Abstract
Objectives
To examine which composite measures are most sensitive to change when measuring psoriatic arthritis (PsA) disease activity, analyses compared the responsiveness of composite measures used in a 48-week randomized, controlled trial of MTX and etanercept in patients with PsA.
Methods
The trial randomised 851 patients to receive weekly: MTX (20 mg/week), etanercept (50 mg/week) or MTX plus etanercept. Dichotomous composite measures examined included ACR 20/50/70 responses, minimal disease activity (MDA) and very low disease activity (VLDA). Continuous composite measures examined included Disease Activity Score (28 joints) using CRP (DAS28-CRP), Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), Disease Activity for Psoriatic Arthritis (DAPSA) and Psoriatic Arthritis Disease Activity Score (PASDAS).
Results
At week 24, etanercept-treated groups were significantly more effective than MTX monotherapy to achieve ACR 20 (primary end point) and MDA (key secondary end point). When examining score changes from baseline at week 24 across the five continuous composite measures, PASDAS demonstrated relatively greater changes in the etanercept-treated groups compared with MTX monotherapy and had the largest effect size and standardized response. Joint count changes drove overall score changes at week 24 from baseline in all the continuous composite measures except for PASDAS, which was driven by the Physician and Patient Global Assessments.
Conclusion
PASDAS was the most sensitive continuous composite measure examined with results that mirrored the protocol-defined primary and key secondary outcomes. Composite measures with multiple domains, such as PASDAS, may better quantify change in PsA disease burden.
Trail registration
https://ClinicalTrials.gov, number NCT02376790.
Keywords: psoriatic arthritis, methotrexate, etanercept, composite measures
Rheumatology key messages
The PASDAS composite measure performed more effectively in this trial compared with joint-focused composite measures.
DAS28-CRP, CDAI, SDAI and DAPSA changes are driven by joint counts; PASDAS changes by global assessments.
PASDAS may be the most effective continuous composite measure for evaluating psoriatic arthritis disease activity.
Introduction
Psoriatic arthritis (PsA) is a systemic, immune-mediated disease with multiple manifestations including arthritis, psoriasis, enthesitis and dactylitis [1]. Because composite measures provide a summary outcome across multiple disease manifestations at a specific time point [2], they may be particularly useful for evaluating disease activity and advising treatment decisions in conditions such as PsA that have multi-organ system manifestations [3]. Composite measures initially used for PsA included the ACR response criteria [4] and Disease Activity Score (28 joints) (DAS28) [5, 6], which were developed for use in rheumatoid arthritis, with an emphasis on peripheral arthritis [7]. Composite measures later developed for PsA included the Disease Activity [Index] in PsA (DAPSA) [8], which evaluates a 66/68-joint count that is recommended for PsA; the PsA Disease Activity Score (PASDAS) [9], which includes the 66/68-joint count as well as non-articular domains such as enthesitis and dactylitis; and the Composite Psoriatic Disease Activity Index (CPDAI) [10], which includes non-articular domains such as enthesitis, dactylitis and skin disease [9–11]. DAS28, DAPSA, CPDAI and PASDAS are all continuous measures where remission is defined as a level below a set cutoff value. Minimal disease activity (MDA) is another composite measure developed for use in PsA that accounts for more than joint involvement alone but is a dichotomous measure representing a state of disease activity [12, 13].
Several prior interventional trials have reported PASDAS to be the most sensitive to change of those composite measures examined [3, 14]. However, it has yet to be definitively determined which composite measures have the greatest sensitivity for measuring change in PsA disease activity and the strongest relationship to patient outcomes. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and Outcome Measures in Rheumatology (OMERACT) were unable to reach a consensus in 2018 as to which composite measures to recommend for use in PsA and instead recommended that these measures be further compared and validated [7].
The objective of the study described here was to compare the responsiveness and psychometric properties (e.g. effect size and standardized response) of composite measures commonly used in PsA. To address this objective, the performance characteristics of the composite measures used in The Study of Etanercept And Methotrexate in Combination or as Monotherapy in Subjects with Psoriatic Arthritis (SEAM-PsA) were examined. This phase 3, double-blind, 48-week, randomized, controlled trial (RCT) was the first study to directly compare MTX with etanercept in patients with PsA by investigating the efficacy of MTX monotherapy compared with both etanercept monotherapy and MTX combined with etanercept [15, 16]. SEAM-PsA is an ideal dataset for examining PsA composite measures as it is a robust trial with over 280 patients in each of the three treatment groups and collected data based on five continuous composite measures [DAS28, Clinical Disease Activity Index (CDAI), Simplified Disease Activity Index (SDAI), DAPSA and PASDAS].
To more thoroughly examine the composite measure data collected during the SEAM-PsA trial, the exploratory analyses described here investigated the relative performance of all five continuous composite measures used in SEAM-PsA and analysed which individual components within each composite measure contributed the most input to the change in composite measure outcomes. Because previous studies comparing PsA composite measures used data from placebo-controlled trials [3, 14], this is the first analysis comparing the performance of composite measures in a PsA trial with an active-comparator design.
Methods
Study design and patient population
The SEAM-PsA study design has been published [15, 16]. In brief, this 48-week, double-blind, international RCT enrolled patients ≥18 years old with active PsA [based on Classification Criteria for Psoriatic Arthritis (CASPAR)] [17], naïve to etanercept and other biologic agents and with no prior use of MTX for PsA (prior treatment with MTX was allowed for psoriasis). At screening and baseline, patients had to have ≥ three tender and ≥ three swollen joints (66/68-joint count) and an active psoriatic skin lesion ≥2 cm in diameter. Patients were randomized 1:1:1 to receive weekly treatment with: (i) oral MTX (target of 20 mg/week) plus subcutaneous placebo, (ii) subcutaneous etanercept (50 mg/week) plus oral placebo, or (iii) subcutaneous etanercept (50 mg/week) plus oral MTX (target of 20 mg/week). MTX, supplied as 2.5-mg tablets in capsules, was initiated at 10 mg/week and titrated up to 20 mg/week over a 4-week period. The MTX dose could be reduced to as low as 10 mg/week in response to MTX-related intolerability or toxicities.
