Abstract
Objectives: Our primary objective was to explore the effect of a eucaloric ketogenic diet (EKD) on mortality, admission to the intensive care unit, and need for non-invasive ventilation in hospitalized patients with COronaVIrus Disease 19 (COVID-19), in comparison to a eucaloric standard diet. Secondary objectives were verification of the safety and feasibility of the diet and its effects on inflammatory parameters, particularly interleukin-6.
Methods: The study is a retrospective analysis of 34 patients fed with an EKD in comparison to 68 patients fed with a eucaloric standard diet, selected and matched using propensity scores 1:2 to avoid the confounding effect of interfering variables. Our hypothesis was that an EKD would reduce mortality, admission to the intensive care unit, and need for non-invasive ventilation in patients with COVID-19.
Results: The preliminary multivariate analysis showed a statistically significant difference in survival (P = 0.046) and need for the intensive care unit (P = 0.049) for the EKD compared with a eucaloric standard diet. Even considering the EKD start day as a time-dependent variable, the results maintain a positive trend for application of the diet, and it is not possible to reject the null hypothesis (P < 0.05). Interleukin-6 concentrations between t0 and t7 (7 d after the beginning of the diet) in the ketogenic nutrition group show a trend that is almost significant (P = 0.062). The EKD was safe and no adverse events were observed.
Conclusions: These results show a possible therapeutic role of an EKD in the clinical management of COVID-19. Currently, a prospective controlled randomized trial is running to confirm these preliminary data.
Keywords: Ketogenic diet, COVID-19, Cytokine storm syndrome, IL-6, Warburg effect, Hyperglycemia, Aerobic glycolysis, SARS-CoV-2
Introduction
COVID-19 is a pandemic disease caused by the SARS-CoV-2 virus, characterized by respiratory and gastrointestinal symptoms [1] and an all-cause in-hospital mortality rate of 43.6% (120/275), according to recent published experience [2], between February 25 and March 25, 2020.
In a subgroup of patients, a cytokine storm syndrome (CSS) characterized by fulminant and fatal hypercytokinemia associated with multiorgan failure seems to be one of the most important precipitating factors of the disease [3]. Moreover, several risk factors, such as age, obesity, and multiorgan comorbidity, have been associated with severe infection and worse outcomes [4], [5], [6].
Currently, there is no proven drug for the treatment of COVID-19 CSS. From the beginning of the pandemic, approaches aimed at the control of hyperinflammation due to CSS have been antiinflammatory therapies such as corticosteroids, interleukin-6 inhibitors, anti-granulocyte-macrophage colony stimulating factor, and programmed cell death protein 1. Recently, BB1 checkpoint inhibitors, hydroxychloroquine, cytokine adsorption devices, and intravenous immunoglobulin [7], [8], [9] have been proposed. According to the World Health Organization, systemic steroids are the only proven therapy in critical and severe COVID-19 [10], so any possible alternative treatment should be investigated. Recently, we proposed an immunometabolic hypothesis identifying a treatment capable of reducing the state of hyperinflammation associated with SARS-CoV-2 infection [11].
There is increasing evidence that macrophages, including resident alveolar macrophages and macrophages recruited from blood, have a crucial role in the pathogenesis of acute respiratory distress syndrome (ARDS) [12]. In a healthy state, alveolar macrophages located in the interface between air and cellular tissue are the prevalent population in the alveolus. The M2 phenotype is the main form of these cells, with immunosuppressive functions and optimally fueled by free fatty acids. During an injury, as the exudative phase of ARDS, peripheral blood monocytes are recruited into the alveolar lumen, then turn into macrophages with M1 phenotype, and finally release various potent proinflammatory mediators (macrophage inflammatory protein-2 and interleukin-8) which attract neutrophils into the alveolar space and are responsible for the tissue damage that happens in ARDS.
Together with neutrophils, pulmonary activated platelets play a crucial thrombo-inflammatory role by forming platelet–neutrophil complexes and monocyte–platelet aggregates, causing the development of a procoagulant and proinflammatory environment [13]. During M1 phenotype activation, there is a metabolic shift from oxidative phosphorylation to aerobic glycolysis (the Warburg effect), with a consequent increase in lactate production [14], which leads to a decrease in type I interferon, a well-known defense mechanism against viruses [15].
