Abstract
Objectives
An efficient pharmacological response to MTX treatment in RA patients relies on the retention and accumulation of intracellular MTX-polyglutamates catalysed by the enzyme folylpolyglutamate synthetase (FPGS). We recently identified a partial retention of FPGS intron 8 (8PR) as a prominent splice variant conferring FPGS dysfunction and decreased MTX polyglutamylation in acute lymphoblastic leukaemia. Here, we explored the association between FPGS 8PR levels and lack of MTX responsiveness in RA patients.
Methods
Thirty-six patients undergoing MTX treatment were enrolled from the Combinatie behandeling Reumatoide Artritis (COBRA)-light trial. RNA was isolated from blood samples at baseline, 13 weeks and 26 weeks of therapy, from patients in either COBRA-light (n = 21) or COBRA (n = 15) treatment arms. RT-qPCR analysis was used to assess RNA levels of FPGS 8PR over wild-type FPGS (8WT).
Results
In the COBRA-light treatment arm, higher baseline ratios of 8PR/8WT were significantly associated with higher 44-joint disease activity score (DAS44) at 13 and 26 weeks. Higher baseline ratios of 8PR/8WT also trended towards not obtaining low disease activity (DAS <1.6) and becoming a EULAR non-responder at 13 and 26 weeks. In the COBRA-treatment arm, a significant association was observed between high baseline 8PR/8WT ratios and higher DAS44 score at 26 weeks. Higher 8PR/8WT ratios were associated with non-response at week 26 based on both low disease activity and EULAR criteria.
Conclusion
This study is the first to associate alterations in FPGS pre-mRNA splicing levels with reduced responsiveness to MTX treatment in RA patients.
Trial registration
ISRCTN55552928.
Keywords: RA, MTX, treat-to-target, disease activity, drug response
Rheumatology key messages
Baseline folylpolyglutamate synthetase (FPGS) with partial retention of intron 8 (8PR)/wild-type (8WT) ratios are significantly associated with week 13 and week 26 DAS44 in COBRA-light treated RA patients.
Baseline FPGS 8PR/8WT ratios are significantly associated with DAS44 at 26 weeks in COBRA-treated RA patients.
Baseline FPGS 8PR/WT ratios were significantly different between low disease activity/EULAR non-responders and responders.
Introduction
The clinical and pharmacological efficacy of both low-dose (10–25 mg/week) MTX treatment in RA patients [1–7] and high-dose (>500 mg/m2) MTX treatment in leukaemia patients [8–10] critically depends on the intracellular bioactivation of MTX to MTX-polyglutamate forms (MTX-PG), a metabolic conversion catalysed by the enzyme folylpolyglutamate synthetase (FPGS) [11]. Proper polyglutamylation results in enhanced intracellular retention because long-chain MTX-PGs, other than MTX in its monoglutamate form, are no longer substrates of ATP-dependent drug efflux transporters [12, 13]. Furthermore, long chain MTX-PGs enhance the pharmacological efficacy of this antifolate by exerting more potent inhibition of intracellular target enzymes in folate metabolism, an essential process for the provision of nucleotides for DNA replication. The anti-leukaemic effect of MTX is primarily exerted by inhibition of dihydrofolate reductase and thymidylate synthase, leading to blockade of DNA replication and consequently cell death. On the other hand, the mechanism of action of low dose MTX in RA is thought to be associated with the inhibition of key enzymes in de novo purine biosynthesis (i.e. 5-aminoimidazole-4-carboxamide ribonucleotide transformylase) by MTX-PGs and the downstream non-lytic release of the anti-inflammatory purine adenosine [14]. However, beyond adenosine release, other factors may contribute to the mechanism of action of low dose MTX [1, 15].
Despite the long standing success of MTX in RA treatment, ∼30% of RA patients experience inadequate responses to MTX treatment due to intolerance or inefficacy at both early and later stages of treatment [1, 15–17]. This in turn necessitates a switch to other chemical and/or biological DMARDs. From this perspective, studies aiming to identify biomarkers for predicting lack of MTX response in RA are of great clinical relevance [18, 19]. A number of recent studies defined clinical, demographic, psychosocial and laboratory variables as predictors that may contribute to the lack of MTX response in early RA [20–23]. With respect to MTX-PG as a possible predictive factor, the accumulation of MTX-PGs has been analysed in red blood cells of RA patients during MTX therapy [24–32]. These studies revealed steady-state levels after 3 months’ therapy [26, 30, 32], associations with a decrease in DAS28 scores [29, 30], but also large inter-patient variabilities between RA patients treated with similar doses of MTX [28–31, 33] that may be accounted for by variable catalytic activities of FPGS [31].