On or after week 24, patients with inadequate responses (defined as <20% improvements from baseline in tender and swollen joint counts) received rescue therapy with etanercept plus MTX until week 48. Because rescue therapy was administered on or after week 24, efficacy analyses described here focused on week-24 outcomes.
The SEAM-PsA RCT was conducted in accordance with the Helsinki Declaration with all patients providing written, informed consent and each participating site obtaining protocol approval by an institutional review board.
Study endpoints
The five dichotomous composite measures employed were: ACR 20 [4], ACR 50 [18], ACR 70 [18], MDA [13] and very low disease activity (VLDA) [19]. The five continuous composite measures employed were: DAS28-CRP [5, 6], CDAI [20], SDAI [21], DAPSA [8] and PASDAS [9]. Supplementary Table S1, available at Rheumatology online lists the components of each composite measure and how results for each measure are calculated. The primary end point was the percentage of patients achieving a 20% improvement in ACR criteria (ACR 20 response) at week 24; the key secondary end point was the percentage of patients achieving MDA at week 24. Secondary endpoints included the percentages of patients achieving ACR 50, ACR 70 and VLDA at week 24 as well as changes from baseline at week 24 in DAS28-CRP, CDAI, SDAI, DAPSA and PASDAS. A publication of the primary results of this trial reported that at week 24, etanercept monotherapy and etanercept plus MTX were statistically significantly more effective than MTX monotherapy in the percentage of patients achieving an ACR 20 response (primary end point) and MDA (key secondary end point) [15]. Mean improvements from baseline at week 24 in PASDAS scores were greater in the etanercept-treated groups compared with MTX monotherapy, while only modest differences were evident between the three treatment groups for the DAPSA score mean change from baseline [15]. Safety results at week 48 indicated no new safety signals associated with use of etanercept or MTX; rates of nausea were higher in the two MTX-treated groups compared with etanercept monotherapy [15].
Statistical analyses
SEAM-PsA was powered to examine the ACR 20 primary end point and the MDA key secondary end point at week 24. All other efficacy endpoints were analysed as observed and without adjustment for multiplicity; therefore, P-values for these other efficacy endpoints are considered descriptive. The Cochran-Mantel-Haenszel test was used for all between-treatment comparisons and was adjusted using stratification factors of baseline body mass index status of ≤30 kg/m2 or >30 kg/m2 and prior use of a non-biologic disease-modifying antirheumatic drug.
To compare the performance of the continuous composite measures (DAS28-CRP, CDAI, SDAI, DAPSA and PASDAS), exploratory analyses examined the effect sizes and standardized responses at week 24 in each treatment group. To calculate the effect size of each continuous composite measure, the following formula was used: (baseline mean − post baseline mean)/s.d. of baseline mean [22]. To calculate the standardized response of each composite measure, the following formula was used: (baseline mean − post baseline mean)/s.d. of change from baseline for that visit in the same treatment group [22].
Exploratory analyses also examined the contribution of the change of individual components within each continuous composite measure to the overall changes in each composite score from baseline to week 24 using the following formula: [change from baseline in each component score/change from baseline in overall score]. For the PASDAS composite measure, analyses of drivers of composite results were performed on the full analysis set (all randomized patients), in a patient subgroup with Leeds Enthesitis Index of >0 at baseline, and in another patient subgroup with Tender Dactylitis Count >0 at baseline. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
In SEAM-PsA, 284 patients were randomised to MTX monotherapy, 284 to etanercept monotherapy and 283 to MTX plus etanercept (combination therapy); 691 patients (81.2% of those enrolled) completed the trial [15]. Baseline disease characteristics and disease activity were generally well balanced across the three treatment groups (Table 1). In this trial, 90.7% of patients were white and the mean (s.d.) age was 48.4 (13.1) years. Most patients were early in the course of their disease as the mean (s.d.) duration of PsA was 3.2 (6.3) years (median 0.6 years), with 56% of patients having disease duration of ≤2 years. Forty-two patients had received prior MTX for psoriasis. During study weeks 4–24, the mean MTX dose maintained by patients in the MTX-treated groups was >18.8 mg (median 20 mg).
Table 1.