Materials and methods
Participants
This study is a retrospective analysis of people with SARS-CoV-2 disease who were admitted to IRCCS San Martino Hospital between February and July 2020, with a peak in hospital admission in March 2020, and who underwent a ketogenic diet. In this regard, in the Infectious Disease Unit a ketogenic diet entered the routine protocol of the ward in the absence of indications or contraindications for a specific diet in COVID-19, according to its inflammatory role.
All participants signed consent for the use of personal treatment data and informed consent to undergo any type of therapy during their hospitalization. They were also informed that a ketogenic diet was part of the routine protocol of the ward, in the absence of contraindications, and had the possibility of accepting or refusing immediately or later, in case of poor palatability or taste. The pharmacologic protocol was not conditioned by the choice of diet.
The exclusion criteria for the eucaloric ketogenic diet (EKD) were type I diabetes mellitus; insulin-dependent type II diabetes or type II diabetes in treatment with sulfonylureas, repaglinide, glucagon-like peptide-1 analogs, sodium-glucose cotransporter-2 inhibitors, or recent arteriosclerotic cardiovascular disease (within 1 mo); food allergies to the diet components; any metabolic disorder that could affect gluconeogenesis; clinical history of severe hypertriglyceridemia with or without pancreatitis; and pregnancy or lactation.
Meanwhile, a randomized controlled trial with the purpose of studying the EKD in a larger sample of individuals in the whole hospital, randomizing the nutritional treatment, was submitted and approved in June by the ethics committee (KETOCOV-1 Register number CER Liguria: 198/2020 - DB id 10517; ClinicalTrials.gov identifier 04492228), and was begun at the end of September 2020 with the recrudescence of the infection in Italy.
Considering the approval of the randomized controlled trial by the ethics committee, at the end of the first wave of the pandemic in July the data of patients treated in the Infectious Disease Unit who followed the routine diet protocol with the EKD for a minimum period of 2 wk were analyzed.
To avoid the confounding effect of interfering variables between the two diet groups, a 1:2 propensity score-matching analysis was performed with patients treated in other facilities and made available to a single management software, to have adequate controls for a valid statistical analysis (see later).
Inclusion criteria for the data analysis were as follows: documented diagnosis of COVID-19 defined by a positive reverse-transcription polymerase chain reaction assay result of a respiratory sample, P/F ratio >100 (arterial oxygen concentration divided by fraction of inspired oxygen) or mild to moderate ARDS [16], and age older than 18 y.
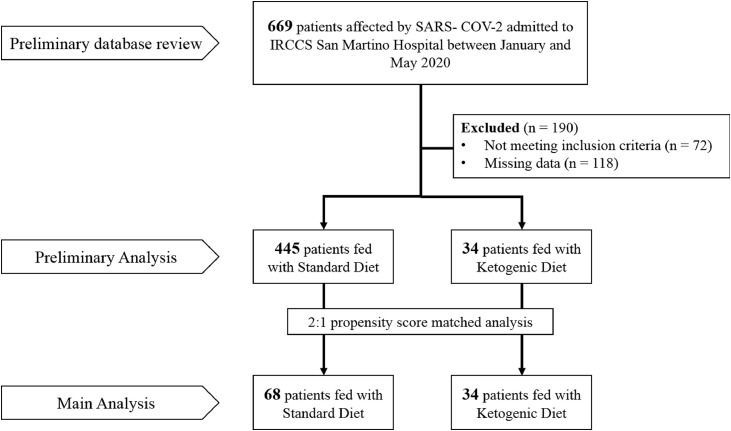
The initial sample of 669 patients was reduced to 479 (297 men and 182 women) because 190 either had missing data (118) or did not meet the inclusion criteria (72). After the propensity score-matching analysis, 34 EKD patients were included in the study and compared with 68 standard-diet (SD) patients.
All participants were investigated for demographic data and the presence of the following comorbidities: diabetes, hypertension, arteriosclerotic cardiovascular disease, heart failure, chronic pulmonary disease, solid and hematological neoplasia, ulcerative disease, moderate or severe liver disease, dementia, collagen diseases, metastatic neoplasia, and hemiplegia.
