Abstract
Objective
The 2019 novel coronavirus disease (COVID-19) is threatening global health and is especially pronounced in patients with chronic metabolic syndromes. Meanwhile, a significant proportion of patients present with digestive symptoms since angiotensin-converting enzyme 2 (ACE2), which is the receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is highly expressed in the intestine. The aim of this study was to evaluate the effects of a high-fat diet (HFD) and a maternal HFD on the intestinal ACE2 levels in adults and neonates.
Methods
We examined intestinal ACE2 protein levels in mice with diet-induced obesity (DIO) and neonatal mice exposed to a maternal HFD. We also investigated Ace2 mRNA expression in intestinal macrophages.
Results
Intestinal ACE2 protein levels were increased in DIO mice but decreased in offspring exposed to a maternal HFD compared with chow-fed controls. Ace2 mRNA expression in intestinal macrophages was detected and downregulated in DIO mice. Additionally, higher intestinal ACE2 protein levels were observed in neonates than in adult mice.
Conclusions
The influence of an HFD on intestinal ACE2 protein levels is opposite in adults and neonates. Macrophages might also be involved in SARS-CoV-2 intestinal infection. These findings provide some clues for the outcomes of patients with COVID-19 with metabolic syndromes.
Keywords: ACE2, Intestine, High-fat diet, Macrophage, COVID-19
Introduction
The 2019 novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, is prevalent worldwide [1]. In patients with COVID-19 infection, gastrointestinal (GI) reactions are commonly reported, and SARS-CoV-2 RNA has been detected in feces and GI tissues [2,3], indicating the existence of GI infections and fecal–oral transmission. Accumulating evidence indicates that individuals with metabolic diseases experience more severe outcomes from COVID-19 [4,5]. Angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for SARS-CoV-2 [6] and, according to the Human Protein Atlas (HPA) database, it is highly expressed in the GI tract [7]. Exploring the factors affecting the expression of intestinal ACE2 may predict the outcomes of intestinal infection with SARS-CoV-2. It has been reported that ACE2 levels in adipose tissue are upregulated in obese/diabetic states [8,9]. It is thus far unclear whether metabolic disorders may alter the expression of ACE2 in the intestine. We undertook this study to investigate the alteration of intestinal ACE2 expression in mice fed short- and long-term high-fat diets (HFDs) as well as pups exposed to maternal HFDs.
Recent studies have reported that SARS-CoV-2 can directly attack immune cells. In the lungs, ACE2-positive alveolar macrophages can be infected by SARS-CoV-2 [10]. Intestinal macrophages also play an important role in gut homeostasis; however, most intestinal studies in COVID-19 focus on epithelial cells, and it is unclear whether intestinal macrophages express ACE2 and contribute to GI infection. Here, we also sought to determine whether intestinal macrophages may express ACE2 and whether it is altered in diet-induced obesity (DIO).
Materials and methods
Animals
C57BL/6 mice were obtained from the Animal Core Facility of Nanjing Medical University and group housed under a 12-h light/dark cycle at 23°C with ad libitum access to food and water. For the HFD study, male mice were kept on a normal chow diet (XietongShengwu, 1010013) until 8 wk of age, and then fed either a chow diet or an HFD (58% HFD with sucrose, Research Diets D12331) for 12 and 48 wk. For the maternal HFD study, 8-wk-old female mice were exposed to either a normal chow diet or an HFD for 12 wk. A normal chow diet–fed male mouse was added to each cage for breeding and they remained in the cages either with chow diet or HFD. Resulting male offspring were sacrificed at postnatal day 12 (PD12). All the animal experiments were approved by the Committee on Animal Care of Nanjing Medical University, and complied with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Isolation of primary microglia and intestinal macrophages
Intestinal macrophages and microglia were isolated as described by us and others with minor modifications [11,12]. For intestinal macrophages, mice were sacrificed, and the intestine was carefully removed, cut longitudinally, and cleaned in Hank's balanced salt solution (HBSS). Tissues were cut into pieces and washed twice in RPMI-1640 medium (Gibco, Waltham, MA, USA) supplemented with 5% fetal bovine serum (Gibco) and 5 mM EDTA at 37°C and shaken for 20 min to remove epithelial cells. Tissue were then digested in Accutase solution (Millipore, CA, USA) for 40 min, and passed through a 70-µm filter. For microglia, brains were homogenized in RPMI-1640 medium. Resuspended brain and gut lysates were layered on a 30%/70% (v/v) Percoll gradient and centrifuged without a brake at 500g for 30 min. The mononuclear cells were collected from the gradient interface. After 30 min of incubation, the non-adherent dead cells and lymphocytes were washed away with HBSS. Attached live cells were harvested for Ace2 expression analysis and immunofluorescent staining.