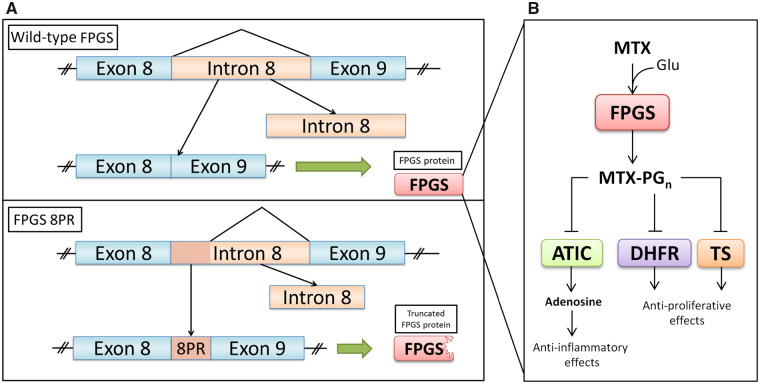
In leukaemia cells, loss of FPGS activity has been recognized as a common mechanism of MTX resistance [10, 11, 13]. Stark et al. [34] reported that loss of FPGS activity can occur due to aberrant pre-mRNA splicing of FPGS, giving rise to splice variants undergoing premature translation termination and a significant loss of FPGS activity (Fig. 1). Recent studies by Wojtuszkiewicz et al. [35] corroborated these findings by showing that multiple MTX-induced pre-mRNA FPGS splice variants in human acute lymphoblastic leukaemia (ALL) cell lines and patients contribute to decreased FPGS activity. Among the FPGS splice variants detected, the most frequent alteration included the partial retention of intron 8 (8PR). This splice variant results in a truncated and inactive FPGS protein [18] (Fig. 1). Quantification of FPGS 8PR as a ratio relative to the wild-type transcript (FPGS 8PR/8WT) in cohorts of paediatric and adult ALL cell specimens revealed large inter-patient variation [35]. In a clinical follow-up study, high FPGS 8PR levels appeared to be associated with diminished accumulation of MTX-PGs in leukaemic blast cells and decreased overall survival in ALL patients [36]. These results prompted us to explore whether FPGS splicing alterations would also impact responsiveness of low dose MTX therapy for RA patients, based on the disease activity score of 44 joints (DAS44).
Fig. 1.
Schematic representation of FPGS pre-mRNA splicing (A) and mode of action of MTX (B)
Canonical FPGS pre-mRNA splicing involves the complete removal of intron 8 prior to translation into a functional FPGS enzyme (A, top). FPGS polyglutamylates MTX by sequentially adding glutamate moieties, forming MTX-PGn. The latter inhibits ATIC, exerting its anti-inflammatory effect, and also blocks DHFR and TS, thereby eliciting its anti-proliferative effect (B). Aberrant splicing of FPGS results in a partially retained intron 8 (8PR), which leads to an interruption of the main open reading frame, resulting in a premature termination codon and possibly a truncated FPGS protein, which loses its catalytic activity (A). 8PR: partial retention of intron 8; ATIC: 5-aminoimidazole-4-carboxamide ribonucleotide transformylase; DHFR: dihydrofolate reductase; FPGS: folylpolyglutamate synthetase; PGn: polyglutamate with n (1–6) glutamate molecules; TS: thymidylate synthase.
Here, we demonstrate that whole blood cell levels of FPGS 8PR are significantly associated with MTX response in early RA patients, underscoring the impact of pre-mRNA splicing alterations for MTX drug response in RA.
Methods
Study population
A total of 162 patients participated in the 'Combination treatment of Rheumatoid Arthritis' (in Dutch ‘Combinatie behandeling Reumatoide Artritis’) COBRA-light trial (trial registration number: ISRCTN55552928), a non-inferior open label randomized trial, of which detailed information has been published earlier [37, 38]. In short, patients with early RA according to the revised ACR criteria [39] were recruited between March 2008 and March 2011. Patients were randomized to either the COBRA strategy (initial 60 mg/day prednisolone, tapered to 7.5 mg/day in 7 weeks; 7.5 mg/week MTX; and 1 g/week sulfasalazine) or to the COBRA-light strategy (initial 30 mg/day prednisolone, tapered to 7.5 mg/day in 9 weeks, and 10 mg/week MTX, increased to 25 mg/week in 8 weeks) (Supplementary Fig. S2, available at Rheumatology online) [37, 38]. Treatment adjustments were protocolized up to 52 weeks and based on the DAS44 every 3 months. In the COBRA strategy, MTX was given at a stable dose in the first 13 weeks. If DAS44 was ≥1.6 at 13 weeks, the MTX dosage was increased biweekly up to 25 mg/week. In the COBRA-light strategy, MTX was given subcutaneously if DAS44 was ≥1.6 at week 13. The study was approved by the Medical Ethics Committees at each participating centre, and executed in accordance with the Declaration of Helsinki/Good Clinical Practice. All patients gave written informed consent before inclusion.
Out of 36 patients enrolled in the COBRA-light trial, whole blood RNA was isolated PAXgene Blood RNA tubes (PreAnalytiX, Hombrechtikon, Switzerland). From each patient, RNA was isolated at both baseline (before treatment) and 13 weeks after treatment.
The RA patients were enrolled either in the COBRA-light or the COBRA trial arm (Supplementary Fig. S1, available at Rheumatology online). Patient characteristics are shown in Table 1.
Table 1.