The Charlson Comorbidity Index, which has been used as a measure of 1-y mortality risk [12], was calculated for all participants. The laboratory data and P/F ratio were taken into account on the day of hospital admission for the control group (standard diet) and the day before administration of the ketogenic diet for the EKD group. Laboratory data required by the ward doctor included routine blood tests (blood cell count, azotemia, creatinine, aspartate aminotransferase, creatinine phosphokinase, lactate dehydrogenase, albumin, triacylglycerols, interleukin-6 [IL-6], polymerase chain reaction, ferritin, lipid profile, fibrinogen, blood sugar, basal hemoglobin A1c, basal vitamin D), basal urine test, and complete urine analysis.
The study was conducted in accordance with the Declaration of Helsinki. Figure 1 reports a detailed scheme of the participants and the analysis steps.
Fig. 1.
Plan of the study.
Diet administration
Participants included in the analysis belonged to two different dietary groups: the standard diet group, fed the eucaloric standard diet (ESD), and the ketogenic diet group, fed the EKD. According to the Reference Intake of Nutrients and Energy for Italian Population and the Italian guidelines for a healthy diet [17,18], the standard oral diet was based on the Mediterranean style [19] and characterized by 30 kcal/kg/d (ranging from 1800 to 2100 kcal), protein intake of 16–20%, lipid intake of 26%–30%, and carbohydrate intake of 42%–50%.
The EKD ranged from 1800 to 2100 kcal and was characterized by very low carbohydrates (<30 g, 5%–6% of total energy) to induce ketosis, with a polyunsaturated/unsaturated/saturated fat ratio of 3:2:1. The protein content of the EKD was similar to that of an average Mediterranean diet (17%–18% of total calories). The average protein intake for the ESD was 86.5 ± 5.5 g, whereas for the EKD it was 89.4 ± 2.5 g.
Endpoints and outcomes
The primary outcomes were 30-d mortality, intensive care unit (ICU) admission, and need for continuous positive airway pressure; they were also considered together in a combined outcome. Secondary outcomes were the effects of the EKD on biological and inflammatory parameters, particularly IL-6.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics, version 25.0 (SPSS Inc., Chicago, IL, USA). Kolmogorov–Smirnov analysis was used to test the normality of the variables. The results of continuous variables are expressed as median and interquartile range. For categorical variables, contingency tables were used to indicate the frequency and percentage in the population. For the comparison of continuous variables between different groups of participants, non-parametric Kruskal–Wallis or Mann–Whitney tests were used when appropriate. Nominal variables were examined with Pearson's χ2 test, and Spearman's rank correlation index was used for correlations with continuous variables.
To adjust for baseline differences that are intrinsic in non-randomized studies, EKD participants (n = 34) were matched 1:2 to ESD participants (n = 68) by a propensity score (PS; Table 1 ). Preliminarily, covariates or factors entered into the model were identified by univariate statistical analysis, and the probability value for inclusion was P ≤ 0.05 between 445 and 34 individuals fed the ESD and EKD, respectively. The PS was estimated by a logistic regression model including variables that were significantly different between the two groups: Charlson Comorbidity Index, lymphocyte count, aspartate aminotransferase, and albumin as continuous variables, and the presence of diabetes, chronic pulmonary disease, hematological neoplasia, and therapy with corticosteroids, remdesivir, and tocilizumab as categorical data. Caliper levels of 0.8 were considered for PS.
Table 1.