Baseline characteristics and disease activity
| MTX monotherapy (n = 284) |
Etanercept monotherapy (n = 284) |
Combination therapy (n = 283) |
|
|---|---|---|---|
| Age in years, mean (s.d.) | 48.7 (13.1) | 48.5 (13.5) | 48.1 (12.7) |
| Female sex, n (%) | 160 (56.3) | 133 (46.8) | 139 (49.1) |
| White race, n (%) | 255 (89.8) | 252 (88.7) | 265 (93.6) |
| Duration of PsA in years, mean (s.d.) [n]a | 3.6 (6.8) [231] | 3.1 (6.0) [222] | 3.0 (6.0) [231] |
| Median (Q1, Q3) [n] | 0.9 (0.1, 3.3) [231] | 0.6 (0.1, 3.0) [222] | 0.5 (0.1, 3.0) [231] |
| Prior use of nonbiologic DMARD, n (%) | 38 (13.4) | 26 (9.2) | 43 (15.2) |
| Body mass index (kg/m2), mean (s.d.) [n] | 30.6 (7.1) [284] | 30.4 (6.6) [283] | 30.0 (6.7) [283] |
| ≤30 kg/m2, n (%) | 146 (51.4) | 153 (53.9) | 160 (56.5) |
| >30 kg/m2, n (%) | 138 (48.6) | 130 (45.8) | 123 (43.5) |
| CRP, mean (s.d.) mg/L [n] | 10.5 (16.3) [284] | 10.7 (15.6) [282] | 8.7 (11.6) [283] |
| mTSS, mean (s.e.) [n] | 2.76 (0.12) [269] | 2.97 (0.13) [273] | 2.70 (0.12) [274] |
| Psoriasis-affected BSA, mean % (s.d.) | 12.7 (18.8) | 10.8 (14.7) | 10.7 (15.6) |
| sPGA, mean (s.d.) [n] | 2.6 (1.1) [281] | 2.6 (1.0) [284] | 2.5 (1.0) [283] |
| Swollen Joint Count (66 joints), mean (s.d.) [n] | 12.9 (9.9) [284] | 11.5 (9.6) [283] | 11.2 (9.1) [282] |
| Tender Joint Count (68 joints), mean (s.d.) [n] | 20.9 (15.0) [284] | 18.8 (14.5) [283] | 20.0 (15.3) [282] |
| Swollen Joint Count (28 joints), mean (s.d.) [n] | 7.7 (5.4) [284] | 6.8 (5.4) [283] | 6.7 (5.0) [282] |
| Tender Joint Count (28 joints), mean (s.d.) [n] | 10.9 (7.4) [284] | 9.5 (7.0) [283] | 9.9 (7.4) [282] |
| Tender Dactylitis Count, mean (s.e.) [n] | 2.3 (0.2) [284] | 2.2 (0.2) [283] | 2.4 (0.3) [282] |
| Leeds Dactylitis Index Score >0 at baseline, n (%) | 98 (34.5) | 96 (33.8) | 90 (31.8) |
| Mean (s.e.) [n] for patients with >0 at baseline | 164.9 (26.9) [98] | 147.6 (20.8) [96] | 138.2 (23.9) [90] |
| Leeds Enthesitis Index Score, mean (s.e.) [n] | 1.5 (0.1) [284] | 1.6 (0.1) [283] | 1.7 (0.1) [282] |
| SPARCC Enthesitis Score >0 at baseline, n (%) | 191 (67.3) | 189 (66.5) | 196 (69.3) |
| Mean (s.e.) [n] for patients with >0 at baseline | 5.7 (0.3) [191] | 5.5 (0.3) [189] | 5.9 (0.3) [196] |
| Physician Global Assessment (0–100), mean (s.d.) [n] | 58.6 (19.4) [284] | 58.3 (18.2) [284] | 58.0 (17.8) [282] |
| Patient Global Assessment (0–100), mean (s.d.) [n] | 60.7 (22.5) [283] | 62.9 (22.1) [284] | 61.0 (20.8) [282] |
| Patient Global Assessment of pain (0–100), mean (s.d.) [n] | 56.1 (21.7) [283] | 56.5 (22.3) [284] | 55.7 (21.6) [282] |
| SF-36 PCS, mean (s.d.) [n] | 35.6 (8.4) [282] | 37.8 (8.4) [284] | 37.4 (9.2) [282] |
| DAS28-CRP, mean (s.d.) [n]; scores range from 2 to 10 | 4.93 (1.11) [283] | 4.80 (1.13) [281] | 4.75 (1.12) [281] |
| CDAI, mean (s.d.) [n]; scores range from 0 to 76 | 30.51 (13.26) [283] | 28.45 (12.89) [283] | 28.55 (12.71) [281] |
| SDAI, mean (s.d.) [n]; scores range from 0 to 86 | 31.56 (13.52) [283] | 29.52 (13.19) [281] | 29.43 (12.90) [281] |
| DAPSA, mean (s.e.) [n]; scores range from 0 to 144 + CRP | 46.5 (1.4) [283] | 43.4 (1.4) [281] | 43.8 (1.4) [281] |
| PASDAS, mean (s.e.) [n]; scores range from 0 to 10 | 6.09 (0.07) [282] | 6.05 (0.07) [279] | 6.04 (0.07) [280] |
[n] is the number of patients analyzed for mean values (if the number is different from the full analysis set). BSA: body surface area; CDAI: Clinical Disease Activity Index; DAPSA: Disease Activity Index for Psoriatic Arthritis; DAS28-CRP: Disease Activity Score (28 joints) using CRP; DMARD: disease-modifying antirheumatic drug; mTSS: van der Heijde modified Total Sharp Score; PASDAS: Psoriatic Arthritis Disease Activity Score; PsA: psoriatic arthritis; Q1: first quartile; Q3: third quartile; SDAI: Simplified Disease Activity Index; SF-36 PCS: Short Form 36 (health survey) Physical Component Summary; SPARCC: Spondyloarthritis Research Consortium of Canada; sPGA: static Physician Global Assessment.
Efficacy outcomes from the dichotomous and continuous composite measures
Table 2 summarizes the percentages of patients who achieved dichotomous composite measure outcomes (ACR 20/50/70, MDA and VLDA) at week 24 [15] and the mean changes from baseline at week 24 in the continuous composite measures (DAS28-CRP, CDAI, SDAI, DAPSA and PASDAS). As previously reported, more patients achieved ACR/MDA/VLDA responses at week 24 in the etanercept-treated groups compared with MTX monotherapy [15] (Table 2). Similarly, greater mean changes from baseline at week 24 were evident in the etanercept-treated groups compared with MTX monotherapy in the DAS28-CRP and PASDAS continuous composite measures (Table 2). Among the continuous composite measures, differences between the etanercept-treated groups and MTX monotherapy were most pronounced in PASDAS (P <0.001 for MTX monotherapy vs etanercept monotherapy and P <0.001 for MTX monotherapy vs combination therapy) and were consistent with the efficacy results obtained using the dichotomous composite measures (Table 2). Mean changes from baseline at week 24 in the articular-focused measures of CDAI, SDAI and DAPSA were similar across all three treatment groups though numerically higher in the etanercept-treated groups compared with MTX monotherapy (Table 2). The efficacy outcomes of the composite measures at weeks 12 and 48 (data not shown) produced comparable trends to those seen at week 24, indicating that the composite measures had stable performance characteristics over time in this trial. The only difference was a greater improvement that occurred in the etanercept-containing arms during the first 3 months, which resulted in a larger difference between the MTX monotherapy and etanercept-containing arms at week 12.
