Abstract
Background
It has been reported that lncRNA SNHG10 (SNHG10) promotes the progression of liver cancer and osteosarcoma; however, the role of SNHG10 in acute myeloid leukemia (AML) remains unknown. This study was to explore the role of SNHG10 in AML.
Methods
The expression of SNHG10 and miR-621 in BM mononuclear cells (BMMNCs) isolated from 60 AML patients and 60 healthy controls were determined by RT-qPCR. Correlation between SNHG10 and miR-621 was analyzed by Pearson correlation coefficient. The overexpression of SNHG10 and miR-621 in transfected AML cells was detected by RT-qPCR, and the regulatory relationship between them was explored. Methylation-specific PCR (MSP) was applied to detect the methylation of miR-621 induced by the overexpression of SNHG10. CCK-8 assay was conducted to evaluate cell proliferation.
Results
In this study, we found that the expression of SNHG10 was upregulated and the expression of miR-621 was downregulated in AML samples. Moreover, SNHG10 and miR-621 were inversely correlated across AML samples, and a high level of SNHG10 predicted poor survival of AML patients. Bioinformatics analysis showed that SNHG10 could be targeted by miR-621. In AML cells, miR-621 overexpression downregulated the expression of SNHG10, while SNHG10 overexpression could not affect the expression of miR-621. However, it was found that the reduction of miR-621 caused by SNHG10 overexpression might be due to the increase of miR-621 methylation. In addition, SNHG10 overexpression significantly promoted BMMNC proliferation, whereas miR-621 overexpression inhibited BMMNC proliferation and abolished the effect of SNHG10 overexpression on BMMNC proliferation.
Conclusion
miR-621 targets SNHG10 to suppress cell proliferation in AML.
Keywords: acute myeloid leukemia, miR-621, SNHG10, proliferation
Introduction
Acute myeloid leukemia (AML) is a type of blood cancer derived from bone marrow and characterized by the rapid reproductivity and abnormal functions of white blood cells.1 AML is the most common subtype of acute leukemia that affects about 20, 000 new cases in the United States every year.2 Due to its extreme aggressiveness, even after active treatment, no more than 30% of AML patients can survive longer than 5 years after the initial diagnosis.3,4 Chemotherapy is the most frequently used treatment for AML. In some cases, targeted therapy drugs and stem cell transplants can also be applied.5,6 However, due to chemoresistance, treatment outcomes are usually poor in almost all cases.5 Therefore, novel treatment approaches are urgently needed.
Targeted therapies are emerging anti-cancer approaches that aim to suppress cancer development mainly by regulating gene expression networks related to cancers.7 Previous studies have shown that the initiation and development of cancer require the involvement of critical molecular players.8,9 In-depth molecular researches on the pathogenesis of AML provide new targets for the therapy of AML.10 Non-coding RNAs (ncRNAs), including lncRNAs and miRNAs, are not involved in protein-coding but it can regulate gene expression to participate in various human diseases.11–13 Also, ncRNAs have played crucial roles in AML. For example, it is reported that miR-125B and miR-29B are two regulatory targets for NRF2 and both of them are abnormally expressed in AML.14 Moreover, anti-miR-125B or elevating miR-29B induced more cell death in AML.14 In addition, a study proposed that serum exosome miRNA could be considered as a minimally invasive early biomarker of AML.15 However, the precise regulation by ncRNAs in cancer biology remains hardly known. A recent research has reported that lncRNA SNHG10 can promote the progression of liver cancer16 and osteosarcoma.17 However, the role of SNHG10 in AML is still elusive.
miR-621 has been demonstrated to be downregulated in several kinds of cancer. It has been reported that miR-621 inhibits the proliferation and metastasis of bladder cancer by suppressing Wnt/β-catenin signaling pathway.18 Low expression of miR-621 has been demonstrated to be associated with poor prognosis in HCC patients.19,20 Besides, miRNA-621 acts as a tumor suppressor in HCC progression by downregulating CAPRIN1 expression.20 Previous bioinformatics analysis shows that SNHG10 can target miR-621.21 This study was therefore conducted to analyze the regulatory relationship between SNHG10 and miR-621 and their roles in AML.
