Abstract
Background
There is no data regarding COVID-19 in Multiple Sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) patients in Latin America.
Objective
The objective of this study was to describe the clinical characteristics and outcomes of patients included in RELACOEM, a LATAM registry of MS and NMOSD patients infected with COVID-19.
Methods
RELACOEM is a longitudinal, strictly observational registry of MS and NMOSD patients who suffer COVID-19 and Dengue in LATAM. Inclusion criteria to the registry were either: (1) a biologically confirmed COVID-19 diagnosis based on a positive result of a COVID-19 polymerase chain reaction (PCR) test on a nasopharyngeal swab; or (2) COVID-19–typical symptoms (triad of cough, fever, and asthenia) in an epidemic zone of COVID-19. Descriptive statistics were performed on demographic and clinical variables. The cohort was later stratified for MS and NMOSD and univariate and multivariate logistic regression analysis was performed to identify variables associated with hospitalizations/intensive critical units (ICU) admission.
Results
145 patients were included in the registry from 15 countries and 51 treating physicians. A total of 129 (89%) were MS patients and 16 (11%) NMOSD. 81.4% patients had confirmed COVID-19 and 18.6% were suspected cases. 23 (15.8%) patients were hospitalized, 9 (6.2%) required ICU and 5 (3.4 %) died due to COVID-19. In MS patients, greater age (OR 1.17, 95% CI 1.05 – 1.25) and disease duration (OR 1.39, 95%CI 1.14-1.69) were associated with hospitalization/ICU. In NMOSD patients, a greater age (54.3 vs. 36 years, p=<0.001), increased EDSS (5.5 vs 2.9, p=0.0012) and disease duration (18.5 vs. 10.3 years, p=0.001) were significantly associated with hospitalization/ICU.
Conclusion
we found that in MS patients, age and disease duration was associated with hospitalization and ICU admission requirement, while age, disease duration and EDSS was associated in NMOSD.
Keywords: multiple sclerosis, NMOSD, COVID-19, registries, Latin America
1. Introduction
Coronavirus disease (COVID-19) is caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). The first cases were reported in Wuhan City, China, in late December 2019 producing an epidemic illness that spread rapidly throughout the world and was declared a pandemic by the World Health Organization (WHO) on March 11th, 2020 (WHO, 2020b).
Multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) patients treated with disease-modifying therapies (DMTs) are more vulnerable to infections and their serious complications than the general population (Winkelmann et al., 2016). According to our current knowledge, DMTs could predispose MS patients to a higher risk of either community-acquired or opportunistic infections (Grebenciucova and Pruitt, 2017; Luna et al., 2020). Traditional immunosuppressive treatments and the emerging NMOSD treatments are also associated with an increased risk of infections (viral and bacterial) (Holmoy et al., 2020). Based on this background, it would be reasonable to hypothesize that these therapies may increase the risk of COVID-19 infection predisposing towards a severe infection and worse outcomes (Brownlee et al., 2020). On the other hand, SARS Cov 2 being an emerging pathogen, the need for data on the impact of this particular virus on these patients grew up rapidly. Different scientific societies worldwide are trying to explain and determine the characteristics of COVID-19 in people with MS and NMOSD (Giovannoni et al., 2020; Louapre et al., 2020a; MSIF, 2020). As an attempt to gather information from many countries and regions, the MS International Federation and the MS Data Alliance, acting under the umbrella of the European Charcot Foundation, coordinated an initiative with multiple data and analytical partners to establish a global data-sharing initiative to investigate COVID-19 in people with MS (Peeters et al., 2020). Despite previous initiatives, there is currently a lack of information about COVID-19 in patients from Latin America (LATAM). The objective of this study was to describe the clinical characteristics and outcomes of MS and NMOSD patients included in RELACOEM (Registro Latinoamericano de Covid-19 y esclerosis múltiple), a LATAM registry of MS and NMOSD patients infected with COVID-19.