Baseline characteristics
| Demographic and disease activity at baseline | COBRA | COBRA-light |
|---|---|---|
| (n = 15) | (n = 21) | |
| Age, mean (s.d.), years | 54 (11) | 50 (11) |
| Women, n (%) | 9 (60) | 16 (76) |
| Disease duration, median (IQR), weeks | 27 (12–40) | 21 (7–31) |
| RF-positive, n (%) | 11 (73) | 13 (62) |
| Anti-CCP positive, n (%) | 12 (80) | 15 (71) |
| DAS44, mean (s.d.) | 4.2 (0.8) | 3.95 (0.8) |
| DAS44 CRP, mean (s.d.) | 4.1 (0.8) | 3.78 (0.7) |
| Tender joints, median (IQR), n | 15 (12–29) | 14 (9–22) |
| Swollen joints, median (IQR), n | 13 (10–18) | 12 (9–14) |
| HAQ, mean (s.d.) | 1.30 (0.64) | 1.19 (0.72) |
| CRP, median (IQR), mg/l | 26 (10–26) | 18 (3–8) |
| ESR, median (IQR), mm/h | 33 (16–43) | 33 (14–46) |
| Patient global assessment, median (IQR), mm (0–100) | 63 (51–68) | 62 (49–79) |
| Patient assessment of pain, median (IQR), mm (0–100) | 59 ( 51–67) | 61 (50–78) |
| Physician assessment disease activity, median (IQR), mm (0–100) | 42 (35–50) | 43 (31–53) |
Tender joints: 53 joints; swollen joints: 44 joints. DAS44: 44-joint DAS.
Outcome measure
Disease activity of RA patients was based on the DAS44 scores [40]. Responsiveness for this study was defined as i) baseline DAS44 scores; ii) DAS44 as a continuous outcome measured at week 13 and 26; iii) the change in DAS44 after 26 weeks of treatment, defined as the ΔDAS44 between week 26 and baseline; iv) low disease activity (LDA) defined as having a DAS44 ≤ 1.6 (evaluated at 3 and 6 months after start), and v) and being a EULAR good responder.
Quantitative real-time PCR
RNA was isolated from using blood PAXgene tubes according to the manufacturer’s protocol (BD Biosciences). RNA (0.25 µg) was reverse transcribed to cDNA using Moloney Murine Leukaemia Virus (M-MLV; Thermo Fisher Scientific, Waltham, MA, USA) in a reaction buffer containing random hexamer primers (Roche, Basel, Switzerland), dNTPs (Roche), and a ribonuclease inhibitor RNasin (Promega, Madison, WI, USA).
Quantification of FPGS 8PR and 8WT levels was performed using RT-qPCR with a LightCycler 480 (Roche). The expression of β-glucuronidase (GusB) was used as a reference. Primer sequences derived from Wojtuszkiewicz et al. [35, 36] are shown in Supplementary Table S1, available at Rheumatology online. To quantify the levels of FPGS 8PR and FPGS WT in clinical samples, 5 µl of 2.5 ng/µl cDNA was used. Pre-mRNA levels of 8PR and 8WT derived from CCRF-CEM T cell leukaemia cells [35, 41] were used as a positive control to correct for any differences between qRT-PCR plates. qRT-PCR pre-mixes were prepared with 10 µl LightCycler 480 SYBR Green I Master mix (2× concentrated, Roche), 1 µl forward primer (5 µM, Biolegio B.V., Nijmegen, The Netherlands), 1 µl reverse primer (5 µM, Biolegio B.V.) and 3 µl sterile ddH2O per reaction. Fifteen microlitres qRT-PCR pre-mix was distributed into a LightCycler 480 Multiwell Plate 96 (white, Roche) on ice, followed by the addition of cDNA. The 96-well plate was sealed and centrifuged briefly. Relative expression of the genes was calculated using the Advanced Relative Quantification option (LightCycler 480 software, version 1.5.1.62, Roche).
Statistical analyses
Both treatment arms were analysed separately due to their different MTX treatment dose schedules. Linear regression was used to establish an association between the levels of FPGS 8PR/8WT and the continuous (Δ)DAS44 scores, in case of normally distributed outcome. Normality of distribution was tested by the Shapiro–Wilk normality test. Regression models include baseline DAS44 scores as covariate to correct for baseline DAS44, which is a known predictor for non-response to MTX. Welch’s t-test was performed to account for unequal variances. A two-sided level of significance was considered at P < 0.05. All statistical analyses were performed with SPSS Statistics software Version 26 (IBM Corp., Armonk, NY, USA) or with GraphPad Prism Version 8.2.1 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Study population
Table 1 provides demographic information on the 36 patients included in this substudy. These characteristics are comparable to that of the total study population [37, 38]. Mean baseline and FPGS 8PR/WT at week 13 were not significantly different between both treatment arms or between male and female gender (P > 0.05). Impact of ethnicity could not be evaluated because the patient population was 89% Caucasian. Of the 36 patients with whole blood PAXgene Blood RNA tubes, we excluded one sample from one patient in the COBRA-light arm at week 13 due to insufficient RNA. Of the 15 patients receiving COBRA treatment, five did not obtain LDA after 13 weeks, and received MTX dose increases.