Patients and Methods
Research Subjects
From August 2018 to January 2020, 60 AML patients (37 males and 23 females, 50 to 73 years, 61.6 ± 5.9 years) and 60 healthy controls (37 males and 23 females, 50 to 73 years, 61.7 ± 5.8 years) were enrolled in Guizhou Provincial People’s Hospital. Guizhou Provincial People’s Hospital Ethics Committee (IRB: 00000430) has approved this study before the enrollment of patients and this study was conducted in accordance with the Declaration of Helsinki. The criteria of the selection of healthy controls were: 1) All healthy controls received systemic physiological exam and all of them were enrolled with normal physiological condition. The criteria of AML patients enrolled were: 1) All AML patients were newly diagnosed cases. 2) Patients with AML did not suffer from other severe pathological diseases. In view of the fact that other clinical disorders and treatments may also affect the expressions of the genes we focused on, this study excluded those patients with other clinical diseases or patients who had accepted other treatments. All patients and healthy people had signed written informed consent.
BM Mononuclear Cells (BMMNCs) and AML Cell Lines
Bone marrow (BM) was collected from both AML patients and healthy controls before the initiation of therapy. Lymphocyte Separation Medium (TBD Sciences) was used to isolate BM mononuclear cells (BMMNCs) from BM of all patients and healthy controls. All samples of BMMNCs were subjected to RNA isolation immediately after collection. Kasumi-3 and Kasumi-6 (ATCC, USA) two human AML cell lines were used in the following experiments. RPMI-1640 containing 10% FBS was used as culture medium which was supplemented with penicillin/streptomycin (100 U/mL). The cells were cultivated in an incubator under the condition of 95% humidity and 5% CO2 at 37 °C.
Cell Transfection
Kasumi-3 and Kasumi-6 cell transfections were carried out with 2 × 106 cells using the Amaxa Nucleofector Kit II (Cambridge, UK). Negative control (NC) miRNA and miR-621 mimic were purchased from Sigma-Aldrich (USA). PcDNA3.1 vector (Invitrogen) was used as the backbone of SNHG10 overexpression. Cells were electro-transfected with pcDNA3.1 (1μg), pcDNA3.1-SNHG10 (1μg), NC-miRNA (50 nM) or miR-621 mimic (50 nM) cells. Transfected cells were incubated at 37°C for 24 h and used to perform the subsequent experiments.
RNA Preparation
RiboZol reagent (Invitrogen) was used to isolate RNA from BMMNC samples and Kasumi-3 and Kasumi-6 cells cultured in vitro. Genomic DNA was removed from all RNA samples by incubating DNase I (Sigma-Aldrich) at 37 °C for 100 min. RNA samples were separated using a 5% Urine-PAGE gel to check RNA integrity. RNA quality was determined based on OD 260/280 ratio.
RT-qPCR
RNA samples with OD 260/280 ratio close to 2.0 were used as templates to synthesize cDNA samples by SSRT IV system (Invitrogen). To determine the expression of SNHG10, RT-qPCR was performed using QuantiFast SYBR Green PCR Kit (QIAGEN) with 18S rRNA as template. Mature miRNAs and poly (A) were added into the tubes; then, miRNA reverse transcriptions and miRNA qPCRs were performed to determine the expression level of mature miR-621. The internal control of miR-621 was U6. Each experiment included three technical replicates. The expression levels of SNHG10 or miR-621 were normalized to the corresponding internal control using the method of 2−ΔΔCT. The primer design was as follows:
5′-GTTGGTCTCTTGGGAGGTAG-3′ (forward) and 5′-CGCCACGACGAACTGCATGC-3′ (reverse) for SNHG10; 5’-AATGTCACCGTTGTCCAGTTG-3’ (forward) and 5’-GTGGCTGGGGCTCTACTT C-3’ (reverse) for GAPDH; 5’-GGCTAGCAACAGCGCTTACCT-3’ for miR-621; 5’-CGCAAGGATGACACGCAAATTC-3’ for U6.
Methylation-Specific PCR (MSP)
Kasumi-3 and Kasumi-6 cells transfected with pcDNA3.1-SNHG10 or empty pcDNA3.1 vector were subjected to genomic DNA isolations, which were performed using a Genomic DNA Extraction Kit (ab156900, Abcam). EZ DNA Methylation Lighting Kit (ZYMO research) was used to convert DNA samples, followed by detecting the methylation of the miR-621 gene using Taq 2X Master Mix (M0270, NEB). MSP primers were 5′-CATCAGGACGACCAGTAACAT-3′ (forward) and 5′-CGACTTGACATTCATCA-3′ (reverse).
