Abstract
Background
Many people are now living longer with HIV due to access to antiretroviral treatment. In turn, there has been an increase in the burden of hypertension and diabetes. The paucity of data on the burden of hypertension and diabetes in adults living with HIV in South Africa is a public health concern. The paper aimed to describe the prevalence and factors associated with hypertension and diabetes among adults living with HIV (ALHIV).
Methods
This was a secondary data analysis of the population based on the South African National HIV Prevalence, Incidence, Behaviour and Communication surveys for 2005, 2008 and 2017. Descriptive statistics were used to summarise the characteristics of the study sample. Bivariate and multivariate logistic regression analyses were used to determine factors associated with hypertension and diabetes.
Results
The total study population of ALHIV aged 25 years and older was 978, 1023 and 2483 for 2005, 2008 and 2017. The prevalence of hypertension showed an increasing trend at 11.8% in 2005, 9.5% in 2008 and 14.3% in 2017. The prevalence of diabetes was 3.3% in 2005, 2.8% in 2008 and 3.2% in 2017. Increased odds of hypertension among adults living with HIV were consistently associated with being female and the age group 45 years older across all the survey years, including pensioners and the sick, living in urban areas, high risk of hazardous alcohol consumption, diabetes and heart disease. Increased odds of diabetes were consistently associated with hypertension across all the survey years, including age group 45 years and older, and poor health. While having a secondary level of education and above was protective against diabetes.
Conclusion
The study showed that the prevalence of hypertension is high and has increased over time among adults living with HIV while the prevalence of diabetes has remained constant. Findings identified factors consistently associated with the prevalence of both diseases overtime, including contemporary risk factors that should be targeted in the integrated management of chronic disease and HIV care model.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-021-10502-8.
Keywords: Hypertension, Diabetes, HIV, South Africa, Prevalence, Factors, Trends
Background
At 7.9 million, South Africa has the largest population of people living with HIV (PLHIV) in the world [1, 2]. The successful roll-out of the anti-retroviral therapy (ART) programme since 2004 has contributed to improvements in the life expectancy and viral load suppression in PLHIV; and deaths due to opportunistic infections have also declined [3, 4]. This has transformed HIV infection into a chronic disease and evidence shows that as a consequence of a combination of factors including ageing, being on ART, HIV infection and certain lifestyle choices, their risk of developing non-communicable diseases (NCDs) including hypertension or diabetes increases [5]. In addition, the growing double burden of the HIV and NCD co-epidemics has put significant pressure not only on the affected individuals but also on the struggling health system, existing health programmes and the economy in South Africa [6–10].
Studies that have been done to characterise the burden of hypertension and diabetes in PLHIV highlight the implication of not only collecting prevalence estimates but also adequately addressing multimorbidity in PLHIV and ensuring adequate availability of treatment to reduce the risk [7]. However, these studies present varied findings, suggesting that results and conclusions may not necessarily be extrapolated from one country to the next and not one intervention fits all [11–18]. A meta-analysis and systematic review across sub-Saharan Africa reported prevalence estimates for hypertension in PLHIV ranging between 5.2 and 50.0% and that of diabetes to range between 0.5 and 36.6%. In South Africa, studies have also reported prevalence estimates in PLHIV for hypertension as high as 38.6% and diabetes as high as 8.66% [19, 20]. However, these estimates cannot be generalised to the entire South African population. Common risk factors of these NCDs in PLHIV have also been reported to include ageing, family history, use of ART, HIV infection, urbanisation, alcohol drinking and high body mass index in studies conducted in Ethiopia, Nigeria, Malawi, Tanzania, Zimbabwe and South Africa [13, 21–27]. Also, further studies indicated that hypertension and diabetes can also act as risk factors of each other [12].
Improved understanding of the magnitude of hypertension and diabetes and associated factors among PLHIV in South Africa is vital for informing public health interventions in the country. This paper investigates trends, prevalence and associated factors of hypertension and diabetes among adults living with HIV (ALHIV) in South Africa using the 2005, 2008, 2012, and 2017 nationally representative population-based HIV Prevalence, Incidence, Behaviour and Communication Surveys.
Methodology
Study design and sample
This secondary analysis utilised data collected from the South African National HIV Prevalence, Incidence, Behaviour, and Communication (SABSSM) surveys in 2005, 2008 and 2017. This was a multi-stage stratified cluster randomised cross-sectional population-based survey undertaken by the Human Sciences Research Council (HSRC). The details of the study and sampling method are described in detail elsewhere [1, 28–30]. Briefly, in the 2005 and 2008 surveys, in each household a maximum of three people were selected randomly to participate in the study, each representing the 2–14 years, 15–24 years and 25 years and older age groups [28, 29]. In the 2012 and 2017 surveys, all household members were eligible to participate in the survey [1, 30]. The analysis for this paper was restricted to ALHIV aged 25 years and older who responded to the questions on the primary outcomes, hypertension and diabetes.
Study variables
The primary outcome variables were hypertension and diabetes. These variables were self-reported by the survey participants when asked the question “Do you currently have any of the following illnesses?”. Both outcome variables were coded 1 for the answer “yes”, and 0 for the answer “no”. The explanatory variables used in this study were grouped into three categories namely socio-demographic, behavioural and health-related factors. The socio-demographic factors included sex (male and female), age (25–34 yrs., 35–44 yrs. and 45+ yrs.), race (Non- Black African including White, Coloured and Indian; and Black African), marital status (never married and ever married), employment status (unemployed, employed and other (incl. Old age pensioner, sick/disabled and unable to work), educational level (no or up to primary and secondary and above), locality type (urban, urban formal, urban informal, tribal area/rural informal and rural formal) and province. The behavioural factors included hazardous alcohol consumption using the Alcohol Use Disorders Identification Test score (no risk (do not drink alcohol), low risk (0–7), medium risk (8–15), high risk (16–19) and addiction likely (20–40). Hazardous alcohol consumption was defined as a habit or amount of alcohol intake that raises the risk of adverse health effects [31]. The health-related factors included the exposure to ARVs (ARV exposed, ARV naive), viral load suppressed, perception of general health (excellent, good, fair, and poor), cancer, mental distress, tuberculosis, heart disease, diabetes and health care access (public care, and private care).
Statistical analysis
All analyses were adjusted for complex survey design, clustering and any bias due to non-response using sample weights and the svyset and svy commands in STATA version 15 [32]. Frequency tables with percentages and median with interquartile ranges (IQR) were used to describe the characteristics of the study sample and trends in the prevalence of hypertension and diabetes in the study population. Pearson’s chi-squared test was used for comparison of categorical variables. Bivariate logistic regression analysis was done and explanatory variables that had a p-value < 0.25 were included in the multivariate logistic regression model to determine the associated factors. The Wald test was used to keep variables that had a p-value < 0.05 through a process of removing, refitting, and verifying until all significant variables were kept in the final main effects model. Variables which had very wide confidence intervals were removed from the model regardless of the p-value. The Variance Inflation Factor test was done to check for multicollinearity at a cut off level of 5. A post-estimation test using the Hosmer-Lemeshow Goodness of Fit (GOF) test was done to test whether the final model was a good fit [33]. If the GOF test failed multiple times, the preliminary final model was used instead. Crude and adjusted odds ratios (aOR), 95% confidence intervals (CI) and p-values ≤0.05 are reported to indicate the level of significance.
Results
Trends in the prevalence of hypertension and diabetes
A total of 978, 1023 and 2483 study participants aged 25 years and older were included for 2005, 2008 and 2017 respectively. Of these, the overall prevalence of hypertension was 11.8% in 2005, 9.5% in 2008 and 14.3% in 2017. While the overall prevalence of diabetes was 3.3% in 2005, 2.8% in 2008 and 3.2% in 2017 (Tables 1 and 2). There was an increase in the trend of hypertension over the 3 years, whereas that of diabetes remained stagnant. The median age (IQR) for those who were hypertensive increased over the period from 37 (33–47) years in 2005, to 44 (34–50) years in 2008 and 48 (38–55) years in 2017 (Table 1). The median age for diabetes also increased from 35 (29–44) years in 2005 to 47 (37–53) years in 2008 and 49 (42–57) years in 2017. Additionally, there was a decreased trend in hypertension and diabetes in the 25–34 age group over the 3 years, while the trend in the 45+ years age group increased.