2. Methods
2.1. Data collection
RELACOEM is a longitudinal, strictly observational registry of MS and NMOSD patients infected with COVID-19 in LATAM. The registry is open to all practicing neurologists and to MS specialists and their teams in LATAM. It tracks the outcomes in a web-based platform that allows researchers to enroll and follow up their infected patients. The registry is facilitated by the Latin American Committee for Treatment and Research in MS (LACTRIMS). Patients included in the registry are required to provide an oral or signed consent form (pending on each approval) authorizing release of their coded medical information anonymized to the central registry of the COVID-19 and MS global data sharing initiative (Peeters et al., 2020). Each patient included in this study was followed up by their treating physician by telephone contact or video consultation with the patient or family member and in some cases, face-to-face consultation after discharge for COVID-19. The incorporated data was requested directly from patients or medical centers. Patients were enrolled as of March 2020 and the first cut-off for this analysis was made on August 30th, 2020. A core questionnaire regarding COVID-19 infection as well as relevant demographic and neurological information is completed by treating clinicians and shared into the global platform. For COVID-19 condition, the status reported by clinicians in the dataset was defined as confirmed, based on a positive diagnostic test, or suspected, based on clinician judgement of exposure and/or typical symptoms (Peeters et al., 2020).
2.2. Population of interest
Inclusion criteria for the analysis were MS and NMOSD patients and at least 1 of the following criteria: (1) a biologically confirmed COVID-19 diagnosis based on a positive result of a COVID-19 polymerase chain reaction (PCR) test on a nasopharyngeal swab; (2) Suspected COVID-19 cases according to the WHO definition (WHO, 2020a). The exclusion criteria for the analysis were MS and NMOSD patients with incomplete data during follow-up.
2.3. Definition of variables and study endpoints
Age, gender, and ethnicity were collected for included patients. MS phenotype was grouped into relapsing-remitting MS (RRMS) and progressive MS (SPMS, PPMS). Treatment for MS or NMOSD was collected considering the current treatment (if any) and last dose received by the patient at COVID-19 infection. Disability was assessed by the Expanded Disability Status Scale (EDSS). Obesity was defined by body mass index (BMI) >30. Presence of comorbidities includes conditions such as cardiovascular disease, arterial hypertension, diabetes, chronic liver disease, kidney disease, other neurological/neuromuscular disorder, lung disease, or presence of a malignant tumor. Regarding COVID-19 outcomes, hospitalization, ICU admission, need for artificial ventilation, and death were collected. The primary end point was the combination of hospitalization and/or ICU admission (hospitalization/ICU).
2.4. Statistical analysis
Descriptive statistics were performed on demographic and clinical variables for the entire cohort. Demographic data were then analyzed for patients that required hospitalization or ICU. The cohort was later stratified by MS and NMOSD and univariate and multivariate logistic regression analysis were performed to identify variables associated with hospitalizations/ICU in the evaluated cohort. Group comparisons were performed using the Mann-Whitney U test for numerical and ordinal variables and the Fisher test or χ2 test when appropriate for categorical variables. Any 2-sided p < .05 was considered statistically significant. Multivariate logistic regression model was performed to determine which variables were independently associated with hospitalization/ICU. Variable selection was done through backward elimination and forward selection by minimizing Akaike information criterion. Results were expressed as odds ratios (ORs) and 95% CIs. Data analyses were performed in Stata 15.1 Software.
3. Results
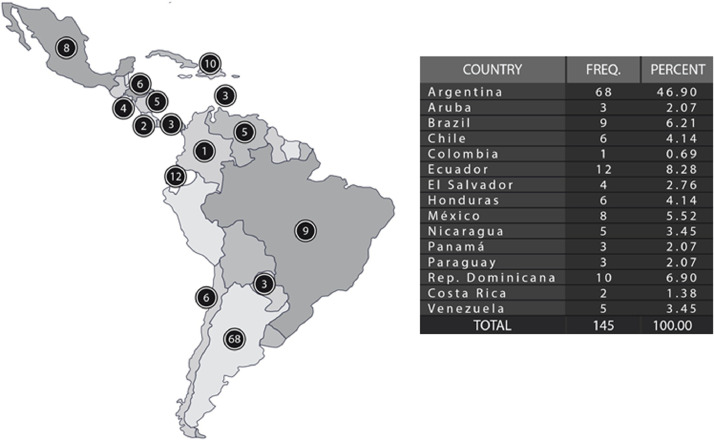
As of October 1st, 2020, 145 patients were included in the registry from 15 countries and 51 treating physicians (Fig. 1 ). Contributions per country are shown in Table 1 . A total of 129 (89%) were MS patients and 16 (11%) NMOSD. The mean (SD) age for the entire cohort was 41 (13) years, 71.7% were female, median EDSS 4 (range 0-8.5) and 17.3% were obese. Regarding COVID-19 status, 81.4% were confirmed and 18.6% were suspected cases. During the evolution, 23 (15.8%) patients were hospitalized, 9 (6.2%) required ICU and 5 (3.4 %) died due to COVID-19. Complete baseline aspects of included patients are shown in Table 2 .
