Abstract
Coronaviruses share conservative spike protein (S) on their enveloped membrane surface, where S1 subunit recognizes and binds the cellular receptor, and the S2 subunit mediates membrane fusion. This similarity raises the question: does coronaviral infection by one create protection to others? Convalescent SARS-CoV-2 (COVID-19) sera were tested for cross reactivity with peptides from Middle East respiratory syndrome coronavirus (MERS-CoV) which shares 74% homology. Our results showed significant cross-reactivity with a peptide of the heptad repeat 2 (HR2) domain of the MERS-CoV spike protein. Sera samples of 47 validated seropositive convalescent COVID-19 patients and 40 sera samples of control patients, collected in pre-COVID time were used to establish cross-bind reactivity with the MERS-CoV peptide. Significantly stronger binding (p < 0.0001) was observed for IgG antibodies in convalescent COVID-19 patients compared to the control group. In ELISA, MERS-CoV peptide helps to discriminate post-COVID-19 populations and non-infected ones by the presence of antibodies in blood samples. This suggests that polyclonal antibodies established during SARS-CoV-2 infection can recognize and probably decrease severity of MERS-CoV and other coronaviral infections. The high homology of the spike protein domain also suggests that the opposite effect can be true: coronaviral infections produce cross-reactive antibodies effective against SARS-CoV-2. The collected data prove that despite the core HR2 region is hidden in the native viral conformation, its exposure during cell entry makes it highly immunogenic. Since inhibitory peptides to this region were previously described, this opens new possibilities in fighting coronaviral infections and developing vaccines effective even after possible viral mutations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00005-021-00607-8.
Keywords: SARS-CoV-2, COVID-9, Coronavirus, Antibodies, Immunoglobulins
Introduction
Coronaviruses such as the Middle East respiratory syndrome coronavirus (MERS-CoV), Severe acute respiratory syndrome coronavirus (SARS-CoV-1), and the recently emerged SARS-CoV-2 are sharing several similar protein regions which are involved in the recognition of the host cells. The SARS-CoV-2 genome (30 kb in size) encodes a large, non-structural polyprotein [open reading frame (ORF)1a/b] that is further proteolytically cleaved to generate 15/16 proteins, four structural proteins, and five accessory proteins (ORF3a, ORF6, ORF7, ORF8 and ORF9). The four structural proteins consist of the spike (S) surface glycoprotein, the membrane (M) protein, the envelope (E) protein, and the nucleocapsid (N) protein, which is essential for SARS-CoV-2 assembly and infection. The MERS-CoV genome structure is encoding 10 proteins; two replicase polyproteins (ORFs 1ab and 1a), three structural proteins (E, N, and M), a surface (spike) glycoprotein (S), and five non-structural proteins (ORFs 3, 4a, 4b, and 5) (Liu et al. 2004; Mackay and Arden 2015).
The spike surface glycoprotein (S) plays a key role in mediating virus attachment and fusion and is indeed present in all human infecting coronaviruses. They can be cleaved by host proteases into an N-terminal S1 subunit and a membrane-bound C-terminal S2 region. To engage a host receptor, the receptor-binding domain (RBD) of the S1 subunit undergoes conformational movements, which transiently hide or expose the determinants of receptor binding (Wrapp et al. 2020; Xia et al. 2019). The heptad repeat 1 (HR1) region in S2 subunits forms a homotrimeric structure, exposing three highly conserved hydrophobic grooves on the surface resulting in binding of three HR2 regions and the formation of a six-helix bundle (6-HB) structure. 6-HB is responsible for a close approximation of viral and host membranes and their subsequent merging. Binding of HR1 and HR2 domains results in the 6-HB needed for merging with the host cell membrane. Thus targeting the HR2 domain specifically binding HR1 could inhibit the viral cell entry (Xia et al. 2014). Further optimisation of the peptide sequence has also the potential of generating pan-coronaviral inhibitors, like EK1 (Xia et al. 2019) able to inhibit SARS-CoV-2 pseudovirus infection (Xia et al. 2020).
The structure of HR2 is poorly resolved during crystallographic assessment due to a high level of flexibility (Yuan et al. 2017). Unlike highly mutable RBD, the HR1 and HR2 domains are highly conservative between coronaviruses, so form a perfect target for viral neutralization and generation of immunity that latter can be used for viral testing. Since HR2 and HR1 domains are merged and surface-exposed after S protein cleavage it is likely that these domains are highly immunogenic.