Table 2.
Composite endpoint responses at week 24
| Composite endpoint responsea | MTX monotherapy (n = 284) |
Etanercept monotherapy (n = 284) |
P-valueb for MTX monotherapy vs etanercept monotherapy | Combination therapy (n = 283) |
P-valueb for MTX monotherapy vs combination therapy |
|---|---|---|---|---|---|
| ACR 20, n/N (%) | 144/284 (50.7) | 173/284 (60.9) | P =0.029 | 184/283 (65.0) | P =0.005 |
| ACR 50, n/N (%) | 77/252 (30.6) | 114/257 (44.4) | P=0.006 | 117/256 (45.7) | P<0.001 |
| ACR 70, n/N (%) | 35/253 (13.8) | 75/257 (29.2) | P<0.001 | 71/256 (27.7) | P<0.001 |
| MDA, n/N (%) | 65/284 (22.9) | 102/284 (35.9) | P =0.005 | 101/283 (35.7) | P =0.005 |
| VLDA, n/N (%) | 12/252 (4.8) | 39/257 (15.2) | P<0.001 | 37/258 (14.3) | P<0.001 |
| DAS28-CRP, mean (s.e.) change from baseline [n]a | −1.55 (0.08) [251] | −1.97 (0.08) [253] | P<0.001 | −1.86 (0.08) [256] | P=0.01 |
| CDAI, mean (s.e.) change from baseline [n] | −15.74 (0.85) [249] | −17.12 (0.78) [257] | P=0.26 | −16.43 (0.85) [256] | P=0.59 |
| SDAI, mean (s.e.) change from baseline [n] | −15.96 (0.86) [248] | −17.75 (0.81) [253] | P=0.15 | −17.01 (0.87) [256] | P=0.41 |
| DAPSA, mean (s.e.) change from baseline [n] | −22.59 (1.4) [251] | −24.99 (1.3) [253] | P=0.24 | −24.92 (1.4) [256] | P=0.23 |
| PASDAS, mean (s.e.) change from baseline [n] | −1.98 (0.10) [246] | −2.64 (0.10) [250] | P<0.001 | −2.63 (0.11) [255] | P<0.001 |
[n] is the number of patients examined for mean values if different from the primary analysis set.
P-values in bold had statistical significance; all others are unadjusted and are italicized.
CDAI: Clinical Disease Activity Index; DAPSA: Disease Activity Index for Psoriatic Arthritis; DAS28-CRP: Disease Activity Score (28 joints) using CRP; MDA: Minimal Disease Activity; PASDAS: Psoriatic Arthritis Disease Activity Score; SDAI: Simplified Disease Activity Index; VLDA: Very Low Disease Activity.
Relative performance of the continuous composite measures
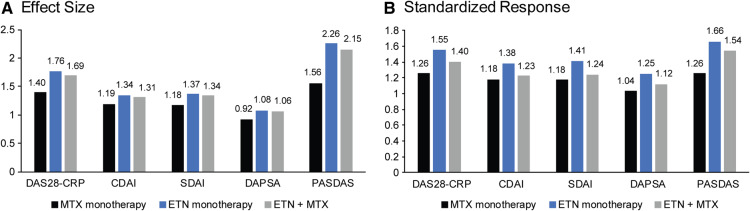
To examine the relative performance of the continuous composite measures, effect sizes and standardized responses were calculated for each measure. Results indicated that the etanercept-treated groups had numerically larger effect sizes and standardized responses than MTX monotherapy across all five continuous composite measures (Fig. 1A and B).
Fig. 1.
Effect size and standardized response of the composite measures by treatment group at week 24.
Effect Size = (baseline mean − post baseline mean)/s.d. of baseline mean. Standardized Response = (baseline mean − post baseline mean)/ s.d. of change from baseline for that visit in the same treatment group. CDAI: Clinical Disease Activity Index; DAPSA: Disease Activity Index for Psoriatic Arthritis; DAS28-CRP: Disease Activity Score (28 joints) using CRP; ETN: etanercept; PASDAS: Psoriatic Arthritis Disease Activity Score; SDAI: Simplified Disease Activity Index.
The most pronounced difference between the etanercept-treated groups and MTX monotherapy was with the PASDAS composite measure. For the effect sizes (Fig. 1A), the difference between etanercept monotherapy and MTX monotherapy ranged from 0.15–0.36 across the four joint-focused measures and was 0.7 with PASDAS; the difference between combination therapy and MTX monotherapy ranged from 0.12–0.29 across the four joint-focused measures and was 0.59 with PASDAS. The difference in standardized responses between etanercept monotherapy and MTX monotherapy (Fig. 1B), ranged from 0.20–0.29 across the four joint-focused measures and was 0.4 with PASDAS; the difference between combination therapy and MTX monotherapy ranged from 0.05–0.14 across the four joint-focused measures and was 0.28 with PASDAS. These data further indicate that of the five continuous composite measures examined, PASDAS generated results that most closely mirrored those obtained with the dichotomous composite measures.
