Abstract
Abstract Objective
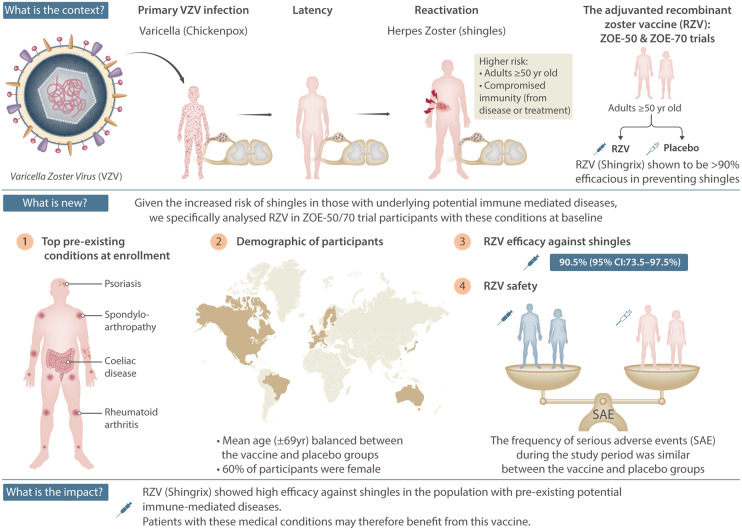
In the ZOE-50 (NCT01165177) and ZOE-70 (NCT01165229) phase 3 clinical trials, the adjuvanted recombinant zoster vaccine (RZV) demonstrated ≥90% efficacy in preventing herpes zoster (HZ) in all age groups ≥50 years. Given the increased HZ risk associated with certain underlying autoimmune diseases or their treatment regimes, we conducted a post hoc analysis of RZV’s efficacy against HZ and safety profile [specifically, the occurrence of serious adverse events (SAEs)] in ZOE-50/70 participants who reported pre-existing potential immune-mediated diseases (pIMDs) at enrolment and were not on immunosuppressive therapies.
Methods
Adults aged ≥50 (ZOE-50) and ≥70 (ZOE-70) years were randomized to receive two doses of RZV or placebo 2 months apart. In this subgroup analysis of participants with at least one pIMD at enrolment, the efficacy was calculated for two-dose recipients who did not develop confirmed HZ before 30 days post-dose 2. SAE occurrence was evaluated for all participants who received at least one dose.
Results
Of the 14 645 RZV and 14 660 placebo recipients from the ZOE-50/70 studies, 983 and 960, respectively, reported at least one pre-existing pIMD at enrolment and were included in these analyses. The most frequent pre-existing conditions were psoriasis, spondyloarthropathy and RA. Efficacy against HZ was 90.5% (95% CI: 73.5, 97.5%) overall with the lowest being 84.4% (95% CI: 30.8, 98.3%) in the 70–79-year-old age group. SAEs and fatal SAEs were similar between RZV and placebo recipients.
Conclusion
In ZOE-50/70 participants with pre-existing pIMDs, RZV was highly efficacious against HZ and SAE incidence was similar between RZV and placebo recipients.
Trial registration
ClinicalTrials.gov, https://clinicaltrials.gov, NCT01165177 (ZOE-50), NCT01165229 (ZOE-70).
Keywords: varicella-zoster virus, potential immune-mediated diseases, rheumatoid arthritis, psoriasis, spondyloarthropathy, vaccines
Graphical Abstract
Rheumatology key messages
The adjuvanted recombinant zoster vaccine is 90.5% efficacious in individuals with ≥1 potential immune-mediated disease.
Serious adverse event incidence was similar between vaccine and placebo recipients with potential immune- mediated diseases.
People suffering from immune-mediated diseases may benefit from vaccination with RZV.
Introduction
Primary infection with varicella-zoster virus typically in childhood causes varicella, or chickenpox. Varicella-zoster virus establishes lifelong latency and upon its reactivation may cause herpes zoster (HZ), a typically painful, dermatomal rash, which most often occurs in older adults [1, 2]. The most common complication of HZ is postherpetic neuralgia, consisting of neuropathic pain that may persist many months after the rash resolves [3]. An adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK) was highly efficacious against both HZ and postherpetic neuralgia in two pivotal phase 3 randomized controlled trials (ZOE-50 and ZOE-70) [4, 5], and is currently licensed in several regions worldwide for use in adults aged ≥50 years.