Demographic and clinical characteristics of participants after propensity score-matched main analysis
| Variable | Standard diet (n = 68) | Ketogenic diet (n = 34) | All participants (N = 102) | P |
|---|---|---|---|---|
| Demographic statistics | ||||
| Age, y | 67 (54–77) | 67 (52–76) | 67 (53–77) | 0.943 |
| Sex, M/F | 40/28 (58.8/41.2) | 23/11 (67.6/32.4) | 63/39 (61.8/38.2) | 0.387 |
| Comorbidities | ||||
| Diabetes mellitus | 7 (10.3) | 1 (2.9) | 8 (7.8) | 0.193 |
| Hypertension | 32 (47.1) | 15 (44.1) | 47 (46.1) | 0.779 |
| Arteriosclerotic cardiovascular disease | 27 (39.7) | 13 (38.2) | 40 (39.2) | 0.886 |
| Heart failure | 3 (4.4) | 2 (5.9) | 5 (4.9) | 0.746 |
| Pulmonary disease | 4 (5.9) | 0 (0.0) | 4 (3.9) | 0.149 |
| Solid neoplasia | 7 (10.3) | 1 (2.9) | 8 (7.8) | 0.193 |
| Hematological neoplasia | 4 (5.9) | 5 (14.7) | 9 (8.8) | 0.139 |
| Ulcerative disease | 3 (4.4) | 0 (0.0) | 3 (2.9) | 0.214 |
| Moderate to severe liver disease | 2 (2.9) | 1 (2.9) | 3 (2.9) | 1.000 |
| Dementia | 3 (4.4) | 1 (2.9) | 4 (3.9) | 0.718 |
| Collagenopathy | 1 (1.5) | 0 (0.0) | 1 (1.0) | 0.477 |
| Metastatic neoplasia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Hemiplegia | 1 (1.5) | 1 (2.9) | 2 (2.0) | 0.614 |
| Charlson Comorbidity Index | 3 (1–5) | 3 (2–5) | 3 (1–5) | 0.838 |
| Clinical features | ||||
| P/F ratio | 281 (205–323) | 312 (166–384) | 286 (188–348) | 0.312 |
| Laboratory values | ||||
| White blood cells, million/m3 | 7.26 (5–10.58) | 6.78 (5.3–8.96) | 7.02 (5.09–10.06) | 0.531 |
| Lymphocytes, 100/m3 | 0.9 (0.6–1.3) | 1.05 (0.74–1.32) | 1.00 (0.69–1.32) | 0.514 |
| Platelets, 100/m3 | 213 (151–274) | 233 (144–305) | 221 (149–293) | 0.616 |
| Aspartate aminotransferase, UI/L | 31 (23–58) | 47 (24–73) | 36 (23–66) | 0.118 |
| Alanine aminotransferase, UI/L | 38 (23–47) | 34 (24–46.5) | 34 (23–47) | 0.650 |
| Ferritin, μg/L | 737 (329–1204.5) | 773 (326–1257) | 771 (326–1207) | 0.646 |
| Interleukin-6, pg/mL | 46.2 (27.85–94.35) | 36.2 (18.3–108) | 45.4 (21.5–101) | 0.647 |
| Albumin, g/L | 27.05 (23.5–30) | 30.75 (28.9–34.1) | 28.95 (24.85–34.05) | 0.065 |
| Concomitant pharmacotherapy | ||||
| Corticosteroid | 51 (75.0) | 29 (85.3) | 80 (78.4) | 0.233 |
| Antibiotic | 37 (54.4) | 22 (64.7) | 59 (57.8) | 0.321 |
| Hydroxychloroquine | 42 (61.8) | 19 (55.9) | 61 (59.8) | 0.568 |
| Remdesivir | 2 (2.9) | 3 (8.8) | 5 (4.9) | 0.195 |
| Tocilizumab | 37 (54.4) | 23 (67.6) | 60 (58.8) | 0.200 |
All values are expressed as median (interquartile range) or number (percentage)
An exploratory Cox regression analysis ignoring the risk of immortality bias was proposed to estimate the effect of the EKD in comparison to the ESD on all endpoints (30-d mortality, ICU admission, need for continuous positive airway pressure, and the composite endpoint). Later, a Cox regression analysis considering the beginning of EKD as a time-dependent covariate was proposed to estimate the effect of the different dietary regimens on all primary endpoints, avoiding the immortality bias. In fact, because all ESD participants began their diet at hospital admission, but EKD participants started their diet a few days later, the ketogenic diet start was considered a time-varying covariate to avoid immortality bias. The results are reported as hazard ratios (HRs) and 95% confidence intervals (CIs). The period from hospitalization to the onset of each outcome was considered for survival analysis.
Results
The demographic and clinical characteristics of the 102 participants are reported in Table 1. The median (interquartile range) age was 67 (53–77) y, and no significant differences between groups were detected for demographic characteristics, comorbidity history, laboratory measures, concomitant pharmacotherapy, or P/F ratios.
In our experience, the overall COVID-19 mortality was 21.6% (22/102); in the ESD and EKD groups, it was respectively 27.9% (19/68) and 8.8% (3/34). A total of 14 of the 102 participants (13.7%) were admitted to the ICU; by group, the rate was 19.1% (13/68) for the ESD group and 2.9% (1/34) for the EKD group. The explorative Cox regression analysis showed a significant association of both survival (P < 0.027) and the need for the ICU (P < 0.025) with the different diets (Table 2 ).