Cell Proliferation Assay
After transfection as mentioned above, the proliferation of Kasumi-3 and Kasumi-6 cells was analyzed by Cell Counting Kit-8 (CCK-8) kit (Dojindo). A 96-well cell culture plate was used to cultivate cells (5000 cells per well). Cells were cultivated in the aforementioned medium under aforementioned conditions. CCK-8 solution was added into each well to reach 10% at 2 h before the measurement of OD values. OD values (450 nm) were measured every 24 h for a total of 4 days.
Statistical Analysis
Gene expression levels in BMMNCs from both AML patients and healthy controls were the average values of three technical replicates and compared by unpaired t-test. The data of multiple cell transfection groups were showed as mean ± SD and then compared by ANOVA Tukey’s test. Linear regression was analyzed by Pearson correlation coefficient. P < 0.05 is presented as statistical significance. Kaplan–Meier curve was used to analyze the association between RNA expressions and patients’ survival.
Results
The Expression of SNHG10 and miR-621 Was Significantly Altered in AML
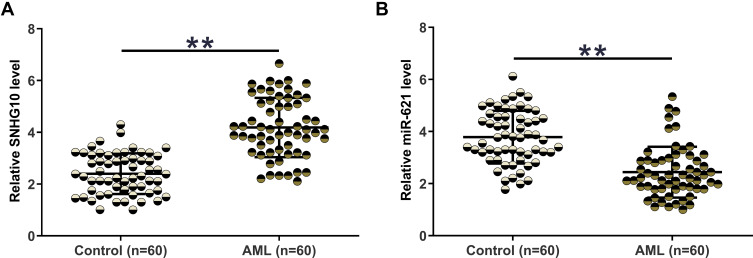
The expression of SNHG10 and miR-621 in BMMNCs from both AML patients (n = 60) and healthy controls (n = 60) was determined by RT-qPCR. The expression level of SNHG10 in the AML group was significantly higher than that in the control group (Figure 1A, p < 0.01). In contrast, compared with controls, AML patients exhibited a lower expression level of miR-621 (Figure 1B, p < 0.05).
Figure 1.
The expression of SNHG10 and miR-621 was significantly altered in AML. The expression of SNHG10 (A) and miR-621 (B) in BMMNCs from both AML patients (n = 60) and healthy controls (n = 60) was determined by RT-qPCR. The expression levels of SNHG10 and miR-621 in BMMNCs were expressed as average values of three technical replicates. **p < 0.01.
SNHG10 and miR-621 Were Inversely Correlated
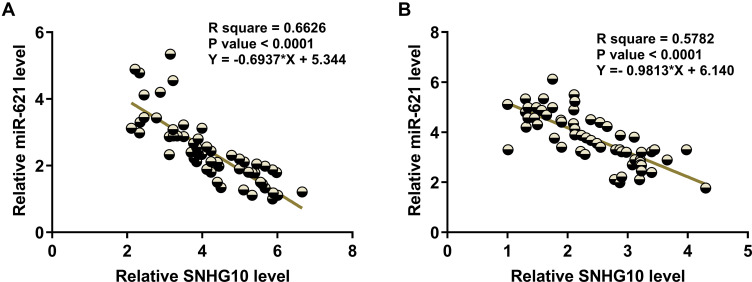
Linear regression analysis of the correlation between SNHG10 and miR-621 in BMMNC samples from AML patients (Figure 2A) and healthy controls (Figure 2B) showed that both SNHG10 and miR-621 were negatively correlated in AML patients and healthy controls.
Figure 2.
SNHG10 and miR-621 were inversely correlated. Correlations between SNHG10 and miR-621 across samples of BMMNCs from both AML patients (A) and healthy controls (B) were analyzed by linear regression. SNHG10 and miR-621 were inversely correlated with each other.
High Level of SNHG10 is Associated with Poor Survival of AML Patients
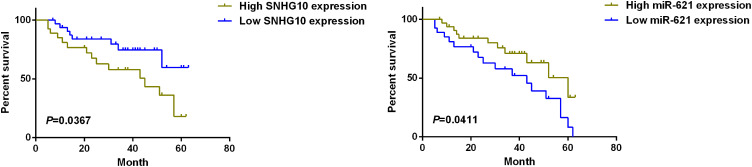
Long-term follow-up showed that the survival rate of the high SNHG10 expression group was significantly lower than that of the low SNHG10 expression group (Figure 3). In contrast, the survival rate of the low miR-621 expression group was significantly lower than that of the high miR-621 expression group (Figure 3).
