Abstract
Objectives
Associations between BMI and health-related quality of life (HRQoL) in SLE have been implied, but data are scarce. We determined the impact of overweight and obesity on HRQoL in a large SLE population.
Methods
We pooled cross-sectional baseline data from the BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) trials (N = 1684). HRQoL was evaluated using the 36-item Short Form Health Survey (SF-36), Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale and the European Quality of Life 5-dimension questionnaire (EQ-5D). Comparisons between BMI groups were conducted using the Mann–Whitney U test and adjustments using linear regression. Clinical relevance was determined by minimal clinically important differences (MCIDs).
Results
In total, 43.2% of the patients had BMI above normal and 17.4% were obese. Overweight and obese patients reported worse SF-36 physical component summary (PCS), physical functioning, role physical, bodily pain and FACIT-Fatigue scores than normal weight patients. Divergences were greater than corresponding MCIDs and more prominent with increasing BMI. Despite no clinically important difference in SF-36 mental component summary scores across BMI categories, patients experienced progressively diminished vitality and social functioning with increasing BMI. In linear regression analysis, BMI above normal and obesity were associated with worse PCS (standardized coefficient β = −0.10, P < 0.001 and β = −0.17, P < 0.001, respectively), FACIT-Fatigue (β = −0.11, P < 0.001 and β = −0.16, P < 0.001) and EQ-5D (β = −0.08, P = 0.001 and β = −0.12, P < 0.001) scores, independently of demographic and disease-related factors. The impact of BMI on the PCS and FACIT-Fatigue was more pronounced than that of SLE activity.
Conclusion
Patients with SLE and BMI above normal experienced clinically important HRQoL diminutions in physical aspects, fatigue and social functioning. A survey of potential causality underlying this association is warranted.
Keywords: SLE, health-related quality of life, obesity, patient-reported outcomes
Rheumatology key messages
Overweight and obese patients with SLE experienced clinically important impairments regarding physical HRQoL and fatigue.
SLE patients’ physical HRQoL, fatigue and social functioning were gradually worse with increasing BMI.
The impact of BMI on physical HRQoL and fatigue was greater than that of SLE activity.
Introduction
SLE is a chronic multisystem autoimmune disease that most commonly affects women of childbearing age. Despite considerable advances on improving life expectancy and preventing organ damage accrual over the past decades [1], patients with SLE still experience a substantially impaired health-related quality of life (HRQoL) compared with the general population, and constitutional symptoms such as fatigue remain frequent complaints [2–4].
Factors contributing to HRQoL diminutions in patients with SLE include fatigue, pain, depression and increased BMI [2, 5–7]. Obesity is associated with poor functional capacity, high concentrations of inflammatory markers and high disease activity [8–10]. In juvenile-onset SLE, obesity has detrimental effects on overall HRQoL [6]. In adult SLE patients, higher BMI is associated with an impaired physical HRQoL [5, 6], while the effect regarding mental aspects remains controversial, with inconsistent reports from different cohorts [5, 6, 9]. Overall, data are scarce and conflicting and the clinical significance of the associations between BMI and HRQoL has not been thoroughly investigated.
In the present study, the aim was to determine the impact of overweight and obesity on physical and mental HRQoL aspects in the large SLE populations of the BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) clinical trials.
Methods
Study design and population
This is a post hoc analysis of prospectively collected data from two phase 3 randomized clinical trials of belimumab in SLE, i.e. the BLISS-52 [11] and BLISS-76 [12] trials. The study design was cross-sectional. We utilized pooled baseline data from the two trials (n = 1684), i.e. data collected prior to the intended trial intervention.
The BLISS-52 trial comprised 865 adult seropositive (ANA titre ≥1:80 and/or anti-dsDNA antibody ≥30 IU/ml) patients with active SLE, defined as a Safety of Estrogen in Lupus National Assessment–SLEDAI (SELENA-SLEDAI) [13] score ≥6 at enrolment despite standard of care therapy, the latter comprising fixed doses of corticosteroids, NSAIDs, antimalarial agents or immunosuppressants for at least 30 days before the first study dose. Organ damage was assessed using the SLICC/ACR Damage Index (SDI) [14]. The BLISS-76 trial included 819 patients with SLE and had a similar design to that of the BLISS-52 trial, but a longer follow-up until week 76.
The similarity in the design allowed us to analyse pooled data from the two trials. After exclusion of three patients with no available BMI data, the total number of patients qualifying for analysis was 1681. However, since BLISS‐52 was primarily conducted in Latin America, Asia Pacific and Eastern Europe while BLISS‐76 was primarily conducted in North America and Europe, we also performed separate analyses of data from the BLISS-52 and BLISS-76 trials. Data were made available by GlaxoSmithKline (Uxbridge, UK) through the Clinical Study Data Request Consortium.
The study was conducted in compliance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all study participants prior to enrolment in the BLISS programmes. Ethical permission for the present investigation was obtained by the Swedish ethical review authority (ref. 2019-05498).
Measurements of HRQoL
SLE patients’ perception of HRQoL was determined using generic instruments, i.e. the Medical Outcomes Study (MOS) 36-item Short Form Health Survey (SF-36) [15], the three-level European Quality of Life 5-dimension (EQ-5D) health questionnaire [16] and the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale [17]. The SF-36 questionnaire consists of 36 questions, grouped in eight subscales, i.e. physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), social functioning (SF), vitality (VT), role emotional (RE) and mental health (MH). The responses to the SF-36 were scored with the SF-36v2 manual [18], yielding subscale scores from 0 to 100. Next, the SF-36 subscales were computed according to a three-step procedure, including Z-score transformation and weighting based on the general US population, to generate two summary measures, the physical component summary (PCS) and the mental component summary (MCS). Although all subscales are weighted in the derivation of PCS and MCS, PF, RP, BP and GH are referred to as the physical aspects and SF, VT, RE and MH as the mental aspects of the SF-36. In terms of interpretation, higher scores on SF-36 subscales and component summaries represent a better HRQoL.
The FACIT-Fatigue scale is a generic 13-item questionnaire that assesses the impact of fatigue over the preceding 7 days. The scores generated have a span from 0 to 52, with higher scores representing greater fatigue.