Table 2.
Cox regression of primary outcomes in EKD group versus ESD group (unadjusted for the risk of immortality bias)
| Outcome | P | HR | 95.0% CI for HR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Death | 0.046 | 0.289 | 0.086 | 0.977 |
| Intensive care unit | 0.049 | 0.130 | 0.017 | 0.996 |
| CPAP | 0.476 | 0.802 | 0.436 | 1.472 |
| Composite endpoint | 0.082 | 0.602 | 0.340 | 1.066 |
CI, confidence interval; CPAP, continuous positive airway pressure; EKD, eucaloric ketogenic diet; ESD, eucaloric standard diet; HR, hazard ratio.
Statistical significant values are in boldface.
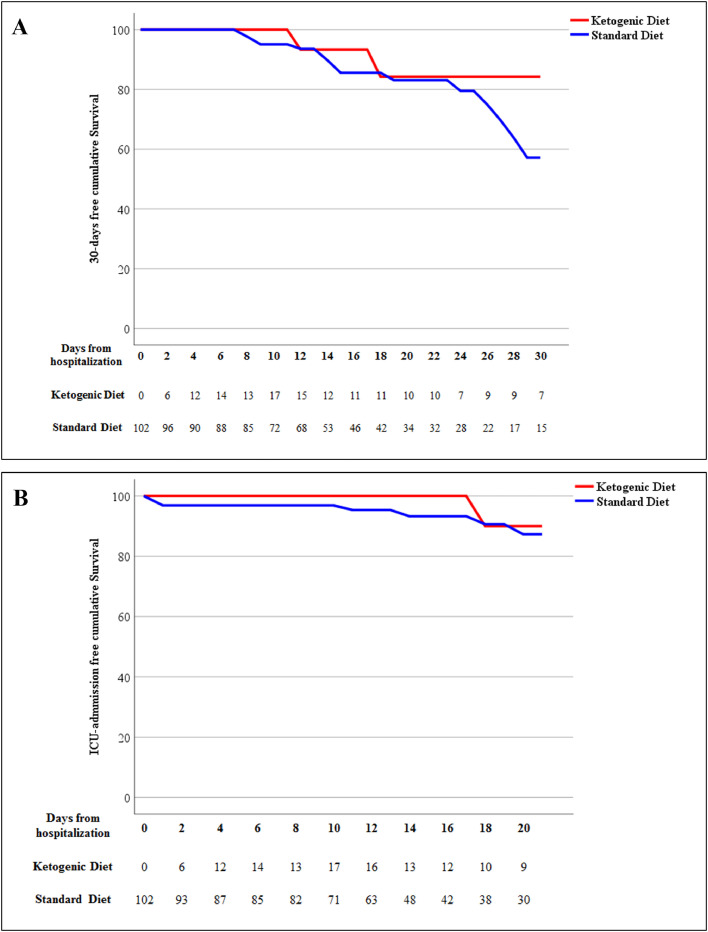
The findings of the main Cox regression analysis are reported in Table 3 . The 30-d mortality in the survival analysis showed a trend of lower risk in the EKD group than the ESD group (HR, 0.416; 95% CI, 0.122–1.413), although this result did not reach statistical significance (P = 0.160; Fig. 2 A). Moreover, the EKD group had a trend of association with lower admission to the ICU than the ESD group (HR, 0.357; 95% CI, 0.045–2.847; P = 0.331; Fig. 2B). No significantly different risks were detected between the two groups in the need for continuous positive airway pressure (EKD versus ESD: HR, 0.968, 95% CI, 0.289–3.242; P = 0.958) or the composite endpoint (EKD versus ESD: HR, 0.674; 95% CI, 0.233–1.949, P = 0.446).
Table 3.
Cox regression with time-dependent covariate of primary outcomes in EKD group versus ESD group (adjusted for the risk of immortality bias)
| Outcome | P | HR | 95.0% CI for HR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Death | 0.160 | 0.416 | 0.122 | 1.413 |
| Intensive care unit | 0.331 | 0.357 | 0.0045 | 2.847 |
| CPAP | 0.958 | 0.968 | 0.289 | 3.242 |
| Composite endpoint | 0.446 | 0.674 | 0.233 | 1.949 |
CI, confidence interval; CPAP, continuous positive airway pressure; EKD, eucaloric ketogenic diet; ESD, eucaloric standard diet; HR, hazard ratio
Fig. 2.
