Dear Editor, SSc is a heterogeneous disease in which vasculopathy and fibrosis affect multiple organ systems such as the skin, gastrointestinal tract, kidneys, heart and lungs [1]. The clinical course of the disease can vary from rapidly progressive, resulting in generalized fibrosis of the vital organs, to a more indolent form developing over an extended period of time. For both physicians and patients, SSc represents a major clinical challenge, as prediction of the disease course remains difficult. Disease progression has been shown to occur often [2, 3]. However, many studies have been performed in selected subgroups of patients or have focused on specific organ domains.
In order to improve prediction of the individual disease course, we need accurate and complete information on the occurrence rate of disease progression per organ system during follow-up, preferably in an unselected cohort of SSc patients. This is crucial, as in many SSc cohorts, mild cases are not extensively followed, making these cohorts ungeneralizable to the entire SSc population. The Leiden Combined Care In SSc Cohort [4] has, from its beginning, included patients in accordance with the ACR/EULAR 2013 criteria [5]. All included patients undergo extensive annual assessment irrespective of symptoms and, as such, data from this cohort can provide information on disease progression in an unselected SSc cohort. We analysed the occurrence of disease progression in 492 SSc patients fulfilling the 2013 ACR/EULAR SSc criteria who underwent at least two complete assessments for organ involvement [4]. Of the included patients, 79% (n = 389) were female, the mean age at baseline was 55 years (SD 14), the median disease duration since first non-Raynaud symptom was 3.2 years (interquartile range 0.9–10.3), 39% (n = 194) were ACA positive, 24% (n = 116) were anti-topoisomerase antibody (ATA) positive, 6% (n = 27) were anti-RNA polymerase III (anti-RNPIII) positive and 12% (n = 61) were ANA negative. Twenty-four percent (n = 118) of the patients had diffuse cutaneous SSc at baseline and 37% (n = 183) had signs of interstitial lung disease (ILD) at baseline.
Disease progression was defined as progression in one or more organ systems, death or the start of immunosuppressive treatment, and was evaluated annually. For ILD, pulmonary artery hypertension (PAH), modified Rodnan skin score (mRSS) and renal crisis, progression was defined as described previously [6, 7] (see Supplementary Table S1 for a detailed explanation). Cardiac progression, gastrointestinal progression and myositis were each defined using a combination of variables (see Supplementary Table S1 for detailed definitions).
In 492 SSc patients [2109 time points, follow-up range 2–10 years (see Supplementary Table S2 for differences in follow-up per subgroup)], disease progression was observed in 52% (n = 257) after a median follow-up duration of 4 years (range 1–8 years), including cardiac progression in 29% (n = 142), lung progression in 23% (n = 114), skin progression in 16% (n = 79) and gastrointestinal (GI) progression in 12% (n = 60). Death [12% (n = 60)], development of PAH [4% (n = 20)], myositis [3% (n = 14)] and renal crisis [1% (n = 5)] occurred less frequently. Forty-eight percent of the patients (n = 235) did not show any progression during a median follow-up of 3 years (range 1–9) (82% female, 18% ATA positive).
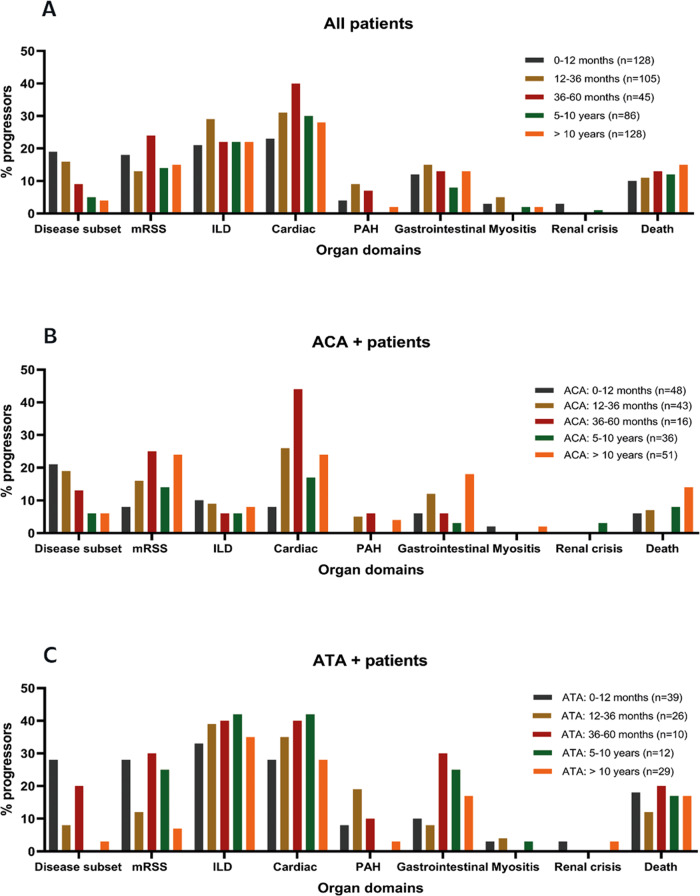
The current literature indicates SSc disease progression occurring most often early in the disease course, specifically in ATA-positive patients. Therefore we evaluated disease progression during follow-up stratified for disease duration, autoantibody subgroup and disease subset (Fig. 1 and Supplementary Figure S1). In our cohort, 56% of observed progression occurred within 5 years since the first non-Raynaud symptom. While progression in skin involvement occurred more frequently in early disease, the proportion of patients with lung, heart or GI progression was relatively stable over time. In total, 24% (n = 63/257) of first-time organ progression occurred after 10 years since the first non-Raynaud symptom. When stratifying for both autoantibody and disease duration, we saw a striking difference in the occurrence of skin progression: while in ATA patients this typically occurred early, the proportion of patients with skin progression increased over time in the ACA group. Cardiopulmonary progression was more frequent in ATA patients (58% of ATA patients showed cardiopulmonary progression), but was also frequently observed in ACA patients (26% of ACA patients showed cardiopulmonary progression) and was independent of disease duration in both groups. In ACA-positive patients, the percentage of progressors was often highest in the subgroup with long-standing disease. Unfortunately, we were underpowered to draw firm conclusions on progression rates in anti-RNPIII-positive patients and ANA-negative patients (Supplementary Figure S1). In addition to antibody specificity, the pattern of skin involvement has been identified as an important clinical biomarker for risk of disease progression. Of the patients that presented with lcSSc, 17% progressed to dcSSc, most frequently within 5 years since the first non-Raynaud symptom. Any progression (excluding progression to dcSSc) occurred in 47% of lcSSc and in 72% of dcSSc (supplementary figure S1) patients. Finally, one might hypothesize that the follow-up duration might be different depending on the clinical severity of the disease. However, in our cohort, clinical follow-up was remarkably comparable for the clinical subgroups we evaluated (Supplementary Table S2), which supports that patients were evaluated annually independent of disease severity.
Fig. 1.
Percentage of progressors per subdomain
(A) Stratified for disease duration since first non-Raynaud symptom. (B) Stratified for ACA-positive patients and disease duration. (C) Stratified for ATA-positive patients and disease duration.
In conclusion, our data confirm that the proportion of SSc patients that experience disease progression over time is substantial, also when applying 2013 ACR criteria that were designed to diagnose SSc earlier. We show that among ACA-positive patients, skin progression does occur, typically in long-standing disease. Finally, our data indicate that cardiopulmonary progression can occur at any time during follow-up, independent of disease duration, underlining the importance of identifying biomarkers for risk stratification that can guide follow-up. The challenge remains to identify individual patients with a low risk of progression and in whom annual complete assessment might be redundant. This justifies our ongoing research in identifying autoantibody levels and characteristics that could contribute to personalized risk stratification [7, 8].
Funding: The authors received no specific funding for this work.
Disclosure statement: The authors declare no competing interests. At the Leiden University Medical Center, SSc patients are included in a prospective observational cohort study (Combined Care in Systemic Sclerosis), which is approved by the Ethics Committee (P09.003). The cohort study is designed in accordance with the ethical principles of the Declaration of Helsinki. All patients gave written informed consent.
Data availability statement
Data are available upon request
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Denton CP, Khanna D.. Systemic sclerosis. Lancet 2017;390:1685–99. [DOI] [PubMed] [Google Scholar]
- 2. Becker M, Graf N, Sauter R. et al. Predictors of disease worsening defined by progression of organ damage in diffuse systemic sclerosis: a European Scleroderma Trials and Research (EUSTAR) analysis. Ann Rheum Dis 2019;78:1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu W, Jordan S, Becker MO. et al. Prediction of progression of interstitial lung disease in patients with systemic sclerosis: the SPAR model. Ann Rheum Dis 2018;77:1326–32. [DOI] [PubMed] [Google Scholar]
- 4. Meijs J, Schouffoer AA, Ajmone Marsan N. et al. Therapeutic and diagnostic outcomes of a standardised, comprehensive care pathway for patients with systemic sclerosis. RMD Open 2016;2:e000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Hoogen F, Khanna D, Fransen J. et al. Classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galie N et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903–75. [DOI] [PubMed] [Google Scholar]
- 7. Boonstra M, Ninaber MK, Ajmone Marsan N. et al. Prognostic properties of anti-topoisomerase antibodies in patients identified by the ACR/EULAR 2013 systemic sclerosis criteria. Rheumatology 2019;58:730–2. [DOI] [PubMed] [Google Scholar]
- 8. NM van Leeuwen JB, Grummels A, Wortel C. et al. Anticentromere antibody levels and isotypes associate with disease severity in systemic sclerosis. Arthritis Rheumatol 2019;71(Suppl 10):abstract 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request