Western blotting
The ileum and colon were dissected and rinsed in phosphate-buffered saline to remove lumenal content. Tissues were lysed in radioimmunoprecipitation buffer (Beyotime, Los Angeles, CA, USA) supplemented with cocktail protease inhibitor (Thermo Fisher Scientific, Waltham, MA, USA). 30 ug proteins were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel, and transferred onto nitrocellulose membranes (Millipore). The membranes were blocked with 5% milk (Phygene, FuZhou, CN) for 1 h at room temperature, then incubated with the following primary antibodies overnight at 4°C: anti-ACE2 (1:1000; R&D Systems, Minneapolis, MN, USA), anti-GAPDH (1:5000;Affinity Biosciences, Cincinnati, OH, USA), and anti β-actin antibody (1:500; Santa Cruz, Dallas, TX, USA).
Real-time quantitative PCR and agarose gel electrophoresis
Total RNA was extracted from primary cells or gut tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and subjected to reverse transcription with HiScript-II-Q RT SuperMix for quantitative polymerase chain reaction (qPCR; Vazyme, Nanjing, China). Real-time qPCR was carried out using SYBR Green Master Mix(Vazyme) on QuantStudio 5 (Applied Biosystems, Foster City, CA, USA). The qPCR reaction was carried out according to the following conditions: predenaturation 5 min at 95°C, 95°C 10s followed by 60°C 30s for 40 cycles, melting curve stage 95°C 15s — 60°C 60s — 95°C 15s.
The following primers were used: Ace2: 5′-CTGGGCAGAAGTTGCTCAAG-3′ (forward) and 5′-TGGGCTCCATTCAGTGTTCC-3′ (reverse); β-actin:5′-CGCAGCCACTGTCGAGTC-3′ (forward) and 5′-GTCATCCATGGCGAACTGGT-3′ (reverse). Equal volume of qPCR products were subjected to 2% agarose gel electrophoresis to confirm the products sizes.
Immunohistochemistry and immunofluorescence
The intestines were fixed by 4% paraformaldehyde (PFA) 48 h and dehydrated by 30% sucrose solution, and then embedded with optimum cutting temperature (Sakura, Torrence, CA, USA). 8 µm cyro sections were cut by cryostat microtome and sticked to the precoated glass slides. Cyro sections of the ileum and colon were incubated with anti-ACE2 antibody (1:1000, R&D Systems) overnight at 4°C. Sections were then incubated with biotin-conjugated secondary antibody for 1 h at room temperature, followed by avidin-biotin-horseradish peroxidase complex. The reaction was visualized by 1% diaminobenzidine with 0.01% hydrogen peroxide and then counterstained with hematoxylin. Air-dried sections were further dehydrated in alcohol gradient and 100% xylene. Isolated intestinal macrophages on coverslips were fixed with 4% PFA, washed and incubated with anti-iba1 antibody (1:800, Wako, Osaka, Japan) overnight at 4°C, followed by 594 Alexa secondary antibody and mounted with antifading mounting medium with diamidino-2-phenylindole (DAPI). Stained sections and cells were imaged with a fluorescence microscope (Nikon, Melville, NY, USA).
Statistical analysis
Data analysis and plots were performed using Prism 8.0 (GraphPad Software). Two groups were compared using a two-tailed unpaired Student's t test. P < 0.05 was considered statistically significant. All data are shown as mean ± SD.