Individuals with underlying autoimmune diseases such as RA and systemic lupus erythematosus are known to be at increased risk for developing HZ and postherpetic neuralgia [2, 6, 7]. In addition to the underlying autoimmune disease, treatment modalities for these conditions also confer an increased risk of infections and complications, including HZ. For example, corticosteroids and Janus kinase inhibitors, used in the treatment of RA and other autoimmune conditions [8, 9], are known to contribute significantly to the increased HZ incidence in this population [10–18]. Thus, individuals with autoimmune diseases may benefit from vaccination against HZ.
In the ZOE-50/70 studies, adults aged ≥50/70 years were eligible for participation if they were not considered immunocompromised by disease or treatment at enrolment and fulfilled all other inclusion criteria. The inclusion/exclusion criteria allowed for enrolment of a broad population aged ≥50 years, including individuals with potential immune-mediated diseases (pIMDs) [4, 5], which included autoimmune diseases and other inflammatory and/or neurological disorders of interest that may or may not have an autoimmune aetiology [19]. A total of 29 305 adults aged ≥50 years received at least one dose of RZV or placebo and were included in the pooled safety analyses. Pre-existing pIMDs at enrolment were reported by 983 (6.7%) RZV and 960 (6.5%) placebo recipients. In this subset of participants, most [940 (95.6%) in the RZV and 912 (95.0%) in the placebo group] did not experience a possible exacerbation of a pre-existing disease or a new onset of a different pIMD during the study period [20].
Here we present a post hoc analysis performed to evaluate RZV efficacy in preventing HZ and occurrence of serious adverse events (SAEs) in the pooled ZOE-50/70 population with pre-existing pIMDs.
Methods
The ZOE-50 and ZOE-70 trials (ZOE-50/70) were large phase 3 clinical trials evaluating the efficacy and safety of RZV, conducted in parallel at the same centres in Asia, Australia, Europe, North America and Latin America in adults aged ≥50 and ≥70 years, respectively. The studies were designed to allow analyses of pooled vaccine efficacy and safety data. Protocol summaries are available at http://www.gsk-clinicalstudyregister.com (study IDs 110390 and 113077). Anonymized individual participant data and study documents can be requested for further research at www.clinicalstudydatarequest.com (study IDs 110390 and 113077).
Individuals aged ≥50 years were eligible for participation unless they had a history of HZ, were previously vaccinated against varicella or HZ, or were considered immunocompromised by underlying disease or immunosuppressive treatments. More specifically, individuals who had received immunosuppressants or immune-modifying drugs for >15 consecutive days within 6 months prior to the first vaccine dose were excluded from participation in the ZOE-50/70 studies. Prednisone <20 mg/day, or equivalent, and inhaled/topical steroid use was allowed during the study. Detailed inclusion and exclusion criteria have been described elsewhere [4, 5]. As prednisone/prednisolone is the mainstay treatment for polymyalgia rheumatica [21], the average daily dose was calculated for ZOE-50/70 participants with polymyalgia rheumatica who received prednisone/prednisolone.
At enrolment, medical conditions for each ZOE-50/70 participant were retrieved from individual medical records and/or by interview, and were recorded in participants’ medical history. Participants with at least one pIMD at enrolment were identified by searching in the clinical database with a customized Medical Dictionary for Regulatory Activities (MedDRA) query for pIMDs reported in their medical history [19]. Medical conditions that were to be identified as pIMDs by this query are provided in Supplementary Table S1, available at Rheumatology online.
ZOE-50/70 participants were randomized 1:1 to receive two doses of either RZV or placebo 2 months apart and followed up for safety and the occurrence of HZ. Suspected HZ cases were confirmed by either polymerase chain reaction or unanimous agreement among the five members of a HZ ascertainment committee based on available clinical information [4, 5]. Vaccine efficacy in preventing HZ was defined as 1 minus the ratio of HZ incidences (RZV over Placebo), multiplied by 100.