The three-level EQ-5D health questionnaire consists of two distinct indices, i.e. a visual analogue scale (VAS) measuring patients’ health perception from 0 (worst health perception) to 100 (best health perception) and a questionnaire consisting of five questions addressing self-care, mobility, daily activity, pain/discomfort and anxiety/depression. Patients’ responses to these five questions are next summarized into a utility index score. In the present study, EQ-5D utility index scores were calculated based on the valuation of EQ-5D health states from a general US population sample [19]. In terms of interpretation, higher utility index scores represent a better HRQoL.
BMI and HRQoL
The patients were stratified into four groups based on their BMI according to cut-off values established by the World Health Organization (WHO): underweight (BMI <18.5 kg/m2), normal weight (18.5≤ BMI <25 kg/m2), overweight (25≤ BMI <30 kg/m2) and obesity (BMI ≥30 kg/m2) [20]. Further, we divided the obesity group into three subgroups: type I obesity (30≤ BMI <35 kg/m2), type II obesity (35≤ BMI <40 kg/m2) and type III obesity (BMI ≥40 kg/m2). In certain analyses, we grouped overweight and obese individuals into a subset herein termed ‘BMI above normal’ patients.
Next we determined minimal clinically important differences (MCIDs) for scores in the different HRQoL instruments, based on previously reported cut-offs. In cases of varying thresholds in the literature, the most stringent one was used. Thus the MCID for SF-36 PCS and MCS scores was defined as ≥2.5, while the corresponding MCID for SF-36 subscale scores was defined as ≥5.0 [21]. The MCID for FACIT-Fatigue scores was set to ≥4 [22, 23]. For the EQ-5D VAS, the MCID was defined as ≥10 [24] and for the EQ-5D utility index score it was set to ≥0.082 [25].
Statistical analysis
Data are presented as number (percentage) or mean (s.d.). For comparisons between BMI groups, the non-parametric Mann–Whitney U test was used. The Pearson’s χ2 test was used to investigate contingent associations between binomial variables. Finally, linear regression analysis was used to evaluate associations of demographic and clinical factors with different HRQoL aspects. Multiple linear regression models were created for assessment of independence and confounding potentiality. P-values <0.05 were considered statistically significant. Statistical analyses were performed using the SPSS software version 25 (IBM, Armonk, NY, USA). GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) was used for the construction of graphs.
Results
Patient characteristics
Table 1 summarizes demographic and SLE disease characteristics across BMI groups. Supplementary Table S1, available at Rheumatology online, shows the corresponding data for the BMI above normal group and the different obesity subgroups.
Table 1 Demographic and clinical characteristics of patients
| Characteristics | Normal weight (N = 874) | Underweight (N = 80) |
Overweight (N = 434) |
Obese (N = 293) |
|||
|---|---|---|---|---|---|---|---|
| P-value | P-value | P-value | |||||
| Demographics | |||||||
| Age, years, mean (s.d.) | 35.4 (10.9) | 30.0 (9.3) | <0.001 | 40.2 (11.5) | <0.001 | 43.4 (10.7) | <0.001 |
| Sex (female), n (%) | 831 (95.1) | 78 (97.5) | 0.328 | 397 (91.5) | 0.010 | 276 (94.2) | 0.554 |
| Ethnic origin, n (%) | |||||||
| Asian | 240 (27.5) | 35 (43.8) | 0.002 | 64 (14.7) | <0.001 | 14 (4.8) | <0.001 |
| Black/African American | 47 (5.4) | 6 (7.5) | 0.428 | 49 (11.3) | <0.001 | 44 (15.0) | <0.001 |
| Indigenous Americana | 206 (23.6) | 11 (13.8) | 0.045 | 107 (24.7) | 0.665 | 50 (17.1) | 0.020 |
| White/Caucasian | 378 (43.2) | 28 (35.0) | 0.153 | 210 (48.4) | 0.079 | 179 (61.1) | <0.001 |
| Clinical characteristics and concomitant treatments | |||||||
| SELENA-SLEDAI score, mean (s.d.) | 9.6 (3.6) | 10.4 (4.3) | 0.174 | 9.8 (3.8) | 0.529 | 9.7 (3.4) | 0.972 |
| SLE duration, years, mean (s.d.) | 6.3 (6.3) | 4.9 (5.5) | 0.056 | 6.4 (6.5) | 0.957 | 7.1 (6.6) | 0.055 |
| SDI score, mean (s.d.) | 0.63 (1.07) | 0.61 (1.0) | 0.880 | 0.82 (1.30) | 0.006 | 1.19 (1.54) | <0.001 |
| Glucocorticoid use, n (%) | 784 (89.7) | 74 (92.5) | 0.426 | 368 (84.8) | 0.002 | 229 (77) | <0.001 |
| Prednisone dose, mg/day, mean (s.d.) | 11.3 (8.5) | 11.2 (8.4) | 0.785 | 10.8 (9.3) | 0.101 | 9.2 (8.0) | <0.001 |
| IS useb, n (%) | 405 (46.3) | 33 (41.3) | 0.382 | 215 (49.5) | 0.275 | 161 (54.9) | 0.011 |
| Azathioprine | 204 (23.3) | 13 (16.3) | 0.148 | 100 (23.0) | 0.904 | 72 (24.6) | 0.667 |
| Methotrexate | 113 (12.9) | 9 (11.3) | 0.667 | 53 (12.2) | 0.714 | 56 (19.1) | 0.009 |
| Mycophenolic acid | 87 (10.0) | 10 (12.5) | 0.471 | 58 (13.4) | 0.064 | 32 (10.9) | 0.636 |
| AMA use, n (%) | 593 (67.8) | 57 (71.3) | 0.532 | 265 (61.1) | 0.015 | 181 (61.8) | 0.057 |
P-values are derived from Pearson’s χ2 or Mann–Whitney U tests; the normal weight group was the reference comparator.
Alaska Native or American Indian from North, South or Central America.
Excluding antimalarial agents.
AMA: antimalarial agents; IS: immunosuppressants.
A total of 727 of 1681 patients (43.2%) had a BMI over the normal range, while 293 participants (17.4%) were obese. The prevalence of BMI above normal and obesity were highest among Black/African American patients (63.7% and 30.1%, respectively), with the corresponding proportions among White/Caucasian being 48.9% and 22.5%, among Native American 42.0% and 13.4% and among Asian patients 22.1% and 4.0%, respectively.