Fig. 1.
Patients included in the registry by country.
Table 1.
Baseline characteristics of the entire cohort.
| N= 145 | |
|---|---|
| Mean age, SD, (range) | 41+-13 (18-75) |
| Female, n (%) | 104 (71.7) |
| Ethnicity, n (%) | |
| Caucasian | 55 (38) |
| Black | - |
| African American | 50 (34.5) |
| Asian | 3 (2) |
| Unknown/not reported | 37 (25.5) |
| MS phenotype, n (%) | |
| CIS | 3 (2) |
| RRMS | 115 (79.3) |
| SPMS | 9 (6.2) |
| PPMS | 2 (1.4) |
| NMOSD | 16 (11.1) |
| Median EDSS, range | 4 (0-8.5) |
| Comorbidity, n (%) | |
| Cerebrovascular disease | 2 (1.4) |
| Hematological disease | 5 (3.4) |
| Coronary heart disease | 2 (1.4) |
| Hypertension | 21 (14.5) |
| Diabetes | 8 (5.5) |
| Chronic liver disease | 2 (1.4) |
| Chronic kidney disease | 2 (1.4) |
| HIV | 2 (1.4) |
| Chronic pulmonary disease | 16 (11) |
| Obesity | 25 (17.3) |
| Mean disease duration SD (years) | 12.6 +-4.5 |
| Current smoker, n (%) | 7 (4.8) |
| Current MS treatments, n (%) | |
| Interferon | 21 (16.2) |
| Glatiramer acetate | 2 (1.5) |
| Fingolimod | 30 (23.2) |
| Dimethyl fumarate | 13 (10) |
| Teriflunomide | 12 (9.3) |
| Cladribine | 4 (3.1) |
| Natalizumab | 8 (6.2) |
| Ocrelizumab | 10 (7.7) |
| Alemtuzumab | 5 (3.8) |
| Rituximab | 12 (9.3) |
| No treatment | 12 (9.3) |
| Current NMOSD treatments n (%) | |
| Azathioprine | 2 (12.5) |
| Mofetil mycophenolate | 2 (12.5) |
| Rituximab | 11 (68.7) |
| No treatment | 1 (6.2) |
| Covid-19 status, n (%) | |
| Suspected | 27 (18.6) |
| Confirmed | 118 (81.4) |
SD= standard deviation; CIS= clinically isolated syndrome; RRMS= relapsing remitting multiple sclerosis; SPMS= secondary progressive multiple sclerosis; PPMS= primary progressive multiple sclerosis; NMOSD= neuromyelitis optica spectrum disorders; DMT= disease modifying treatment;
Table 2.
Demographic and clinical characteristics of hospitalization, ICU admission and dead of COVID included patients.
| Hospitalization N=23 | ICU admission N=9 | Dead N=5 | |
|---|---|---|---|
| Mean age, SD (years) | 50.4+-10 | 52+-11 | 56+-10 |
| Median EDSS (SD) | 4+-2.5 | 5+-2.3 | 6+-1.2 |
| Mean disease duration, SD (years) | 15+-8 | 17+-3 | 18+-14 |
| Female, N (%) | 16 (70) | 7 (77.8) | 5 (100) |
| MS phenotype | |||
| RRMS | 10 (43.5) | 1 (11.1) | 0 |
| Progressive | 4 (17.4) | 1 (11.1) | 0 |
| NMOSD | 9 (39.1) | 7(77.7) | 5 (100) |
| Obesity | 6 (26.1) | 4 (44.4) | 2 (40) |
| Diabetes | 4 (17.4) | 2 (22.2) | 2 (40) |
| DMT | 15 (65.3) | 8 (88.9) | 5 (100) |
SD= standard deviation; RRMS= relapsing remitting multiple sclerosis; SPMS= secondary progressive multiple sclerosis; PPMS= primary progressive multiple sclerosis; NMOSD= neuromyelitis optica spectrum disorders; DMT= disease modifying treatment; ICU= intensive critical unit.