The analysis of vaccine efficacy in the subset of pooled ZOE-50/70 participants with pre-existing pIMDs at enrolment [overall and by age group (50–59, 60–69, 70–79 and ≥80 years)] was performed in the modified total vaccinated cohort, which included all participants who received both doses of study vaccine and did not develop a confirmed HZ episode before 30 days post-dose 2. This allowed participants to achieve full protection resulting from a two-dose schedule. For the efficacy assessment, only the first confirmed HZ episode in a participant was considered, and the follow-up period for the efficacy assessment ceased at the time of the first occurrence. Safety was assessed in the TVC, which included participants who received at least one dose: SAEs were recorded from RZV or placebo dose 1 through 12 months post-last dose, and fatal SAEs were recorded during the entire study period (median follow-up duration of 4.4 years in the TVC [20]).
All statistical analyses were performed using the SAS Drug Development software package (SAS Institute Inc., Cary, NC, USA).
Results
Study population results
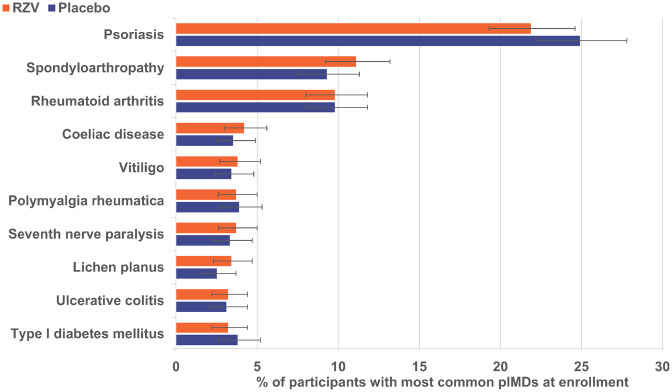
From 14 645 RZV and 14 660 placebo recipients included in the pooled TVC of the ZOE-50/70 studies, 983 and 960, respectively, reported at least one pIMD at enrolment [20]. The mean age of participants having at least one pIMD at enrolment was 68.8 and 69.4 years in the RZV and placebo groups, respectively, and more than half (RZV: 59.9%, Placebo: 60.8%) were female (Table 1). The most frequent pre-existing pIMDs reported at enrolment were psoriasis, spondyloarthropathy, RA and coeliac disease; their prevalence was balanced between study groups (Fig. 1 and Supplementary Table S2, available at Rheumatology online). For the period ranging from 6 months before first study vaccine administration up to study end, prednisone/prednisolone use was reported by 27 out of 36 (75.0%) and 23 out of 37 (62.2%) polymyalgia rheumatica patients from the RZV and placebo groups, with average doses of 13 and 9 mg/day, respectively.
Table 1.
Demographic characteristics of pooled ZOE-50/70 participants with pre-existing pIMDs (TVC)
| Characteristic | RZV (n = 983) | Placebo (n = 960) |
|---|---|---|
| Age at first dose, mean (s.d.), years | 68.8 (9.6) | 69.4 (9.5) |
| Age category, n (%) | ||
| 50–59 years | 230 (23.4) | 208 (21.7) |
| 60–69 years | 165 (16.8) | 156 (16.3) |
| 70–79 years | 450 (45.8) | 468 (48.7) |
| ≥80 years | 138 (14.0) | 128 (13.3) |
| Sex, n (%) | ||
| Female | 589 (59.9) | 584 (60.8) |
| Male | 394 (40.1) | 376 (39.2) |
| Geographic ancestry, n (%) | ||
| White – Caucasian/European | 830 (84.4) | 829 (86.4) |
| Asian – East Asian | 64 (6.5) | 50 (5.2) |
| Asian – Japanese | 30 (3.1) | 27 (2.8) |
| African/African American | 11 (1.1) | 7 (0.7) |
| White – Arabic/North African | 9 (0.9) | 6 (0.6) |
| Other | 39 (4.0) | 41 (4.3) |
n: number of participants included in each group; n (%): number (percentage) of participants in a category; pIMD: potential immune-mediated disease; Placebo: participants receiving placebo; RZV: participants receiving the adjuvanted recombinant zoster vaccine; TVC: total vaccinated cohort; ZOE-50/70: RZV efficacy studies in adults ≥50 and ≥70 years of age, respectively.