There was no difference in SELENA-SLEDAI scores across different BMI groups. However, overweight [0.82 (s.d. 1.30), P = 0.006] and obese patients [1.19 (s.d. 1.54), P < 0.001] had higher SDI scores compared with SLE patients of normal weight [0.63 (s.d. 1.07)].
The frequency of patients receiving antimalarial agents was lower within the overweight group (61.1%) compared with the normal weight group (67.8%; P = 0.015). In contrast, the proportion of patients receiving immunosuppressive agents was higher within the obesity (54.9%) compared with the normal weight group (46.3%; P = 0.011). Notably, obese patients were on lower daily prednisone equivalent doses [9.2 mg/day (s.d. 8.0)] than patients with normal BMI [11.3 mg/day (s.d. 8.5), P < 0.001; Table 1].
MOS SF-36
Overweight and obese patients reported lower PCS, PF, RP and BP scores compared with normal weight patients; the differences were greater than the corresponding MCIDs and more prominent with increasing BMI (Fig. 1). The obese group had lower GH scores [36.5 (s.d. 18.6)] compared with normal weight patients [42.4 (s.d. 18.9), P < 0.001; Supplementary Table S2, available at Rheumatology online] and the differences were greater than the MCIDs in the type 2 and 3 but not the type 1 obesity subgroups. All three obesity subgroups reported worse PCS, PF, BP and GH scores compared with the overweight group (Fig. 1). In the BLISS-52 trial, we observed clinically important differences between patients with BMI above normal and normal weight patients in PF and RP and between obese and normal weight patients in PCS, PF, RP and BP (Supplementary Table S3, available at Rheumatology online). In the BLISS-76 trial, patients with BMI above normal and obese patients experienced worse PCS, PF, RP and BP than normal weight patients, with the differences being greater than the corresponding MCIDs (Supplementary Table S4, available at Rheumatology online).
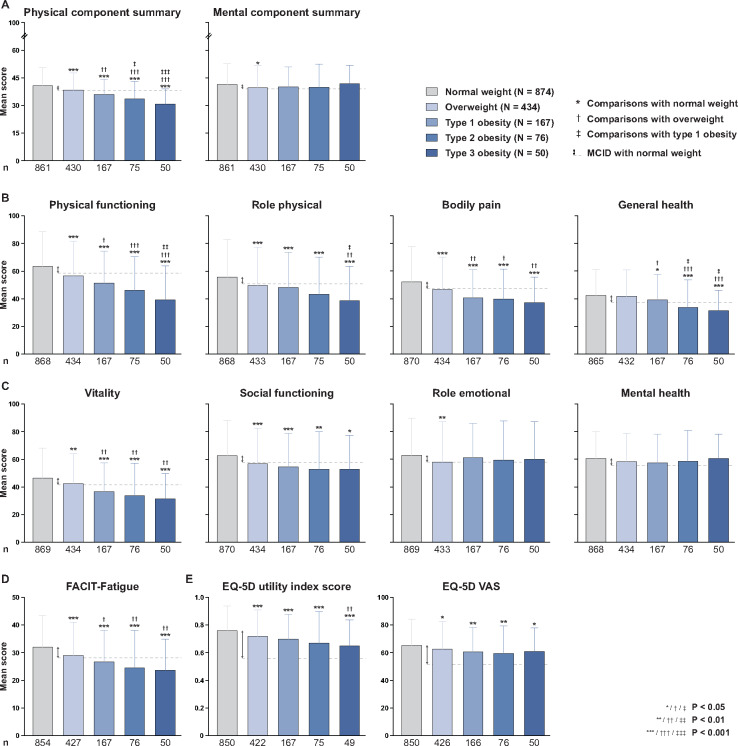
Fig. 1.
HRQoL indices across different BMI groups of SLE patients
Groups are based on BMI measures, according to cut-off values established by the World Health Organization. The heights of the boxes represent mean scores and the whiskers indicate s.d. Upper bounds of bars below the dashed lines represent means that differ from the mean scores in the corresponding normal weight group by more than the MCID, the latter denoted by double-sided arrows. Symbols above the whiskers indicate statistically significant differences. Since the number of observations in the subgroups may differ from the total number of patients due to missing data, the number of available observations (n) is provided.
When mental HRQoL was analysed, overweight patients had lower MCS scores [39.8 (s.d. 11.8)] than normal weight subjects [41.5 (s.d. 11.2), P = 0.029], but the difference was not clinically important (<MCID), whereas obese patients did not show differences compared with the other groups (Fig. 1). However, we found progressively reduced VT and SF scores with increasing BMI, with all obesity subgroups displaying clinically meaningful differences (≥MCID) compared with the normal weight group. MH scores did not differ across the BMI groups (Fig. 1). In analysis stratified by trial, the difference in MCS and MH scores exceeded the MCID in the BLISS-52 trial (Supplementary Table S3, available at Rheumatology online) but not in the BLISS-76 trial (Supplementary Table S4, available at Rheumatology online) in the comparison between obese and normal weight patients, while VT and SF scores showed greater differences than the corresponding MCID in both trials.
No differences were seen in SF-36 component summary or subscale scores between underweight and normal weight SLE patients (Supplementary Table S5, available at Rheumatology online).
FACIT-Fatigue
All groups of BMI above normal reported significantly worse FACIT-Fatigue scores compared with the normal weight group [32.1 (s.d. 11.5)] and displayed a gradual impairment with increasing BMI (Fig. 1). The differences were greater than the MCID for all obesity subgroups (type 1, 26.8 (s.d. 11.3); type 2, 24.6 (s.d. 13.6); type 3, 23.7 (s.d. 11.3), P < 0.001 for all), but not for the overweight group [29.0 (s.d. 12.1), P < 0.001]. Moreover, the differences were greater than the MCID for patients with BMI above normal and obese patients in the pooled dataset (Supplementary Table S2, available at Rheumatology online) and the BLISS-76 trial (Supplementary Table S4, available at Rheumatology online), but not in the BLISS-52 trial (Supplementary Table S3, available at Rheumatology online).
We found no difference in FACIT-Fatigue scores between underweight and normal weight individuals (Supplementary Table S5, available at Rheumatology online).