To estimate the risk of hospitalization/ICU, the cohort was stratified by MS and NMOSD patients (Table 3 and 4 ). From 129 MS patients, 15 patients (11.6%) required hospitalization/ICU admission. Older age (OR 1.17, 95% CI 1.05 – 1.25) and longer disease duration (OR 1.39, 95%CI 1.14-1.69) were associated with hospitalization/ICU. There was no association between gender (female OR 0.54, 95% CI .016-1.82), RRMS phenotype (OR 0.89, 95%CI 0.15-5.5), obesity (OR 1.26, 95%CI 0.46-3.85) and DMT use (OR 0.44, 95%CI 0.03-5.81) with hospitalization/ICU requirement in the multivariate analysis. We did not observe risk association with specific treatments in MS patients (Table 3). No deaths from COVID-19 occurred in MS patients.
Table 3.
Risk factors of hospitalization and ICU in MS patients, N=129.
| Non hospitalization /ICUN= 114 | Hospitalization ICUN=15 | P | OR | 95%CI | |
|---|---|---|---|---|---|
| Female | 69 (60.5) | 8 (53.3) | 0.32 | 0.54 | 0.16-1.82 |
| Age | 40.3+-13 | 50.9+-12 | 0.006 | 1.17 | 1.05-1.25 |
| RRMS | 107 (82.9) | 11 (73.3) | 0.22 | 0.89 | 0.15-5.5 |
| Median EDSS (SD) | 3.5+-2.1 | 4+-2.6 | 0.29 | 0.23 | 0.2-3.5 |
| Obese | 18 (15.7) | 1 (6.7) | 0.40 | 1.26 | 0.46-3.85 |
| Disease duration | 9.5-+5 | 15+-3 | 0.001 | 1.39 | 1.14-1.69 |
| Current smoker | 7 (6.1) | 0 | - | - | - |
| DMT | 92(94) | 14 (93.3) | 0.37 | 0.44 | 0.03-5.81 |
SD= standard deviation; RRMS= relapsing remitting multiple sclerosis; DMT= disease modifying treatment; ICU= intensive critical unit
Table 4.
Hospitalization and ICU in NMOSD patients, N=16.
| Non hospitalization /ICUN=7 | Hospitalization ICUN=9 | P | OR | 95%CI | |
|---|---|---|---|---|---|
| Female | 6 (85.7) | 8 (88.9) | 0.87 | - | - |
| Age | 36+-3 | 54+-3 | <0.001 | - | - |
| Median EDSS, SD | 3 +-0.5 | 5.5+-1 | 0.0012 | - | - |
| Obese | 1 (14.3) | 5 (55.5) | 0.09 | - | - |
| Disease duration | 10.3+-5 | 18.5+-5 | 0.001 | - | - |
| Current smoker | 0 | 0 | - | - | - |
| Azathioprine | 1 (14.2) | 1 (11.1) | 0.72 | - | - |
| MMF | 1 (14.2) | 1 (11.1) | 0.25 | - | - |
| Rituximab | 4 (71.6) | 7 (77.7) | 0.37 | - | - |
SD= standard deviation; NMOSD= neuromyelitis optica spectrum disorders; ICU= intensive critical unit.
From 16 NMOSD included patients, 9 patients (56%) required hospitalization/ICU admission and 5 patients (31.2%) died from COVID-19. Due to the low number of patients, a univariate analysis was done to identify differences between groups.