DAS44 as continuous outcome
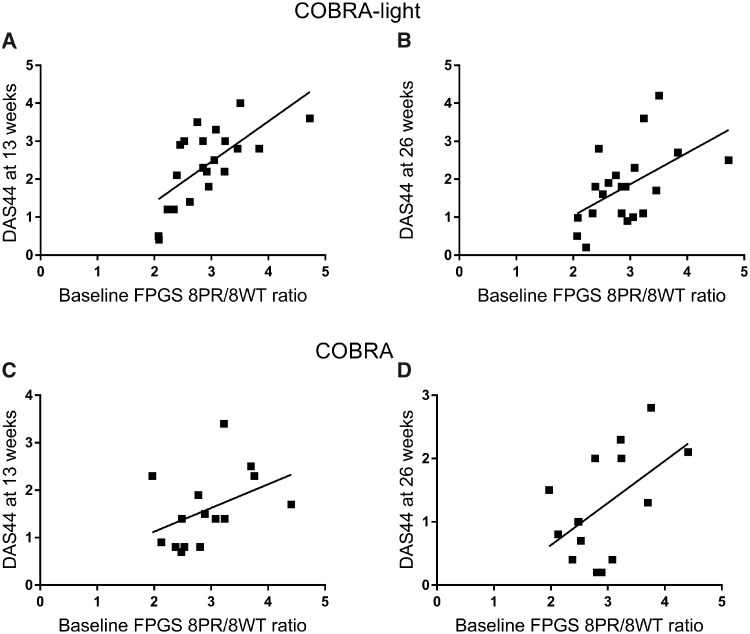
Baseline whole blood FPGS 8PR/8WT ratios were determined and were not associated significantly with baseline DAS44 in COBRA-light and COBRA patients [β −0.21 (95% CI: −1.2, 0.74), P = 0.58]. Higher baseline whole blood FPGS 8PR/8WT ratios of COBRA-light patients were significantly associated with higher DAS44 scores at 13 weeks [β 0.668 (95% CI: 0.46, 1.63), P = 0.001, Fig. 2A] and 26 weeks [β 0.536 (95% CI: 0.16, 1.51), P = 0.018, Fig. 2B]. Higher baseline ratios of 8PR/WT were also significantly associated with lower ΔDAS44 score at 13 weeks [β 0.59 (95% CI: 0.46, 1.63), P = 0.001] and 26 weeks [β 0.45 (95% CI: 0.16, 1.512), P = 0.02]. A non-significant association was observed between baseline ratios and DAS44 scores at 13 weeks in COBRA treated patients [β 0.60 (95% CI: 0.06, 1.25), P = 0.07, Fig. 2C], and higher baseline 8PR/WT ratios were significantly associated with higher DAS44 scores at 26 weeks [β 0.0 (95% CI: 0.09, 1.42), P = 0.03, Fig. 2D].
Fig. 2.
Baseline whole blood FPGS 8PR/8WT levels and disease activity scores
(A and B) Baseline ratios of FPGS 8PR over 8WT in COBRA-light are plotted against DAS44 scores at 13 weeks [β 0.668 (95% CI: 0.46, 1.63), P = 0.001, A] and 26 weeks [β 0.536 (95% CI: 0.16, 1.51), P = 0.018, B]. (C and D) Baseline ratios of FPGS 8PR over 8WT in COBRA patients are plotted against DAS44 scores at 13 weeks [β 0.51 (95% CI: 0.06, 1.25), P = 0.07, C] and 26 weeks [β 0.60 (95% CI: 0.09, 1.42), P = 0.029, D]. 8PR: partial retention of intron 8; 8WT: wild-type; FPGS: folylpolyglutamate synthetase.
In addition, a non-significant association was found between 8PR/8WT ratios at 13 weeks and DAS44 at 13 weeks [β 0.50 (95% CI: 0.18, 1.12), P = 0.14, Supplementary Fig. S2A, available at Rheumatology online] in COBRA-light patients, and a significant association was found for higher 8PR/WT ratios at week 13 with higher DAS44 at 26 weeks [β 0.69 (95% CI: 0.46, 1.52), P = 0.001, Supplementary Fig. S2B, available at Rheumatology online]. Lower ΔDAS44 scores at 26 weeks were also significantly associated with higher FPGS 8PR/8WT ratios at 13 weeks [β 0.58 (95% CI: 0.46, 1.52), P = 0.001].
For COBRA patients, higher 8PR/WT ratios at 13 weeks were associated with DAS44 scores at 13 weeks [β 0.58 (95% CI: 0.09, 1.33), P = 0.03, Supplementary Fig. S2C, available at Rheumatology online] and 26 weeks [β 0.47 (95% CI: 0.11, 1.35), P = 0.088, Supplementary Fig. S2D, available at Rheumatology online]. In addition, an association was observed between FPGS 8PR/8WT ratios at 13 weeks and ΔDAS44 at 26 weeks in COBRA patients [β 0.33 (95% CI: −0.108, 1.35), P = 0.09]. Although some effects were non-significant, all effects were in the anticipated direction with confidence intervals ruling out negative associations counter to the hypothesis.