Kaplan–Meier estimates (between control and treatment groups) stratified by time-varying start of ketogenic diet for (A) 30-d mortality and (B) need for intensive care unit.
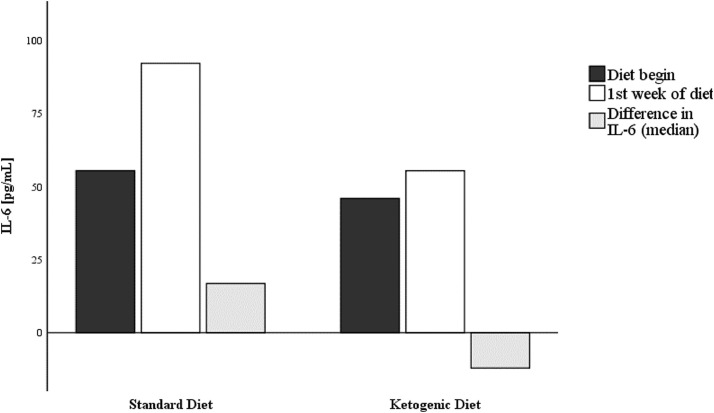
Compared to the ESD group, the EKD group had a median IL-6 difference of −51.8 pg/mL and a mean difference of −169 pg/mL (data from 22 of the 34 sets). After IL-6 imputation into two bins—Δ < 0 and Δ ≥ 0, IL-6 trend, from day 0 to day 7, was almost significantly lower for patients who followed EKD (χ2 = 3.698, df = 1; P = 0.05447; Table 4 , Fig. 3 ).
Table 4.
Variation in interleukin-6 from day 0 to day 7 (adjusted for the risk of immortality bias)
| ESD | EKD | |
|---|---|---|
| Increase | 14 (58.3) | 7 (30.4) |
| Decrease | 10 (41.7) | 16 (69.6) |
EKD, eucaloric ketogenic diet; ESD, eucaloric standard diet
All values are expressed as number (percentage)
p-value = 0.05between ESD and EKD (Pearson's chi-squared test)
Fig. 3.
Variation in interleukin-6 during ketogenic diet versus standard diet.
The EKD was safe, and no adverse events were observed in participants who were fed it. In particular, acidosis was never observed, and arterial pH at baseline was similar in both groups (P = 0.100). At t7 (compared to t0), arterial pH had fallen by a similar percentage in both groups (57% versus 42%, P = 0.484). The median variation of arterial pH was about +0.0075 in the ESD group versus −0.0100 in the EKD group (P = 0.342).
Discussion
Nutritional status appears to be a relevant factor influencing the outcomes of COVID-19, but little information has emerged about the impact of early nutritional support in pre-ICU patients [20]. Surely nutrition plays a pivotal role in the prevention of the comorbidities most frequently associated with COVID-19, such as hypertension, cardiovascular and cerebrovascular disease, and diabetes, which have been noted as respectively twofold, threefold, and twofold higher in ICU/severe cases than in non-ICU/severe ones [21].
Obesity is associated with a worse prognosis in people with COVID-19, especially younger ones [4,5,22]. A hyperinflammatory response has been recognized as the main cause of morbidity and mortality in these people [23].
A genetic substrate of CSS has been recently suggested, with alpha1-antitrypsin deficiency alleles possibly contributing to national differences in COVID-19 infection. The association between alpha1-antitrypsin deficiency and severity and mortality rates has not yet been defined, and the exact pathophysiological mechanism that determines this process is unknown [24]. COVID-19 CSS appears 8–10 d after the onset of symptoms of the disease and is characterized by high fever, dyspnea, bilateral pulmonary infiltrates that can evolve into ARDS, and multisystemic organ failure [25]. Effective treatments for COVID-19 and COVID-19 CSS are needed immediately. Patient timing and selection seem to be particularly crucial in managing the acute phase of COVID-19.