The hospitalized/ICU admitted patients were older (54.3 vs. 36 years, p <0.001), had a higher EDSS (5.5 vs 2.9, p 0.0012) and longer disease duration (18.5 vs. 10.3 years, p 0.001) compared to patients that did not require hospitalization (Table 4). In addition, all deaths occurred in patients under treatment with Rituximab.
4. Discussion
We describe the largest series to date in LATAM of MS and NMOSD patients who developed an infection by COVID-19. In our cohort, the majority patients had a diagnosis confirmed by PCR. COVID-19 symptom profiles were consistent with those described in the general population and no deaths were described in MS patients (all the episodes were identified in NMOSD patients). If we consider the overall mortality of our cohort (including MS and NMOSD patients), it was slightly increased compared to COVID-19 mortality in the general population (PAHO/WHO, 2020). On the other hand, MS patient mortality in our series was lower compared to another MS patient's cohort (Louapre et al., 2020a; Sormani and Italian Study Group on, 2020). During follow-up, the total number of hospitalized patients and those who required admission in the ICU was also like previous publications on MS patients (Louapre et al., 2020a; Sormani and Italian Study Group on, 2020). A multicenter and collaborative French study with 347 MS patients showed that 21.0% had a COVID-19 severity score of 3 or more, and 12 patients (3.5%) died of COVID-19 (Louapre et al., 2020a).
We found an increased proportion of hospitalizations in NMOSD when compared to MS patients. More than half of the included NMOSD patients were hospitalized and most of them were admitted to UCI. One third of NMOSD patients died, all on Rituximab treatment. Reports on COVID-19 infection in patients with NMOSD are scarce, so it is not possible to draw definite conclusions regarding the severity of the infection in these patients. In a previous study in France, out of 15 NMOSD infected patients, 5 patients were hospitalized (all under treatment with rituximab). Only one patient needed mechanical ventilation and no deaths were reported (Louapre et al., 2020b). Iranian cohort, reported that out of 5 NMOSD infected patients, 3 required hospitalization and no deaths were reported (Sahraian et al., 2020).
In our cohort, in MS patients the severity of COVID-19 was associated with older age, higher EDSS and longer disease duration while no association was found with others factors such as gender, obesity and DMT use, neither in general or after stratification by specific treatments. These finding are in line with results of a study from New York, which identified older age, presence of comorbidities, progressive disease, and a non-ambulatory status, but not DMTs, as risk factors for COVID-19 critical illness or related death (Parrotta et al., 2020). In the French COVISEP study, using a multivariate analyses, author showed that age, EDSS, male sex and obesity were independent risk factors of severe forms of COVID-19, but report not association with exposure to DMTs and the level of immunosuppression (Louapre et al., 2020a). In the Italian MuSC-19 study, more than 80% of deaths occurred in persons with advanced disease and disability (median EDSS of 7). Regarding DMTs and the risk of severe COVID-19, recent large analysis showed that MS patients treated with anti-CD20 DMTs (rituximab or ocrelizumab), were at higher risk of more severe COVID-19 compared to other DMTs (Steve Simpson et al., 2020; Sormani and Italian Study Group on, 2020). Even in the group of patients with anti-CD20 therapies, the rate of reported deaths is 3% (Mohn et al., 2020). A recent review of all MS patients and SARS-CoV-2 infection published in the literature so far, found that the numbers of serious or fatal cases remain surprisingly low overall. Considering all published cases of MS patients with COVID-19, the rate of fatal outcome is 4%. Regarding untreated MS patients, of the 83 people for whom the outcome was published, 17% died from COVID-19 and another 7% needed non-invasive or mechanical ventilation (Mohn et al., 2020). However, careful analysis should be done to confirm those findings and emphasize the need for more research.
Although a small number of NMOSD patients were included, elderly patients, increased EDSS and longer disease duration were associated with hospitalization/ICU admission. These associations were also reported in the French NMOSD and COVID-19 study, as well as all patients requiring hospitalization being under treatment with rituximab (Louapre et al., 2020a). This result should be cautiously analyzed because it is possible that a larger cohort could identify a subgroup of patients with risk factors that modify the risk of COVID-19. To date, a few cases of NMOSD patients with COVID-19 infection were reported (Ciampi et al., 2020; Creed et al., 2020; Fan et al., 2020; Sahraian et al., 2020). Although it is possible that only the most severe cases of COVID-19 have been reported in this study, as NMOSD is a rare condition, it will be difficult to obtain robust data to identify the risk factors for the severity of COVID-19 infection. Further research to combine NMSOD cases globally is needed so it would be necessary to share global data.