LDA and EULAR response
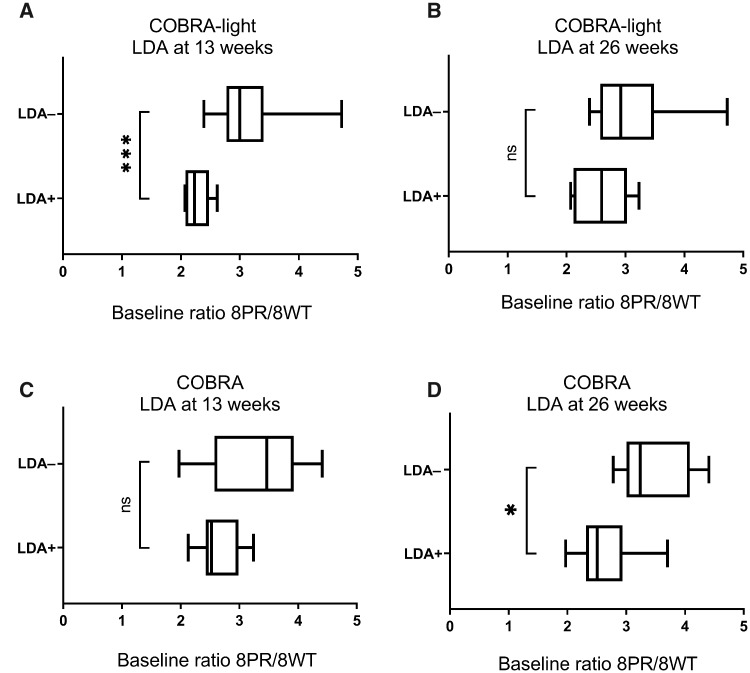
In the COBRA-light arm, mean baseline ratios of 8PR/8WT were significantly higher in patients not obtaining LDA (insufficient response/non-response) compared with patients that reached LDA (responders) at 13 weeks (P = 0.0002, Fig. 3A), but not at week 26 (P = 0.056, Fig. 3B). In addition, mean baseline ratios of 8PR/8WT were significantly higher in EULAR non-responders than in EULAR responders at week 13 (P = 0.008) and at week 26 (P = 0.043). Receiver operating characteristic (ROC) curve analysis for baseline 8PR/8WT ratio predicting non-response (LDA or EULAR) at 13 weeks showed an area under the curve (AUC) of 0.96 for achieving LDA and 0.85 for becoming a EULAR non-responder (Supplementary Fig. S3, available at Rheumatology online).
Fig. 3.
FPGS 8PR/8WT levels and COBRA-light/COBRA patients with LDA.
LDA was defined as DAS44 <1.6. (A and B) COBRA-light baseline FPGS 8PR/8WT ratios are plotted and Welch’s t test (unequal variances assumed) was performed for both groups at 13 weeks (P = 0.0002, A) and 26 weeks (P = 0.056, B). (C and D) COBRA baseline FPGS 8PR/8WT ratios are plotted and Welch’s t test (unequal variances assumed) was performed for both groups at 13 weeks (P = 0.133, C) and 26 weeks (P = 0.036, D). LDA+: patients reaching LDA (responders); LDA−: patients not reaching LDA (insufficient response at 13 weeks or non-response at 26 weeks); *P < 0.05, ***P < 0.001. 8PR: partial retention of intron 8; 8WT: wild-type; FPGS: folylpolyglutamate synthetase; LDA: low disease activity; ns: not significant.
For the COBRA-treated group, baseline ratios of FPGS 8PR/8WT were not significantly different between LDA response groups at 13 weeks (P = 0.133, Fig. 3C), but were significantly higher in patients not obtaining LDA (non-responders) at week 26 (P = 0.036, Fig. 3D). Baseline ratios were also significantly higher in EULAR non-responders at week 13 and week 26 (P = 0.008 and P = 0.043, respectively).
Finally, ROC curve analysis for baseline 8PR/8WT ratio predicting non-response (LDA or EULAR) at 13 weeks showed an AUC of 0.74 for achieving LDA and 0.89 for becoming a EULAR non-responder (Supplementary Fig. S3, available at Rheumatology online).
Discussion
The current study is the first to demonstrate an association of aberrant FPGS pre-mRNA splicing and response to MTX therapy (based on DAS44 scores at week 13 and 26, ΔDAS44) as part of COBRA and COBRA-light treatment protocols for RA patients. In addition, during COBRA-light therapy (which implements MTX dose increases in the first 9 weeks of therapy), significant differences were observed between the mean baseline levels of FPGS 8PR/8WT between LDA responders and non-responders at week 13 and EULAR responders/non-responders at week 13 and 26, while similar observations were made for patients receiving COBRA therapy with significant differences of mean baseline FPGS 8PR/8WT ratios between LDA responders and non-responders at 26 weeks and EULAR responders/non-responders at 13 and 26 weeks.
Alternative splicing has gained recent interest for its contributory role in altered drug metabolism and impaired drug response in cancer treatment [42, 43]. This was illustrated in a recent study demonstrating that alternative splicing is a contributing factor in glucocorticoid drug resistance in leukaemia treatment coming with distinct alternative splicing profiles in either T cell or B cell leukaemia [44]. The association between specific splicing alterations and drug resistance was therapeutically harnessed to target various cancers [45]. Conceivably, similar mechanisms may apply for RA treatment with DMARDs, which are dependent on metabolism/bioactivation, with the anchor drug MTX as a prototypic example. Clinical efficacy of MTX is critically dependent on its conversion to MTX-PGs by FPGS [1, 15, 25, 27–32], and consequently diminished FPGS activity can result in lack of responsiveness to MTX treatment [11].
In the current study we particularly focused on one FPGS pre-mRNA splice variant, i.e. partial retention of intron 8 (8PR), which was recently identified as a dominant variant in leukaemia cells and associated with diminished MTX-polyglutamylation and dismal clinical outcome [34–36]. Remarkably, in the RA setting too, a high ratio of FPGS 8PR/8WT in whole blood of RA patients appears to have a predictive value for MTX responsiveness in both the COBRA-light and COBRA treatment arms. Moreover, differences in the association between the FPGS 8PR splice variant and disease activity between patients receiving COBRA-light and COBRA treatments could be attributed to their distinct dose regimens. The apparent lack of association of the 8PR/8WT ratio at 13 weeks and disease activity at 13 weeks in COBRA patients might be explained by the fact that in the COBRA treatment arm, MTX dose increments start only after 13 weeks of initial treatment and thus MTX accumulation and effect would be limited. This also supports the hypothesis that the levels of the FPGS 8PR splice variant are related to the anti-rheumatic activity of MTX and not to a by-product of reduced inflammation due to either treatment strategy.