Table 1.
Sociodemographic characteristics and hypertension among ALHIV 25 years and older by survey year for 2005, 2008 and 2017 in South Africa
| 2005 | 2008 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Population characteristics | Hypertension | No hypertension | p-value | Hypertension | No hypertension | p-value | Hypertension | No hypertension | p-value |
| Overall | 126 (11.8%) | 820 (88.2%) | 107 (9.5%) | 859 (90.5%) | 372 (14.3%) | 1955 (85.7%) | |||
| Sex | |||||||||
| Female | 97 (13.9%) | 542 (86.1%) | 0.057 | 93 (12.8%) | 578 (87.2%) | < 0.001 | 289 (16.6%) | 1365 (83.4%) | < 0.001 |
| Male | 29 (8.2%) | 278 (91.8%) | 14 (3.0%) | 281 (97.0%) | 83 (10.1%) | 590 (89.9%) | |||
| Age | |||||||||
| Median (IQR) | 37 (33–47) | 34 (29–39) | 44 (34–50) | 34 (29–39) | 48 (38–55) | 36 (31–44) | |||
| 25–34 years | 30 (8.6%) | 396 (91.4%) | 0.009 | 21 (4.9%) | 396 (95.1%) | < 0.001 | 48 (5.8%) | 761 (94.3%) | < 0.001 |
| 35–44 years | 37 (12.0%) | 288 (88.0%) | 31 (7.9%) | 296 (92.1%) | 84 (9.8%) | 647 (90.2%) | |||
| 45+ years | 59 (21.3%) | 136 (78.7%) | 55 (26.1%) | 167 (73.7%) | 240 (30.6%) | 547 (69.4%) | |||
| Race | |||||||||
| Non-Black African | 15 (10.4%) | 65 (89.6%) | 0.757 | 4 (13.9%) | 54 (86.1%) | 0.476 | 41 (20.3%) | 153 (79.7%) | 0.087 |
| Black African | 111 (11.8%) | 754 (88.2%) | 103 (9.4%) | 805 (90.6%) | 331 (14.1%) | 1802 (85.9%) | |||
| Marital status | |||||||||
| Never married | 41 (9.5%) | 437 (90.5%) | 0.121 | 54 (8.5%) | 524 (91.6%) | 0.154 | 192 (11.9%) | 1288 (88.1%) | < 0.001 |
| Ever married | 84 (14.3%) | 380 (85.7%) | 53 (11.7%) | 332 (88.3%) | 180 (18.9%) | 667 (81.1%) | |||
| Highest educational qualification | |||||||||
| None or Primary | 68 (15.1%) | 315 (84.9%) | 0.147 | 56 (13.4%) | 274 (86.6%) | 0.072 | 101 (18.9%) | 441 (81.1%) | 0.002 |
| Secondary and above | 58 (9.74%) | 503 (90.3%) | 50 (8.0%) | 580 (92.0%) | 199 (12.3%) | 1253 (87.7%) | |||
| Employment status | |||||||||
| Unemployed | 59 (9.9%) | 462 (90.1%) | 0.108 | 54 (9.9%) | 423 (90.1%) | 0.314 | 249 (15.4%) | 1199 (84.6%) | < 0.001 |
| Employed | 40 (13.0%) | 291 (87.0%) | 34 (7.9%) | 345 (92.1%) | 99 (11.0%) | 718 (89.0%) | |||
| Other | 26 (20.3%) | 64 (79.7%) | 19 (13.4%) | 90 (86.6%) | 23 (33.7%) | 37 (66.3%) | |||
| Locality | |||||||||
| Urban formal | 57 (12.7%) | 326 (87.3%) | 0.491 | 47 (12.4%) | 323 (87.6%) | 0.072 | 236 (15.9%) | 1027 (84.1%) | 0.008 |
| Urban informal | 34 (14.2%) | 180 (85.8%) | 30 (10.3%) | 208 (89.7%) | |||||
| Tribal area/Rural informal | 28 (10.7%) | 239 (89.3%) | 25 (6.1%) | 238 (93.9%) | 99 (11.6%) | 636 (88.4%) | |||
| Rural formal | 7 (6.2%) | 75 (93.8%) | 5 (5.9%) | 90 (94.1%) | 37 (10.1%) | 292 (89.9%) | |||
| Hazardous alcohol consumption | |||||||||
| No risk | 88 (13.2%) | 558 (86.8%) | 0.085 | 73 (9.7%) | 574 (90.3%) | 261 (14.1%) | 1359 (85.9%) | ||
| Low risk | 24 (6.7%) | 175 (93.3%) | 25 (11.4%) | 181 (88.6%) | 0.230 | 83 (16.8%) | 407 (83.2%) | 0.078 | |
| Medium risk | 12 (16.4%) | 52 (83.6%) | 6 (5.2%) | 62 (94.8%) | 13 (7.1%) | 137 (92.9%) | |||
| High risk | 1 (5.5%) | 11 (94.5%) | 1 (1.2%) | 15 (98.8%) | 8 (22.6%) | 23 (77.4%) | |||
| Addiction likely | 1 (4.4%) | 11 (95.6%) | 1 (1.7%) | 12 (98.3%) | 4 (9.3%) | 25 (90.7%) | |||
| Exposure to ARVs | |||||||||
| ARV exposed | 234 (15.1%) | 1150 (84.9%) | 0.478 | ||||||
| ARV naive | 107 (13.0%) | 600 (87.0%) | |||||||
| Viral load suppressed | |||||||||
| Yes | 254 (15.9%) | 1231 (84.1%) | 0.033 | ||||||
| No | 116 (11.9%) | 703 (88.1%) | |||||||
| Perception of general health | |||||||||
| Excellent | 5 (7.3%) | 88 (92.7%) | 0.003 | 5 (2.2%) | 161 (97.8%) | 0.006 | 42 (10.0%) | 371 (90.0%) | < 0.001 |
| Good | 55 (9.0%) | 498 (91.0%) | 48 (9.9%) | 444 (90.1%) | 186 (13.2%) | 1134 (86.8%) | |||
| Fair | 50 (19.3%) | 182 (80.7%) | 43 (14.5%) | 194 (85.5%) | 105 (18.4%) | 370 (81.6%) | |||
| Poor | 15 (22.9%) | 51 (77.1%) | 11 (10.0%) | 59 (90.0%) | 39 (27.1%) | 78 (72.9%) | |||
| Diabetes | |||||||||
| Yes | 20 (56.1%) | 14 (43.9%) | < 0.001 | 17 (54.0%) | 10 (46.0%) | < 0.001 | 49 (52.7%) | 35 (47.3%) | < 0.001 |
| No | 103 (10.3%) | 804 (89.7%) | 88 (7.7%) | 849 (92.3%) | 322 (13.1%) | 1919 (86.9%) | |||
| Heart disease | |||||||||
| Yes | 7 (28.8%) | 12 (71.2%) | 0.008 | 23 (41.8%) | 25 (58.2%) | < 0.001 | |||
| No | 99 (8.7%) | 845 (91.3%) | 346 (13.6%) | 1922 (86.4%) | |||||
| Cancer | |||||||||
| Yes | 3 (54.5%) | 2 (45.5%) | 0.009 | 3 (65.5%) | 3 (34.5%) | < 0.001 | 4 (66.2%) | 4 (33.8%) | < 0.001 |
| No | 120 (11.5%) | 816 (88.5%) | 102 (8.8%) | 855 (91.2%) | 365 (14.2%) | 1946 (85.8%) | |||
| Mental distress | |||||||||
| Yes | 71 (18.2%) | 327 (81.8%) | 0.004 | 5 (8.6%) | 31 (91.4%) | 0.951 | 128 (16.1%) | 512 (83.9%) | 0.230 |
| No | 53 (7.52%) | 491 (92.5%) | 99 (8.9%) | 826 (91.1%) | 244 (13.7%) | 1443 (86.3%) | |||
| Tuberculosis | |||||||||
| Yes | 9 (15.4%) | 54 (84.6%) | 0.467 | 5 (7.6%) | 44 (92.5%) | 0.757 | 16 (19.5%) | 60 (80.5%) | 0.257 |
| No | 114 (11.5%) | 766 (88.5%) | 100 (9.0%) | 815 (91.0%) | 352 (14.1%) | 1891 (85.9%) | |||
| Health care access | |||||||||
| Public | 109 (12.7%) | 675 (87.3%) | 0.113 | 90 (10.6%) | 649 (89.4%) | 0.104 | 337 (14.7%) | 1766 (85.3%) | 0.296 |
| Private | 15 (7.1%) | 122 (92.9%) | 15 (6.3%) | 186 (93.7%) | 35 (12.7%) | 164 (87.3%) | |||
Table 2.