Degree of individual component contribution to the continuous composite measures
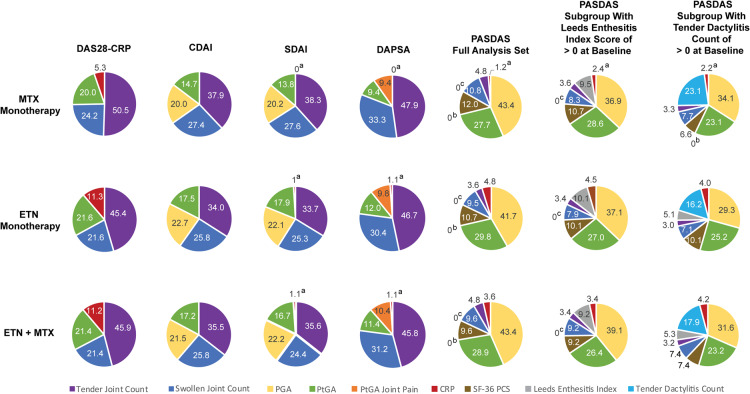
Analyses examined the contribution of each component change to the overall changes in continuous composite scores from baseline at week 24. Across DAPSA, DAS28-CRP, CDAI and SDAI, the main drivers were changes in Tender and Swollen Joint Counts with much less contribution made by changes in Physician and Patient Global Assessments, Patient Global Assessment of Joint Pain, and CRP (Table 3). In contrast, the main drivers of results in PASDAS were changes in the Physician and Patient Global Assessments of disease activity (Table 4). Changes in the Leeds Enthesitis Index and Tender Dactylitis Count contributed little to the PASDAS results when analysing the full patient analysis set. However, the contribution of changes in the Leeds Enthesitis Index to the PASDAS score became more prominent when a patient subset with Leeds Enthesitis Index scores >0 at baseline was analysed (Table 4). Similarly, the contribution of changes in Tender Dactylitis Count to the PASDAS score change notably increased in the patient subgroup with Tender Dactylitis Count >0 at baseline (Table 4). Across the composite measures examined, the contribution of CRP to the overall changes in response to treatment was minor. To more easily visualize the relative contribution of each component to the composite score change at week 24 from baseline, pie charts were generated showing the percentage contribution of each component’s median value to the overall composite score in each treatment group (Fig. 2).
Table 3.
Contribution of each component to the joint-focused composite score change from baseline to week 24
| Median (mean: 95% CI) component contribution to the overall scorea | DAPSA |
DAS28-CRP |
CDAI |
SDAI |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MTX (n = 251) |
ETN (n = 253) |
MTX+ETN (n = 256) |
MTX (n = 251) |
ETN (n = 253) |
MTX+ETN (n = 256) |
MTX (n = 249) |
ETN (n = 257) |
MTX+ETN (n = 256) |
MTX (n = 248) |
ETN (n = 253) |
MTX+ETN (n = 256) |
|
| Tender Joint Countb | 0.46 (0.61:0.18, 1.03) | 0.43 (0.69:0.26, 1.11) | 0.44 (0.4:0.10, 0.69) | 0.48 (0.48:0.34, 0.61) | 0.44(0.68:0.32, 1.04) | 0.45 (0.55:0.30, 0.80) | 0.36 (0.38:0.20, 0.56) | 0.33 (0.33:0.29, 0.38) | 0.33 (−0.02:−0.46, 0.42) | 0.36 (0.43:0.24, 0.61) | 0.32 (0.33:0.29, 0.38) | 0.32 (0.56:−0.13, 1.25) |
| Swollen Joint Countc | 0.32 (0.28:0.02, 0.55) | 0.28 (0.20:0.07, 0.33) | 0.30 (0.52:−0.00, 1.03) | 0.23 (0.22:0.17, 0.27) | 0.21 (0.30:0.17, 0.43) | 0.21 (0.42:0.14, 0.69) | 0.26 (0.33:0.25, 0.40) | 0.25 (0.23:0.19, 0.27) | 0.24 (0.31:0.16, 0.47) | 0.26 (0.33:0.25, 0.41) | 0.24 (0.23:0.19, 0.27) | 0.22 (0.34:0.19, 0.49) |
| Physician Global Assessment | — | — | — | — | — | — | 0.19 (0.27:0.15, 0.39) | 0.22 (0.28:0.17, 0.40) | 0.20 (0.45:0.20, 0.70) | 0.19 (0.42:−0.00, 0.84) | 0.21 (0.11:−0.09, 0.32) | 0.20 (−0.02:−0.63, 0.59) |
| Patient Global Assessment | 0.09 (0.12:−0.02, 0.27) | 0.11 (0.05:−0.15, 0.24) | 0.11 (0.04:−0.08, 0.15) | 0.19 (0.15:−0.00, 0.30) | 0.21 (−0.04:−0.46, 0.37) | 0.21 (−0.09:−0.65, 0.46) | 0.14 (0.03:−0.19, 0.24) | 0.17 (0.15:0.03, 0.27) | 0.16 (0.26:0.11, 0.40) | 0.13 (−0.17:−0.59, 0.26) | 0.17 (0.29:0.10, 0.48) | 0.15 (0.07:−0.14, 0.29) |
| Patient Global Assessment Joint Pain | 0.09 (0.09:−0.03, 0.21) | 0.09 (0.11:0.06, 0.16) | 0.10 (0.05:−0.08, 0.17) | — | — | — | — | — | — | — | — | — |
| C-Reactive Protein |
0.0 (−0.11:−0.37, 0.16) | 0.01 (−0.04:−0.16, 0.08) | 0.01 (0.00:−0.03, 0.04) | 0.05 (0.16:0.05, 0.26) | 0.11 (0.07:−0.03, 0.16) | 0.11 (0.13:0.03, 0.23) | — | — | — | 0.0 (−0.01:−0.05, 0.04) | 0.01 (0.03:0.01, 0.06) | 0.01 (0.05:0.02, 0.08) |
Contributions to overall score are calculated by: [change from baseline in each of the component scores/change from baseline in the overall score]. Positive values indicate changes in the same direction as the change in overall score. Negative values indicate changes in the opposite direction as the change in overall score. Both an individual’s [change in scaled component/change in composite score] for all components and the average across all patients for a given treatment group and a visit’s [change in scaled component/change in composite score] for all components sum to 1. n = number of patients examined.
Represents a 28-joint count except for DAPSA, which had a 68-joint count.