This similarity can result in the development of cross-reactive antibodies and protection against other coronaviruses, in case of being infected by another virus species. In this work, we would like to establish if SARS-CoV-2 results in the production of antibodies, that are also recognizing MERS-CoV antigens.
Materials and Methods
Patient Cohorts
All analyses of human materials were performed following the institutional guidelines and with the approval of the Ethics Committees of Danylo Halytsky Lviv National Medical University (DH LNMU No. 5/2017-02-23). The current study involved 47 samples from SARS-CoV-2 infected patients treated in the Lviv Regional Clinical Infection Hospital of Infectious Disease/Department of Infectious Diseases, Danylo Halytsky Lviv National Medical University (Lviv, Ukraine) during April–May 2020. Written informed consent was obtained from every person before blood collection. All patients were PCR-positive upon hospitalization. Sera collection took place at least 3 weeks after the appearance of clinical symptoms when a person was recovered. Recovery was assessed by (a) disappearance of clinical symptoms, (b) two consecutive negative PCR tests made within 2 days difference. All sera collected were tested positive for anti-SARS-CoV-2 antibodies using reference detection test from Xema (Xema Medica, Ukraine/Finland), in this test a mixture of spike and nucleocapside antigens served as and antigen. Cohort of patients consisted from 29 males and 18 females, the disease course was mild in 11 persons (23.4%), moderate in 28 (59.6%) and severe in 8 cases (17.0%); the later were associated with age > 60 years old and accompanying chronic illnesses. For most cases incubation period lasted 5–14 days. None of the patients had a history of MERS-CoV infection.
A group of normal healthy donors (NHD), who have donated blood between June and November 2019 (pre-COVID-19) served as controls, they were both age- and sex-matched to fit the study group. None of the healthy donors had a history of MERS-CoV infection. Informed written consent for blood withdrawal was obtained from each patient and NHD.
ELISA Tests
Sera samples from convalescent COVID-19 patients and NHDs were frozen at − 20 °C. For testing anti-MERS response immunosorbent NUNC maxisorp plates (Thermo Scientific, Waltham, USA) were coated with NH2-CCTTTTTTSLTQINTTLLDLEYEMKKLEEVVKKLEESYIDLKEL-COOH peptide (50 μL of a 4 μg/mL solution) in a carbonate-bicarbonate buffer (100 mM, pH 9.6). For testing anti-SARS-CoV-2 response immunosorbent NUNC MaxiSorp plates (Thermo Scientific, Waltham, USA) were coated with recombinant 194 a.a. protein corresponding to the RBD domain of spice protein of SARS-CoV-2, (Exploregen LLC, UA). All serum samples were diluted 1:1000 in the carbonate-bicarbonate buffer and incubated at 37 °C for 1 h, after that the plates were washed again. Goat anti-human IgG (H + L)-horseradish peroxidase (109-035-003, Jackson ImmunoResearch) was diluted in washing buffer (1:25,000), added to the plates, and incubated at room temperature for 1 h. After the corresponding washings, the assay was developed with 3,3′,5,5′-tetramethylbenzidine (TMB) containing an excess of H2O2 as a substrate (50 µL/well). The reaction was stopped with 50 µl/well of sulfuric acid (1 M). The absorbance was read at 450 nm/600 nm using a Perkin Elmer BioAssay reader HST700 (Waltham, USA). anti-SARS-CoV-2 ELISA was additionally tested with reference positive sera for COVID-19 diagnostics, anth their signal was in the renge of 0.45–0.60 optical density units. The coefficient of variation (CV) between replicates was controlled to be < 3%. Other ELISA parameters were controlled according to the best practice of ELISA analysis (Crowther 2009; Kiessig et al. 1993) and our previous reports (Biermann et al. 2018; Bozhenko et al. 2020).
Bioinformatics
The protein homology searches were done using blast (National Center for Biotechnology Information and Protein Data Bank [PDB]) databases. To include the regions with resolved structures in our searches we had used SEQATOMS (http://www.bioinformatics.nl/tools/seqatoms/) (Brandt et al. 2008). Protein structures were visualized using PyMOL (https://pymol.org/). Multiple sequence alignments were done using CLUTALW (Larkin et al. 2007).