Figure 3.
High level of SNHG10 is associated with poor survival of AML patients. Kaplan–Meier curve for the analysis of the association between the survival of AML patients and the expression level of SNHG10 or miR-621. Patients with high lncRNA SNHG10 expression or low miR-621 expression had poorer survival.
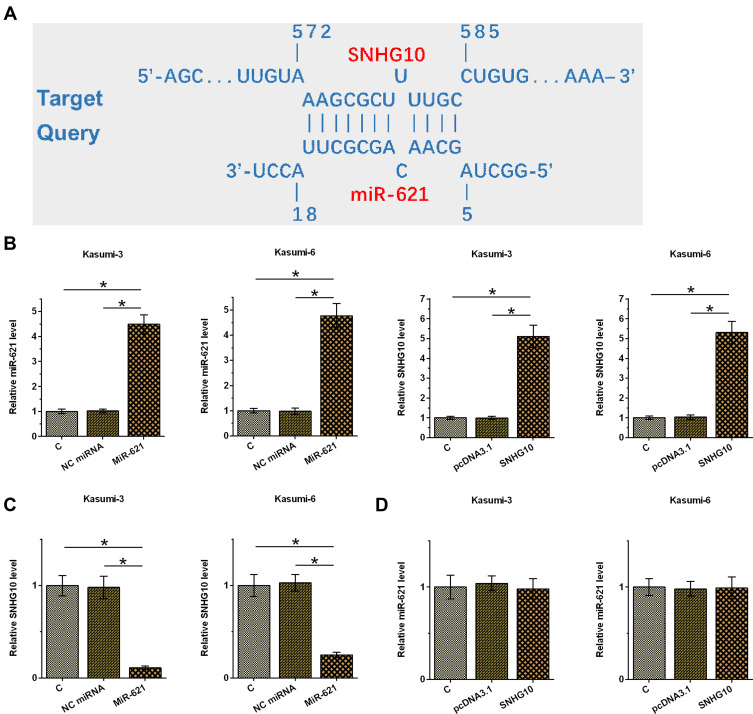
SNHG10 Targeted miR-621 in AML Cells to Downregulate Its Expression
IntaRNA 2.0 (http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp) was used for bioinformatics analysis and showed that miR-621 could be targeted by SNHG10 (Figure 4A). Kasumi-3 and Kasumi-6 cells were transfected with miR-621 mimic and pcDNA3.1-SNHG10, and then the transfections were determined and confirmed by RT-qPCR (Figure 4B, p < 0.05). Moreover, it was found that upregulation of miR-621 downregulated the expression of SNHG10 (Figure 4C, p < 0.05), while upregulation of SNHG10 failed to affect the expression of miR-621 (Figure 4D).
Figure 4.
SNHG10 targeted miR-621 to downregulate its expression in AML cells. Bioinformatics analysis performed using IntaRNA 2.0 (A). Kasumi-3 and Kasumi-6 cells were transfected with miR-621 mimic and pcDNA3.1-SNHG10, followed by the confirmation of transfections using RT-qPCR (B). The effect of miR-621 overexpression on SNHG10 (C), and the effect of SNHG10 overexpression on miR-621 (D) were also analyzed by RT-qPCR. Mean ± SD values were used to express data from 3 biological replicates. *p < 0.05.
Overexpression of SNHG10 Promoted miR-621 Methylation
MSP was used to detect the methylation of miR-621 induced by SNHG10 overexpression in both Kasumi-3 and Kasumi-6 cells. Elevating SNHG 10 significantly increases the methylation of the miR-621 gene (Supplementary Figure 1), which suggested the regulation of miR-621 by SNHG10 might be achieved through methylation of the miR-621 gene.