Fig. 1.
Ten most common pIMDs in both groups at enrolment (TVC)
Participants with pre-existing pIMDs were identified by querying the global medical history of the participants included in TVC with a customized Medical Dictionary for Regulatory Activities query for pIMDs [19]. Error bars represent 95% CI. pIMD: potential immune-mediated disease; Placebo: participants receiving placebo; RZV: participants receiving the adjuvanted recombinant zoster vaccine; TVC: total vaccinated cohort.
Efficacy in ZOE-50/70 participants with at least one pIMD at enrolment
Vaccine efficacy against HZ in pooled ZOE-50/70 participants with at least one pIMD at enrolment was 90.5% (95% CI: 73.5, 97.5%). Point estimates for efficacy were ≥84.4% across age groups (Table 2).
Table 2.
Vaccine efficacy against HZ in pooled ZOE 50/70 participants with pre-existing pIMDs (mTVC)
| RZV |
Placebo |
Vaccine efficacyb, % (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | No. of confirmed HZ cases | Sum of follow-up yearsa | Incidence per 1000 person-years | n | No. of confirmed HZ cases | Sum of follow-up yearsa | Incidence per 1000 person-years | ||
| Overall | 936 | 4 | 3611.7 | 1.1 | 923 | 38 | 3408.8 | 11.1 | 90.5 (73.5, 97.5) |
| 50–59 YOA | 222 | 1 | 885.6 | 1.1 | 201 | 11 | 775.6 | 14.2 | 92.8 (50.5, 99.8) |
| 60–69 YOA | 159 | 0 | 638.3 | 0.0 | 151 | 8 | 588.8 | 13.6 | 100 (54.9, 100) |
| 70–79 YOA | 427 | 2 | 1623.0 | 1.2 | 450 | 13 | 1647.3 | 7.9 | 84.4 (30.8, 98.3) |
| ≥80 YOA | 128 | 1 | 464.8 | 2.2 | 121 | 6 | 397.0 | 15.1 | 86.2 (-13.5, 99.7) |
Vaccine efficacy was estimated against first or only HZ episode occurring during the entire study period. All efficacy estimates were adjusted by region; overall efficacy estimates were also adjusted by age strata.
The follow-up period ceased when the first confirmed HZ episode occurred.
Calculated with the Poisson method. HZ: herpes zoster; mTVC: modified total vaccinated cohort; n: number of participants included in each group; pIMD: potential immune-mediated disease; Placebo: participants receiving placebo; RZV: participants receiving the adjuvanted recombinant zoster vaccine; YOA: years of age; ZOE-50/70: RZV efficacy studies in adults ≥50 YOA and ≥70 YOA, respectively.
SAEs in ZOE-50/70 participants with at least one pIMD at enrolment
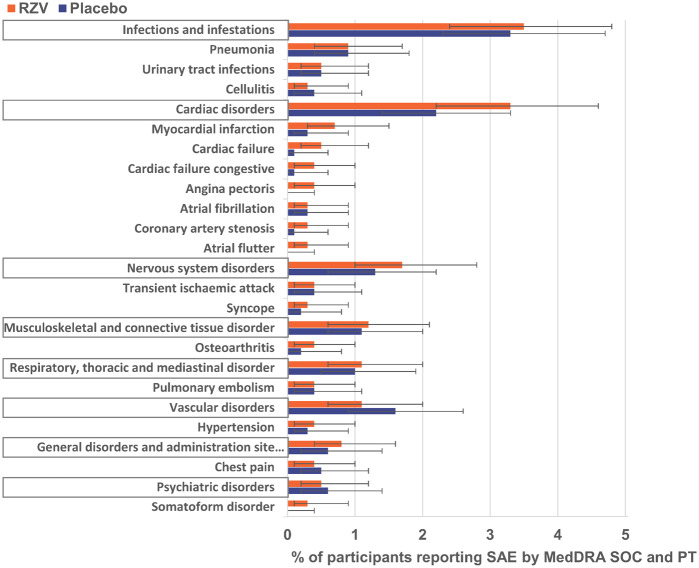
From first dose through 365 days post-last dose, at least one SAE was reported by 144 (14.6%) RZV and 112 (11.7%) placebo recipients with at least one pre-existing pIMD (Supplementary Table S3, available at Rheumatology online). The most frequently reported SAEs by MedDRA system organ class were infections and infestations, followed by cardiac disorders. The most frequently reported SAEs in both treatment groups by MedDRA preferred term (PT) were pneumonia, myocardial infarction, urinary tract infection and cardiac failure (Fig. 2 and Supplementary Table S3, available at Rheumatology online).