Sociodemographic characteristics and diabetes among ALHIV 25 years and older by survey year for 2005, 2008 and 2017 in South Africa
| 2005 | 2008 | 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Population characteristics | Diabetes | No diabetes | p-value | Diabetes | No diabetes | p-value | Diabetes | No diabetes | p-value |
| Overall | 34 (3.3%) | 907 (96.7%) | 29 (2.8%) | 937 (97.2%) | 87 (3.2%) | 2242 (96.8%) | |||
| Sex | |||||||||
| Female | 22 (2.5%) | 614 (97.5%) | 0.198 | 26 (3.5%) | 645 (96.5%) | 0.227 | 63 (3.6%) | 1592 (96.4%) | 0.208 |
| Male | 12 (4.5%) | 293 (95.5%) | 3 (1.5%) | 292 (98.5%) | 24 (2.5%) | 650 (97.5%) | |||
| Age | |||||||||
| Median (IQR) | 35 (29–44) | 34 (29–40) | 47 (37–53) | 34 (29–40) | 49 (42–57) | 37 (32–46) | |||
| 25–34 years | 10 (3.1%) | 415 (96.9%) | 0.866 | 5 (1.1%) | 411 (98.9%) | < 0.001 | 4 (0.7%) | 804 (99.3%) | < 0.001 |
| 35–44 years | 12 (3.1%) | 312 (96.9%) | 9 (2.2%) | 317 (97.8%) | 21 (2.8%) | 710 (97.2%) | |||
| 45+ years | 12 (4.1%) | 180 (95.9%) | 15 (9.2%) | 209 (90.8%) | 62 (6.8%) | 728 (93.2%) | |||
| Race | |||||||||
| Non-Black African | 3 (0.9%) | 76 (99.1%) | 0.030 | 3 (8.0%) | 55 (92.0%) | 0.103 | 10 (5.6%) | 183 (94.4%) | 0.184 |
| Black African | 31 (3.3%) | 830 (96.7%) | 26 (2.7%) | 882 (97.3%) | 77 (3.1%) | 2059 (96.9%) | |||
| Marital status | |||||||||
| Never married | 11 (3.2%) | 465 (96.8%) | 0.944 | 11 (2.0%) | 566 (98.0%) | 0.097 | 38 (2.8%) | 1441 (97.2%) | 0.250 |
| Ever married | 23 (3.3%) | 438 (96.7%) | 18 (4.5%) | 368 (95.5%) | 49 (3.9%) | 801 (96.1%) | |||
| Highest educational qualification | |||||||||
| None or Primary | 12 (3.0%) | 370 (97.0%) | 0.915 | 12 (4.5%) | 318 (95.5%) | 0.157 | 34 (7.1%) | 509 (92.9%) | < 0.001 |
| Secondary and above | 22 (3.5%) | 535 (96.5%) | 16 (2.2%) | 614 (97.9%) | 37 (1.7%) | 1416 (98.3%) | |||
| Employment status | |||||||||
| Unemployed | 17 (3.0%) | 501 (97.0%) | 0.915 | 17 (2.4%) | 460 (97.6%) | 0.528 | 56 (3.4%) | 1394 (96.6%) | 0.582 |
| Employed | 13 (3.7%) | 317 (96.3%) | 9 (2.9%) | 370 (97.1%) | 29 (3.0%) | 788 (97.0%) | |||
| Other (incl. Old age pensioner. Sick/disabled and unable to work) | 4 (3.4%) | 85 (96.6%) | 3 (5.0%) | 106 (95.0%) | 2 (1.4%) | 58 (98.6%) | |||
| Locality | |||||||||
| Urban formal | 18 (4.2%) | 361 (95.8%) | 0.271 | 11 (3.1%) | 359 (96.9%) | 0.797 | 56 (3.5%) | 1209 (96.5%) | 0.241 |
| Urban informal | 10 (4.8%) | 203 (95.2%) | 6 (2.0%) | 231 (98.0%) | |||||
| Tribal area/Rural informal | 6 (2.1%) | 261 (97.9%) | 9 (2.5%) | 255 (97.53%) | 24 (2.8%) | 711 (97.2%) | |||
| Rural formal | 0 (0.0%) | 82 (100%) | 3 (4.6%) | 92 (95.4%) | 7 (1.4%) | 322 (98.6%) | |||
| Hazardous alcohol consumption | |||||||||
| No risk | 22 (3.4%) | 623 (96.7%) | 0.040 | 22 (3.2%) | 625 (96.8%) | 0.679 | 62 (3.2%) | 1559 (96.9%) | 0.548 |
| Low risk | 5 (1.1%) | 191 (98.9%) | 5 (1.9%) | 201 (98.1) | 19 (3.0%) | 472 (97.0%) | |||
| Medium risk | 6 (9.6%) | 57 (90.4%) | 0 (0.0%) | 68 (100%) | 4 (5.3%) | 146 (94.7%) | |||
| High risk | 0 (0.0%) | 12 (100%) | 1 (2.4%) | 15 (97.6%) | 1 (0.2%) | 30 (99.8%) | |||
| Addiction likely | 1 (6.0%) | 11 (94.0%) | 0 (0.0%) | 13 (100%) | 0 (0.0%) | 29 (100%) | |||
| Exposure to ARVs | |||||||||
| ARV exposed | 56 (3.2%) | 1331 (96.8%) | 0.675 | ||||||
| ARV naive | 26 (3.3%) | 680 (96.7%) | |||||||
| Viral load suppressed | |||||||||
| Yes | 56 (3.0%) | 1430 (97.0%) | 0.392 | ||||||
| No | 30 (3.5%) | 790 (96.5%) | |||||||
| Perception of general health | |||||||||
| Excellent | 1 (1.5%) | 92 (98.5%) | 0.001 | 1 (0.9%) | 165 (99.1%) | 0.031 | 6 (1.2%) | 406 (98.8%) | < 0.001 |
| Good | 14 (1.8%) | 537 (98.2%) | 10 (2.0%) | 481 (98.0%) | 42 (2.6%) | 1278 (97.5%) | |||
| Fair | 15 (8.4%) | 214 (91.6%) | 15 (6.2%) | 223 (93.8%) | 22 (4.1%) | 457 (95.9%) | |||
| Poor | 4 (3.9%) | 62 (96.1%) | 3 (2.7%) | 67 (97.3%) | 17 (13.9%) | 99 (86.1%) | |||
| Hypertension | |||||||||
| Yes | 20 (15.6%) | 103 (84.4%) | < 0.001 | 17 (16.1%) | 88 (83.9%) | < 0.001 | 49 (11.3%) | 322 (88.7%) | < 0.001 |
| No | 14 (1.6%) | 804 (98.4%) | 10 (1.4%) | 849 (98.7%) | 35 (1.7%) | 1919 (98.3%) | |||
| Heart disease | |||||||||
| Yes | 5 (22.1%) | 15 (77.9%) | < 0.001 | 4 (5.5%) | 44 (94.5%) | 0.298 | |||
| No | 23 (2.4%) | 920 (97.6%) | 83 (3.1%) | 2188 (96.9%) | |||||
| Cancer | |||||||||
| Yes | 3 (54.5%) | 2 (45.5%) | < 0.001 | 2 (48.7%) | 4 (51.3%) | < 0.001 | 3 (41.5%) | 5 (58.5%) | < 0.001 |
| No | 31 (2.9%) | 905 (97.1%) | 25 (2.3%) | 933 (97.7%) | 84 (3.1%) | 2230 (96.9%) | |||
| Mental distress | |||||||||
