Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disorder in western countries and an increasing cause of end-stage liver disease and hepatocellular carcinoma. NAFLD is known to coexist in patients with inflammatory bowel disease (IBD). This study aims to examine the prevalence of NAFLD, as well as trends in NAFLD-associated fibrosis, in a well-characterized IBD cohort utilizing a validated noninvasive test.
Methods
We conducted a single-center retrospective chart review of patients at a large academic IBD center between 2007 and 2017. Patients with IBD and concurrent hepatic steatosis were identified. Charts were reviewed for baseline characteristics and laboratory data in order to calculate and trend NAFLD progression over time by a noninvasive marker, the NAFLD fibrosis score (NFS).
Results
Of 207 patients with IBD and concurrent NAFLD, NFS was able to be calculated for 138 patients at index diagnosis. A subsequent NFS was able to be calculated at 5-year follow-up for 56 patients. Over 5 years, 9 patients (16%) had worsening in NFS category, 4 patients (7%) had improvement in NFS category, and the remaining 43 patients (77%) stayed within their index NFS category.
Conclusions
IBD patients with NAFLD tend to have stable liver disease over 4–6 years, and the risk of liver disease progression is low. This is the first study to document the progression of NAFLD by noninvasive testing over time.
Keywords: Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis, Nonalcoholic fatty liver disease, NAFLD fibrosis score
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease in western countries and a leading cause of end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) [1]. The coexistence of NAFLD in patients with inflammatory bowel disease (IBD) has been described in published case reports as early as 1873 when CH Thomas described a patient with ulcerative colitis (UC) and hepatomegaly with steatosis [2]. Today, the prevalence of NAFLD in the US population has been estimated to be between 10 and 40% [3–5]. A meta-analysis investigating 19 existing studies, which included 5620 subjects, reported an overall pooled prevalence of NAFLD of 27.5% in patients with IBD [6].
IBD patients with concomitant NAFLD present a unique challenge. In the general population, overall mortality is not significantly increased in the presence of isolated fatty liver based on data from the National Health and Nutrition Examination survey (NHANES) [7]. This is not the case in IBD. Patients with IBD experience higher mortality from NAFLD, with standardized mortality ratio of 2.26 and 2.82 in patients with UC and CD, respectively, when compared to the general population [8].
The pathogenesis of NAFLD development and progression to nonalcoholic steatohepatitis (NASH) in IBD is poorly understood. The risk factors for NAFLD in the general population, including obesity, hypertension, and insulin resistance, are also known to contribute to the risk of NAFLD development in patients with IBD. Some groups have suggested that weight gain secondary to disease control by biologic therapies could contribute to NAFLD development [9]. It has also been theorized that biologic medications such as anti-tumor necrosis factor α (TNF-α) agents or frequent steroid use may have their own independent effect on NAFLD [10], though studies show no significant association between the use of IBD therapeutics and the risk of developing NAFLD [11]. In addition, the chronic inflammatory state in IBD is known to induce release of cytokines and adipokines which may lead to increased hepatic inflammation and fibrosis [12]. However, existing literature suggests that symptom severity of IBD by validated scoring systems such as the Harvey–Bradshaw index (HBI) does not correlate with the severity of concurrent NAFLD as defined by the validated NAFLD fibrosis score (NFS). This is shown to be true in both patients with CD and UC [13].
This study aims to examine the prevalence of NAFLD, as well as trends in NAFLD-associated fibrosis, in a well-characterized IBD cohort utilizing a validated noninvasive test.
Methods
We conducted a single-center retrospective chart review of patients seen at our IBD center between 2007 and 2017 to identify patients with IBD and concurrent hepatic steatosis.
NAFLD was defined as the presence of hepatic steatosis without concomitant heavy alcohol use, viral hepatitis, or evidence of other primary liver disease. In this study, hepatic steatosis was determined retrospectively on the basis of either an imaging study reporting fatty infiltration of the liver or biopsy results which documented hepatic steatosis. Heavy alcohol use was defined as consumption of greater than 14 drinks per week in men and greater than 7 drinks per week in women by self-report. Race was classified into Caucasian, African-American, Asian, and other.
