, sources and ecological risk assessment. Environ. Pollut. 2019;249:1071–1080. doi: 10.1016/j.envpol.2018.10.115. [DOI] [PubMed] [Google Scholar]
- Chakraborty P., Shappell N.W., Mukhopadhyay M., Onanong S., Rex K.R., Snow D. Surveillance of plasticizers, bisphenol A, steroids and caffeine in surface water of river Ganga and Sundarban wetland along the Bay of Bengal: Occurrence, sources, estrogenicity screening and ecotoxicological risk assessment. Water Res. 2021;190:116668. doi: 10.1016/j.watres.2020.116668. [DOI] [PubMed] [Google Scholar]
- Chen C., Gao G., Xu Y., Pu L., Wang Q., Wang L., Wang W., Song Y., Chen M., Wang L. SARS-CoV-2–positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann. Intern. Med. 2020;172:832–834. doi: 10.7326/M20-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collivignarelli M.C., Abbà A., Bertanza G., Pedrazzani R., Ricciardi P., Miino M.C. Lockdown for CoViD-2019 in Milan: what are the effects on air quality? Sci. Total Environ. 2020;732:139280. doi: 10.1016/j.scitotenv.2020.139280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collivignarelli M.C., Collivignarelli C., Miino M.C., Abbà A., Pedrazzani R., Bertanza G. SARS-CoV-2 in sewer systems and connected facilities. Process. Saf. Environ. Prot. 2020;143:196–203. doi: 10.1016/j.psep.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel A., Thomsen A., McArdell C.S., Joss A., Giger W. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ. Sci. Technol. 2005;39:3981–3989. doi: 10.1021/es048550a. [DOI] [PubMed] [Google Scholar]
- GreaterChennnaiCorporation . Vol. 724. Greater Chennai Corporation; 2018. City Disaster Managment Plan.https://www.chennaicorporation.gov.in/ Available at: [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10. [Google Scholar]
- Hai F.I., Yang S., Asif M.B., Sencadas V., Shawkat S., Sanderson-Smith M., Gorman J., Xu Z.-Q., Yamamoto K. Carbamazepine as a possible anthropogenic marker in water: occurrences, toxicological effects, regulations and removal by wastewater treatment technologies. Water. 2018;10:107. [Google Scholar]
- Hallo A., Rojas A., Hallo C. Perspective from ecuador, the second country with more confirmed cases of coronavirus disease 2019 in south america: a review. Cureus. 2020;12 doi: 10.7759/cureus.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. MedRxiv. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;138875 doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDT Calculations: Converting from nanograms to copy number. 2013. https://sg.idtdna.com/pages/education/decoded/article/calculations-converting-from-nanograms-to-copy-number Available at: Accessed on January 2021.
- Jones-Lepp T., Stevens R. Pharmaceuticals and personal care products in biosolids/sewage sludge: the interface between analytical chemistry and regulation. Anal. Bioanal. Chem. 2007;387:1173–1183. doi: 10.1007/s00216-006-0942-z. [DOI] [PubMed] [Google Scholar]
- Kadi N., Khelfaoui M. Population density, a factor in the spread of COVID-19 in Algeria: statistic study. Bull. Natl. Res. Cent. 2020;44:1–7. doi: 10.1186/s42269-020-00393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R., Wani S., Singh A., Lal K. 2012. Wastewater Production, Treatment and Use in India, National Report Presented at the 2nd Regional Workshop on Safe Use of Wastewater in Agriculture. [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci.Tot.Environ. 2020;139076 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., M. Yasojima . K. Komori . Y. Suzuki . H. Tanaka Removal characteristics of human antibiotics during wastewater treatment in Japan. Water Pract. Technol. 2006;1(3) doi: 10.2166/wpt.2006.059. [DOI] [Google Scholar]
- Kotwani A., Holloway K. Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect. Dis. 2011;11:99. doi: 10.1186/1471-2334-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Keisuke, Nakada Norihide, Hanamoto Seiya, Inaba Manami, Katayama Hiroyuki, Thuan Do An, Thi Viet Nga Tran, Oguma Kumiko, Hayashi Takeshi, Takizawa Satoshi. Pepper mild mottle virus as an indicator and a tracer of fecal pollution in water environments: comparative evaluation with wastewater-tracer pharmaceuticals in Hanoi, Vietnam. Sci. Total Environ. 2015:506–507. doi: 10.1016/j.scitotenv.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Kwon J.-W., Rodriguez J.M. Occurrence and removal of selected pharmaceuticals and personal care products in three wastewater-treatment plants. Arch. Environ. Contam. Toxicol. 2014;66:538–548. doi: 10.1007/s00244-013-9979-0. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;139652 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Ferraro G.B., Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci. Total Environ. 