| Yes | 19 (5.0%) | 376 (95.0%) | 0.061 | 3 (8.9%) | 33 (91.1%) | 0.053 | 25 (3.4%) | 615 (96.6%) | 0.789 |
| No | 15 (2.1%) | 527 (97.9%) | 24 (2.3%) | 902 (97.7%) | 62 (3.1%) | 1627 (96.9%) | |||
| Tuberculosis | |||||||||
| Yes | 4 (7.4%) | 57 (92.6%) | 0.134 | 4 (7.1%) | 45 (92.9%) | 0.116 | 8 (5.9%) | 68 (94.1%) | 0.142 |
| No | 30 (3.0%) | 850 (97.0%) | 24 (2.5%) | 892 (95.5%) | 79 (3.1%) | 2168 (96.9%) | |||
| Health care access | |||||||||
| Public | 28 (3.2%) | 753 (96.8%) | 0.559 | 21 (2.3%) | 719 (97.7%) | 0.190 | 69 (2.8%) | 2034 (97.2%) | 0.002 |
| Private | 6 (4.4%) | 130 (95.6%) | 7 (5.1%) | 193 (94.9%) | 17 (8.1%) | 184 (91.9%) | |||
In 2005, 2008 and 2017 females had a higher prevalence of hypertension at 13.9, 12.8 and 16.6% respectively compared to males. Black Africans had the highest prevalence of hypertension at 11.8% in 2005 whereas, non-black Africans had the highest prevalence in 2008 and 2017 with 13.9 and 20.3% respectively. The prevalence estimates for diabetes were as follows: Black Africans with 3.3% in 2005, Non-Black Africans with 8.0% in 2008 and Non-Black Africans with 5.6% in 2017. In 2017, those who were exposed to ARVs had a high prevalence of hypertension at 15.1% and a marginally lower prevalence of diabetes at 3.2% compared to those who were naïve to ARVs (Tables 1 and 2).
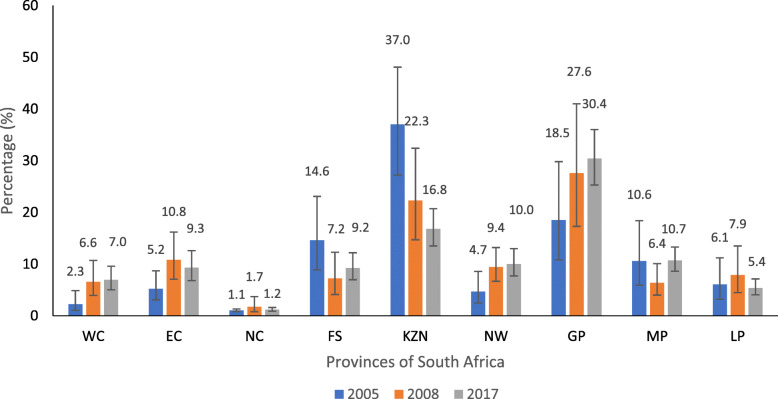
The prevalence distribution of hypertension was highest in the KwaZulu Natal province at 37.0% in 2005 and for 2008 and 2017, the Gauteng province had the highest proportion of 27.6 and 30.4% respectively (Fig. 1).
Fig. 1.
Prevalence of hypertension among ALHIV by provinces in 2005, 2008 & 2017
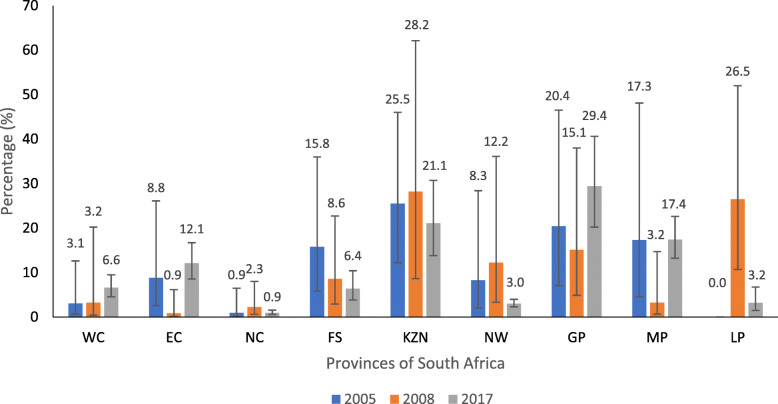
The illustration shows that diabetes was highest in the KwaZulu Natal province in 2005 and 2008 at 25.5 and 28.2% respectively, and in 2017, the Gauteng Province had the highest estimation at 29.4% (Fig. 2).
Fig. 2.
Prevalence of diabetes among ALHIV by provinces in 2005, 2008 & 2017
Factors associated with hypertension
The factors associated with hypertension in 2005 included being female (aOR = 2.59; 95% CI = 1.26 to 5.32), the age group 45+ years (aOR = 4.03; 95% CI = 1.96 to 8.28), those with diabetes (aOR = 11.73; 95% CI = 4.02 to 34.20), and those with mental distress (aOR = 2.97; 95% CI = 1.59 to 5.54) (Table 3). In 2008, factors associated with hypertension included being female (aOR = 4.31; 95% CI = 1.94 to 9.58), the age group 45+ years (aOR = 8.44; 95% CI = 3.92 to 18.15), those who perceived their health to be good (aOR = 3.72; 95% CI = 1.00 to 13.81), those who perceived their health to be fair (aOR = 5.44; 95% CI = 1.40 to 21.12), those who perceived their health to be poor (aOR = 4.82; 95% CI = 1.05 to 22.20), those who lived in an urban formal area (aOR = 2.95; 95% CI = 1.34 to 6.53), those with diabetes (aOR = 6.16; 95% CI = 1.68 to 22.59) and those who accessed public health care services (aOR = 2.58; 95% CI = 1.02 to 6.51) (Table 4). While in 2017 factors associated with hypertension included being female (aOR = 2.33; 95% CI = 1.60 to 3.42), the age group 45+ years (aOR = 7.32; 95% CI = 4.78 to 11.21), pensioners and the sick (aOR = 2.27; 95% CI = 1.09 to 4.73), those who lived in an urban area (aOR = 1.61; 95% CI = 1.16 to 2.23), those who had a high risk of hazardous alcohol consumption (aOR = 4.43; 95% CI = 1.67 to 11.76), those with diabetes (aOR = 5.17; 95% CI = 2.69 to 9.96) and those with heart disease (aOR = 3.36; 95% CI = 1.59 to 7.10) (Table 5).
Table 3.
Factors associated with hypertension for 2005
| Variables in the model | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Sex | ||
| Male | Reference | Reference |
| Female | 1.81 (0.98–3.37) | 2.59 (1.26–5.32) * |
| Age groups | ||
| 25–34 years | Reference | Reference |
| 35–44 years | 1.46 (0.73–2.92) | 1.72 (0.81–3.64) |
| 45+ years | 2.90 (1.43–5.89) ** | 4.03 (1.96–8.28) *** |
| Race | ||
| Non-Black African | Reference | Reference |
| Black African | 1.15 (0.47–2.85) | 0.96 (0.34–2.81) |
| Hazardous alcohol consumption | ||
| No risk | Reference | Reference |
| Low risk | 0.47 (0.23–0.95) * | 0.56 (0.26–1.24) |
| Medium risk | 1.28 (0.55–2.98) | 1.48 (0.60–3.69) |
| High risk | 0.38 (0.05–3.18) | 0.38 (0.04–3.37) |
| Addiction likely | 0.29 (0.03–2.44) | 0.18 (0.01–2.92) |
| Diabetes | ||
| No | Reference | Reference |
| Yes | 11.16 (4.14–30.05) *** | 11.73 (4.02–34.20) *** |
| Mental distress | ||
| No | Reference | Reference |
| Yes | 2.73 (1.54–4.85) ** | 2.97 (1.59–5.54) ** |
*p < 0.05, **p < 0.01, ***p < 0.001
Table 4.