Degree of hepatic fibrosis was estimated using the NAFLD fibrosis score (NFS) as a noninvasive marker for fibrosis assessment. The NFS is a validated scoring system that has been shown to accurately stratify patients with NAFLD into those with and without advanced fibrosis [14]. Variables included in this scoring system are age (years), body mass index (kg/m2), impaired fasting glucose/diabetes (yes or no), platelet count (× 103/μL), albumin (g/dL), and AST (µL)/ALT (µL) ratio. These variables are used as independent indicators of advanced liver fibrosis to create a numeric score which correlates to a hepatic disease severity category. Although liver biopsy remains the gold standard for the evaluation of fibrosis, it is invasive and not without major risks. Imaging modalities such as transient elastography (FibroScan) and magnetic resonance elastography are noninvasive, well-validated fibrosis assessment methods that are not readily available. However, NFS was chosen for this study as it can be calculated from patient characteristics and laboratory values which are frequently obtained at the time of office visit, and may be monitored serially. In this study, NFS was calculated twice, at the first evidence of NAFLD, considered the “index image” or “index biopsy” and 5 years later. Patients were then divided into score categories with correlated fibrosis severity. NFS < – 1.455 indicates a scoring category of F0–F2 or “no fibrosis, mild fibrosis, or moderate fibrosis.” NFS between −1.455 and 0.675 indicates a scoring category of “indeterminant disease.” Lastly, NFS > 0.675 indicates a scoring category of F3–F4 or “advanced fibrosis to cirrhosis.” The scoring system provides negative predictive value between 88 and 93%, and a positive predictive value between 82 and 90% [14].
All statistical analyses were conducted using SAS version 9.4 for Windows (SAS Institute, Inc, Cary, NC). Chi-square and Fisher’s exact tests were used for bivariate analyses to evaluate relationships between BMI, type of IBD, and medication with NAFLD.
Results
Between 2007 and 2017, 1672 unique patients with IBD presented to our center to initiate care. Of those patients, 236 were found to have hepatic steatosis by imaging (n = 233) or biopsy (n = 3). Exclusions were made for patients with hepatitis B or C, HIV, heavy alcohol use, existing or known methotrexate related liver injury, sarcoidosis of the liver, and primary sclerosing cholangitis. Subsequently, 207 patients with NAFLD were included in further examination (Table 1, Fig. 1).
Table 1.
Patient characteristics in all IBD patients and those with NAFLD at index and at 5-year follow-up
| Demographics | All IBD patients (n = 1672) | IBD patients with NAFLD (n = 207) | IBD patients with NAFLD and index NFS (n = 138) | IBD patients with NAFLD and 5-year follow-up (n = 56) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 752 (45) | 96 (46) | 68 (49) | 25 (45) |
| Female | 920 (55) | 111 (54) | 70 (51) | 31 (55) |
| Mean age (y ± SD) | 48 ± 15.6 | 50.6 ± 13.6 | 49.6 ± 13.3 | 52.1 ± 12.4 |
| BMI (mean ± SD), n (%) | 25.9 ± 6.8 | 29.5 ± 8 | 29.7 ± 7.8 | 30.8 ± 8.3 |
| Underweight < 18.5 | 54 (3) | 12 (6) | 7 (5) | 2 (3) |
| Normal 18.5–24.9 | 420 (25) | 42 (20) | 33 (24) | 11 (20) |
| Overweight 25–29.9 | 245 (15) | 42 (20) | 32 (23) | 19 (34) |
| Obese > 30 | 213 (13) | 79 (38) | 66 (48) | 24 (43) |
| Blank | 740 (44) | 32 (16) | 0 (0) | 0 (0) |
| Race, n (%) | ||||
| Caucasian | 1325 (79) | 162 (78) | 105 (76) | 42 (75) |
| African-American | 260 (16) | 35 (17) | 26 (19) | 13 (23) |
| Asian | 51 (3) | 3 (2) | 2 (1) | 1 (2) |
| Other | 36 (2) | 7 (3) | 5 (4) | 0 (0) |
| IBD type, n (%) | ||||
| Crohn’s disease | 897 (55) | 131 (63) | 86 (62) | 39 (70) |
| Ulcerative colitis | 453 (27) | 39 (19) | 24 (17) | 10 (18) |
| Undetermined type | 67 (4) | 9 (4) | 5 (4) | 0 (0) |
| Microscopic | 30 (1) | 3 (1) | 0 (0) | 0 (0) |
| Eosinophilic | 1 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unclassified | 224 (13) | 26 (13) | 23 (17) | 7 (12) |
| Medication history, n (%) | ||||
| 5-ASA use | 334 (20) | 36 (17) | 23 (17) | 10 (18) |
| Immunosuppressant use | 790 (47) | 106 (51) | 65 (47) | 35 (63) |
| Biologic use | 745 (45) | 116 (56) | 75 (54) | 37 (66) |
Fig. 1.