EQ-5D
Overweight and obese patients had worse EQ-5D VAS and utility index scores compared with normal weight subjects. The type 3 obesity subgroup reported worse EQ-5D utility index scores [0.65 (s.d. 0.19)] compared with the overweight group [0.72 (S.D. 0.19), P = 0.006]. The corresponding differences in the type 1 and 2 obesity subgroups were not statistically significant. None of the differences in the EQ-5D VAS or utility index scores between BMI groups were greater than the corresponding MCIDs (Fig. 1). Comparisons between patients with a BMI above normal and normal weight patients as well as between obese and normal weight patients are presented in Supplementary Table S2 for the pooled dataset, Supplementary Table S3 for the BLISS-52 trial and Supplementary Table S4 for the BLISS-76 trial, available at Rheumatology online.
No difference was noted in the EQ-5D VAS or utility index scores between underweight and normal weight subjects (Supplementary Table S5, available at Rheumatology online).
Linear regression analysis
We next assessed independence and confounding potentiality using multiple linear regression analysis. Based on reports from previous literature, selected covariates included age, sex, ethnicity, SELENA-SLEDAI score, SLE disease duration, SDI score, prednisone (or equivalent) dose and use of antimalarial or immunosuppressive agents [2, 5, 6, 26, 27].
First, we investigated associations between BMI and HRQoL using BMI as a continuous variable in the multivariable models (Fig. 2A–5A;Supplementary Fig. S1A, available at Rheumatology online; Supplementary Table S6, available at Rheumatology online). Higher BMI was independently associated with more severely impaired physical aspects of HRQoL, i.e. lower PCS (standardized coefficient β = −0.20, P < 0.001), PF (β = −0.23, P < 0.001), RP (β = −0.12, P < 0.001), BP (β = −0.12, P < 0.001) and GH (β = −0.13, P < 0.001) scores (Supplementary Table S6, available at Rheumatology online). In contrast, no association was found between BMI and SF-36 MCS scores (β = −0.04, P = 0.264; Fig. 4A), but we found a negative impact of BMI on VT (β = −0.16, P < 0.001; Fig. 5A) and SF (β = −0.11, P = 0.001; Fig. 4A).
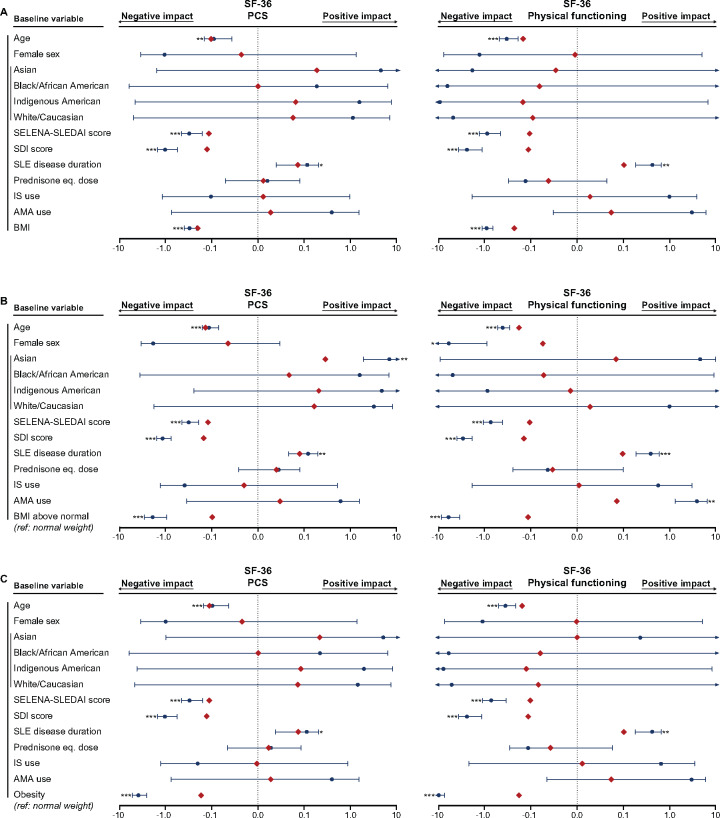
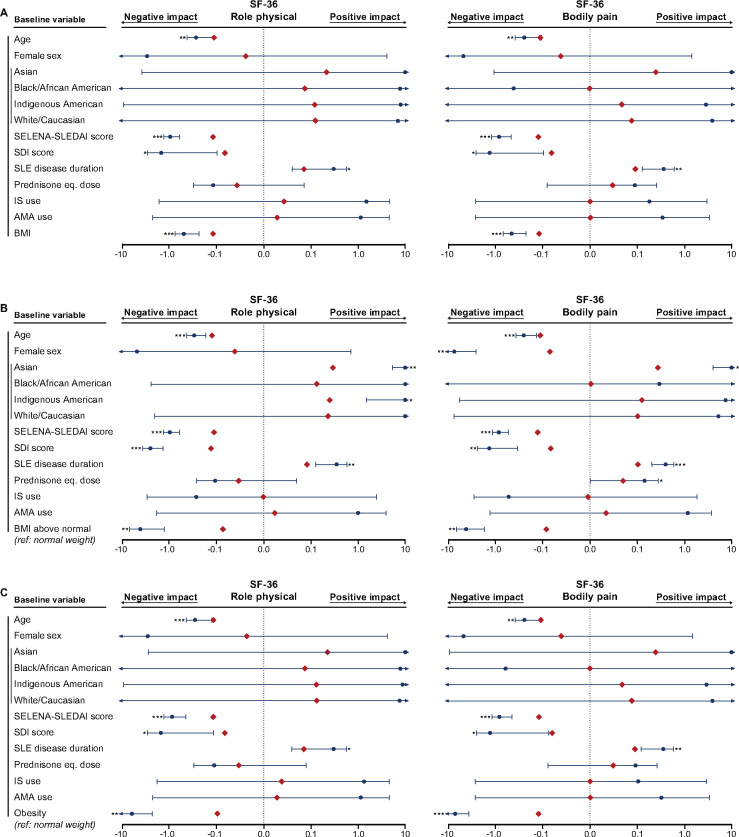
Fig. 2.