Factors associated with hypertension for 2008
| Variables in the model | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Sex | ||
| Male | Reference | Reference |
| Female | 4.81 (2.10–11.02) *** | 4.31 (1.94–9.58) *** |
| Age groups | ||
| 25–34 years | Reference | Reference |
| 35–44 years | 1.68 (0.76–3.71) | 2.22 (0.99–4.97) |
| 45+ years | 6.95 (3.34–14.45) *** | 8.44 (3.92–18.15) *** |
| Race | ||
| Non-African | Reference | Reference |
| African | 0.65 (0.19–2.17) | 0.67 (0.14–3.28) |
| Locality | ||
| Tribal area/Rural informal | Reference | Reference |
| Rural formal | 0.96 (0.25–3.66) | 0.93 (0.35–2.46) |
| Urban informal | 1.77 (0.90–3.51) | 2.40 (1.06–5.43) * |
| Urban formal | 2.20 (1.13–4.28) * | 2.95 (1.34–6.53) ** |
| Hazardous alcohol consumption | ||
| No risk | Reference | Reference |
| Low risk | 1.20 (0.61–2.36) | 1.44 (0.66–3.12) |
| Medium risk | 0.50 (.018–1.42) | 0.78 (0.26–2.35) |
| High risk | 0.11 (0.01–0.97) * | 0.15 (0.01–1.78) |
| Addiction likely | 0.16 (0.02–1.47) | 0.25 (0.03–1.97) |
| Perception of general health | ||
| Excellent | Reference | Reference |
| Good | 4.92 (1.49–16.22) ** | 3.72 (1.00–13.81) * |
| Fair | 7.56 (2.33–24.58) ** | 5.44 (1.40–21.12) * |
| Poor | 4.95 (1.30–18.80) * | 4.82 (1.05–22.20) * |
| Diabetes | ||
| No | Reference | Reference |
| Yes | 14.03 (5.27–37.35) *** | 6.16 (1.68–22.59) ** |
| Heart disease | ||
| No | Reference | Reference |
| Yes | 4.26 (1.33–13.70) * | 1.13 (0.23–5.52) |
| Health care access | ||
| Private | Reference | Reference |
| Public | 1.76 (0.88–3.51) | 2.58 (1.02–6.51) * |
*p < 0.05, **p < 0.01, ***p < 0.001
Table 5.
Factors associated with hypertension for 2017
| Variables in the model | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Sex | ||
| Male | Reference | Reference |
| Female | 1.76 (1.29–2.41) *** | 2.33 (1.60–3.42) *** |
| Age groups | ||
| 25–34 years | Reference | Reference |
| 35–44 years | 1.78 (1.11–2.83) * | 1.83 (1.12–3.00) * |
| 45+ years | 7.24 (4.88–10.74) *** | 7.32 (4.78–11.21) *** |
| Race | ||
| Non-Black African | Reference | Reference |
| Black African | 0.65 (0.39–1.07) | 0.61 (0.33–1.13) |
| Employment status | ||
| Unemployed | Reference | Reference |
| Employed | 0.68 (0.48–0.98) * | 0.76 (0.50–1.15) |
| Other (incl. Old age pensioner, sick/disabled and unable to work) | 2.80 (1.45–5.40) ** | 2.27 (1.09–4.73) * |
| Locality | ||
| Rural informal | Reference | Reference |
| Rural (farms) | 0.85 (0.51–1.42) | 1.58 (0.86–2.90) |
| Urban | 1.44 (1.08–1.91) * | 1.61 (1.16–2.23) ** |
| Exposure to ARVs | ||
| ARV naive | Reference | Reference |
| ARV exposed | 1.20 (0.85–1.68) | 0.97 (0.67–1.40) |
| Hazardous alcohol consumption | ||
| No risk | Reference | Reference |
| Low risk | 1.23 (0.88–1.71) | 1.26 (0.83–1.93) |
| Medium risk | 0.47 (0.24–0.91) * | 0.61 (0.29–1.30) |
| High risk | 1.78 (0.57–5.57) | 4.43 (1.67–11.76) ** |
| Addiction likely | 0.78 (0.31–1.94) | 1.10 (0.43–2.78) |
| Diabetes | ||
| No | Reference | Reference |
| Yes | 7.38 (4.41–12.35) *** | 5.17 (2.69–9.96) *** |
| Heart disease | ||
| No | Reference | Reference |
| Yes | 4.57 (2.35–8.88) *** | 3.36 (1.59–7.10) ** |
*p < 0.05, **p < 0.01, ***p < 0.001
Factors associated with diabetes
The factors associated with diabetes in 2005 included being Black African (aOR = 5.70; 95% CI = 1.10 to 29.44) and those with hypertension (aOR = 11.71; 95% CI = 3.78 to 36.29) (Table 6). In 2008, factors associated with diabetes included the age group 45+ years (aOR = 4.44; 95% CI = 1.00 to 19.73), those who had hypertension (aOR = 7.56; 95% CI = 2.29 to 24.92) and those who had heart disease (aOR = 6.92; 95% CI = 1.62 to 29.62) (Table 7). While in 2017 factors associated with diabetes included the age group 45+ years (aOR = 7.90; 95% CI = 2.11 to 29.58), those who reported having poor health (aOR = 6.48; 95% CI = 1.65 to 25.41), those who had hypertension (aOR = 4.60; 95% CI = 2.34 to 9.07), those who had secondary and above education (aOR = 0.31; 95% CI = 0.16 to 0.59) and those who accessed public health care (aOR = 0.25; 95% CI = 0.11 to 0.60) (Table 8).
Table 6.
Factors associated with diabetes for 2005
| Variables in the model | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Sex | ||
| Male | Reference | Reference |
| Female | 0.55 (0.22–1.39) | 0.41 (0.14–1.20) |
| Age groups | ||
| 25–34 years | Reference | Reference |
| 35–44 years | 1.00 (0.35–2.89) | 0.88 (0.29–2.66) |
| 45+ years | 1.32 (0.44–3.93) | 0.58 (0.19–7.75) |
| Race | ||
| Non-Black African | Reference | Reference |
| Black African | 3.77 (1.04–13.65) * | 5.70 (1.10–29.44) * |
| Perception of general health | ||
| Excellent | Reference | Reference |
| Good | 1.20 (0.15–9.78) | 1.41 (0.26–7.62) |
| Fair | 5.91 (0.71–48.97) | 5.16 (0.92–29.01) |
| Poor | 2.61 (0.25–27.00) | 2.30 (0.33–16.05) |
| Hypertension | ||
| No | Reference | Reference |
| Yes | 11.16 (4.14–30.05) *** | 11.71 (3.78–36.29) *** |
| Mental distress | ||
| No | Reference | Reference |
| Yes | 2.47 (0.93–6.54) | 1.09 (0.40–2.92) |
| Health facility of choice | ||
| Private | Reference | Reference |
| Public | 0.73 (0.25–2.14) | 0.42 (0.15–1.11) |
*p < 0.05, **p < 0.01, ***p < 0.001
Table 7.