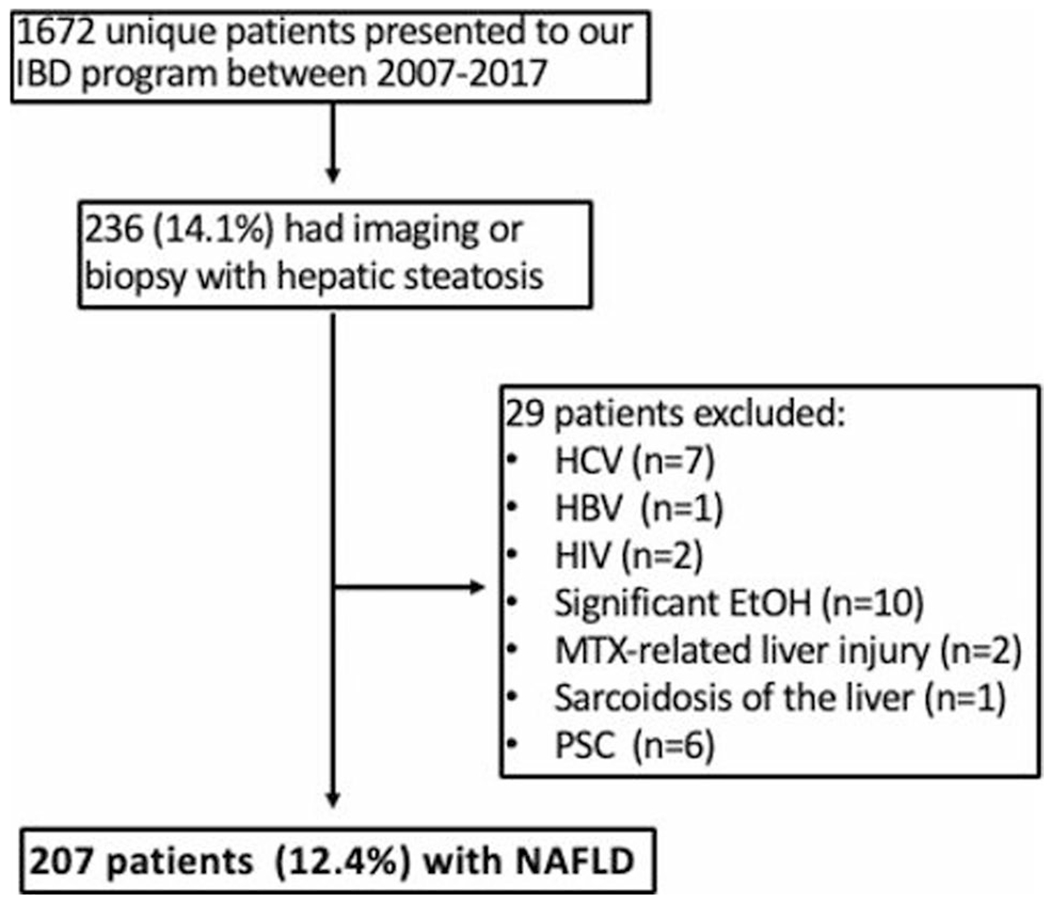
NAFLD inclusion criteria for IBD population. Nonalcoholic fatty liver disease (NAFLD), inflammatory bowel disease (IBD), hepatitis c virus (HCV), hepatitis b virus (HBV), human immunodeficiency virus (HIV), alcohol (EtOH), methotrexate (MTX), primary sclerosing cholangitis (PSC)
Of the 207 patients with NAFLD, 111 (54%) were female, mean age was 50.6 ± 13.6 years, and mean IBD disease duration was 20.5 ± 11.8 years. Mean BMI (29.5 ± 8 kg/m2, “overweight”) was associated with higher rates of NAFLD (p < 0.01). NAFLD rates were significantly higher in patients with CD when compared to patients with UC (p = 0.02). There was no association found between NAFLD and use of aminosalicylates, immunosuppressants, or biologics (p = 0.34, p = 0.49, p = 0.13). One hundred sixty-two (78%) of the patients were Caucasian, 35 (17%) were African-American, 3 (2%) were Asian, and 10 (5%) were other races (Table 1).