Results
ACE2 protein levels are increased in the ileum of DIO mice
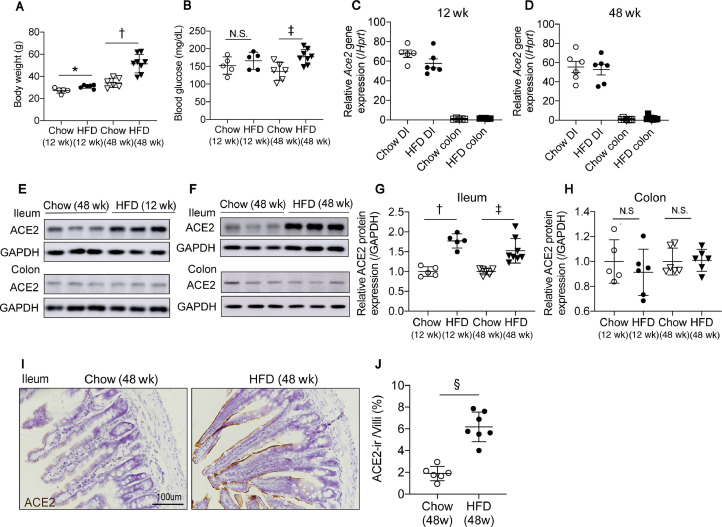
We first characterized the expression of ACE2 in the gut of mice fed short-term (12 wk) and long-term (48 wk) HFD. Both 12- and 48-wk HFD-fed mice gained significant body weight, and 48-wk HFD-fed mice had hyperglycemia (Fig. 1 A,B). The ileum and colon of mice were analyzed for relative Ace2 mRNA expression and ACE2 protein level expression. qPCR shows that Ace2 mRNA expressions are much higher in the ileum than colon as literature reported, however, there is no difference between chow-fed and HFD fed groups at either time points (Fig. 1C,D). Protein levels of ACE2 were analyzed by Western blot. Consistent with the HPA database and qPCR results, we observed that ACE2 is highly expressed in the ileum and is expressed at low levels in the colon, suggesting that the small intestine could be more prone to infection by COVID-19. Notably, ACE2 protein levels were much higher in the ileum of the HFD groups at both 12 and 48 wk (Fig. 1E–H). Compared with 12-wk HFD feeding, ACE2 protein levels in the ileum were not further elevated by 48-wk HFD feeding. In the colon, ACE2 protein levels did not differ between the chow and HFD groups. The higher surface protein level of ACE2 in the ileum of mice fed a 48-wk HFD was confirmed by immunohistochemistry (Fig. 1I,J). These results show that ACE2 is highly expressed in the ileum and upregulated in DIO mice but is not further increased by age or prolonged HFD feeding.
Fig. 1.
ACE2 expression is increased in the ileum of mice fed on HFD. (A) Body weight of mice fed an HFD for 12- and 48-wk. (B) Basal blood glucose level is elevated in mice fed an HFD for 48-wk. (C,D) qPCR shows relative Ace2 mRNA levels are much higher in the ileum than the colon but do not differ between chow- or HFD-fed animals at both time points. (E,F) Western blot using ACE2 antibody indicates that ACE2 protein levels are increased in the ileum of mice fed HFD for 12- and 48-wk compared with chow-fed mice. ACE2 protein levels in the colon did not differ between HFD- and chow-fed mice. (G,H) Quantification of Western blot intensities in (E,F) estimating the amount of ACE2 protein levels in the ileum. (I) Significantly higher ACE2 level in the ileum of mice fed an HFD for 48-wk by immunohistochemical analysis. (J) The surface ACE2-ir area percentage in each villi quantified by imaged J. Data are presented as means ± SD. *P < 0.05, †P <0.001, ‡P < 0.01, vs controls. ACE, angiotensin-converting enzyme; ACE-ir, angiotensin-converting enzyme immunoreactive; DI, diet induced; HFD, high-fat diet.