Represents a 28-joint count except for DAPSA, which had a 66-joint count.
CDAI: Clinical Disease Activity Index; DAPSA: Disease Activity Index for Psoriatic Arthritis; DAS28-CRP: Disease Activity Score (28 joints) using CRP; ETN: etanercept; SDAI: Simplified Disease Activity Index.
Table 4.
Contribution of each component to PASDAS composite score change from baseline to week 24
| Median (mean: 95% CI) component contribution to the overall scorea | PASDAS full analysis set |
PASDAS: subgroup with leeds enthesitis index >0 at baseline |
PASDAS: subgroup with tender dactylitis count >0 at baseline |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MTX (n = 246) |
ETN (n = 250) |
MTX+ETN (n = 255) |
MTX (n = 161) |
ETN (n = 165) |
MTX+ETN (n = 177) |
MTX (n = 87) |
ETN (n = 86) |
MTX+ETN (n = 86) |
|
| PGA | 0.36 (−0.47: −1.93, 0.99) | 0.35 (0.08: −0.48, 0.63) | 0.36 (0.41: 0.21, 0.61) | 0.31 (−0.90: −3.12, 1.33) | 0.33 (−0.08: −0.91, 0.76) | 0.34 (0.35: 0.09, 0.61) | 0.31 (−1.85: −6.00, 2.29) | 0.29 (0.21: 0.11, 0.31) | 0.30 (0.29: 0.23, 0.35) |
| PtGA | 0.23 (0.41: −0.13, 0.94) | 0.25 (0.31: 0.19, 0.43) | 0.24 (0.47: −0.04, 0.99) | 0.24 (0.55: −0.27, 1.36) | 0.24 (0.28: 0.12, 0.45) | 0.23 (0.58: −0.17, 1.32) | 0.21 (1.00: −0.44, 2.44) | 0.25 (0.26: 0.22, 0.31) | 0.22 (0.27: 0.14, 0.39) |
| SF-36 PCS | 0.10 (0.01: −0.26, 0.28) | 0.09 (−0.43: −1.24, 0.37) | 0.08 (0.15: 0.01, 0.29) | 0.09 (−0.06: −0.45, 0.33) | 0.09 (−0.69: −1.91, 0.52) | 0.08 (0.21: 0.04, 0.39) | 0.06 (0.15: 0.04, 0.25) | 0.10 (0.10: 0.08, 0.12) | 0.07 (0.15: −0.01, 0.31) |
| Swollen Joint Count (66) | 0.09 (0.10: 0.05, 0.15) | 0.08 (0.03: −0.07, 0.13) | 0.08 (−0.06: −0.33, 0.21) | 0.07 (0.07: −0.01, 0.14) | 0.07 (−0.01: −0.16, 0.14) | 0.08 (−0.11: −0.49, 0.28) | 0.07 (0.13: 0.05, 0.20) | 0.07 (0.06: 0.04, 0.08) | 0.07 (0.12: 0.06, 0.18) |
| Tender Joint Count (68) | 0.04 (0.02: −0.01, 0.05) | 0.03 (0.05: −0.02, 0.12) | 0.04 (−0.02: −0.18, 0.13) | 0.03 (0.03: 0.01, 0.05) | 0.03 (0.05: −0.05, 0.16) | 0.03 (−0.07: −0.29, 0.15) | 0.03 (0.02: −0.01, 0.05) | 0.03 (0.02: 0.01, 0.04) | 0.03 (0.01: −0.01, 0.04) |
| Leeds Enthesitis Index | 0.0 (0.68: −0.22, 1.58) | 0.0 (0.54: −0.48, 1.56) | 0.0 (−0.07: −0.32, 0.18) | 0.08 (1.04: −0.34, 2.41) | 0.09 (0.83: −0.71, 2.37) | 0.08 (−0.11: −0.46, 0.25) | 0.0 (0.71: −0.68, 2.11) | 0.05 (0.04: −0.01, 0.09) | 0.05 (0.05: 0.04, 0.07) |
| Tender Dactylitis Count | 0.0 (0.47: −0.13, 1.08) | 0.0 (0.25: −0.00, 0.51) | 0.0 (0.17: −0.09, 0.43) | 0.0 (0.55: −0.32, 1.42) | 0.0 (0.37: −0.02, 0.76) | 0.0 (0.20: −0.17, 0.58) | 0.21 (0.97: −0.65, 2.58) | 0.16 (0.27: 0.11, 0.42) | 0.17 (0.20: 0.14, 0.25) |
| C-Reactive Protein |
0.01 (−0.23: −0.53, 0.08) | 0.04 (0.17: −0.00, 0.35) | 0.03 (−0.04: −0.15, 0.06) | 0.02 (−0.28: −0.74, 0.17) | 0.04 (0.24: −0.03, 0.50) | 0.03 (−0.07: −0.22, 0.07) | 0.02 (−0.13: −0.48, 0.21) | 0.04 (0.04: 0.02, 0.05) | 0.04 (−0.09: −0.36, 0.18) |
Contributions to overall score are calculated by [change from baseline in each of the component scores/change from baseline in the overall score]. Positive values indicate changes in the same direction as the change in overall score. Negative values indicate changes in the opposite direction as the change in overall score. Both an individual’s [change in scaled component/change in composite score] for all components and the average across all patients for a given treatment group and a visit’s [change in scaled component/change in composite score] for all components sum to 1. n = number of patients examined.
ETN: etanercept; PASDAS: Psoriatic Arthritis Disease Activity Score; PGA: Physician Global Assessment; PtGA: Patient Global Assessment; SF-36 PCS: Short Form 36 (health survey) Physical Component Summary.
Fig. 2.