Associations of BMI with SF-36 PCS and PF
The forest plots illustrate results from multiple linear regression models. (A) BMI was analysed as a continuous variable. Separate models for the (B) BMI above normal and (C) obesity groups were created, with the normal weight group as the reference comparator in both cases. The dark blue circles represent the unstandardized coefficients and the whiskers represent the 95% CIs. The red diamonds represent the standardized coefficients. Asterisks indicate statistically significant associations. Level of significance: *P < 0.05, **P < 0.01, ***P < 0.001. AMA: antimalarial agents.
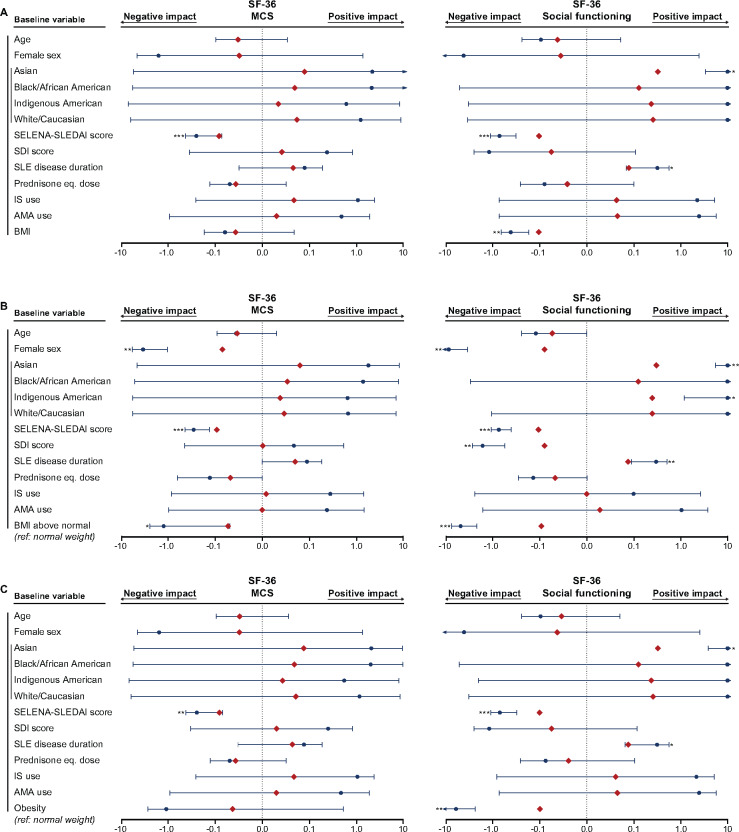
Fig. 4.
Associations of BMI with SF-36 MCS and SF
The forest plots illustrate results from multiple linear regression models. (A) BMI was analysed as a continuous variable. Separate models for the (B) BMI above normal and (C) obesity groups were created, with the normal weight group as the reference comparator in both cases. The dark blue circles represent the unstandardized coefficients and the whiskers represent the 95% CIs. The red diamonds represent the standardized coefficients. Asterisks indicate statistically significant associations. Level of significance: *P < 0.05, **P < 0.01, ***P < 0.001. IS: immunosuppressants; AMA: antimalarial agents.
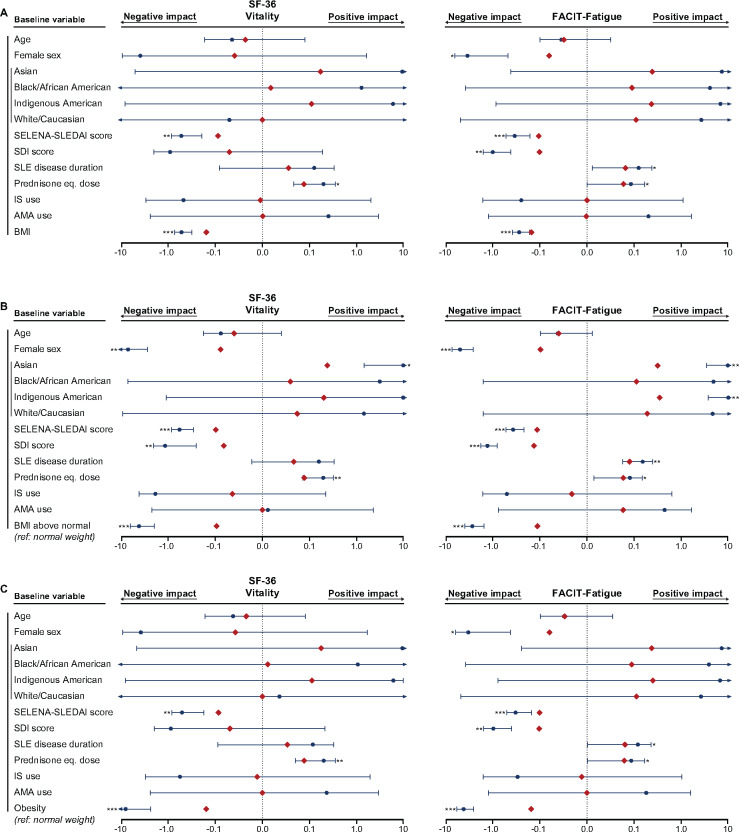
Fig. 5.
Associations of BMI with SF-36 VT and FACIT-Fatigue
The forest plots illustrate results from multiple linear regression models. (A) BMI was analysed as a continuous variable. Separate models for the (B) BMI above normal and (C) obesity groups were created, with the normal weight group as the reference comparator in both cases. The dark blue circles represent the unstandardized coefficients and the whiskers represent the 95% CIs. The red diamonds represent the standardized coefficients. Asterisks indicate statistically significant associations. Level of significance: *P < 0.05, **P < 0.01, ***P < 0.001. IS: immunosuppressants; AMA: antimalarial agents.
Next we created separate models for the BMI above normal group and the obesity group, with the normal weight group as the reference comparator in both cases (Fig. 2B and C–Fig. 5B and C; Supplementary Fig. S1B–C, available at Rheumatology online; Supplementary Table S7, available at Rheumatology online; Supplementary Table S8, available at Rheumatology online). Obesity was independently associated with lower scores in all components related to physical HRQoL, whereas BMI above normal was negatively associated with PCS as well as all physical SF-36 aspects except GH (Figs 2 and 3). Notably, obesity was the covariate showing the most pronounced associations with HRQoL diminutions in physical aspects, yielding greater absolute values of β coefficients compared with age, SELENA-SLEDAI score, SDI score and disease duration. We found no association between obesity and MCS scores (β = −0.04, P = 0.182; Fig. 4; Supplementary Table S8, available at Rheumatology online), but BMI above normal (β = −0.05, P = 0.041) was associated with lower MCS scores (Supplementary Table S7, available at Rheumatology online). Furthermore, we observed a negative impact of BMI above normal and obesity on VT (β = −0.09, P < 0.001 and β = −0.16, P < 0.001, respectively; Fig. 5) and SF (β = −0.09, P < 0.001 and β = −0.10, P = 0.001, respectively; Fig. 4).