Ace2 mRNA expression is downregulated in intestinal macrophages of mice fed a HFD
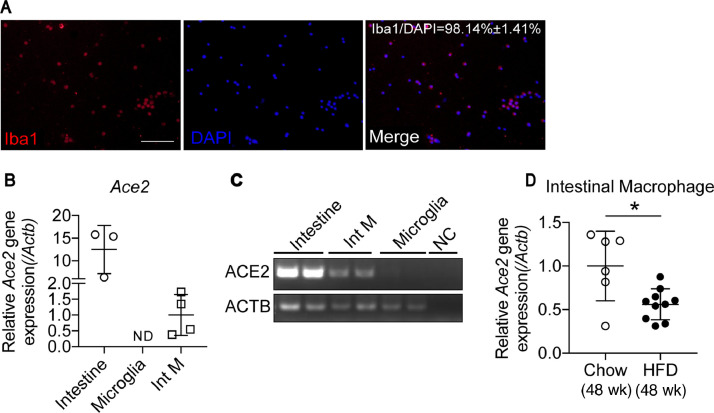
It has been reported that alveolar macrophages and Kupfer cells express high levels of Ace2 and therefore could be targeted by SARS-CoV-2 [13]. Considering that little attention has been given to intestinal macrophages, we then sought to determine whether intestinal macrophages may express Ace2 and could be modulated in a DIO model. The purity of isolated intestinal macrophages was 98.14 ± 1.4%, as indicated by the ratio of ionized calcium-binding adaptor molecule 1 and DAPI counts (Fig. 2 A). Ace2 gene expression in intestinal macrophages was confirmed by quantitative reverse transcription PCR(qRT-PCR) and the visualization of qPCR products on agarose gels (Fig. 2C). Total RNA extracted from a piece of small intestine was used as the positive control. Meanwhile, in another type of tissue resident macrophage, microglia in the brain, Ace2 gene expression was undetectable(Fig, 2B,C) suggesting that Ace2 is diversely distributed in macrophages in different organs. We next checked Ace2 mRNA expression in intestinal macrophages isolated from mice fed an HFD or chow diet. Unexpectedly, the Ace2 mRNA level was significantly decreased in intestinal macrophages of mice fed an HFD for 48 wk (Fig. 2D), which was opposite to the increased Ace2 expression in the whole tissue of the ileum. These results suggest that intestinal macrophages may take part in the pathologic process of COVID-19, but the potential mechanisms need further study.
Fig. 2.
Ace2 mRNA expression is downregulated in Int M of mice fed an HFD. (A) Purity of the isolated Int M is indicated by the ratio of Iba1 and DAPI positive counts. (B) Ace2 mRNA are detected in Int M but not in the microglia. (C) Ace2 mRNA expression in Int M are validated by qPCR products separated on agarose gel electrophoresis. (D) Ace2 relative mRNA expression is decreased in Int M of mice fed an HFD for 48 wk compared with chow. Data presented as mean ± SD.*P < 0.01 vs the controls. Scale bar = 100 um in A. ACE, angiotensin-converting enzyme; DAPI, diamidino-2-phenylindole; HFD, high-fat diet; Iba1, ionized calcium-binding adaptor molecule 1; Int M, intestinal macrophages; NC, negative control; ND, not detectable. qPCR, quantitative polymerase chain reaction.
ACE2 protein is highly expressed in the neonatal intestine and is decreased by maternal HFD feeding
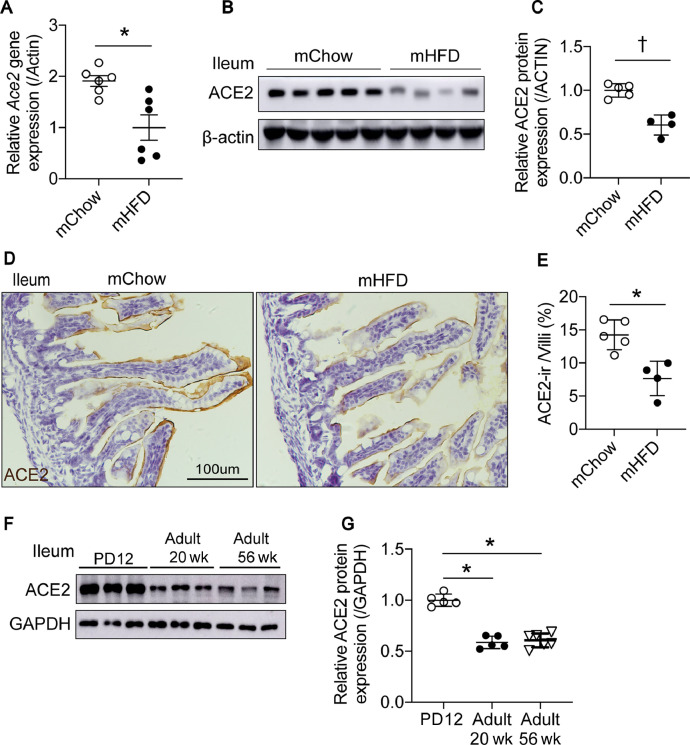
Considering that an increasing number of women have metabolic syndromes in their child-bearing years and that there are some cases of SARS-CoV-2 infection in newborns, we next evaluated the effects of maternal HFD on neonatal intestinal Ace2 expression. Surprisingly, on PD12, in the ileum of the male offspring exposed to maternal HFD, Ace2 mRNA levels and protein levels were both significantly decreased compared with that in the maternal chow-fed