Factors associated with diabetes for 2008
| Variables in the model | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Sex | ||
| Male | Reference | Reference |
| Female | 2.31 (0.57–9.32) | 1.28 (0.28–5.77) |
| Age groups | ||
| 25–34 years | Reference | Reference |
| 35–44 years | 2.03 (0.46–8.98) | 1.95 (0.43–8.86) |
| 45+ years | 9.14 (2.67–31.27) ** | 4.44 (1.00–19.73) * |
| Race | ||
| Non-African | Reference | Reference |
| African | 0.32 (0.08–1.35) | 0.39 (0.09–1.81) |
| Hypertension | ||
| No | Reference | Reference |
| Yes | 14.03 (5.27–37.35) *** | 7.56 (2.29–24.92) *** |
| Heart disease | ||
| No | Reference | Reference |
| Yes | 11.71 (3.02–45.39) *** | 6.92 (1.62–29.62) ** |
*p < 0.05, **p < 0.01, ***p < 0.001
Table 8.
Factors associated with diabetes for 2017
| Variables in the model | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Sex | ||
| Male | Reference | Reference |
| Female | 1.47 (0.80–2.70) | 1.80 (0.86–3.78) |
| Age groups | ||
| 25–34 years | Reference | Reference |
| 35–44 years | 3.88 (1.01–14.93) * | 6.38 (1.80–22.62) ** |
| 45+ years | 9.88 (2.96–32.95) *** | 7.90 (2.11–29.58) ** |
| Race | ||
| Non-African | Reference | Reference |
| African | 0.54 (0.211–1.36) | 0.52 (0.15–1.82) |
| Educational level | ||
| None or Primary | Reference | Reference |
| Secondary and above | 0.22 (0.13–0.39) *** | 0.31 (0.16–0.59) *** |
| Exposure to ARVs | ||
| ARV naive | Reference | Reference |
| ARV exposed | 0.98 (0.54–1.77) | 0.94 (0.47–1.85) |
| Perception of general health | ||
| Excellent | Reference | Reference |
| Good | 2.18 (0.86–5.52) | 1.15 (0.41–3.26) |
| Fair | 3.56 (1.30–9.81) * | 1.04 (0.32–3.35) |
| Poor | 13.46 (4.63–39.15) *** | 6.48 (1.65–25.41) ** |
| Hypertension | ||
| No | Reference | Reference |
| Yes | 7.38 (4.41–12.35) *** | 4.60 (2.34–9.07) *** |
| Heart disease | ||
| No | Reference | Reference |
| Yes | 1.79 (0.59–5.46) | 0.77 (0.18–3.31) |
| Healthcare access | ||
| Private | Reference | Reference |
| Public | 0.34 (0.17–0.70) ** | 0.25 (0.11–0.60) ** |
*p < 0.05, **p < 0.01, ***p < 0.001
Discussion
This study found an overall prevalence of hypertension among ALHIV in 2017 of 14.3%, and this was an increase since 2005. This hypertension prevalence is comparable to other African studies that also report estimates ranging between 10.2 and 17.4% [12, 18, 34–36]. On the other hand, the overall prevalence of diabetes was 3.2% in 2017, which remained the same as in 2005 and 2008. This prevalence is lower than previously reported in other African countries with estimates ranging between 5.6 and 8.66% [12–14, 20, 36, 37]. This may have been attributed to the participants not being aware of diabetes-related symptoms leading to the observed underestimation and this is supported by an IDF report stating that Africa has the highest proportion of undiagnosed diabetes [38]. Nevertheless, as PLHIV are more likely to interact with health facilities where hypertension screening is routine, case-finding and linkage to treatment can occur [39]. However different measures for diabetes will need to be implemented as routine vital checks at health facilities do not typically include diabetes tests unless patients have been recently diagnosed and appear “unwell” [39]. The findings also show that in 2005 most hypertensive and diabetic ALHIV were from the KwaZulu-Natal province. However, for 2017, the Gauteng province had the highest proportions of hypertensive and diabetic participants. Comparative studies to determine possible reasons for the shift from 2005 to 2017 could not be found at the time of this study. Nonetheless, according to the 2005 SABSSM survey, the KwaZulu-Natal province had a significantly higher number of PLHIV than the other provinces thus supporting our findings [28]. The shift in 2017 may have been due to urbanisation, migration and a rise in the elderly population which may be attributed to ART in PLHIV [40].
Our study also found that females, aged 45 years and older, and those who resided in an urban area had the highest prevalence of both NCDs for all 3 years. The high prevalence in women may be as a result of an underestimation in men reflecting more on their health-seeking behaviour than disease prevalence itself [14, 18, 34, 35, 41, 42]. The impact of urbanisation has also been documented in studies such as the 2012 SANHANES study and the South African cross-sectional study on HIV positive educators which suggest that urban living leads to easy access to unhealthy diets and a sedentary lifestyle [34, 35]. In 2008 and 2017, we further see that those who had primary education and below also had a high prevalence of hypertension and diabetes as supported by Ntuli et al. [43]. Wang et al. suggest that not only an unhealthy diet but even a lack of regular and effective physical exercise and higher alcohol intake can be correlated with low levels of schooling [44]. The presence of comorbidities was also evident with higher proportions of individuals with cancer, heart disease, TB and mental distress reporting having hypertension and diabetes. This is consistent with studies which observed similar findings globally and in Africa [34, 45–47]. This could mean that PLHIV who have a comorbidity require close monitoring to ensure that their health conditions are effectively managed thereby reducing the risk of acquiring more NCDs.
Factors that increased the odds of hypertension for all 3 years in our study included being female, aged 45 years and older, or having diabetes. Antonello et al., Malaza et al., and Zungu et al. suggest that increased odds in females were mainly attributed to women having higher BMI’s and hip to weight ratios than men and women being more physically inactive, all of which are considered risk factors [35, 41, 48]. Ageing has also been well described to result in gradual vascular stiffening and the prolonged use of ART particularly protease inhibitors is related to the production of vascular reactive oxygen species resulting in hypertension [17, 23, 24]. While diabetes has been suggested by Petrie et al., and Gazzaruso et al. to result from hypertension-linked insulin resistance due to multiple pathophysiological mechanisms influencing microvascular and endothelial functioning [24, 25]. Mental distress also increased the odds of having high blood pressure in 2005. An American study found that one’s mental health affects one’s ability to maintain a healthy lifestyle, seek early treatment for comorbid conditions, or consistently adhere to treatment programs [45]. Living in an urban area was another significant factor associated with hypertension in 2008 and 2017. Findings from previous studies suggest that urbanisation and the associated lifestyle change including poor diet and low physical activity are risk factors of hypertension [49]. While having a high risk of hazardous alcohol consumption or having heart disease were also additional associated factors for hypertension found in 2017. In Zimbabwean and Brazilian studies alcohol consumption increased the risk of hypertension, however, there have been several hypotheses proposed for this association [50, 51]. Some researchers have to an extent been able to characterize the pathogenesis due to harmful alcohol drinking, citing that a reduction in vasodilators like nitric oxide occurs leading to an inflammatory lack of relaxation and oxidative injury of the blood vessel lining [52]. Other researchers have suggested the link between harmful alcohol consumption and the eventual accumulation of triglycerides and total cholesterol, both of which have been documented to have a link to hypertension [53]. In addition, according to the WHO, heart disease can result from hypertension in the general population [54]. Hypertension and diabetes have long been documented to be a major modifiable risk factor for heart disease due to the progressive damage to the heart that occurs over time [55, 56]. Lastly, in 2017, the employment category “other” which included pensioners, sick and disabled people, was also associated with hypertension. The reasoning for this includes that pensioners are presumably older hence the effect of age may have attributed to this finding; also, sick and disabled people are more likely to be non-mobile and be physically inactive both of which again are characteristics that have been documented to increase the risk of hypertension.