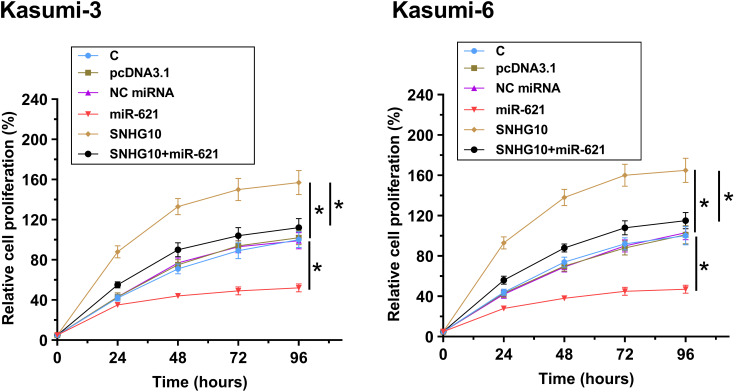
MiR-621 Targeted SNHG10 to Inhibit the Proliferation of AML Cells
CCK-8 assay was used to analyze the roles of miR-621 and SNHG10 in the proliferation of Kasumi-3 and Kasumi-6 cells. The results showed that miR-621 overexpression inhibited Kasumi-3 and Kasumi-6 cell proliferation, while SNHG10 overexpression promoted Kasumi-3 and Kasumi-6 cell proliferation. Moreover, the effects of SNHG10 overexpression on Kasumi-3 and Kasumi-6 cell proliferation could be abolished by miR-621 overexpression (Figure 5, p < 0.05).
Figure 5.
SNHG10 targeted miR-621 to inhibit the proliferation of AML cells. The effects of miR-621 and SNHG10 on regulating the proliferation of Kasumi-3 and Kasumi-6 cells were analyzed by CCK-8 assay. Mean ± SD values were used to express data from 3 biological replicates. *p < 0.05.
Discussion
In this study, we explored the interaction and the internal relationship between miR-621 and SNHG10 in AML. We found that SNHG10 was upregulated and miR-621 was downregulated in AML. In addition, SNHG10 might be targeted by miR-621 to suppress the proliferation of AML cells.
The function of SNHG10 has only been investigated in liver cancer,16 bladder cancer18 and osteosarcoma.17 In liver cancer, SNHG10 is overexpressed and its homolog SCARNA13 forms a positive feedback loop to promote the initiation and metastasis of tumor.16 In non-small cell lung cancer (NSCLC), SNHG10 has been found to be downregulated.22 Moreover, SNHG10 may downregulate miR-21 in NSCLC to suppress cancer cell proliferation. Thus, SNHG10 shows different functions in different cancers. In this study, we found that SNHG10 was upregulated in AML. SNHG10 overexpression increased the proliferation rate of AML cells. Therefore, SNHG10 may play an oncogene role in AML.
miR-621 has been reported to be a tumor suppressor in both breast cancer and liver cancer.19–21 In breast cancer, miR-621 was downregulated and its overexpression enhanced p53 activity and downregulated FBXO11 to increase the sensitivity of cancer cells to chemotherapy.21 miR-621 was also downregulated in liver cancer and targeted CAPRIN1 and SETDB1 to suppress cancer cell proliferation and increase the sensitivity of cancer cells to radiotherapy.19,20 However, based on our knowledge, the role of miR-621 in AML is still unclear. Our study reveals a decrease of miR-621 in AML and its inhibitory effects on cell cancer proliferation. Therefore, miR-621 is likely a tumor suppressor in AML. It is worth noting that SNHG10 overexpression could inhibit the expression of miR-621, but miR-621 overexpression failed to affect the expression of SNHG10. This might be related to the increase in the methylation of the miR-621 gene by SNHG10 overexpression.
Up to now, all previous studies focused on the interaction between miR-621 and protein-coding genes.19,20,22 Our study is the first to report that miR-621 can target an lncRNA to suppress cancer cell proliferation, which fulfilled our understandings of the interactions between different ncRNAs. In conclusion, SNHG10 is upregulated in AML, and miR621 is downregulated in AML. SNHG10 may be targeted by miR-621 to suppress AML cell proliferation.
Funding Statement
This work was supported by Qiankehe Project (Grant No. GZSYBS [2019] 03).
Ethics Approval
This study was approved by the Ethics Committee of Guizhou Provincial People’s Hospital and conformed to the provisions of the Declaration of Helsinki. IRB number of this study is IRB00000430.
Disclosure
Hongqian Zhu reports grants from Qiankehe Project during the conduct of the study. The authors report no other potential conflicts of interest in this work.