Data Analysis
ELISA testing was performed in duplicate using two technical replicates for each analysis (CV always < 3%). The data were normalized between plates using positive controls and corrected for background signal of secondary antibodies on each plate, then the mean values were calculated. The mean values were used to construct data on the figures. For comparisons between two groups, the Mann–Whitney U-test for numerical variables was used. A receiver operating characteristic (ROC) curve was generated. The area under the ROC was calculated to estimate the specificity, sensitivity, and usefulness of the binding assays. All analyses were performed using Excel 2016 (Microsoft Corp., Redmond, WA, USA) and Prism 8.2 (GraphPad, San Diego, USA) software. A p value of ≤ 0.05 was considered statistically significant. Four levels of significance are depicted in the figures by asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Results
Selection’s Rationale of S1 MERS Peptide
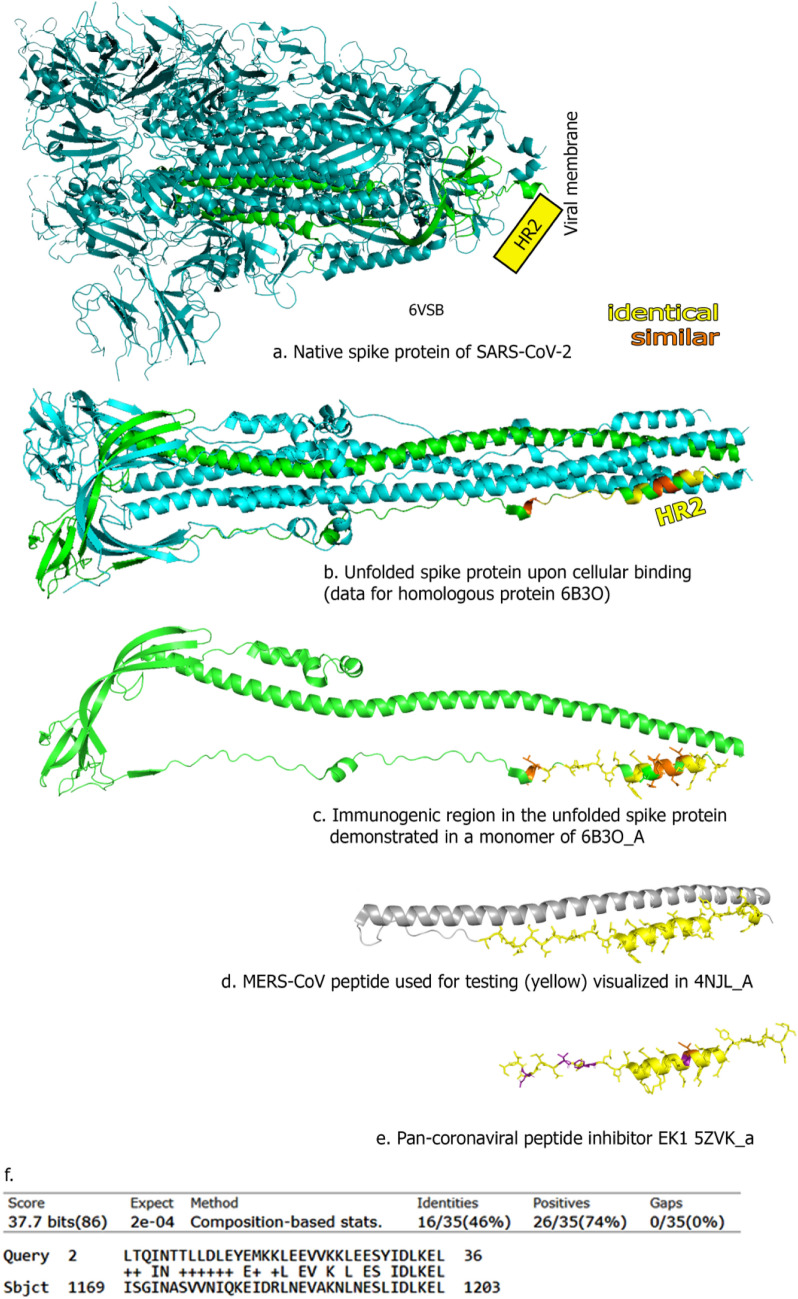
The complete crystal structure of the HR2 domain of SARS-CoV-2 remains currently unavailable due to its conformation changes and problems in stabilization (Wrapp et al. 2020) (Fig. 1a). Using the SEQATOMS algorithm (Brandt et al. 2008) we selected the most complete structure of the corresponding region containing defined atomic coordinates, namely one proposed by Walls et al. (2017), a model for HR1 HR2 rearrangements and unfolding accompanying viral entry into host cells for other coronaviruses. This structure demonstrates exposure of the HR2 domains upon cellular binding in trimeric (Fig. 1b) and monomeric form (Fig. 1c). To evaluate the cross-reactivity we selected the HR2-specific peptide of the spike protein of MERS-CoV reported to possessed high immunogenic potential (Du et al. 2013; Mou et al. 2013; Wang et al. 2013). Indeed, ongoing studies show that it can be also successfully used to raise MERS-recognizing antibodies in the presence of neutrophil extracellular traps (NET) forming nanoadjuvants (Bilyy et al. 2019). The selected HR2 peptide of the spike protein of MERS-CoV [depicted yellow, Fig. 1d using PDB deposited crystal structure of 4NJL_A, (Lu et al. 2014)] shared significant similarities in 3D structure between (the only) known crystal structure of unfolded HR2 domain (Fig. 1c) and with pan-coronaviral inhibitor peptide EK1, using crystal structure 5ZVK_a (Xia et al. 2019) (Fig. 1e). Performed protein BLAST analysis revealed 46% identity and 76% similarity in amino acid sequence of the MERS peptide with the corresponding peptide of the SARS-CoV-2 spike protein (sequence ID QKJ68605.1) (Fig. 1f). The obtained data of closed structural similarity between viral HR2 domains of S protein inspired us to permorm ELISA tests for cross-reactivity of produced antibodies.
Fig. 1.