The common significant factor associated with diabetes across the years was having hypertension and the supporting reasons and studies are identical to those mentioned above. In 2008 other additional factors included being 45 years and above and having heart disease; both established risk factors in other studies [49]. In 2017, being 45 years and above, and reporting poor health was significantly associated with diabetes in the study population. Also, having secondary and above education level lowered one’s odds of having diabetes. Other studies with a similar finding reported that education increased awareness and therefore the individuals were more likely to make better health-related decisions [18]. The above findings from our study, thus, offer various avenues for possible interventions which may include active awareness campaigns targeted at men, those who have educational qualifications below secondary level or those residing in urban areas. Also, as the HIV population is ageing, closely monitoring the impact of antiretroviral medication could be considered. Lastly, these findings could allow for the refinement of the integrated chronic care model allowing for increased case finding.
Limitations
This study had a few limitations. Due to the cross-sectional nature of the surveys, causality cannot be determined in the study population. Questionnaire data used were self-reported and therefore prone to social desirability, recall bias and underreporting. The analysis did not account for unmeasured and unreported risk factors and other confounders that may have had an impact on the outcome variables. Also, as hypertension and diabetes were not measured or diagnostically determined, misclassification bias may have been introduced. Lastly, wide confidence intervals for some variables were observed and this may have been as a result of a small sample answering particular questions, therefore, conclusions drawn from the data need to be replicated with a larger sample size. Nevertheless, the surveys were nationally representative and can be generalised to adults aged 25 years and above living with HIV with hypertension and diabetes.
Conclusion
Population-based nationally representative surveys are important for developing sound health decisions and policies that would affect the country. This study identified factors consistently associated with the prevalence of hypertension and diabetes among ALHIV overtime. Additional contemporary risk factors were also identified. Therefore, these findings contribute to understanding the trend and identifying modifiable factors responsible for hypertension and diabetes among ALHIV adults from 2005 to 2017. This may help inform the development of integrated chronic disease management model to reduce the co-morbid burden of disease and associated adverse health outcomes.
Supplementary Information
Additional file 1: Table S1. Characteristics of adults living with HIV in South Africa: 2005, 2008 & 2017. Figure S1. Distribution of study participants by province in 2005, 2008 & 2017.
Acknowledgements
Not applicable.
Abbreviations
- ALHIV
Adults living with HIV
- ART
Antiretroviral Therapy
- AUDIT
Alcohol Use Disorder Identification Test
- CDC
Centers for Disease Control and Prevention
- CI
Confidence Interval
- HIV
Human immunodeficiency virus
- HSRC
Human Sciences Research Council
- IDF
International Diabetes Federation
- NCDs
Non-communicable diseases
- OR
Odds ratios
- PLHIV
People Living with HIV
- REC
Research Ethics Committee
- SABSSM
South African National HIV Prevalence, Incidence, Behaviour and Communication survey
- SANHANES
South African National Health and Nutrition Examination Survey
- WHO
World Health Organization
Authors’ contributions
NC, NZ and CC conceptualized the study. NC, MM and NZ contributed to the methodology. NC did the formal analysis. NZ and CC supervised the study. NC wrote the original draft. All authors reviewed, edited and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The dataset(s) are accessible on request on the HSRC data research repository http://datacuration.hsrc.ac.za/.
Declarations
Ethics approval and consent to participate
Ethical clearance for the secondary analysis was obtained from the University of the Witwatersrand Human Research Ethics Committee (certificate M191181). Ethical approval for the primary studies was obtained from the HSRC Research Ethics Committee (2005 certificate REC: 5/24/05/04; 2008 certificate REC: 2/23/10/07; 2017 certificate REC: 4/18/11/15) and the USA’s Centers for Disease Control and Prevention (CDC). Voluntary informed written consent was submitted by all individuals who agreed to participate in the studies. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simbayi LC, Zuma K, Zungu N, Moyo S, Marinda E, Jooste S, et al. South African national HIV prevalence, incidence, behaviour and communication survey, 2017. Cape Town: HSRC Press; 2019. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS . HIV estimates with uncertainty bounds 1990-2019 Geneva. 2020. [Google Scholar]
- 3.Evans D. Ten years on ART – where to now? 2013. [DOI] [PubMed] [Google Scholar]
- 4.Haal K, Smith A, van Doorslaer E. The rise and fall of mortality inequality in South Africa in the HIV era. SSM Popul Health. 2018;5:239–248. doi: 10.1016/j.ssmph.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS . Chronic care of HIV and non-communicable diseases: how to leverage the HIV experience. Geneva: UNAIDS; 2011. [Google Scholar]
- 6.Patel P, Rose CE, Collins PY, Nuche-Berenguer B, Sahasrabuddhe VV, Peprah E, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. AIDS. 2018;32(Suppl 1):S5–S20. doi: 10.1097/QAD.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamkuemah M, Gausi B, Oni T. Missed opportunities for NCD multimorbidity prevention in adolescents and youth living with HIV in urban South Africa. BMC Public Health. 2020;20(1):821. doi: 10.1186/s12889-020-08921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tollman SM, Kahn K, Sartorius B, Collinson MA, Clark SJ, Garenne ML. Implications of mortality transition for primary health care in rural South Africa: a population-based surveillance study. Lancet. 2008;372(9642):893–901. doi: 10.1016/S0140-6736(08)61399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erzse A, Stacey N, Chola L, Tugendhaft A, Freeman M, Hofman K. The direct medical cost of type 2 diabetes mellitus in South Africa: a cost of illness study. Glob Health Action. 2019;12(1):1636611. doi: 10.1080/16549716.2019.1636611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaziano TA, Steyn K, Cohen DJ, Weinstein MC, Opie LH. Cost-effectiveness analysis of hypertension guidelines in South Africa. Circulation. 2005;112(23):3569–3576. doi: 10.1161/CIRCULATIONAHA.105.535922. [DOI] [PubMed] [Google Scholar]
- 11.Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa—a systematic review and meta-analysis. Syst Rev. 2019;8(1):4. doi: 10.1186/s13643-018-0927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ataro Z, Ashenafi W, Fayera J, Abdosh T. Magnitude and associated factors of diabetes mellitus and hypertension among adult HIV-positive individuals receiving highly active antiretroviral therapy at Jugal Hospital, Harar, Ethiopia. Hiv Aids (Auckl) 2018;10:181–192. doi: 10.2147/HIV.S176877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekrikpo UE, Akpan EE, Ekott JU, Bello AK, Okpechi IG, Kengne AP. Prevalence and correlates of traditional risk factors for cardiovascular disease in a Nigerian ART-naive HIV population: a cross-sectional study. BMJ Open. 2018;8(7):e019664. doi: 10.1136/bmjopen-2017-019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magodoro IM, Esterhuizen TM, Chivese T. A cross-sectional, facility based study of comorbid non-communicable diseases among adults living with HIV infection in Zimbabwe. BMC Res Notes. 2016;9:379. doi: 10.1186/s13104-016-2187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey SL, Ayles H, Beyers N, Godfrey-Faussett P, Muyoyeta M, du Toit E, et al. Diabetes mellitus in Zambia and the Western cape province of South Africa: prevalence, risk factors, diagnosis and management. Diabetes Res Clin Pract. 2016;118:1–11. doi: 10.1016/j.diabres.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gbadamosi MA, Tlou B. Prevalence of abnormal glucose metabolism among adults attending an outpatient department at a tertiary referral hospital in Swaziland: a cross-sectional study. BMC Public Health. 2020;20(1):392. doi: 10.1186/s12889-020-08489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peck RN, Shedafa R, Kalluvya S, Downs JA, Todd J, Suthanthiran M, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 2014;12(1):125. doi: 10.1186/s12916-014-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwarisiima D, Balzer L, Heller D, Kotwani P, Chamie G, Clark T, et al. Population-Based Assessment of Hypertension Epidemiology and Risk Factors among HIV-Positive and General Populations in Rural Uganda. PLoS One. 2016;11(5):e0156309. doi: 10.1371/journal.pone.0156309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magula NP, Motala AA, Lalloo UG. The prevalence and incidence of diabetes mellitus and other disorders of glycaemia in south African black patients on antiretroviral therapy. Int J Infect Dis. 2014;21:10. doi: 10.1016/j.ijid.2014.03.425. [DOI] [Google Scholar]