As a reservation, the findings of this study should be considered as preliminary, warranting additional (prospective) studies addressing the following points. First, this study attributes the association of 8PR/8WT ratios and poor outcome to a direct effect of MTX treatment due to its direct biological relation to MTX metabolism. However, it may also be argued that changes in FPGS 8PR/8WT ratios reflect inflammation/immune activation-induced alterations in folate metabolism observed during RA onset and progression [46]. As such, it is conceivable that splicing alterations impacting critical metabolic and signalling pathways in RA disease have potential value for treatment response predictions beyond MTX. To establish this further, future sufficiently powered studies should include control groups of both healthy individuals and RA patients receiving different treatment strategies to confirm the current findings. In addition, due to the limited sample size, further studies need to be performed to assess the impact of ethnicity and other potential parameters on FPGS 8PR/8WT expression and MTX response. Furthermore, one cannot rule out the possibility that co-medication of MTX in the COBRA and COBRA-light treatment arms may contribute to the lack of responsiveness, as both prednisolone and sulfasalazine were tapered down during therapy [37]. Since patients received the same cumulative dose of prednisolone in both treatment arms, whereas associations between FPGS 8PR/8WT levels and DAS44 scores clearly differed, this supports the notion that the level of the 8PR/8WT splice variant is associated with the response to MTX. For sulfasalazine, however, drug interactions have been reported for folate-dependent enzymes [47] and the reduced folate carrier (SLC19A1) [48], the primary influx transporter of MTX [11]. However, there is no direct evidence that sulfasalazine, as part of the COBRA treatment arm, confers any inhibitory activity on FPGS itself. To further pinpoint the importance of FPGS 8PR/8WT ratio and MTX response, future dedicated studies in RA patients receiving MTX monotherapy are warranted. Another limitation of the current study was that no data were available on FPGS catalytic activity and MTX-PGn levels in either erythrocytes or peripheral blood mononuclear cells to directly link FPGS 8PR/WT ratios with altered MTX cellular pharmacology and response to MTX.
From a potential diagnostic perspective, it is of interest that associations of FPGS 8PR with lack of response to MTX could be identified in RNA extracted from whole blood of RA patients isolated with PAXgene tubes. Despite the limited sample size, we were able to construct a preliminary ROC curve with high sensitivity and specificity for prediction of LDA or EULAR non-response at 13 weeks using baseline FPSG 8PR/8WT ratios as predictor. However, there are several issues to be addressed before an analysis of FPGS 8PR may qualify as a bona fide prognostic marker. First, since total RNA is derived from leukocytes, it remains to be determined which immune cells (T cells, B cells and monocytes) differentially contribute to the expression of the FPGS 8PR splice variant. Second, although FPGS 8PR level has been associated with loss of FPGS activity and reduced accumulation of MTX-PGs in leukaemia cells [35, 36], this functional association needs to be confirmed in immune cells relevant to RA pathophysiology. Recently, the technical feasibility of FPGS catalytic activity and MTX-PGs analysis in PBMCs derived from RA patients was demonstrated by sensitive mass spectrometry [49, 50]. This type of analysis may complement MTX therapeutic drug monitoring initiatives and determine whether alterations in FPGS splicing play a role in inter-patient variability of MTX-polyglutamylation and therapy response [28–31, 33]. Finally, these parameters deserve further consideration to improve the current predictive models for MTX response or lack of responsiveness in RA patients [20–22, 33]. In conclusion, ratios of FPGS 8PR/8WT seem to precede poorer response [based on (Δ)DAS44], and significant differences can be found between (LDA/EULAR) responders and non-responders in MTX-treated RA patients that warrant further investigation into its causal effect and potential value as a predictive biomarker for MTX response.