Fig. 3.
Associations of BMI with SF-36 RP and BP
The forest plots illustrate results from multiple linear regression models. (A) BMI was analysed as a continuous variable. Separate models for the (B) BMI above normal and (C) obesity groups were created, with the normal weight group as the reference comparator in both cases. The dark blue circles represent the unstandardized coefficients and the whiskers represent the 95% CIs. The red diamonds represent the standardized coefficients. Asterisks indicate statistically significant associations. Level of significance: *P < 0.05, **P < 0.01, ***P < 0.001. IS: immunosuppressants; AMA: antimalarial agents.
Likewise, there was an association between diminished FACIT-Fatigue scores and high BMI, independent of SLE disease duration and prednisone equivalent dose positively impacting on FACIT-Fatigue scores and female sex, SELENA-SLEDAI score and SDI score negatively impacting on FACIT-Fatigue scores (Fig. 5). Furthermore, higher BMI was consistently associated with lower EQ-5D utility index scores in all models (Supplementary Fig. S1, available at Rheumatology online). High BMI was associated with low EQ-5D VAS scores when BMI was treated as a continuous variable (β = −0.07, P = 0.045), but statistical significance was not reached when BMI was treated as a dichotomous variable assessing the impact of BMI above normal (β = −0.05, P = 0.063) and obesity (β = −0.06, P = 0.061).
Higher SELENA-SLEDAI scores were associated with lower SF-36 PCS and MCS scores as well as lower FACIT-Fatigue scores in all models. We also found that SDI scores were negatively associated with SF-36 PCS scores and FACIT-Fatigue scores, but no association was seen with SF-36 MCS scores. Finally, older age was associated with impairments in physical aspects of SF-36, i.e. PCS, PF, RP and BP (Figs 2 and 3), but no association was found with regard to mental aspects of SF-36, with the exception of RE (β = −0.10, P = 0.005).
Discussion
In the present post hoc analysis of the BLISS-52 and BLISS-76 trials, we demonstrated that overweight and obese patients with SLE experience clinically important impairments of HRQoL, especially with regard to physical aspects and fatigue. The observed associations were independent of age, sex, ethnic origin, disease duration, disease activity, organ damage accrual and current immunosuppressive treatment. Interestingly, we found that HRQoL diminutions were more prominent with increasing BMI with regard to physical domains, social functioning and fatigue.
The physical aspects of HRQoL showed the most prominent diminutions in patients with BMI above normal. These diminutions were clinically important regarding all outcomes in the obesity subgroups and all except SF-36 GH in overweight patients. Moreover, study participants with a gradually higher BMI experienced a gradually worse HRQoL in all physical SF-36 items. Importantly, these associations were independent of demographic and disease-related factors in linear regression analysis. Our findings are in conformity with previous studies of adult [5, 6] and juvenile [28] SLE populations, which, irrespective of the tool used for the HRQoL evaluation, reported poor physical performance in obese patients.
With regard to mental aspects of HRQoL, vitality and social functioning were substantially impaired in patients with BMI above normal. This association was more prominent with increasing BMI, including increasing degree of obesity. Again, it is worth noting that the observed associations were independent of the impact of disease activity, organ damage and current immunosuppressive treatment. Overweight patients reported poorer SF-36 MCS and RE compared with normal weight individuals, but these differences did not reach the cut-off of clinical importance and were absent in the comparison between obese and normal weight individuals. The lower numbers of patients in the groups of obesity-level BMI may constitute a possible explanation. Nonetheless, the divergent findings within mental aspects of HRQoL are in line with previous observations both in the general [29–31] and SLE [5, 6, 9] populations. de Zwaan et al. [29] found no association between BMI and SF-36 MCS scores and Busutil et al. [30] reported no association between BMI and 12-item General Health Questionnaire scores, a self-reported measure for mental well-being [32], both in the general population. Zhu et al. [5] showed that overweight and obese patients with SLE had lower SF-36 MCS, VT, SF, RE and MH scores compared with the normal weight group, whereas Tamayo et al. [6] found no association between BMI and SF-36 MCS scores in a German cohort of SLE patients.
Associations between BMI and the mental compartment of HRQoL have to be interpreted in the context of the interplay of psychosocial factors influencing HRQoL in opposing directions; such factors may include stress, depressive symptoms, chronic pain and psychological adjustment processes in patients with long-standing disease [33]. Nonetheless, as previously proposed by Busutil et al. [30], the potential lack of sensitivity to detect impairment in mental aspects of HRQoL with a generic measure such as the SF-36 as a possible explanation underlying our observations warrants investigation.
Overweight and obese patients reported poor FACIT-Fatigue scores, experiencing a gradual impairment with increasing BMI. As expected, these observations were consistent with the results derived from the analysis of the SF-36 VT subscale. The differences in the overweight and obese groups vs normal weight individuals were clinically important; it is worth noting that the MCID in this study was set to 4 points on the FACIT-Fatigue scale [22], albeit lower MCIDs have also been estimated in certain SLE populations [23]. In all models, the impact of high BMI on fatigue was independent of the effect of disease activity, organ damage and concomitant drug use, and even more prominent. These findings have to be interpreted with caution since the degree of SLE activity in this study was limited by the inclusion and exclusion criteria of the BLISS trials, in particular SELENA-SLEDAI scores ≥6 and no severe active renal or neuropsychiatric SLE. Nonetheless, the consistency of this association is of particular importance in light of fatigue being the most frequent complaint in patients with SLE, reported by up to 92% of patients in different cohorts [3, 34] and up to 23% of patients in remission [35], pointing to the need for exploration of the underlying reasons at a biological level.