Index NFS was able to be calculated for 138 (66%) patients based on laboratory results within ± 1 year of index image or index biopsy. Of the 138 patients, the majority (78%) had index imaging with CT, while the rest were diagnosed by ultrasound, MRI, or liver biopsy. Mean BMI at index NFS calculation was 30.1 ± 8.0 kg/m2. Ninety patients (65%) had NFS < – 1.455 (no fibrosis, mild fibrosis, or moderate fibrosis), 42 (31%) had NFS between – 1.455 and 0.675 (indeterminant disease), and 6 (4%) had NFS > 0.675 (advanced fibrosis or cirrhosis). Fifty-six patients had sufficient laboratory data to calculate NFS in a 5 ± 1-year follow-up. Compared to the index group of IBD patients with NAFLD for which an NFS was calculated (n = 138), older age was found to be statistically significant (p < 0.05) in the 5-year follow-up group (n = 56). BMI, disease type (CD vs. UC), and gender showed no statistically significant difference. Of these 56 patients, 9 (16%) had worsening in NFS category, 4 (7%) had improvement in NFS category, and the remaining 43 (77%) stayed within their index NFS category (Table 2, Fig. 2). Average BMI at the 5-year time point was 31.5 ± 7.9 kg/m2. Of the 9 patients (16%) that had worsening in NFS category at 5 years, 3 (33%) were female, and 9 (100%) were Caucasian. Six (66%) patients had hypertension, and 3 (33%) had both hypertension and diabetes. The average change in BMI of this group of 9 patients was + 1.3 kg/m2.
Table 2.
NAFLD fibrosis score categories at index and at 5-year follow-up