Similarity of HR2 regions between different coronaviruses. a Image of Spike protein (S1) of SARS-CoV-2. b S1 upon host cell interaction: conformational changes in trimer are occurring, exposing previously hidden HR2 domain regions (Wrapp et al. 2020), c monomeric part of (b), exposing domain with structural similarity (yellow—identical, orange—similar amino acids) towards corresponding MERS peptide, depicted on (d) and pan. (f). Protein BLAST analysis of used MERS protein is showing similarity towards the sequence in the genome of SARS-CoV-19. Sequence ID: QKJ68605.1. Crystal structure of HR2 domain for SARS-CoV-2 is currently not available, thus it is represented as a rectangle in (a) based on the last connected coordinates available in 6VSB structure
Antibody Cross-Reactivity towards Coronaviral HR2 Domains
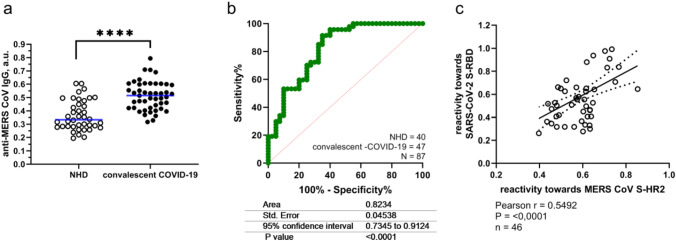
Having established the structural similarity between the S1 MERS peptide with the genome of SARS-CoV-2, sera of convalescent SARS-CoV-2 infected patient, who have never suffered from MERS-CoV infection before, have been collected and tested for the presence of antibodies. The S1 MERS-CoV specific peptide, NH2-CCTTTTTTSLTQINTTLLDLEYEMKKLEEVVKKLEESYIDLKEL-COOH, which we previously successfully used to raise anti-MERS antibodies while testing novel NET-stimulating adjuvants (Bilyy et al. 2019), was immobilized on ELISA plates and incubated with sera samples. As can be seen from Fig. 2a, stronger binding of IgG from sera of SARS-CoV-2 convalescent patients is observed when compared to sera of patients without previous SARS-CoV-2 infection (p < 0.0001, n = 87). Minor differences were detected for IgM binding (p = 0.016) and no difference was detected for IgA or IgE antibody subclasses (Supplementary Information Fig. S1).
Fig. 2.
Antibody cross-reactivity between coronaviruses: sera of convalescent COVID-19 patients possess antibodies recognizing MERS-specific peptide of HR2 spike protein domain. a Difference between IgG levels recognizing peptide of HR2 domain in S protein of MERS in convalescent SARS-CoV-2 patients and non-infected healthy donors. b ROC curve for discrimination of “anti-coronaviral” IgG antibodies using indicated MERS peptide as antigen. c IgG reactivity in convalescent plasma of SARS-CoV-2 patients towards HR2 domain in S protein of MERS vs RBD part of S protein of SARS-CoV-2. ****p < 0.0001
Using this S1 MERS-CoV specific peptide, discrimination of persons that have suffered from SARS-CoV-2 infection and those who were not in contact with the virus resulted in a predictive value (area under ROC curve) equal to 0.823 (Fig. 2b), with a specificity and sensitivity of ~ 60% (95% confidence). SARS-CoV-2 infection results in the generation of antibodies with significantly strong cross-reactive towards a MERS-specific peptide with 76% homology. Highly conservative regions of the exposed domain suggest that the opposite can be true—the coronaviral disease can result in some antibodies able to recognize SARS-CoV-2 epitops circulating in the blood.