Fig. 2.
SAEs in participants with pre-existing pIMDs reported through 365 days post-last vaccine dose (TVC)
SAEs by MedDRA SOC (framed) and PT are presented here. Per PT, only SAEs reported by ≥0.3% of RZV recipients are presented here. Error bars represent 95% CI. MedDRA: Medical Dictionary for Regulatory Activities; pIMD: potential immune-mediated disease; Placebo: participants receiving placebo; PT: preferred term; RZV: participants receiving the adjuvanted recombinant zoster vaccine; SAE: serious adverse event; SOC: system organ class; TVC: total vaccinated cohort; YOA: years of age.
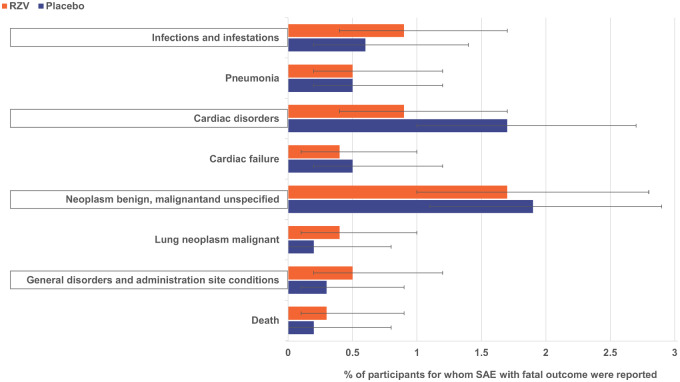
From first dose through 365 days post-last dose, fatal SAEs were reported for 12 (1.2%) RZV and 9 (0.9%) placebo recipients with at least one pre-existing pIMD (Supplementary Table S3, available at Rheumatology online). In the RZV group, the following 12 SAEs with fatal outcome by MedDRA PT were recorded: acute myocardial infarction, myocardial infarction, large intestinal obstruction, sudden death, neutropenic sepsis, pneumonia, skull fracture, ovarian cancer, prostate cancer, rectal adenocarcinoma, cerebrovascular accident, pneumonia aspiration and two pancreatic carcinomas. In the placebo group, the nine SAEs with fatal outcome recorded were: acute myocardial infarction, chronic hepatic failure, large cell lung cancer, metastases to central nervous system, cerebrovascular accident, azotaemia, pulmonary fibrosis and two pancreatic carcinomas. There was no pattern regarding the causes of death for these participants and these were independent from underlying pIMDs (Supplementary Table S1, available at Rheumatology online) and expected for an elderly population.
From first vaccination through study end, fatal SAEs were reported for 50 (5.1%) RZV and 63 (6.6%) placebo recipients (Supplementary Table S3, available at Rheumatology online). The most frequent of these by MedDRA PT were pneumonia, cardiac failure and lung neoplasm malignant (Fig. 3 and Supplementary Table S3, available at Rheumatology online).
Fig. 3 SAEs with fatal outcome in participants with pre-existing pIMDs reported during the entire study period (TVC)
SAEs by MedDRA SOC (framed) and PT are presented here. Per PT, only SAEs reported by ≥0.3% of RZV recipients are presented here. Error bars represent 95% CI. MedDRA: Medical Dictionary for Regulatory Activities; pIMD: potential immune-mediated disease; Placebo: participants receiving placebo; PT: preferred term; RZV: participants receiving the adjuvanted recombinant zoster vaccine; SAE: serious adverse event; SOC: system organ class; TVC: total vaccinated cohort.