group (Fig. 3 A–C), which was opposite to the results in the adult DIO mice (Fig. 1E–H). Immunohistochemistry also confirmed the decreased ACE2 surface protein levels in the intestinal epithelium of neonatal mice exposed to maternal HFD (Fig. 3D,E). Additionally, we compared intestinal ACE2 protein levels at different ages in chow-fed mice. The highest expression of intestinal ACE2 protein was detected in neonates compared with 20- and 56-wk-old mice (Fig. 3F,G). Taken together, these results suggest that neonates with high levels of intestinal ACE2 have a high risk for inducing SARS-CoV-2 tropism, although maternal HFD may not be a predisposing factor for COVID-19 in neonates.
Fig. 3.
ACE2 protein levels are lower in the ileum of the offspring exposed to maternal HFD. (A) qPCR shows relative Ace2 mRNA levels are lower in ileum of the postnatal day 12 (PD12) male pups exposed to maternal HFD. (B) Western blot using ACE2 antibody shows ACE2 relative protein levels in the ileum of the PD12 male pups exposed to maternal HFD are lower than maternal chow fed pups. (C) Quantification of western blot intensities in (B) by image J. (D) Decreased ACE2 surface protein expression in the ileum of the offspring exposed to maternal HFD is shown by immunohistochemistry. (E) ACE2-ir area percentage in each villus quantified by imaged J. (F) Western blot shows ACE2 relative protein levels are higher in the ileum during the postnatal period compared with 20- and 56-wk-old mice. (G) Quantification of Western blot intensities in (F) by image J. Data presented as means ± SD.*P < 0.01, †P <0.001 vs controls. ACE, angiotensin-converting enzyme; ACE-ir, angiotensin-converting enzyme immunoreactive; HFD, high-fat diet; mChow, maternal chow; mHFD, maternal high-fat diet; PD, postnatal day; qPCR, quantitative polymerase chain reaction.
Discussion
In this study, we reported that chronic HFD feeding increased intestinal ACE2 protein expression, which may partly contribute to the higher susceptibility of individuals with obesity to COVID-19. ACE2 was reported to be expressed in alveolar macrophages in the lung and CD169-positive macrophages in the spleen and lymph nodes, strengthening the evidence that SARS-CoV-2 can directly attack macrophages to trigger more severe symptoms [10,14]. We also found that Ace2 is expressed in intestinal macrophages, consistent with a recent report that Ace2 is expressed in CD11b-enriched cells isolated from patients [15]. Furthermore, we also observed that Ace2 expression is undetectable in microglia. The latest research reported that SARS-CoV-2 can invade the central nervous system, infecting patients’ neural progenitor cells and brain organoids [16]. Thus, microglia in the brain might not be infected by viruses and may be able to execute their normal functions. This evidence suggests that the Ace2 expression levels in myeloid cells in various organs are differentially regulated.