To the best of our knowledge, this is the first study conducted in LATAM evaluating risk of COVID-19 in patients with MS and NMOSD. We are aware this study has limitations. First, it may include a potential referral bias toward severe COVID-19 or risk factors in included patients. Unlike previous studies mentioned above, we did not find a relationship between the severity of the infection and some comorbidities like obesity and the exposure to DMTs in patients with MS nor NMOSD. This could be related to the number of patients included and the frequency of events used to perform the analysis. Regarding MS patients, it is important to note that some immunosuppressive treatments, notably cladribine and alemtuzumab, are not commonly used in LATAM (mainly due to access and cost problems). Furthermore, therapies targeting IL-6 receptor or C5 complement are not currently approved in LATAM for NMOSD treatment. Therefore, their potential effect on the cytokine storm during COVID-19 could not be studied. Also, although it is a large cohort from LATAM countries it does not necessarily reflect the entire LATAM population (e.g. there was scarce participation of Brazil in the study). In addition, the number of patients from some countries was limited, reducing the statistical power of the analyses and result interpretation. Another limitation of our registry is that information is likely to comprise a greater proportion of severe cases requiring medical attention, potentially missing milder cases.
It is important to highlight that RELACOEM is a study carried out within the framework of a LATAM registry of prevalent infections (and not only COVID-19) in patients with MS and NMOSD that emerged during the pandemic. In this way, it sets precedents to begin working in this direction and to be able to develop longitudinal studies with greater statistical power from our region.
In conclusion, we found that in MS patients, age and disease duration were associated with hospitalization and ICU admission requirement, while age, disease duration and EDSS were associated in NMOSD. DMTs use was not associated with more severe evolution of COVID-19 infection. The current data on the cases published so far shows that COVID-19 severity or death rate among MS patients is comparatively low and that it is probably not the DMTs themselves that pose a risk (Mohn et al., 2020). Our results are in line with several cohort studies and future analysis with an increased statistical power will clarify the behavior of COVID-19 in MS and NMOSD patients from our region.
Funding
The author(s) disclosed receipt of the financial support from Biogen, for the creation and maintenance of the central platform.
Author statement
Ricardo Alonso: Management and coordination responsibility for the research activity planning and execution. Conceptualization and formulation overarching research goals and aims. Development or design of methodology. Preparation, creation.
Berenice Silva Design of methodology
Orlando Garcea Design of methodology
Juan I. Rojas: Management and coordination responsibility for the research activity planning and execution. Conceptualization and formulation overarching research goals and aims. Development or design of methodology. Preparation, creation.
Patricio E. Correa Diaz: Provision of study patients
Giordani Rodrigues dos Passos: Provision of study patients
Deyanira A. Ramirez Navarro: Provision of study patients
Luis A. Garcia Valle: Provision of study patients
Luis C. Rodriguez Salinas: Provision of study patients
Laura Negrotto: Provision of study patients. Critical review and commentary
Geraldine Luetic: Provision of study patients.
Verónica A. Tkachuk: Provision of study patients.
Jimena Míguez: Provision of study patients.
Fernando Hamuy Diaz de Bedoya: Provision of study patients.
Lorna Galleguillos Goiry: Provision of study patients.
Nicia E. Ramírez Sánchez: Provision of study patients.
Marcos Burgos: Provision of study patients.
Judith Steinberg: Provision of study patients.
Maria E. Balbuena: Provision of study patients.
Priscilla Monterrey Alvarez: Provision of study patients.
Pablo A. López: Provision of study patients. Critical review and commentary
María C. Ysrraelit: Provision of study patients. Critical review and commentary
Rosalba A. León: Provision of study patients.
Aron Benzadon Cohen: Provision of study patients.
Fernando Gracia: Provision of study patients.
Omaira Molina: Provision of study patients.