Median percent contribution of each component to composite score change from baseline to week 24
Effect Size = (baseline mean − post baseline mean)/s.d. of baseline mean. Standardized Response = (baseline mean − post baseline mean)/ s.d. of change from baseline for that visit in the same treatment group. CDAI: Clinical Disease Activity Index; DAPSA: Disease Activity Index for Psoriatic Arthritis; DAS28-CRP: Disease Activity Score (28 joints) using CRP; ETN: etanercept; PASDAS: Psoriatic Arthritis Disease Activity Score; SDAI: Simplified Disease Activity Index.
Mean values generated for the contribution of each component to the change in continuous composite scores at week 24 from baseline showed a lack of consistency between treatment groups for each component (Tables 3 and 4). Large CIs associated with the mean values suggest that data were skewed and/or that the variation in patient disease characteristics was high. In contrast, median values for the contribution of each component to composite score changes from baseline to week 24 were fairly similar between treatment groups for each of the components.
Discussion
The analyses described here compared the responsiveness of the DAS28-CRP, CDAI, SDAI, DAPSA and PASDAS continuous composite measures used in the SEAM-PsA trial. Overall, the PASDAS results were the most consistent with the ACR 20 and MDA results. In addition to most closely mirroring the protocol-defined primary and key secondary endpoints, PASDAS also showed the largest treatment differences and largest standardized response in this study. These findings indicate that of the continuous composite measures examined, PASDAS may have the strongest ability to detect treatment-associated changes in PsA disease activity. Several other trials in which different PsA continuous composite measures [including DAS28, DAPSA, PASDAS, CPDAI and GRAppa Composite Score (GRACE)] were compared have also concluded that PASDAS performed the best of the measures examined [3, 11, 14, 23]. In contrast to these previously published analyses that examined data from placebo-controlled studies, SEAM-PsA is one of the first active-comparator studies in PsA that utilized composite measures to examine disease activity. Though composite measures may perform differently in active-comparator studies compared with placebo-controlled RCTs, data from SEAM-PsA were consistent with previous studies in indicating that PASDAS was the most responsive of the composite measures examined.
Our analysis of which individual components drive the results for the five continuous composite measures showed that DAPSA, DAS28-CRP, CDAI and SDAI were mainly driven by joint counts, while PASDAS was mainly driven by the global assessments. When examining the contribution of each individual component change to the overall changes in composite scores from baseline to week 24, there were differences in the consistency of results across treatment groups when comparing mean and median values. The wide CIs associated with the mean changes may be owing to inclusion, in this global study, of patients that had different disease patterns and/or variability in disease duration before receiving treatment. The fact that different people completed the assessments may also be a contributing factor. The median values for each individual component change showed more consistency between treatment groups, suggesting that the use of medians may better represent group data. The median changes indicated that the type of treatment received in each treatment group appeared to have little effect on the relative contributions of changes in individual components to the overall changes in composite scores from baseline to week 24.
Though DAPSA and PASDAS were both developed specifically for use in PsA [8, 9], the SEAM-PsA results described here indicated that PASDAS was consistently the measure most sensitive to change and that DAPSA was not able to discriminate between the treatment groups. DAPSA was designed to measure peripheral arthritis and does not include a measure of enthesitis or dactylitis. In contrast, PASDAS includes both specific assessments of enthesitis and dactylitis severity as well as global assessments, which are likely to reflect disease activity in multiple domains. That PASDAS may be adaptive to the influence of various PsA manifestations was reflected in how those patient subgroups with enthesitis or dactylitis count >0 at baseline produced results showing a higher input of the enthesitis or dactylitis component changes to the overall composite PASDAS score change at week 24 (Table 4). Overall, the data presented here suggest that composite measures that include multiple domains, such as PASDAS, are better at quantifying the PsA disease burden as they appear to show the greatest sensitivity to change and better represent the breadth of disease manifestations.
Strengths of the analyses presented here include that the data were from a large, active-comparator, double-blind RCT in PsA with over 280 patients in each treatment group, which allowed for comparisons between the treatment groups. In addition, the trial collected data from 10 composite measures used in PsA (five dichotomous measures and five continuous measures). Key limitations of the analyses include that SEAM-PsA was neither designed nor powered for studying continuous composite outcomes individually (thus, the P-values generated for the composite measure analyses were descriptive). In addition, these analyses may have some limitations regarding their generalizability and may be best applicable to the type of patients included in SEAM-PsA with predominantly polyarticular disease, MTX naïve, and with early PsA disease. Though extrapolating findings from an RCT to the real-world population of patients with PsA can be challenging, results from this trial can inform the planning of future trials (especially active-comparator trials) that use composite measures to evaluate PsA therapies administered to patients with early 1disease.
Supplementary Material
Acknowledgements
Linda Rice, PhD at Amgen Inc, assisted in writing the manuscript and preparing the tables and figures. There is a plan to share data. This may include de-identified individual patient data for variables necessary to address the specific research question in an approved data-sharing request; also related data dictionaries, study protocol, statistical analysis plan, informed consent form, and/or clinical study report. Data sharing requests relating to data in this manuscript will be considered after the publication date. There is no end date for eligibility to submit a data sharing request for these data. Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, endpoints/outcomes of interest, statistical analysis plan, data requirements, publication plan, and qualifications of the researcher(s). In general, Amgen does not grant external requests for individual patient data for the purpose of re-evaluating safety and efficacy issues already addressed in the product labelling. A committee of internal advisors reviews requests. If not approved, requests may be further arbitrated by a Data Sharing Independent Review Panel. Requests that pose a potential conflict of interest or an actual or potential competitive risk may be declined at Amgen’s sole discretion and without further arbitration. Upon approval, information necessary to address the research question will be provided under the terms of a data sharing agreement. This may include anonymized individual patient data and/or available supporting documents, containing fragments of analysis code where provided in analysis specifications. Further details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/.
Funding: This work was supported by Amgen Inc, which sponsored the SEAM-PsA trial, designed the trial in collaboration with academic investigators, oversaw data collection, performed the data analyses, and supported the development of this manuscript.