To determine whether the strong binding with S1 peptide is correlated with a higher amount of anti-SARS-CoV-2 antibodies we used recombinant RBD protein immobilization on ELISA plates to incubate with sera samples to evaluate the amount of formed IgG type antibodies. As can be seen from Fig. 2c, the sera samples having shown stronger binding of IgG antibodies with anti-HR2 MERS spike protein also contained higher IgG reactivity towards anti-RBD spike protein of SARS-CoV-2. The Pearson’s correlation between the two parameters was 0.5492, p < 0.0001. This suggests stronger humoral response towards one of the virus will be associated with the intensity of the immune response towards other coronaviruses.
Discussion
Cross-reactivity between coronaviruses has become a critical question since it brings new promises against COVID-19 protection. On the other hand, this cross-reactivity can be negative, since available cross-reactivity towards coronaviruses will make coronaviruses not the best choice for vectors in vaccines, especially taking into account recent data on broad immune cross-reactivity (Ng et al. 2020). Indeed, it was reported that epitope pools detect CD4+ and CD8+ T cells in 100% and 70% of convalescent COVID-19 patients respectively, recognizing S and M proteins, with at least eight SARS-CoV-2 ORFs targeted. T cell reactivity to SARS-CoV-2 epitopes is also detected in non-exposed individuals (Grifoni et al. 2020; Mateus et al. 2020). In SARS-CoV-2 patients, S-reactive CD4+ T cells equally target N-terminal and C-terminal parts of the spike protein, whereas in healthy donors S-reactive CD4+ T cells react almost exclusively to the C-terminal part. This part is characterized by a higher homology to spike glycoprotein of human endemic “common cold” coronaviruses and contains the S2 subunit of S with the cytoplasmic peptide, the fusion peptide, and the transmembrane domain but not the RBD. S-reactive CD4+ T cells from SARS-CoV-2 patients are further distinct to those from healthy donors as they co-expressed higher levels of CD38 and HLA-DR, indicating their recent in vivo activation (Braun et al. 2020). Potential preexisting cross-reactive T cell immunity to SARS-CoV-2 has broad implications, as it could explain aspects of differential SARS-CoV-2 clinical outcomes, influence epidemiological models of herd immunity, or affect the performance of SARS-CoV-2 candidate vaccines. Pre-existing memory CD4+ T cells that are cross-reactive with comparable affinity to SARS-CoV-2 and the common cold coronaviruses HCoV-OC43, HCoV-229E, HCoV-NL63, or HCoV-HKU1. Thus, variegated T cell memory to coronaviruses that cause the common cold may underline at least some of the extensive heterogeneity observed in COVID-19 disease (Mateus et al. 2020).
Based on our data and those of others, it would be reasonable to assume that the exposure of the C-terminal of the spike protein upon merging with the host cell makes it an excellent immunogenic target. It conservative nature could also allow the development of cross-viral immunity (cellular and humoral) which circumvent the high mutation rate in regions such as ACE2.
Our study was performed on the cohort of 47 patients, thus more detailed information for cross-coronaviral immunity against different strains is needed. However, after the current report was published as preprint (Rabets et al. 2020), there were a series of works confirming our idea, that humoral cross-coronaviral immunity is important in defining COVID (Beretta et al. 2020; Lipsitch et al. 2020; Yaqinuddin 2020).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof. Jean Dubuission and Dr. Karin Seron, University of Lille, CNRS, INSERM, CHU Lille, Institut Pasteur de Lille, U1019-UMR 8204-CIIL-Center for Infection and Immunity of Lille, F-59000 Lille, France for providing immunogenic MERS peptides amd Dr. Yuri Lebedin and Xema Medica Co, Ukraine/Finland for providing refence sera and tests. Financial support from the Cedars-Sinai Medical Center’s International Research and Innovation in Medicine Program, the Association for Regional Cooperation in the Fields of Health, Science and Technology (RECOOP HST Association) RCSS grant 020, and BMYSRG 015; Grant of Ministry of Healthcare of Ukraine 0119U101338 and National Research Foundation of Ukraine 2020.02/0131; Volkswagen-Stiftung grant No 97744. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant agreements No 861878 and 872331. Financial support by ANR project “nanoMERS” (ANR-18-CE09-0021) is acknowledged.