| NFS category | Index (n = 138), n (%) | 5 years (n = 56), n (%) |
|---|---|---|
| F0–F2 | 90 (65) | 32 (57) |
| Indeterminant | 42 (31) | 19 (34) |
| F3–F4 | 6 (4) | 5 (9) |
NAFLD nonalcoholic fatty liver disease, NFS nonalcoholic fatty liver disease fibrosis score, F0–F2 category: no fibrosis, mild fibrosis, or moderate fibrosis, F3–F4 advanced fibrosis to cirrhosis
Fig. 2.
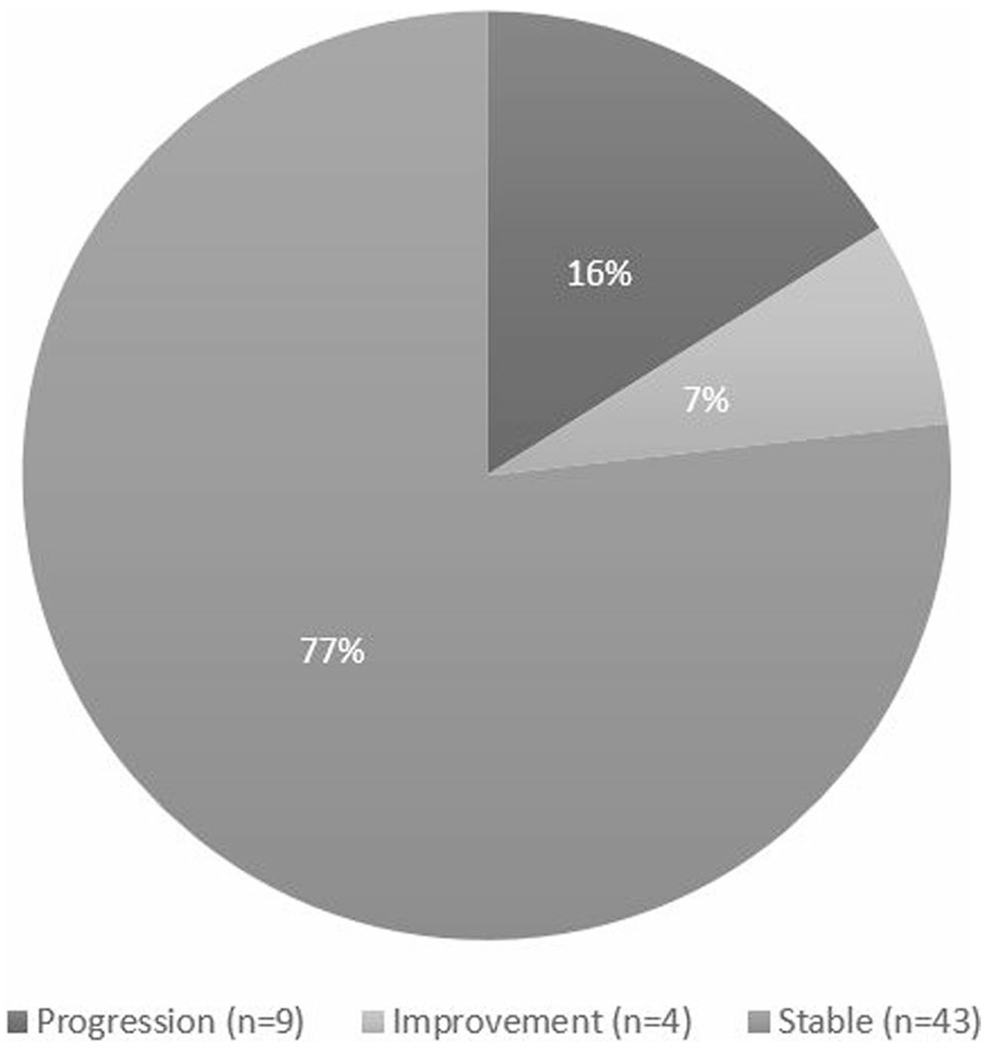
NFS change at 5 years: 56 patients had sufficient laboratory data to calculate NFS initially and at 5-year follow-up. The majority of IBD patients had stable NAFLD by NFS
Medication exposure was reviewed retrospectively for the 56 patients with index NFS and 5-year NFS (Fig. 3). Of these 56 patients, ever-exposure to biologic therapy, methotrexate therapy, and thiopurine therapy was identified. In patients ever exposed to biologic therapy, 39 (80%) had stable NFS at 5 years, 5 (10%) had progression of NFS at 5 years, and 5 (10%) had improvement in NFS at 5 years. All patients have had exposure to systemic corticosteroid. In patients ever exposed to methotrexate, 13 (76%) had stable NFS at 5 years, 3 (18%) had progression of NFS at 5 years, and 1 (6%) had improvement in NFS at 5 years. In patients ever exposed to thiopurine therapy, 25 (73%) had stable NFS at 5 years, 5 (15%) had progression of NFS at 5 years, and 4 (12%) had improvement in NFS at 5 years. In patients without any of the drug exposures, 4 (67%) had stable NFS at 5 years and 2 (33%) had progression of NFS at 5 years.
Fig. 3.