Disclosure statement: L.C.C. has received research grants from AbbVie, Celgene, Lilly, Novartis, and Pfizer and consulting fees from AbbVie, Amgen, Celgene, Galapagos, Gilead, Janssen, Lilly, MSD, Novartis, Pfizer and UCB; J.F.M. has been a consultant and/or investigator for AbbVie, Aclaris, Almirall, Biogen, Celgene, Dermavant, EMD Serono, Incyte, Janssen, Kyowa Kirin Co, Leo Pharma, Lilly, Merck Research Laboratories, Novartis, Pfizer, Sanofi Regeneron, Sun Pharma and UCB. He is also a member of the Burrage Capital Management Boston Advisory Board; P.J.M. has received research grants from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer, Sun and UCB. He has been a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Sun and UCB and a speaker for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Lilly, Novartis, Pfizer and UCB; A.O. has received research grant support from Amgen, Novartis and Pfizer and consulting fees from AbbVie, Amgen, BMS, Celgene, Corrona, Janssen, Lilly, Pfizer and Novartis (Dr Ogdie’s spouse has received royalties from Novartis); D.D.G. has received research grants from AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis, Pfizer and UCB and consulting fees from AbbVie, Amgen, BMS, Celgene, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer and UCB; V.S. has received consulting fees from AbbVie, Amgen, AstraZeneca, Bayer, Bioventus, Blackrock, Boehringer Ingelheim, BMS, Celltrion, Corrona, Crescendo, EMDSerono, Flexion, Genentech/Roche, Glenmark, GSK, Inmedix, Janssen, Kezar, Kypha, Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Samumed, Sandoz, Sanofi, Selecta, Setpoint and UCB; L.L., P.K.Y., D.H.C., G.K. and J.B.C. are employees of Amgen Inc and own Amgen Inc stock; P.S.H. has received research grants from AbbVie, Janssen and Novartis, and consulting fees from AbbVie, Amgen, Celgene, Galapagos, Pfizer and UCB. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2. Shwartz M, Restuccia JD, Rosen AK.. Composite measures of health care provider performance: a description of approaches. Milbank Q 2015;93:788–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helliwell PS, Kavanaugh A.. Comparison of composite measures of disease activity in psoriatic arthritis using data from an interventional study with golimumab. Arthritis Care Res 2014;66:749–56. [DOI] [PubMed] [Google Scholar]
- 4. Felson DT, Anderson JJ, Boers M. et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 5. Prevoo ML, van ‘t Hof MA, Kuper HH. et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 6. Wells G, Becker JC, Teng J. et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coates LC, FitzGerald O, Merola JF. et al. Group for research and assessment of psoriasis and psoriatic arthritis/outcome measures in rheumatology consensus-based recommendations and research agenda for use of composite measures and treatment targets in psoriatic arthritis. Arthritis Rheumatol 2018;70:345–55. [DOI] [PubMed] [Google Scholar]
- 8. Schoels M, Aletaha D, Funovits J. et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. [DOI] [PubMed] [Google Scholar]
- 9. Helliwell PS, FitzGerald O, Fransen J. et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72:986–91. [DOI] [PubMed] [Google Scholar]
- 10. Mumtaz A, Gallagher P, Kirby B. et al. Development of a preliminary composite disease activity index in psoriatic arthritis. Ann Rheum Dis 2011;70:272–7. [DOI] [PubMed] [Google Scholar]
- 11. Wervers K, Luime JJ, Tchetverikov I. et al. Comparison of disease activity measures in early psoriatic arthritis in usual care. Rheumatology 2019;58:2251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gossec L, McGonagle D, Korotaeva T. et al. Minimal disease activity as a treatment target in psoriatic arthritis: a review of the literature. J Rheumatol 2018;45:6–13. [DOI] [PubMed] [Google Scholar]
- 13. Coates LC, Fransen J, Helliwell PS.. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 14. Coates LC, Mahmood F, Emery P, Conaghan PG, Helliwell PS.. The dynamics of response as measured by multiple composite outcome tools in the TIght COntrol of inflammation in early Psoriatic Arthritis (TICOPA) trial. Ann Rheum Dis 2017;76:1688–92. [DOI] [PubMed] [Google Scholar]
- 15. Mease PJ, Gladman DD, Collier DH. et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol 2019;71:1112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mease PJ, Gladman DD, Samad AS. et al. Design and rationale of the Study of Etanercept and Methotrexate in Combination or as Monotherapy in Subjects with Psoriatic Arthritis (SEAM-PsA). RMD Open 2018;4:e000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study (Caspar Study Group). Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 18. Felson DT, Anderson JJ, Lange ML, Wells G, LaValley MP.. Should improvement in rheumatoid arthritis clinical trials be defined as fifty percent or seventy percent improvement in core set measures, rather than twenty percent? Arthritis Rheum 1998;41:1564–70. [DOI] [PubMed] [Google Scholar]
- 19. van Mens LJJ, van de Sande MGH, van Kuijk AWR, Baeten D, Coates LC.. Ideal target for psoriatic arthritis? Comparison of remission and low disease activity states in a real-life cohort. Ann Rheum Dis 2018;77:251–7. [DOI] [PubMed] [Google Scholar]
- 20. Aletaha D, Nell VP, Stamm T. et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smolen JS, Breedveld FC, Schiff MH. et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 2003;42:244–57. [DOI] [PubMed] [Google Scholar]
- 22. Helliwell P, Coates LC, FitzGerald O, Nash P, Soriano ER, Husni ME. et al. Tofacitnib improves composite endpoint measures of disease in patients with psoriatic arthritis. [Poster session] American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting, San Diego, CA, USA, 2017.
- 23. Helliwell P, Coates LC, FitzGerald O. et al. Disease-specific composite measures for psoriatic arthritis are highly responsive to a Janus kinase inhibitor treatment that targets multiple domains of disease. Arthritis Res Ther 2018;20:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.