Supplementary Material
Acknowledgements
We thank all patients, as well as all doctors who enrolled patients in this study, and all research nurses who were involved in patient management. This study was supported in part by a grant from the Dutch Society for Clinical Chemistry (NVKC) to I.B.M. and R.J. The COBRA-light trial was executed within the framework of project T1-106 of the Dutch Top Institute Pharma.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: M.T.N. has received research funding or speaking/consultancy honoraria from Abb Vie, Pfizer, Merck, Roche, BMS, UCB, Eli Lilly, Celgene and Janssen (less than $10 000 each); W.F.L. has received consulting fees, speaking fees and/or honoraria from Amgen, MSD, Pfizer, and Eli Lilly (less than $10 000 each). The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Brown PM, Pratt AG, Isaacs JD.. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol 2016;12:731–42. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, Barton A. et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 3. Aletaha D, Neogi T, Silman AJ. et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 4. Taylor PC, Balsa Criado A, Mongey AB. et al. How to get the most from methotrexate (MTX) treatment for your rheumatoid arthritis patient?—MTX in the treat-to-target strategy. J Clin Med 2019;8:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kremer JM. Toward a better understanding of methotrexate. Arthritis Rheum 2004;50:1370–82. [DOI] [PubMed] [Google Scholar]
- 6. Municio C, Soler Palacios B, Estrada-Capetillo L. et al. Methotrexate selectively targets human proinflammatory macrophages through a thymidylate synthase/p53 axis. Ann Rheum Dis 2016;75:2157–65. [DOI] [PubMed] [Google Scholar]
- 7. Rohr MK, Mikuls TR, Cohen SB, Thorne JC, O’Dell JR.. Underuse of methotrexate in the treatment of rheumatoid arthritis: a national analysis of prescribing practices in the US. Arthritis Care Res (Hoboken) 2017;69:794–800. [DOI] [PubMed] [Google Scholar]
- 8. Bertino JR. Karnofsky memorial lecture. Ode to methotrexate. J Clin Oncol 1993;11:5–14. [DOI] [PubMed] [Google Scholar]
- 9. Masson E, Relling MV, Synold TW. et al. Accumulation of methotrexate polyglutamates in lymphoblasts is a determinant of antileukemic effects in vivo. A rationale for high-dose methotrexate. J Clin Invest 1996;97:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rots MG, Pieters R, Peters GJ. et al. Role of folylpolyglutamate synthetase and folylpolyglutamate hydrolase in methotrexate accumulation and polyglutamylation in childhood leukemia. Blood 1999;93:1677–83. [PubMed] [Google Scholar]
- 11. Raz S, Stark M, Assaraf YG.. Folylpoly-γ-glutamate synthetase: a key determinant of folate homeostasis and antifolate resistance in cancer. Drug Resist Updat 2016;28:43–64. [DOI] [PubMed] [Google Scholar]
- 12. van der Heijden JW, Oerlemans R, Tak PP. et al. Involvement of breast cancer resistance protein expression on rheumatoid arthritis synovial tissue macrophages in resistance to methotrexate and leflunomide. Arthritis Rheum 2009;60:669–77. [DOI] [PubMed] [Google Scholar]
- 13. Gonen N, Assaraf YG.. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Updat 2012;15:183–210. [DOI] [PubMed] [Google Scholar]
- 14. Friedman B, Cronstein B.. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine 2019;86:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van der Heijden JW, Dijkmans BA, Scheper RJ, Jansen G.. Drug Insight: resistance to methotrexate and other disease-modifying antirheumatic drugs—from bench to bedside. Nat Clin Pract Rheumatol 2007;3:26–34. [DOI] [PubMed] [Google Scholar]
- 16. van der Heijde D, Klareskog L, Rodriguez-Valverde V. et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum 2006;54:1063–74. [DOI] [PubMed] [Google Scholar]
- 17. Bakker MF, Jacobs JW, Welsing PM. et al. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med 2012;156:329–39. [DOI] [PubMed] [Google Scholar]
- 18. Yu MB, Firek A, Langridge W.. Predicting methotrexate resistance in rheumatoid arthritis patients. Inflammopharmacology 2018;26:699–708. [DOI] [PubMed] [Google Scholar]
- 19. Ling S, Bluett J, Barton A.. Prediction of response to methotrexate in rheumatoid arthritis. Expert Rev Clin Immunol 2018;14:419–29. [DOI] [PubMed] [Google Scholar]
- 20. Teitsma XM, Jacobs JWG, Welsing PMJ. et al. Inadequate response to treat-to-target methotrexate therapy in patients with new-onset rheumatoid arthritis: development and validation of clinical predictors. Ann Rheum Dis 2018;77:1261–7. [DOI] [PubMed] [Google Scholar]
- 21. Sergeant JC, Hyrich KL, Anderson J. et al. Prediction of primary non-response to methotrexate therapy using demographic, clinical and psychosocial variables: results from the UK Rheumatoid Arthritis Medication Study (RAMS). Arthritis Res Ther 2018;20:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Rotte M, Pluijm SMF, de Jong PHP. et al. Development and validation of a prognostic multivariable model to predict insufficient clinical response to methotrexate in rheumatoid arthritis. PLoS One 2018;13:e0208534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saevarsdottir S, Wallin H, Seddighzadeh M. et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis 2011;70:469–75. [DOI] [PubMed] [Google Scholar]
- 24. Angelis-Stoforidis P, Vajda FJ, Christophidis N.. Methotrexate polyglutamate levels in circulating erythrocytes and polymorphs correlate with clinical efficacy in rheumatoid arthritis. Clin Exp Rheumatol 1999;17:313–20. [PubMed] [Google Scholar]
- 25. Dervieux T, Furst D, Lein DO. et al. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum 2004;50:2766–74. [DOI] [PubMed] [Google Scholar]
- 26. Dalrymple JM, Stamp LK, O’Donnell JL. et al. Pharmacokinetics of oral methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 2008;58:3299–308. [DOI] [PubMed] [Google Scholar]