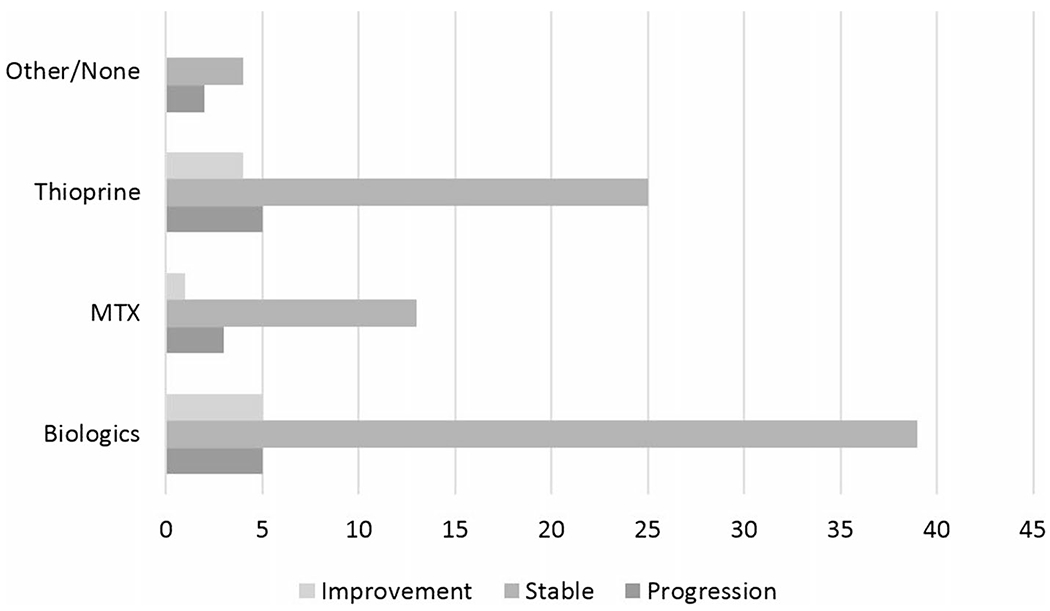
NFS change at 5 years by medication exposure: of the 56 patients with sufficient laboratory data to calculate NFS initially and at 5-year follow-up, ever-exposure to biologic therapy, methotrexate therapy, and thiopurine therapy was identified. There was no apparent effect on NFS according to medication exposure
Discussion
This is the first study to our knowledge that documents progression of NAFLD over time in patients with IBD using NFS, while also confirming previously described prevalence. In this large sample size of patients with IBD, prevalence of NAFLD was found to be 12.4%. Most patients did not have advanced fibrosis or cirrhosis at the time of index diagnosis of NAFLD. Only 4% had advanced disease by NFS criteria at this time point. Most patients remained in their index NFS category at 5-year follow-up.
The prevalence rate of NAFLD in this IBD cohort seen at our institution was 12% which is consistent with rates previously reported in the literature [3–6]. Additionally, using NFS, we have demonstrated the majority of patients in this cohort did not have progression of fibrosis regardless of type of IBD therapy, as shown in prior reports [11, 12]. It has been described in the previous literature that the degree of steatohepatitis and fibrosis can wax and wane overtime. While our study demonstrates overall stability of disease between index and 5 years by NFS, a small cohort of patients are appreciated to have changes in disease despite the short follow-up, representing this known pattern of disease fluctuation [15].
Our study had several limitations. Sufficient laboratory data to calculate NFS were only available for two-thirds of the 207 patients with NAFLD due to the retrospective design of the study. Data for all confounding risk factors of NAFLD including smoking and metabolic syndrome could not be obtained; however, BMI and diabetes were factors used in calculation of NFS. Patients without imaging or biopsy findings to evaluate for steatosis were assumed not to have hepatic steatosis, which may not be an accurate assumption. Additionally, precise time course of medication exposures was not able to be obtained upon chart review. While many patients with IBD do utilize a variety of therapies concurrently and independently, medication exposure durations were not clearly documented for the purposes of retrospective data collection. NFS itself poses a challenge as it is best at identifying patients at the extremes of liver disease. However, one-third of our cohort fell in the “indeterminant” range, making their degree of liver disease unclear in the absence of a secondary method of fibrosis assessment. Additionally, the interval between index NFS and 5-year followup is in fact a range that included plus or minus 1 year, and thus is a limitation to the progression analysis. An important strength of our study is the large sample size population of IBD patients treated at an academic institution with generally more complex phenotypes warranting more intensive IBD therapy. While it is understood that the true population of interest is a smaller cohort of 56 patients, the large starting population of IBD patients, approximately 1600, allows this study to confirm prevalence of NAFLD in an IBD cohort. Longitudinal 5-year assessments add to this study’s strength, as prior investigations were cross-sectional in nature. Utilizing the NFS, which is a validated, clinically relevant tool, also enhances the applicability of our study.