Discussion
This post hoc analysis showed that RZV is highly efficacious in the prevention of HZ and did not identify any imbalance between the vaccine and the placebo group in terms of SAEs in adults ≥50 years of age enrolled in the ZOE-50/70 studies who had at least one pre-existing pIMD at study entry.
The ZOE-50 study showed that RZV is 97.2% (95% CI 93.7, 99.0%) efficacious in preventing HZ in adults aged ≥50 years [4]. Pooled ZOE-50/70 data show that efficacy is maintained at >90% even in the oldest age group (≥80 year-olds) [5], and is not impacted by geographic ancestry/ethnicity, region, gender [22] or comorbidities [23]. The individuals included in our post hoc analysis were similar in terms of demographic characteristics (Table 1) with population in pooled ZOE-50/70, where the mean age of the participants was 68.6 years; 58.2% were female and most of the participants (73.7%) were white [20]. Our analysis shows that efficacy of RZV is also high in individuals having pre-existing pIMDs when enrolled in the ZOE-50/70 studies, underscoring the robustness of RZV in the prevention of HZ. It should, however, be noted that per inclusion/exclusion criteria, study participants were not considered immunocompromised by disease or treatment at enrolment. This includes individuals who received prednisone doses <20 mg/day (e.g. polymyalgia rheumatica patients), which is a rather permissive threshold, given that, more conservatively, doses >10 mg/day are already accounted for as immunosuppressive [24].
In this subset of pooled ZOE-50/70 participants with pre-existing pIMDs at enrolment, the occurrence of SAEs within 1 year post-last study vaccine dose was similar between the RZV [14.6% (95% CI: 12.5, 17.0%)] and the placebo [11.7% (95% CI: 9.7, 13.9%)] groups, and, in the RZV group, it appeared to be higher than in the overall pooled ZOE-50/70 population [RZV: 10.1% (95% CI: 9.6, 10.6%), placebo: 10.4% (95% CI: 9.9, 10.9%)] [20]. In the overall ZOE-50/70 population, the most frequently reported SAEs by MedDRA system organ class were infections and infestations, followed by cardiac disorders [20]. In the subset of participants reporting pIMDs at enrolment, infections and infestations and cardiac disorders were also the most frequently reported SAEs by system organ class. By MedDRA PT, the most frequently reported SAE was pneumonia both in the overall study population [20] and in the subset of participants having pIMDs at enrolment. In the subset of participants reporting pIMDs at enrolment, more RZV than placebo recipients: 32 [3.3% (95% CI: 2.2, 4.6%)] vs 21 [2.2% (95% CI: 1.4, 3.3%)] reported cardiac disorders, but the CIs for the corresponding occurrences are overlapping. No temporal clustering of SAEs was observed.
In the subset of pooled ZOE-50/70 participants with pre-existing pIMDs at enrolment, occurrence of fatal SAEs during the entire study period was similar between the RZV [5.1% (95% CI: 3.8, 6.7%)] and the placebo [6.6% (95% CI: 5.1, 8.3%)] groups and appeared to be similar to the overall pooled ZOE-50/70 population [RZV: 4.3% (95% CI: 4.0, 4.7%), placebo: 4.6% (95% CI: 4.3, 5.0%)] [20]. The reported SAEs in this population were generally consistent with events anticipated to occur in this age group, and the overall SAE profile observed is similar to that observed in the full study population [20].
Occurrence of exacerbations and new onset pIMDs in the subset of pooled ZOE-50/70 participants with pre-existing pIMDs at enrolment has been described previously [20]. Briefly, 27 (2.8%) participants in the RZV group and 27 (2.8%) participants in placebo group reported a possible exacerbation of a pre-existing pIMD and 16 (1.6%) and 23 (2.4%), respectively, reported a new onset of a different pIMD during the whole study period [20]. In addition, our data show a balance between study groups in the frequency and nature of SAEs, confirming the favourable safety profile of RZV in populations with pIMDs.