Declarations
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Beretta A, Cranage M, Zipeto D. Is cross-reactive immunity triggering COVID-19 immunopathogenesis? Front Immunol. 2020;11:567710. doi: 10.3389/fimmu.2020.567710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann MHC, Boeltz S, Pieterse E, et al. Autoantibodies recognizing secondary necrotic cells promote neutrophilic phagocytosis and identify patients with systemic lupus erythematosus. Front Immunol. 2018;9:989. doi: 10.3389/fimmu.2018.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyy R, Paryzhak S, Turcheniuk K, et al. Aluminum oxide nanowires as safe and effective adjuvants for next-generation vaccines. Mater Today. 2019;22:58–66. doi: 10.1016/j.mattod.2018.10.034. [DOI] [Google Scholar]
- Bozhenko M, Boichuk M, Bila G, et al. Freezing influences, the exposure of IgG glycans in sera from multiple sclerosis patients. Ukr Biochem J. 2020;92:21–31. doi: 10.15407/ubj92.02.021. [DOI] [Google Scholar]
- Brandt BW, Heringa J, Leunissen JAM. SEQATOMS: a web tool for identifying missing regions in PDB in sequence context. Nucleic Acids Res. 2008;36:W255–W259. doi: 10.1093/nar/gkn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J, Loyal L, Frentsch M, et al. (2020) Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. MedRxiv. 2020;04(17):20061440. doi: 10.1101/2020.04.17.20061440. [DOI] [Google Scholar]
- Crowther JR. The ELISA guidebook. Humana Press. 2009 doi: 10.1007/978-1-60327-254-4. [DOI] [Google Scholar]
- Du L, Zhao G, Kou Z, et al. Identification of a receptor-binding domain in the S Protein of the novel human coronavirus middle east respiratory syndrome coronavirus as an essential target for vaccine development. J Virol. 2013;87:9939–9942. doi: 10.1128/JVI.01048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessig S, Abel U, Risse P, et al. Problems of cut-off level determination in enzyme immunoassays: The case of TBE-ELISA. Klin Lab. 1993;39:877–886. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lipsitch M, Grad YH, Sette A, et al. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat Rev Immunol. 2020;20:709–713. doi: 10.1038/s41577-020-00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Xiao G, Chen Y, et al. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu Q, Zhu Y, et al. Structure-based discovery of Middle East respiratory syndrome coronavirus fusion inhibitor. Nat Commun. 2014;5:3067. doi: 10.1038/ncomms4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay IM, Arden KE. Middle East respiratory syndrome: an emerging coronavirus infection tracked by the crowd. Virus Res. 2015;202:60–88. doi: 10.1016/j.virusres.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Raj VS, van Kuppeveld FJM, et al. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol. 2013;87:9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, Faulkner N, Cornish GH, et al. (2020) Pre-existing and de novo humoral immunity to SARS-CoV-2 in humans. BioRxiv. 2020;05(14):095414. doi: 10.1101/2020.05.14.095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabets A, Bila G, Grytsko R, et al. (2020) Development of antibodies to pan-coronavirus spike peptides in convalescent COVID-19 patients. MedRxiv. 2020;08(20):20178566. doi: 10.1101/2020.08.20.20178566. [DOI] [Google Scholar]
- Walls AC, Tortorici MA, Snijder J, et al. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc Natl Acad Sci USA. 2017;114:11157–11162. doi: 10.1073/pnas.1708727114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Shi X, Jiang L, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23:986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D, Wang N, Corbet KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Liu Q, Wang Q, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) entry inhibitors targeting spike protein. Virus Res. 2014;194:200–210. doi: 10.1016/j.virusres.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Yan L, Xu W, et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. 2019;5:eaav4580. doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Liu M, Wang C, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqinuddin A. Cross-immunity between respiratory coronaviruses may limit COVID-19 fatalities. Med Hypotheses. 2020;144:110049. doi: 10.1016/j.mehy.2020.110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Cao D, Zhang Y, et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;8:15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.