One could argue that disease activity may constitute a link between BMI and fatigue, with overweight maintaining an inflammatory state [10], subsequently resulting in fatigue. In the present study, however, we found no difference in the degree of SLE disease activity across the different BMI categories. Moreover, the literature has been conflicting regarding the relationship between disease activity and fatigue, with some studies reporting detrimental effects [5, 8, 29] and others showing no association [2, 36], even when the same tools for measuring disease activity were used. Herein the impact of high BMI, in particular obesity, was more pronounced than the impact of disease activity on SF-36 VT and FACIT-Fatigue scores in multivariable regression models. Although the potential bias imposed by the selected SLE population of the BLISS trials has to be reckoned with during interpretation, our findings highlight the importance of including factors such as suboptimal weight in the clinical evaluation for the management of fatigued SLE patients. Indeed, fatigue is acknowledged as one of the most important components of the global SLE burden, which has important implications with regard to socio-economic facets [4]. Results from our study advocate for multidimensional strategies including non-pharmacological approaches, such as weight control, along with pharmacological interventions that have proven efficacy in reducing fatigue [22, 26, 37], towards optimization of patient care and use of societal resources.
Our results were interpreted in the context of previously determined thresholds for clinically important differences, validated for SLE populations [38–40]. The use of validated MCIDs allows clinicians, patients, researchers and policymakers to perform meaningful evaluations of the data, especially when interpretation of the numerical scales of different tools is not intuitive. Furthermore, defining MCIDs is particularly important when large patient samples are analysed, such as the one in the present post hoc analysis, since small and clinically futile numerical differences may reach the level of statistical significance [41, 42]. MCIDs utilized in the present study have been used to assess change over time in previous literature, i.e. improvement or worsening, whereas the comparisons herein were cross-sectional. For this reason, we chose to set the MCIDs to the highest thresholds previously reported. This stringent approach may underestimate some differences, such as the ones observed in the SF-36 MCS, EQ-5D utility index and EQ-5D VAS scores, but ensures the clinical relevance of our findings.
The post hoc nature of the present study was a limitation. The BLISS trials were not designed to evaluate patients’ HRQoL and can be underpowered for detecting differences in certain indices. Moreover, our cross-sectional study was not designed to address the potential causal relationship between body weight and HRQoL. It is important to note that data collected within the frame of the BLISS-52 and BLISS-76 trials may differ from what is encountered in daily clinical practice, even if our investigation was based on baseline data only, i.e. prior to the trial intervention. This selection bias introduced by trial design may limit the generalizability of our findings. For instance, patients were strictly selected to have active SLE, defined as a SELENA-SLEDAI score ≥6, and a stable treatment regimen with a prednisone equivalent dose between 0 and 40 mg/day, antimalarial agents, NSAIDs or conventional immunosuppressants for a period of at least 30 days prior to baseline. Finally, patients with severe active lupus nephritis and central nervous system manifestations were excluded from the BLISS programmes. The potential impact of body weight remains to be addressed in these subsets of SLE patients, especially since the mental compartment of HRQoL is expected to be particularly affected in the latter group.
Nonetheless, the large study population and the homogeneous data collection in the BLISS-52 and BLISS-76 trials allowed us to adjust for multiple factors known to impact SLE patients’ HRQoL and factors with confounding potentiality. To our knowledge, this is one of the largest analyses to date of SLE patients’ BMI in relation to HRQoL.
Conclusion
In the present analysis of 1681 patients with SLE, overweight and obesity were highly associated with clinically important HRQoL diminutions. High BMI was found to particularly impact physical HRQoL aspects, as well as fatigue and social functioning among mental aspects. The observed associations were independent of other factors, including disease activity, organ damage, and current treatment. Notably, the impact of BMI on physical aspects of HRQoL and fatigue was more pronounced than that of SLE disease activity, disease duration and organ damage. Longitudinal investigation to address causality is warranted. As a future perspective, results from such surveys could be a prelude to the implementation of weight control strategies as a complementary intervention to current pharmacological management of lupus patients.
Supplementary Material
Acknowledgements
The authors would like to thank GlaxoSmithKline (Uxbridge, UK) for sharing the data from the BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) trials with the Clinical Study Data Request Consortium, as well as all participating patients. Data will be made available by the corresponding author upon reasonable request. A.G., F.H.B. and I.P. were responsible for study conception. A.G., E.Å., Y.E. and I.P. were responsible for study coordination. A.G., F.H.B., P.J., S.S., S.E. and I.P. were responsible for data processing and statistics. A.G., F.H.B. and I.P. drafted the manuscript. A.G., Y.E., S.P. and I.P. were responsible for interpretation of the results. All authors read and critically revised the manuscript for intellectual content, approved its final version prior to submission and agree to be accountable for all aspects of the work.