The limitations of this post hoc analysis must be considered when interpreting its results. The presented analyses were not controlled for type 1 error. More importantly, the ZOE-50/70 studies were not powered to assess vaccine efficacy or safety in subsets such as study participants with pre-existing pIMDs. Nonetheless, the number of pooled ZOE-50/70 participants with pIMDs at enrolment was substantial and therefore allowed for the estimation of vaccine efficacy against HZ and occurrence of SAEs in this population subset, although not by discrete pre-existing pIMDs reported at enrolment. Pre-existing pIMDs at enrolment were not taken into consideration for randomization. However, the prevalence of each of the most frequent pIMDs was similar between the RZV and placebo groups. In addition, persons with pre-existing pIMDs who were undergoing immunosuppressive treatment were not enrolled in the ZOE-50/70 studies. However, RZV was also shown to be safe, immunogenic and highly efficacious against HZ in populations that are severely immunosuppressed by disease and/or its treatment [25–28].
Conclusions
In the subset of participants from the ZOE-50/70 clinical trials who had at least one pre-existing pIMD at enrolment, RZV was 90.5% efficacious against confirmed HZ cases, and the safety profile in terms of SAE occurrence was similar in the vaccine and placebo groups.
Trademark statement: Shingrix is a trademark of the GSK group of companies.
Supplementary Material
Acknowledgements
The authors thank all study participants, the clinical investigators, and study nurses and GSK study staff involved in the ZOE-50 and ZOE-70 trials. The authors thank Fernanda Tavares Da Silva (GSK) for critically reviewing the manuscript. The authors would also like to thank Modis c/o GSK for editorial assistance and manuscript coordination. Medical writing services were provided by Alpár Pöllnitz and Maria Maior and editorial assistance and publication coordination were provided by Natalia Tumanova and Divya Kesters. The list of contributors who conceived and designed separately the ZOE-50 and ZOE-70 studies, as well as those who collected and interpreted the ZOE-50 and ZOE-70 studies’ data can be found in the contributor sections of the primary publications [4, 5]. All named authors contributed to the generation, analysis and interpretation of the post hoc analyses data and actively participated in writing of the manuscript. All named authors reviewed and approved the final submitted version of the paper. All ZOE-50/70 study group contributors had an opportunity to review a draft of the paper.
Funding: This work was sponsored by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the conduct and analysis of the studies. GlaxoSmithKline Biologicals SA covered the costs associated with the development and the publishing of the present manuscript.
Disclosure statement: A.F.D., D.R., C.H., T.Z. and A.S. were employees of the GSK group of companies at the time the analysis was designed, initiated, and/or conducted. C.H is currently an employee of UCB Pharma. A.F.D., A.S., D.R. and T.Z. own stocks in the GSK group of companies. M.J.L. received fees for serving on advisory boards from Merck Sharp & Dohme, GSK and Curevo.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Yawn BP, Gilden D.. The global epidemiology of herpes zoster. Neurology 2013;81:928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kawai K, Yawn BP.. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc 2017;92:1806–21. [DOI] [PubMed] [Google Scholar]
- 3. Kawai K, Rampakakis E, Tsai TF. et al. Predictors of postherpetic neuralgia in patients with herpes zoster: a pooled analysis of prospective cohort studies from North and Latin America and Asia. Int J Infect Dis 2015;34:126–31. [DOI] [PubMed] [Google Scholar]
- 4. Lal H, Cunningham AL, Godeaux O. et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96. [DOI] [PubMed] [Google Scholar]
- 5. Cunningham AL, Lal H, Kovac M. et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016;375:1019–32. [DOI] [PubMed] [Google Scholar]
- 6. Mareque M, Oyaguez I, Morano R, Casado MA.. Systematic review of the evidence on the epidemiology of herpes zoster: incidence in the general population and specific subpopulations in Spain. Public Health 2019;167:136–46. [DOI] [PubMed] [Google Scholar]