Conclusions
IBD patients with NAFLD tend to have stable liver disease over 4–6 years, and the risk of liver fibrosis progression is low. Hepatic fibrosis progression seems to be independent of drug class used to treat IBD in our study population, consistent with the existing literature. Further research is needed to confirm the findings of this study, and to understand patient phenotypes with IBD that are at higher risk of developing hepatic fibrosis and advanced liver disease.
Acknowledgments
Funding Dr. Gabrielle Ritaccio, Dr. Gianna Stoleru, Dr. Ameer Abutaleb, Dr. Sasan Sakiani, and Dr. Uni Wong have no financial disclosure. Dr. Raymond K. Cross has participated in advisory boards for Abbvie, Janssen, Pfizer, Samsung Bioepis, and Takeda and engaged in consulting for LabCorp. He is also on a Data Safety Monitoring Board for Gilead. Dr. Kirti Shetty has research grant support from Enanta.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical considerations This study was deemed by the Institutional Review Board at University of Maryland as Non-human Subjects Research.
References
- 1.Reeves HL, Zaki MY, Day CP. Hepatocellular carcinoma in obesity, type 2 diabetes, and NAFLD. Dig Dis Sci. 2016;61:1234–1245. [DOI] [PubMed] [Google Scholar]
- 2.Thomas C Ulceration of the colon with a much enlarged fatty liver. Trans Pathol Soc Philos. 1873;4:87–88. [Google Scholar]
- 3.Riegler G, D’Inca R, Sturniolo GC, et al. Hepatobiliary alterations in patients with inflammatory bowel disease: a multicenter study. Caprilli & Gruppo Italiano Studio Colon-Retto. Scand J Gastroenterol. 1998;33:93–98. [DOI] [PubMed] [Google Scholar]
- 4.de Fazio C, Torgano G, de Franchis R, et al. Detection of liver involvement in inflammatory bowel disease by abdominal ultrasound scan. Int J Clin Lab Res. 1992;21:314–317. [DOI] [PubMed] [Google Scholar]
- 5.Bargiggia S, Maconi G, Elli M, et al. Sonographic prevalence of liver steatosis and biliary tract stones in patients with inflammatory bowel disease: study of 511 subjects at a single center. J Clin Gastroenterol. 2003;36:417–420. [DOI] [PubMed] [Google Scholar]
- 6.Zou ZY, Shen B, Fan JG. Systematic review with metaanalysis: epidemiology of nonalcoholic fatty liver disease in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:1764–1772. [DOI] [PubMed] [Google Scholar]
- 7.Le MH, Devaki P, Ha NB, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;12:e0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bewtra M, Kaiser LM, Tenhave T, et al. Crohn’s disease and ulcerative colitis are associated with elevated standardized mortality ratios. Inflamm Bowel Dis. 2013;19:599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas L, Chevalier R, Major BT, et al. Biologic agents are associated with excessive weight gain in children with inflammatory bowel disease. Dig Dis Sci. 2017;62:3110–3116. [DOI] [PubMed] [Google Scholar]
- 10.Lopetuso LR, Mocci G, Marzo M, et al. Harmful effects and potential benefits of anti-tumor necrosis factor (TNF)-α on the liver. Int J Mol Sci. 2018;19:2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapumnuaypol K, Kanjanahattakij N, Pisarcik D, et al. Effects of inflammatory bowel disease treatment on the risk of non-alcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2018;30:854–860. [DOI] [PubMed] [Google Scholar]
- 12.Carr RM, Oranu A, Khungar V. Nonalcoholic fatty liver disease: pathophysiology and management. Gastroenterol Clin North Am. 2016;45:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr RM, Patel A, Bownik H, et al. Intestinal inflammation does not predict nonalcoholic fatty liver disease severity in inflammatory bowel disease patients. Dig Dis Sci. 2017;62:1354–1361. [DOI] [PubMed] [Google Scholar]
- 14.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45: 846–854. [DOI] [PubMed] [Google Scholar]
- 15.Rinella ME, Tacke F, Sanyal AJ, Anstee QM, participants of the AASLD/EASL Workshop. Report on the AASLD/EASL Joint Workshop on Clinical Trial Endpoints in NAFLD. Hepatology. 2019;70:1424–1436. [DOI] [PubMed] [Google Scholar]