It is of great significance to understand the maternal factors affecting the susceptibility of newborns to COVID-19 to prevent more newborns from being infected. In contrast to the results in adult DIO mice, the ACE2 protein levels were significantly decreased in the ileum of offspring exposed to maternal HFD. Thus, maternal HFD may not be a promoting factor for COVID-19 infection in the neonatal intestine and may even reduce susceptibility. However, contrary to recent studies in humans stating that intestinal Ace2 mRNA expression is increased with age [17,18] and the lower disease severity in children, we detected the highest protein levels of intestinal ACE2 in neonates compared with young (20-wk-old) and old (56-wk-old) adult mice. Ortiz et al. also reported that ACE2 proteins are expressed on the cell surface of lung cells and are higher in young children [19]. These conflict conclusions may be partly due to the inconsistence between mRNA and protein results of ACE2. We found that Ace2 mRNA results are not always the same as the Western blot or immunohistochemical results. This discrepancy is more obvious in adult intestine in our study, indicating that in adult intestine, ACE2 protein turnover rate might be slower. Thus, for ACE2, both protein and mRNA levels should be assessed before making conclusions.
This study provided some clues for COVID-19 susceptibility in individuals with metabolic diseases; however, due to biosafety requirements, intestinal Ace2 expression was analyzed in mice without SARS-CoV-2 infection. Whether patients with obesity exhibit more common GI manifestations and higher intestinal viral loads requires additional clinical information. Meanwhile, the role of Ace2 in intestinal macrophages is still poorly understood. Further investigations may shed some light on these points.
Footnotes
This work was funded by Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX20_1418), grants from the National Natural Science Foundation of China (grant nos. 81873654 and 31800971), Natural Science Foundation of Jiangsu Province (grant no. BK20180684), and The Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant no. 18KJA180008). JS was responsible for the visualization, investigation, and writing of the original draft preparation. LC and XAX were responsible for the study investigation. XYW was responsible for the study investigation and validation. MQJ was responsible for the study methodology and resources. HJ was responsible for the methodology. YG was responsible for the study conceptualization, review and Editing of the manuscript, and supervision. The authors have no conflicts of interest to declare.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.04.045. 765–7.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 4.Weis N, Thorsteinsson K, Martinussen C, Madsbad S. The endocrine and metabolic link between COVID-19, diabetes and obesity. Ugeskr Laeger. 2020;182 [PubMed] [Google Scholar]
- 5.Mauvais-Jarvis F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. 2020;69:1857–1863. doi: 10.2337/dbi19-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 8.Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 2016;65:85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Xu YZ, Liu B, Wu R, Yang YY, Xiao XQ, et al. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. ScientificWorldJournal. 2014;2014 doi: 10.1155/2014/603409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Zhong L. Lung adenocarcinoma patients own higher risk of SARS-CoV-2 infection. Preprints. 2020 doi: 10.18632/aging.202375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Vidal-Itriago A, Kalsbeek MJ, Layritz C, Garcia-Caceres C, Tom RZ, et al. Lipoprotein lipase maintains microglial innate immunity in obesity. Cell Rep. 2017;20:3034–3042. doi: 10.1016/j.celrep.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The Short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50 doi: 10.1016/j.immuni.2018.12.018. 432–45.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song X, Hu W, Yu H, Zhao L, Zhao Y, Zhao Y. High expression of angiotensin-converting enzyme-2 (ACE2) on tissue macrophages that may be targeted by virus SARS-CoV-2 in COVID-19 patients. bioRxiv. 2020 [Google Scholar]
- 14.Chen Y, Feng Z, Diao B, Wang R, Wang G. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv. 2020 [Google Scholar]
- 15.Burgueno JF, Reich A, Hazime H, Quintero MA, Fernandez I, Fritsch J, et al. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 2020;26:797–808. doi: 10.1093/ibd/izaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang BZ, Chu H, Han S, Shuai H, Deng J, Hu YF, et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020:1–4. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuille-Dit-Bille RN, Liechty KW, Verrey F, Guglielmetti LC. SARS-CoV-2 receptor ACE2 gene expression in small intestine correlates with age. Amino Acids. 2020;52:1063–1065. doi: 10.1007/s00726-020-02870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowak JK, Lindstrom JC, Kalla R, Ricanek P, Halfvarson J, Satsangi J. Age, inflammation, and disease location are critical determinants of intestinal expression of SARS-CoV-2 Receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.05.030. 1151–4.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz ME, Thurman A, Pezzulo AA, Leidinger MR, Klesney-Tait JA, Karp PH, et al. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine. 2020;60 doi: 10.1016/j.ebiom.2020.102976. [DOI] [PMC free article] [PubMed] [Google Scholar]