There is a consensus that ketosis protects healthy tissues against oxidative stress by simultaneously decreasing production of reactive oxygen species and increasing endogenous antioxidant capacity [22]. It is well known that a ketogenic diet can inhibit inflammation. Studies have shown that it reduces circulating inflammatory markers in humans [26]. A KD, via hydroxybutyrate, is capable of activating hydroxycarboxylic acid receptor 2, a G protein-coupled receptor which inhibits nuclear factor κB in macrophages, dendritic cells, and microglia and reduces neuroinflammation [27], [28].
Finally, from a clinical point of view, previous experience shows clinical improvement in respiratory function with a ketogenic diet. After 10 d of modified protein-saving fasting (a ketogenic diet with very low calorie content), a statistically significant improvement in functional residual capacity and expiratory reserve volume has been observed [28], [29]. In addition, a 20-d ketogenic diet has shown a significant decrease in end-tidal carbon dioxide tension [30].
As reported in the introduction, the use of corticosteroids is currently suggested by World Health Organization guidelines, and it is actually the first approach in severe disease [9]. In our study, corticosteroid treatment was present in 75% of the ESD group and 85.3% of the EKD group. Tocilizumab was present in 54.4% of the ESD group and 67.6% of the EKD group.
The antiinflammatory efficacy of the EKD was almost significant and therefore seems independent of steroid or anticytokine treatment with tocilizumab. In fact, the analysis of the course of IL-6 in the first week of therapy highlights the almost significant variation in IL-6 from the beginning to 7 d after the start of the EKD. Levels of IL-6 did not increase but rather tended to be slightly reduced in the EKD group (Table 4, Fig. 3). This trend underlines the fact that after 1 wk, participants were at increased risk of COVID-19 CSS. Dietary treatment is, in this regard, a possible immunomodulation aimed mainly at the activity of macrophages without interfering with antiviral clinical efficacy.
During the treatment, the participants did not present any adverse events related to diet.
A limitation of the study is the lack of controlled randomization, and it was conducted in a single hospital facility. A further limitation is the number of participants (34 in the EKD group versus 68 in the ESD group), because recruiting was discontinued owing to the absence of patients admitted from the end of July; therefore, it is possible that the study was underpowered.
A strength of the study is the usefulness of the propensity score, which, with the large hospital COVID-19 database (approximately 669 cases), allowed proper 1:2 matching, overcoming the absence of controlled randomization.
The present study provides preliminary data for all participants who were on an EKD during the epidemic period that ended at the end of July. The preliminary multivariate analysis (Table 2) demonstrated a significant association of both survival (P = 0.046) and the need for the ICU (P = 0.049) with the EKD compared with the ESD. Indeed, as reported, the rigorous statistical strategy used for the data analysis—even if retrospective—was based on the use of a Cox regression with time-dependent variables and was developed to minimize the possibility of bias derived from the administration of the ketogenic diet (compared to standard hospital food) at different time distances from initial hospitalization.
Using the start day of the ketogenic diet as a “time-dependent” variable means that participants are all considered at t0 as controls; only when they begin the ketogenic diet are they considered cases. Through this strategy, the effect of an adverse event or early outcome (in the first days) affects the EKD group more, because the group is less numerous; conversely, if such an early occurrence affects an ESD participant, its statistical effect is greatly diluted. In scientific work this strategy is not always applied, and there may be a statistical distortion known as immortality bias in which this potential distortion is not considered and data are analyzed on static groups over the study period. In fact, if we did not consider the ketogenic-diet variable as time dependent, all our results would reach statistical significance (P < 0.05). On the contrary, by eliminating the immortality bias and applying a rigorous statistical analysis, we were not able to reject the null hypothesis (P < 0.05). However, the results maintain a positive trend toward the application of the ketogenic diet.
The reported data are therefore an exact picture of the actual state, and the trends of reduced mortality, reduced need for ICU admission, and reduced IL-6 between 0 and 7 d—although not statistically significant—suggest a possible alternative treatment to the disease, in the absence of side effects, additional costs, and risks for the patient.
Different from other studies in which nutrition is considered as a support to drug therapy, this study is the first to underline the role of clinical nutrition therapy as a pathophysiological support to drug therapy in improving the prognosis of not only COVID-19 but also other infectious diseases in which immunomodulation could have a role in reducing hyperinflammation syndromes.
Conclusions
In conclusion, this retrospective pilot study provides valuable preliminary information regarding the possible role of an EKD in controlling mortality and ICU admission by means of the immunomodulation of COVID-19 CSS.