Funding: This work was supported by the GlaxoSmithKline Investigator-Sponsored Studies programme and grants from the Swedish Rheumatism Association (R-932236), Professor Nanna Svartz Foundation (2019-00290), Ulla and Roland Gustafsson Foundation (2019-12), King Gustaf V 80-year Anniversary Foundation (FAI-2019-0635), Region Stockholm and Karolinska Institutet.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Tselios K, Gladman DD, Sheane BJ, Su J, Urowitz M.. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971–2013). Ann Rheum Dis 2019;78:802–6. [DOI] [PubMed] [Google Scholar]
- 2. Azizoddin DR, Gandhi N, Weinberg S. et al. Fatigue in systemic lupus: the role of disease activity and its correlates. Lupus 2019;28:163–73. [DOI] [PubMed] [Google Scholar]
- 3. Pettersson S, Lövgren M, Eriksson LE. et al. An exploration of patient-reported symptoms in systemic lupus erythematosus and the relationship to health-related quality of life. Scand J Rheumatol 2012;41:383–90. [DOI] [PubMed] [Google Scholar]
- 4. Campbell R Jr, Cooper GS, Gilkeson GS.. Two aspects of the clinical and humanistic burden of systemic lupus erythematosus: mortality risk and quality of life early in the course of disease. Arthritis Rheum 2008;59:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu LW, Zhang T, Pan HF, Li XP, Ye DQ.. BMI, disease activity, and health-related quality-of-life in systemic lupus erythematosus. Clin Rheumatol 2010;29:1413–7. [DOI] [PubMed] [Google Scholar]
- 6. Tamayo T, Fischer-Betz R, Beer S, Winkler-Rohlfing B, Schneider M.. Factors influencing the health related quality of life in patients with systemic lupus erythematosus: long-term results (2001–2005) of patients in the German Lupus Erythematosus Self-Help Organization (LULA Study). Lupus 2010;19:1606–13. [DOI] [PubMed] [Google Scholar]
- 7. Calderon J, Flores P, Aguirre JM. et al. Impact of cognitive impairment, depression, disease activity, and disease damage on quality of life in women with systemic lupus erythematosus. Scand J Rheumatol 2017;46:273–80. [DOI] [PubMed] [Google Scholar]
- 8. Chaigne B, Chizzolini C, Perneger T. et al. Impact of disease activity on health-related quality of life in systemic lupus erythematosus—a cross-sectional analysis of the Swiss Systemic Lupus Erythematosus Cohort Study (SSCS). BMC Immunol 2017;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rizk A, Gheita TA, Nassef S, Abdallah A.. The impact of obesity in systemic lupus erythematosus on disease parameters, quality of life, functional capacity and the risk of atherosclerosis. Int J Rheum Dis 2012;15:261–7. [DOI] [PubMed] [Google Scholar]
- 10. Oeser A, Chung CP, Asanuma Y, Avalos I, Stein CM.. Obesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosus. Arthritis Rheum 2005;52:3651–9. [DOI] [PubMed] [Google Scholar]
- 11. Navarra SV, Guzmán RM, Gallacher AE. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 12. Furie R, Petri M, Zamani O. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petri M, Kim MY, Kalunian KC. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. [DOI] [PubMed] [Google Scholar]
- 14. Gladman D, Ginzler E, Goldsmith C. et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 15. Ware J, Sherbourne C.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 16.EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 17. Webster K, Cella D, Yost K.. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ware J. SF-36 health survey update. Spine 2000;25:3130–9. [DOI] [PubMed] [Google Scholar]
- 19. Shaw JW, Johnson JA, Coons SJ.. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–20. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: World Health Organization, 2000. [PubMed] [Google Scholar]
- 21. Strand V, Crawford B.. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Exp Rev Pharmacoecon Outcomes Res 2005;5:317–26. [DOI] [PubMed] [Google Scholar]
- 22. Strand V, Levy RA, Cervera R. et al. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Ann Rheum Dis 2014;73:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pettersson S, Lundberg IE, Liang MH, Pouchot J, Henriksson EW.. Determination of the minimal clinically important difference for seven measures of fatigue in Swedish patients with systemic lupus erythematosus. Scand J Rheumatol 2015;44:206–10. [DOI] [PubMed] [Google Scholar]
- 24. Bangert E, Wakani L, Merchant M, Strand V, Touma Z.. Impact of belimumab on patient-reported outcomes in systemic lupus erythematosus: review of clinical studies. Patient Relat Outcome Meas 2019;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coretti S, Ruggeri M, McNamee P.. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res 2014;14:221–33. [DOI] [PubMed] [Google Scholar]
- 26. Parodis I, Lopez Benavides AH, Zickert A. et al. The impact of belimumab and rituximab on health-related quality of life in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2019;71:811–21. [DOI] [PubMed] [Google Scholar]
- 27. Alarcon GS, McGwin G Jr, Uribe A. et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum 2004;51:465–74. [DOI] [PubMed] [Google Scholar]
- 28. Mina R, Klein-Gitelman MS, Nelson S. et al. Effects of obesity on health-related quality of life in juvenile-onset systemic lupus erythematosus. Lupus 2015;24:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Zwaan M, Petersen I, Kaerber M. et al. Obesity and quality of life: a controlled study of normal-weight and obese individuals. Psychosomatics 2009;50:474–82. [DOI] [PubMed] [Google Scholar]
- 30. Busutil R, Espallardo O, Torres A. et al. The impact of obesity on health-related quality of life in Spain. Health Qual Life Outcomes 2017;15:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hopman WM, Berger C, Joseph L. et al. The association between body mass index and health-related quality of life: data from CaMos, a stratified population study. Qual Life Res 2007;16:1595–603. [DOI] [PubMed] [Google Scholar]
- 32. Williams P, Goldberg D.. A user’s guide to the General Health Questionnaire. Slough: NFER-Nelson, 1988. [Google Scholar]
- 33. Singer MA, Hopman WM, MacKenzie TA.. Physical functioning and mental health in patients with chronic medical conditions. Qual Life Res 1999;8:687–91. [DOI] [PubMed] [Google Scholar]
- 34. Burgos PI, Alarcon GS, McGwin G Jr. et al. Disease activity and damage are not associated with increased levels of fatigue in systemic lupus erythematosus patients from a multiethnic cohort: LXVII. Arthritis Rheum 2009;61:1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider M, Mosca M, Pego-Reigosa JM. et al. Understanding remission in real-world lupus patients across five European countries. Lupus 2016;25:505–12. [DOI] [PubMed] [Google Scholar]
- 36. Choi ST, KJ Park IH, Lee YW. et al. Subscale analysis of quality of life in patients with systemic lupus erythematosus: association with depression, fatigue, disease activity and damage. Clin Exp Rheumatol 2012;30:665–72. [PubMed] [Google Scholar]
- 37. Parodis I, Sjowall C, Jonsen A. et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev 2017;16:343–51. [DOI] [PubMed] [Google Scholar]
- 38. Thumboo J, Fong KY, Ng TP. et al. Validation of the MOS SF-36 for quality of life assessment of patients with systemic lupus erythematosus in Singapore. J Rheumatol 1996;26:97–102. [PubMed] [Google Scholar]
- 39. Stoll T, Gordon C, Seifert B. et al. Consistency and validity of patient administered assessment of quality of life by the MOS SF-36; its association with disease activity and damage in patients with systemic lupus erythematosus. J Rheumatol 1997;24:1608–14. [PubMed] [Google Scholar]
- 40. Wang SL, Wu B, Zhu LA. et al. Construct and criterion validity of the Euro Qol-5D in patients with systemic lupus erythematosus. PLoS One 2014;9:e98883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC.. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 2007;7:541–6. [DOI] [PubMed] [Google Scholar]
- 42. Crosby RD, Kolotkin RL, Williams GR.. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol 2003;56:395–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.