- 20.Prioreschi A, Munthali RJ, Soepnel L, Goldstein JA, Micklesfield LK, Aronoff DM, et al. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta-analysis. BMJ Open. 2017;7(3):e013953. doi: 10.1136/bmjopen-2016-013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto E. Blood pressure and ageing. Postgrad Med J. 2007;83(976):109–114. doi: 10.1136/pgmj.2006.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in Older Adults. Diabetes Care. 2012;35(12):2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV-infected adults: novel pathophysiologic mechanisms. Hypertension. 2018;72(1):44–55. doi: 10.1161/HYPERTENSIONAHA.118.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazzaruso C, Bruno R, Garzaniti A, Giordanetti S, Fratino P, Sacchi P, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21(7):1377–1382. doi: 10.1097/00004872-200307000-00028. [DOI] [PubMed] [Google Scholar]
- 26.Mutyambizi C, Chola L, Groot W, Pavlova M, Labadarios D, Hongoro C. The extent and determinants of diabetes and cardiovascular disease comorbidity in South Africa – results from the south African National Health and nutrition examination survey (SANHANES-1) BMC Public Health. 2017;17(1):745. doi: 10.1186/s12889-017-4792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Divala OH, Amberbir A, Ismail Z, Beyene T, Garone D, Pfaff C, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health. 2016;16(1):1243. doi: 10.1186/s12889-016-3916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shisana ORT, Simbayi LC, Parker W, Zuma K, Bhana A, Connolly C, Jooste S, Pillay V, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2005. Cape Town: HSRC Press; 2005. [Google Scholar]
- 29.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Pillay-van-Wyk V, et al. South African national HIV prevalence, incidence, behaviour and communication survey 2008: a turning tide among teenagers? Cape Town: HSRC Press; 2009. [Google Scholar]
- 30.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. 2014. [DOI] [PubMed] [Google Scholar]
- 31.Reid MC, Fiellin DA, O'Connor PG. Hazardous and harmful alcohol consumption in primary care. Arch Intern Med. 1999;159(15):1681–1689. doi: 10.1001/archinte.159.15.1681. [DOI] [PubMed] [Google Scholar]
- 32.StataCorp . Stata Statistical Software: Release 15. College Station: StataCorp LLC; 2017. [Google Scholar]
- 33.Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med. 2016;4(6):111. doi: 10.21037/atm.2016.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shisana O, Labadarios D, Rehle T, Simbayi L, Zuma K, Dhansay A, et al. The south African National Health and nutrition examination survey, 2012: SANHANES-1: the health and nutritional status of the nation. 2014. [Google Scholar]
- 35.Zungu NP, Mabaso ML, Kumalo F, Sigida S, Mlangeni L, Wabiri N, et al. Prevalence of non-communicable diseases (NCDs) and associated factors among HIV positive educators: findings from the 2015/6 survey of health of educators in public schools in South Africa. PLoS One. 2019;14(2):e0209756. doi: 10.1371/journal.pone.0209756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Heerden A, Barnabas RV, Norris SA, Micklesfield LK, van Rooyen H, Celum C. High prevalence of HIV and non-communicable disease (NCD) risk factors in rural KwaZulu-Natal, South Africa. J Int AIDS Soc. 2017;20(2):e25012. doi: 10.1002/jia2.25012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu C, Umanski G, Blank A, Meissner P, Grossberg R, Selwyn PA. Comorbidity-related treatment outcomes among HIV-infected adults in the Bronx. NY J Urban Health. 2011;88(3):507–516. doi: 10.1007/s11524-010-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.International Diabetes Federation . IDF Diabetes Atlas, 9th edn Brussels, Belgium. 2019. [Google Scholar]
- 39.Knowledge Translation Unit UoCTLI . Adult primary care guide (APC) 2019/2020. Pretoria: The National Department of Health of South Africa; 2019. [Google Scholar]
- 40.StatsSA . Statistics South Africa. Formal census. 2011. [Google Scholar]
- 41.Antonello VS. Carlos Ferreira Antonello I, Grossmann TK, Tovo CV, Brasil dal Pupo B, de Quadros Winckler L. hypertension—an emerging cardiovascular risk factor in HIV infection. J Am Soc Hypertens. 2015;9(5):403–407. doi: 10.1016/j.jash.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 42.da Cunha GH, Franco KB, Galvão MTG, Lima MAC, Fontenele MSM, Siqueira LR, et al. Diabetes mellitus in people living with HIV/AIDS: prevalence and associated risk factors. AIDS Care. 2020;32(5):600–607. doi: 10.1080/09540121.2019.1695727. [DOI] [PubMed] [Google Scholar]
- 43.Ntuli ST, Maimela E, Alberts M, Choma S, Dikotope S. Prevalence and associated risk factors of hypertension amongst adults in a rural community of Limpopo Province, South Africa. Afr J Prim Health Care Fam Med. 2015;7(1):847. doi: 10.4102/phcfm.v7i1.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Chen J, Wang K, Edwards CL. Education as an important risk factor for the prevalence of hypertension and elevated blood pressure in Chinese men and women. J Hum Hypertens. 2006;20(11):898–900. doi: 10.1038/sj.jhh.1002086. [DOI] [PubMed] [Google Scholar]
- 45.Ojike N, Sowers JR, Seixas A, Ravenell J, Rodriguez-Figueroa G, Awadallah M, et al. Psychological distress and hypertension: results from the National Health Interview Survey for 2004-2013. Cardiorenal Med. 2016;6(3):198–208. doi: 10.1159/000443933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schutte AE, Ware LJ, Huisman HW, Fourie CMT, Greeff M, Khumalo T, et al. Psychological distress and the development of hypertension over 5 years in black south Africans. J Clin Hypertens. 2015;17(2):126–133. doi: 10.1111/jch.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization . Global report on diabetes. Geneva: World Health Organization; 2016. [Google Scholar]
- 48.Malaza A, Mossong J, Bärnighausen T. Newell M-L. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One. 2012;7(10):e47761. doi: 10.1371/journal.pone.0047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price AJ, Crampin AC, Amberbir A, Kayuni-Chihana N, Musicha C, Tafatatha T, et al. Prevalence of obesity, hypertension, and diabetes, and cascade of care in sub-Saharan Africa: a cross-sectional, population-based study in rural and urban Malawi. Lancet Diabetes Endocrinol. 2018;6(3):208–222. doi: 10.1016/S2213-8587(17)30432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chireshe R, Naidoo K, Nyamakura R. Hypertension among human immunodeficiency virus infected patients on treatment at Parirenyatwa hospital: a descriptive study. Afr J Prim Health Care Fam Med. 2019;11(1):e1–e8. doi: 10.4102/phcfm.v11i1.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda MLR, Barcellos NT, Alencastro PR, Wolff FH, Moreira LB, Gus M, et al. Alcohol drinking pattern: a comparison between HIV-infected patients and individuals from the general population. PLoS One. 2016;11(6):e0158535. doi: 10.1371/journal.pone.0158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Husain K, Ferder L, Ansari RA, Lalla J. Chronic ethanol ingestion induces aortic inflammation/oxidative endothelial injury and hypertension in rats. Hum Exp Toxicol. 2011;30(8):930–939. doi: 10.1177/0960327110384520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hao G, Wang Z, Zhang L, Chen Z, Wang X, Guo M, et al. Relationship between alcohol consumption and serum lipid profiles among middle-aged population in China: a multiple-center cardiovascular epidemiological study. Angiology. 2015;66(8):753–758. doi: 10.1177/0003319714549557. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organisation . Fact Sheet – Hypertension. 2019. [Google Scholar]
- 55.Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–1258. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Characteristics of adults living with HIV in South Africa: 2005, 2008 & 2017. Figure S1. Distribution of study participants by province in 2005, 2008 & 2017.
Data Availability Statement
The dataset(s) are accessible on request on the HSRC data research repository http://datacuration.hsrc.ac.za/.