References
- 1.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–1907. doi: 10.1016/S0140-6736(06)69780-8 [DOI] [PubMed] [Google Scholar]
- 2.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson MP, Waters TM, Kaplan EK, McKillop CN, Martin MG. Hospital volume and acute myeloid leukemia mortality in Medicare beneficiaries aged 65 years and older. Blood. 2016;128(6):872–874. doi: 10.1182/blood-2016-05-716662 [DOI] [PubMed] [Google Scholar]
- 4.Bhatt VR, Shostrom V, Giri S, et al. Early mortality and overall survival of acute myeloid leukemia based on facility type. Am J Hematol. 2017;92(8):764–771. doi: 10.1002/ajh.24767 [DOI] [PubMed] [Google Scholar]
- 5.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas D, Majeti R. Biology and relevance of human acute myeloid leukemia stem cells. Blood. 2017;129(12):1577–1585. doi: 10.1182/blood-2016-10-696054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int J Nanomedicine. 2014;9:467–483. doi: 10.2147/IJN.S36654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sison EAR, McIntyre E, Magoon D, Brown P. Dynamic chemotherapy-induced upregulation of CXCR4 expression: a mechanism of therapeutic resistance in pediatric AML. Mol Cancer Res. 2013;11(9):1004. doi: 10.1158/1541-7786.MCR-13-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakshmikuttyamma A, Scott SA, DeCoteau JF, Geyer CR. Reexpression of epigenetically silenced AML tumor suppressor genes by SUV39H1 inhibition. Oncogene. 2010;29(4):576–588. doi: 10.1038/onc.2009.361 [DOI] [PubMed] [Google Scholar]
- 10.Shafer D, Grant S. Update on rational targeted therapy in AML. Blood Rev. 2016;30(4):275–283. doi: 10.1016/j.blre.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. doi: 10.1038/nrg3074 [DOI] [PubMed] [Google Scholar]
- 12.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NM, Zaitseva L, Bowles KM, MacEwan DJ, Rushworth SA. NRF2-driven miR-125B1 and miR-29B1 transcriptional regulation controls a novel anti-apoptotic miRNA regulatory network for AML survival. Cell Death Differ. 2015;22(4):654–664. doi: 10.1038/cdd.2014.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornick NI, Huan J, Doron B, et al. Serum exosome microRNA as a minimally-invasive early biomarker of AML. Sci Rep. 2015;5:11295. doi: 10.1038/srep11295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan T, Yuan K, Yan X, et al. LncRNA SNHG10 facilitates hepatocarcinogenesis and metastasis by modulating its homolog SCARNA13 via a positive feedback loop. Cancer Res. 2019;79(13):3220–3234. doi: 10.1158/0008-5472.CAN-18-4044 [DOI] [PubMed] [Google Scholar]
- 17.Entenmann W, Gränzer W, Müller E, Schmidt E. [A new telemetry system for measuring the pH value (author’s transl)]. Biomed Tech (Berl). 1980;25(9):220–223. doi: 10.1515/bmte.1980.25.9.220 [DOI] [PubMed] [Google Scholar]
- 18.Tian H, Wang X, Lu J, Tian W, Chen P. MicroRNA-621 inhibits cell proliferation and metastasis in bladder cancer by suppressing Wnt/β-catenin signaling. Chem Biol Interact. 2019;308:244–251. doi: 10.1016/j.cbi.2019.05.042 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, You W, Zhou H, et al. Downregulated miR-621 promotes cell proliferation via targeting CAPRIN1 in hepatocellular carcinoma. Am J Cancer Res. 2018;8(10):2116–2129. [PMC free article] [PubMed] [Google Scholar]
- 20.Shao Y, Song X, Jiang W, et al. MicroRNA-621 acts as a tumor radiosensitizer by directly targeting SETDB1 in hepatocellular carcinoma. Mol Ther. 2019;27(2):355–364. doi: 10.1016/j.ymthe.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue J, Chi Y, Chen Y, et al. miRNA-621 sensitizes breast cancer to chemotherapy by suppressing FBXO11 and enhancing p53 activity. Oncogene. 2016;35(4):448–458. doi: 10.1038/onc.2015.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang M, Wang L, Cao C, Song S, Wu F. LncRNA SNHG10 is downregulated in non-small cell lung cancer and predicts poor survival. 2020. [DOI] [PMC free article] [PubMed]