- 7. Forbes HJ, Bhaskaran K, Thomas SL. et al. Quantification of risk factors for postherpetic neuralgia in herpes zoster patients: a cohort study. Neurology 2016;87:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A.. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 2013;72:ii111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz DM, Kanno Y, Villarino A. et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017;16:843–62. [DOI] [PubMed] [Google Scholar]
- 10. Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF.. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol 2019;81:102–10. [DOI] [PubMed] [Google Scholar]
- 11. Yang SC, Lai YY, Huang MC, Tsai CS, Wang JL.. Corticosteroid dose and the risk of opportunistic infection in a national systemic lupus erythematosus cohort. Lupus 2018;27:1819–27. [DOI] [PubMed] [Google Scholar]
- 12. Curtis JR, Xie F, Yang S. et al. Herpes zoster in tofacitinib: risk is further increased with glucocorticoids but not methotrexate. Arthritis Care Res (Hoboken) 2019;71:1249–54. [DOI] [PubMed] [Google Scholar]
- 13. Colombel JF. Herpes zoster in patients receiving JAK inhibitors for ulcerative colitis: mechanism, epidemiology, management, and prevention. Inflamm Bowel Dis 2018;24:2173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamaoka K. Benefit and risk of tofacitinib in the treatment of rheumatoid arthritis: a focus on herpes zoster. Drug Saf 2016;39:823–40. [DOI] [PubMed] [Google Scholar]
- 15. Siegel SAR, Winthrop KL.. In the real world: infections associated with biologic and small molecule therapies in psoriatic arthritis and psoriasis. Curr Rheumatol Rep 2019;21:36. [DOI] [PubMed] [Google Scholar]
- 16. Ma C, Lee JK, Mitra AR. et al. Systematic review with meta-analysis: efficacy and safety of oral Janus kinase inhibitors for inflammatory bowel disease. Aliment Pharmacol Ther 2019;50:5–23. [DOI] [PubMed] [Google Scholar]
- 17. Almanzar G, Kienle F, Schmalzing M. et al. Tofacitinib modulates the VZV-specific CD4+ T cell immune response in vitro in lymphocytes of patients with rheumatoid arthritis. Rheumatology (Oxford) 2019;58:2051–60. [DOI] [PubMed] [Google Scholar]
- 18. Bechman K, Subesinghe S, Norton S. et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford) 2019;58:1755–66. [DOI] [PubMed] [Google Scholar]
- 19. Tavares Da Silva F, De Keyser F, Lambert PH. et al. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine 2013;31:1870–6. [DOI] [PubMed] [Google Scholar]
- 20. Lopez-Fauqued M, Campora L, Delannois F. et al. Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine 2019;37:2482–93. [DOI] [PubMed] [Google Scholar]
- 21. Do-Nguyen D, Inderjeeth CA, Edelman J, Cheah P.. Retrospective analysis of the clinical course of patients treated for polymyalgia. Open Access Rheumatol 2013;5:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willer DO, Oostvogels L, Cunningham AL. et al. Efficacy of the adjuvanted recombinant zoster vaccine (RZV) by sex, geographic region, and geographic ancestry/ethnicity: a post-hoc analysis of the ZOE-50 and ZOE-70 randomized trials. Vaccine 2019;37:6262–7. [DOI] [PubMed] [Google Scholar]
- 23. Oostvogels L, Heineman TC, Johnson RW. et al. Medical conditions at enrollment do not impact efficacy and safety of the adjuvanted recombinant zoster vaccine: a pooled post-hoc analysis of two parallel randomized trials. Hum Vaccin Immunother 2019;15:2865– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allantaz-Frager F, Turrel-Davin F, Venet F. et al. Identification of biomarkers of response to IFNg during endotoxin tolerance: application to septic shock. PLoS One 2013;8:e68218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bastidas A, de la Serna J, El Idrissi M. et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA 2019;322:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dagnew AF, Ilhan O, Lee WS. et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis 2019;19:988–1000. [DOI] [PubMed] [Google Scholar]
- 27. Vink P, Delgado Mingorance I, Maximiano Alonso C. et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer 2019;125:1301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vink P, Ramon Torrell JM, Sanchez Fructuoso A. et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase III, randomized clinical trial. Clin Infect Dis 2020;70:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.