These data must necessarily be supported by further evidence from a larger sample, and the randomized controlled prospective clinical trial that began in September, with the recrudescence of COVID-19 infection in Italy, could be particularly useful.
Footnotes
The article was submitted to the Ligurian Ethical Committee, which approved its publication on November 24, 2020.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.nut.2021.111236.
Appendix. Supplementary materials
References
- 1.Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vena A, Giacobbe DR, Di Biagio A, Mikulska M, Taramasso L, De Maria A, GECOVID Study Group Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26:1537–1544. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, Garg A, Rout A, Lavie CJ. Association of obesity with more critical illness in COVID-19. Mayo Clin Proc. 2020;95:2040–2042. doi: 10.1016/j.mayocp.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95:1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan RE, Adab P, Cheng KK Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 7.Vijayvargiya P, Esquer Garrigos Z, Castillo Almeida NE, Gurram PR, Stevens RW, Razonable RR. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof) Mayo Clin Proc. 2020;95:1454–1466. doi: 10.1016/j.mayocp.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corticosteroids for COVID-19. Available at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Corticosteroids-2020.1. Accessed November 30, 2020.
- 10.Sukkar SG, Bassetti M. Induction of ketosis as a potential therapeutic option to limit hyperglycemia and prevent cytokine storm in COVID-19. Nutrition. 2020;79–80 doi: 10.1016/j.nut.2020.110967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Xiu H, Zhang S, Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris G, Bortolasci CC, Puri BK, Olive L, Marx W, O'Neil A, et al. The pathophysiology of SARS-CoV-2: a suggested model and therapeutic approach. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly B, O'Neill LAJ. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian Z, Travanty EA, Oko L, Edeen K, Berglund A, Wang J, et al. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol. 2013;48:742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 16.ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.SICS Editore; SINU: 2014. LARN: Livelli di assunzione di riferimento di nutrienti ed energia per la popolazione italiana, IV revisione. [Google Scholar]
- 18.Linee guida per una sana alimentazione 2018. Available at: https://www.crea.gov.it/web/alimenti-e-nutrizione/-/linee-guida-per-una-sana-alimentazione-2018. Accessed July 24, 2020.
- 19.Vitiello V, Germani A, Capuzzo Dolcetta E, Donini LM, Del Balzo V. The new modern Mediterranean diet Italian pyramid. Ann Ig. 2016;28:179–186. doi: 10.7416/ai.2016.2096. [DOI] [PubMed] [Google Scholar]
- 20.Laviano A, Koverech A, Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe M, Risi R, Tuccinardi D, Baquero CJ, Manfrini S, Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 2020;36:e3325. doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 23.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapira G, Shomron N, Gurwitz D. Ethnic differences in alpha-1 antitrypsin deficiency allele frequencies may partially explain national differences in COVID-19 fatality rates. FASEB J. 2020;34:14160–14165. doi: 10.1096/fj.202002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langer-Gould A, Smith JB, Gonzales EG, Castillo RD, Figueroa JB, Ramanathan A, et al. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis. 2020;99:291–297. doi: 10.1016/j.ijid.2020.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Stubbs BJ, Koutnik AP, Goldberg EL, Upadhyay V, Turnbaugh PJ, Verdin E, et al. Investigating ketone bodies as immunometabolic countermeasures against respiratory viral infections. Med (N Y) 2020;1:43–65. doi: 10.1016/j.medj.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Müller-Fielitz H, et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 29.Sukkar SG, Signori A, Borrini C, Barisione G, Ivaldi C, Romeo C, et al. Feasibility of protein-sparing modified fast by tube (ProMoFasT) in obesity treatment: a phase II pilot trial on clinical safety and efficacy (appetite control, body composition, muscular strength, metabolic pattern, pulmonary function test) Med J Nutrition Metab. 2013;6:165–176. doi: 10.1007/s12349-013-0126-2. Epub 2013 May 30. PMID: 24027606; PMCID: PMC3764321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubini A, Bosco G, Lodi A, Cenci L, Parmagnani A, Grimaldi K, et al. Effects of twenty days of the ketogenic diet on metabolic and respiratory parameters in healthy subjects. Lung. 2015;193:939–945. doi: 10.1007/s00408-015-9806-7. Epub 2015 Sep 26. Erratum in: Lung. 2016 Nov 1;: PMID: 26410589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.