Magdalena Casas: Provision of study patients. Critical review and commentary
Norma H. Deri: Provision of study patients
Agustín Pappolla: Provision of study patients
Liliana Patrucco: Provision of study patients
Edgardo Cristiano: Provision of study patients
Dario Tavolini: Provision of study patients
Debora Nadur: Provision of study patients
Ana M. Toral Granda: Provision of study patients
Roberto Weiser: Provision of study patients
Fátima Pagani Cassará: Provision of study patients
Vladimiro Sinay: Provision of study patients
Claudia Cárcamo Rodríguez: Provision of study patients
Luciana G. Lazaro: Provision of study patients
María L. Menichini: Provision of study patients
Raúl Piedrabuena: Provision of study patients
Geraldine Orozco Escobar: Provision of study patients
Adriana Carra: Provision of study patients
Anibal Chertcoff: Provision of study patients
Biany Santos Pujols: Provision of study patients
Carlos Vrech: Provision of study patients
Adriana Tarulla: Provision of study patients
René Carvajal: Provision of study patients
Carolina Mainella: Provision of study patients
Jefferson Becker: Provision of study patients
Liesbet M. Peeters: Critical review and commentary
Clare Walton: Critical review and commentary
Marina Alonso Serena Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data
Sebastián Nuñez, Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data
Disclosure of conflicts of interest
Edgardo Cristiano has received fees for consultations as a scientific advisory board member and for travel to meetings, conferences, and clinical trials of the following companies: Avanir, Bayer, Biogen, Merck, Novartis, Roche and Teva.
Juan Ignacio Rojas has received honoraria from Novartis as a scientific advisor. He has received travel grants and attended courses and conferences on behalf of Merck-Serono Argentina, Novartis Argentina.
Liliana Patrucco has received honoraria for scientific and research grants from Teva Tuteur, Merck Serono, Biogen Idec, and Bayer Schering.
Ricardo Alonso and Berenice Silva has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Biogen, Merck Serono, Novartis, Sanofi -Genzyme and Roche.
Orlando Garcea has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Biogen, Merck Serono, Novartis, Sanofi -Genzyme and Roche.
Liesbet M. Peeters has no personal pecuniary interests to disclose, other than being the chair of The MS Data Alliance (MSDA), which receives income from a range of corporate sponsors, recently including: Biogen, BristolMyersSquibb (formerly Celgene), Canopy Growth Corporation, Genzyme, Icometrix, Merck, Mylan, Novartis, QMENTA, Quanterix, Roche.
The rest of authors declares no conflict of interest with the study project.
Acknowledgements
The authors thank everyone who actively participated in any of the teleconferences, webinars, and task force meetings. We would like to thank all the MS patients and healthcare professionals who already contributed to the different data collection efforts.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.102886.
Appendix. Supplementary materials
References
- Brownlee W., Bourdette D., Broadley S., Killestein J., Ciccarelli O. Neurology; 2020. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. [DOI] [PubMed] [Google Scholar]
- Ciampi E., Uribe-San-Martin R., Soler B., Fernandez R., Garcia P., Navarrete-Asenjo C., Tirapegui J.M., Torres R., Polanco J., Suarez F., Cuello M.J., Carcamo C. COVID-19 in MS and NMOSD: A multicentric online national survey in Chile. Mult Scler Relat Disord. 2020;45 doi: 10.1016/j.msard.2020.102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M.A., Ballesteros E., Jr L.J.G., Imitola J. Mild COVID-19 infection despite chronic B cell depletion in a patient with aquaporin-4-positive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2020;44 doi: 10.1016/j.msard.2020.102199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M., Qiu W., Bu B., Xu Y., Yang H., Huang D., Lau A.Y., Guo J., Zhang M.N., Zhang X., Yang C.S., Chen J., Zheng P., Liu Q., Zhang C., Shi F.D. Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Hawkes C., Lechner-Scott J., Levy M., Waubant E., Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenciucova E., Pruitt A. Infections in Patients Receiving Multiple Sclerosis Disease-Modifying Therapies. Current neurology and neuroscience reports. 2017;17(11):88. doi: 10.1007/s11910-017-0800-8. [DOI] [PubMed] [Google Scholar]