2020;750:141711. doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan B., Phillips M., Mowery H., Jones-Lepp T.L. Contamination profiles and mass loadings of macrolide antibiotics and illicit drugs from a small urban wastewater treatment plant. Chemosphere. 2009;75:70–77. doi: 10.1016/j.chemosphere.2008.11.047. [DOI] [PubMed] [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580:176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Medema, G., Been, F., Heijnen, L., & Petterson, S. 2020a. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: Opportunities and challenges. Curr. Opin. Environ. Sci. Health. [DOI] [PMC free article] [PubMed]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020;8:104306. doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S., Huang C.-H., Mukherji S., Padhye L.P. Occurrence and fate of pharmaceuticals in WWTPs in India and comparison with a similar study in the United States. Chemosphere. 2016;159:526–535. doi: 10.1016/j.chemosphere.2016.06.047. [DOI] [PubMed] [Google Scholar]
- Nam S.-W., Choi D.-J., Kim S.-K., Her N., Zoh K.-D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014;270:144–152. doi: 10.1016/j.jhazmat.2014.01.037. [DOI] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;1-4 doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo S., Carrà G., Bottino P., Zanotto E., De Santis M.C., Margaria J.P., Giorgio A., Mandili G., Martini M., Cavallo R. A novel multiplex qRT-PCR assay to detect SARS-CoV-2 infection: high sensitivity and increased testing capacity. Microorganisms. 2020;8:1064. doi: 10.3390/microorganisms8071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. Quantification strategies in real-time PCR. AZ Quantitative PCR. 2004;1:89–113. [Google Scholar]
- Race M., Ferraro A., Galdiero E., Guida M., Núñez-Delgado A., Pirozzi F., Siciliano A., Fabbricino M. Current emerging SARS-CoV-2 pandemic: potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020;109808 doi: 10.1016/j.envres.2020.109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakkini V., Vincent S. A survey of solid waste Management in Chennai (a case study of around Koyambedu market and Madhavaram poultry farms) J. Civil. Eng. Environ. Sci. 2018;4:009–0012. [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, J.A., 2003. Zeolite bed leach septic system and method for wastewater treatment. Google Patents.
- Spongberg A.L., Witter J.D. Pharmaceutical compounds in the wastewater process stream in Northwest Ohio. Sci. Total Environ. 2008;397:148–157. doi: 10.1016/j.scitotenv.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., De Ridder D., Petrovic D., Schrempft S., Marcus K. Repeated seroprevalence of anti-SARS-CoV-2 IgG antibodies in a population-based sample from Geneva, Switzerland. MedRxiv. 2020 doi: 10.1101/2020.05.02.20088898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijekoon K.C., Hai F.I., Kang J., Price W.E., Guo W., Ngo H.H., Nghiem L.D. The fate of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters and pesticides during MBR treatment. Bioresour. Technol. 2013;144:247–254. doi: 10.1016/j.biortech.2013.06.097. [DOI] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. Msystems. 2020;5(4) doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.-M., Moulin L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. MedRxiv. 2020 [Google Scholar]
- Yasojima M., Nakada N., Komori K., Suzuki Y., Tanaka H. Occurrence of levofloxacin, clarithromycin and azithromycin in wastewater treatment plant in Japan. Water Sci. Technol. 2006;53:227–233. doi: 10.2166/wst.2006.357. [DOI] [PubMed] [Google Scholar]
- Ye L., Cornelis P., Guillemyn K., Ballet S., Christophersen C., Hammerich O. Structure revision of N-mercapto-4-formylcarbostyril produced by Pseudomonas fluorescens G308 to 2-(2-hydroxyphenyl) thiazole-4-carbaldehyde [aeruginaldehyde] Nat. Prod. Commun. 2014;9 (1934578X1400900615) [PubMed] [Google Scholar]
- Younes H.A., Mahmoud H.M., Abdelrahman M.M., Nassar H.F. Seasonal occurrence, removal efficiency and associated ecological risk assessment of three antibiotics in a municipal wastewater treatment plant in Egypt. Environ. Nanotechnol. Monit. Manag. 2019;12:100239. [Google Scholar]
- Zhang Y., Geißen S.-U., Gal C. Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere. 2008;73:1151–1161. doi: 10.1016/j.chemosphere.2008.07.086. [DOI] [PubMed] [Google Scholar]
- Zhou H., Wu C., Huang X., Gao M., Wen X., Tsuno H., Tanaka H. Occurrence of selected pharmaceuticals and caffeine in sewage treatment plants and receiving rivers in Beijing, China. Water Environ. Res. 2010;82:2239–2248. doi: 10.2175/106143010x12681059116653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material