- 27. Becker ML, van Haandel L, Gaedigk R. et al. Analysis of intracellular methotrexate polyglutamates in patients with juvenile idiopathic arthritis: effect of route of administration on variability in intracellular methotrexate polyglutamate concentrations. Arthritis Rheum 2010;62:1803–12. [DOI] [PubMed] [Google Scholar]
- 28. Stamp LK, O’Donnell JL, Chapman PT. et al. Methotrexate polyglutamate concentrations are not associated with disease control in rheumatoid arthritis patients receiving long-term methotrexate therapy. Arthritis Rheum 2010;62:359–68. [DOI] [PubMed] [Google Scholar]
- 29. Bulatović Ćalasan M, den Boer E, de Rotte MCFJ. et al. Methotrexate polyglutamates in erythrocytes are associated with lower disease activity in juvenile idiopathic arthritis patients. Ann Rheum Dis 2015;74:402–7. [DOI] [PubMed] [Google Scholar]
- 30. de Rotte MC, den Boer E, de Jong PH. et al. Methotrexate polyglutamates in erythrocytes are associated with lower disease activity in patients with rheumatoid arthritis. Ann Rheum Dis 2015;74:408–14. [DOI] [PubMed] [Google Scholar]
- 31. Yamamoto T, Shikano K, Nanki T, Kawai S.. Folylpolyglutamate synthase is a major determinant of intracellular methotrexate polyglutamates in patients with rheumatoid arthritis. Sci Rep 2016;6:35615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takahashi C, Kaneko Y, Okano Y. et al. Association of erythrocyte methotrexate-polyglutamate levels with the efficacy and hepatotoxicity of methotrexate in patients with rheumatoid arthritis: a 76-week prospective study. RMD Open 2017;3:e000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dervieux T, Furst D, Lein DO. et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis 2005;64:1180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stark M, Wichman C, Avivi I, Assaraf YG.. Aberrant splicing of folylpolyglutamate synthetase as a novel mechanism of antifolate resistance in leukemia. Blood 2009;113:4362–9. [DOI] [PubMed] [Google Scholar]
- 35. Wojtuszkiewicz A, Raz S, Stark M. et al. Folylpolyglutamate synthetase splicing alterations in acute lymphoblastic leukemia are provoked by methotrexate and other chemotherapeutics and mediate chemoresistance. Int J Cancer 2016;138:1645–56. [DOI] [PubMed] [Google Scholar]
- 36. Wojtuszkiewicz A, Assaraf YG, Hoekstra M. et al. The association of aberrant folylpolyglutamate synthetase splicing with ex vivo methotrexate resistance and clinical outcome in childhood acute lymphoblastic leukemia. Haematologica 2016;101:e291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. den Uyl D, ter Wee M, Boers M. et al. A non-inferiority trial of an attenuated combination strategy (‘COBRA-light’) compared to the original COBRA strategy: clinical results after 26 weeks. Ann Rheum Dis 2014;73:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ter Wee MM, den Uyl D, Boers M. et al. Intensive combination treatment regimens, including prednisolone, are effective in treating patients with early rheumatoid arthritis regardless of additional etanercept: 1-year results of the COBRA-light open-label, randomised, non-inferiority trial. Ann Rheum Dis 2015;74:1233–40. [DOI] [PubMed] [Google Scholar]
- 39. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 40. van Gestel AM, Prevoo ML, van 't Hof MA. et al. Development and validation of the European League against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League against Rheumatism Criteria. Arthritis Rheum 1996;39:34–40. [DOI] [PubMed] [Google Scholar]
- 41. Liani E, Rothem L, Bunni MA. et al. Loss of folylpoly-γ-glutamate synthetase activity is a dominant mechanism of resistance to polyglutamylation-dependent novel antifolates in multiple human leukemia sublines. Int J Cancer 2003;103:587–99. [DOI] [PubMed] [Google Scholar]
- 42. Wojtuszkiewicz A, Assaraf YG, Maas MJ. et al. Pre-mRNA splicing in cancer: the relevance in oncogenesis, treatment and drug resistance. Expert Opin Drug Metab Toxicol 2015;11:673–89. [DOI] [PubMed] [Google Scholar]
- 43. Siegfried Z, Karni R.. The role of alternative splicing in cancer drug resistance. Curr Opin Genet Dev 2018;48:16–21. [DOI] [PubMed] [Google Scholar]
- 44. Sciarrillo R, Wojtuszkiewicz A, Kooi IE. et al. Glucocorticoid resistant pediatric acute lymphoblastic leukemia samples display altered splicing profile and vulnerability to spliceosome modulation. Cancers (Basel) 2020;12:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dehm SM. mRNA splicing variants: exploiting modularity to outwit cancer therapy. Cancer Res 2013;73:5309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blits M, Jansen G, Assaraf YG. et al. Methotrexate normalizes up-regulated folate pathway genes in rheumatoid arthritis. Arthritis Rheum 2013;65:2791–802. [DOI] [PubMed] [Google Scholar]
- 47. Baggott JE, Morgan SL, Ha T, Vaughn WH, Hine RJ.. Inhibition of folate-dependent enzymes by non-steroidal anti-inflammatory drugs. Biochem J 1992;282:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jansen G, van der Heijden J, Oerlemans R. et al. Sulfasalazine is a potent inhibitor of the reduced folate carrier: implications for combination therapies with methotrexate in rheumatoid arthritis. Arthritis Rheum 2004;50:2130–9. [DOI] [PubMed] [Google Scholar]
- 49. Muller IB, Hebing RF, Jansen G. et al. Personalized medicine in rheumatoid arthritis: methotrexate polyglutamylation revisited. Exp Rev Prec Med Drug Dev 2018;3:331–4. [Google Scholar]
- 50. Muller IB, Lin M, Struys EA. et al. Development and validation of a sensitive UHPLC-MS/MS-based method for the analysis of folylpolyglutamate synthetase enzymatic activity in peripheral blood mononuclear cells: application in rheumatoid arthritis and leukemia patients. Ther Drug Monit 2019;41:598–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.