- Holmoy T., Hoglund R.A., Illes Z., Myhr K.M., Torkildsen O. Recent progress in maintenance treatment of neuromyelitis optica spectrum disorder. J Neurol. 2020 doi: 10.1007/s00415-020-10235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Creange A., Wahab A., Pelletier J., Heinzlef O., Labauge P., Guilloton L., Ahle G., Goudot M., Bigaut K., Laplaud D.A., Vukusic S., Lubetzki C., De Seze J., Covisep i. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Maillart E., Papeix C., Zeidan S., Biotti D., Lepine Z., Wahab A., Zedet M., Labauge P., Tilikete C., Pique J., Tourbah A., Mathey G., Dimitri Boulos D., Branger P., Kremer L.D., Marignier R., Collongues N., De Seze J. Outcomes of coronavirus disease 2019 in patients with neuromyelitis optica and associated disorders. Eur J Neurol. 2020 doi: 10.1111/ene.14612. [DOI] [PubMed] [Google Scholar]
- Luna G., Alping P., Burman J., Fink K., Fogdell-Hahn A., Gunnarsson M., Hillert J., Langer-Gould A., Lycke J., Nilsson P., Salzer J., Svenningsson A., Vrethem M., Olsson T., Piehl F., Frisell T. Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2020;77(2):184–191. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn N., Konen F.F., Pul R., Kleinschnitz C., Pruss H., Witte T., Stangel M., Skripuletz T. Experience in Multiple Sclerosis Patients with COVID-19 and Disease-Modifying Therapies: A Review of 873 Published Cases. Journal of clinical medicine. 2020;9(12) doi: 10.3390/jcm9124067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MSIF, 2020. The coronavirus and MS – global advice. (Accessed 5 may 2020 2020).
- PAHO/WHO, 2020. PAHO/WHO Response, Report 32. (Accessed 2 november 2020.
- Parrotta E., Kister I., Charvet L., Sammarco C., Saha V., Charlson R.E., Howard J., Gutman J.M., Gottesman M., Abou-Fayssal N., Wolintz R., Keilson M., Fernandez-Carbonell C., Krupp L.B., Zhovtis Ryerson L. COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters L.M., Parciak T., Walton C., Geys L., Moreau Y., De Brouwer E., Raimondi D., Pirmani A., Kalincik T., Edan G., Simpson-Yap S., De Raedt L., Dauxais Y., Gautrais C., Rodrigues P.R., McKenna L., Lazovski N., Hillert J., Forsberg L., Spelman T., McBurney R., Schmidt H., Bergmann A., Braune S., Stahmann A., Middleton R., Salter A., Bebo B.F., Rojas J.I., van der Walt A., Butzkueven H., van der Mei I., Ivanov R., Hellwig K., Sciascia do Olival G., Cohen J.A., Van Hecke W., Dobson R., Magyari M., Brum D.G., Alonso R., Nicholas R., Bauer J., Chertcoff A., de Seze J., Louapre C., Comi G., Rijke N. COVID-19 in people with multiple sclerosis: A global data sharing initiative. Mult Scler. 2020 doi: 10.1177/1352458520941485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraian M.A., Azimi A., Navardi S., Rezaeimanesh N., Naser Moghadasi A. Evaluation of COVID-19 infection in patients with Neuromyelitis optica spectrum disorder (NMOSD): A report from Iran. Mult Scler Relat Disord. 2020;44 doi: 10.1016/j.msard.2020.102245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sormani M.P., Italian Study Group on, C.-i.i.m.s. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steve Simpson et al., 2020. Associations of DMT therapies with COVID-19 severity in multiple sclerosis: an international cohort study MSVirtual 2020.
- WHO, 2020a. WHO COVID-19 Case definition. (Accessed 16 december 2020 2020).
- WHO, 2020b. WHO Director-General's opening remarks at the media briefing on COVID-19 (Accessed 11 march 2020.
- Winkelmann A., Loebermann M., Reisinger E.C., Hartung H.P., Zettl U.K. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12(4):217–233. doi: 10.1038/nrneurol.2016.21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.