Abstract
Insufficient physical activity (PA) and excessive stationary behavior (SB) are contributors to pediatric obesity, though antecedents and consequences of these behaviors in this population are relatively unknown. This pilot study examined affect, loss of control eating (LOCE), overeating, and hunger surrounding PA and SB in 17 youth with overweight/obesity. Participants completed a 14-day ecological momentary assessment (EMA) wearing accelerometers. At the momentary level, higher negative affect and lower positive affect predicted SB increases and PA decreases following EMA prompts; higher positive affect predicted PA increases. Higher LOCE predicted SB increases and PA decreases, while increases in PA and decreases in SB predicted short-term increases in LOCE and overeating. At the individual level, higher SB and lower PA were related to lower positive affect and higher negative affect, LOCE, overeating, and hunger. Findings suggest affect is a relevant antecedent and consequence of PA/SB, and dysregulated eating may acutely impact PA/SB.
Keywords: children, obesity, emotion, physical activity, disordered eating, ecological momentary assessment
Pediatric obesity is a rising public health problem that occurs in approximately 20% of school-aged children and adolescents, and is known to confer risk for a host of adverse long-term physical and psychological health outcomes (Llewellyn et al., 2016; Simmonds et al., 2016; Skinner et al., 2016). Physical inactivity (i.e., an insufficient physical activity level to meet present physical activity recommendations) and sedentary behavior (i.e., any waking behavior characterized by an energy expenditure ≤1.5 metabolic equivalents while in a sitting, reclining or lying posture) are two factors that contribute to the development and maintenance of pediatric obesity (Janssen et al., 2004; Krebs & Jacobsen, 2003; Tremblay et al., 2017; Trost et al., 2001), though interventions targeting physical activity in children generally demonstrate only modest and relatively short-lived effects (Sims et al., 2015). This is particularly concerning given that physical activity can exert beneficial effects on both emotional functioning and eating behaviors, each of which is relevant for weight regulation and eating disorder prevention. In order to develop more precise and effective prevention and intervention efforts, it is therefore necessary to (1) identify and target the momentary processes that promote or impede physical activity and sedentary behaviors among youth with overweight and obesity in daily life, and (2) examine potential interrelationships between physical activity and other health-related behaviors that contribute to weight regulation.
Affect and Physical Activity
While earlier health behavior theories heavily emphasized the role of conscious cognitions, increasing theoretical and empirical work has demonstrated that affective processes are highly relevant to a range of health behaviors (e.g., Conner et al., 2015; Edwards et al., 2017; Williams & Evans, 2014). Broadly, affect refers to consciously accessible feeling states, which, in contrast to emotions and mood, do not require cognitive appraisal or reflection (Fredrickson, 2001; Ekkekakis, 2013). Affective experiences have been conceptualized based on the dimensions of valence (i.e., the extent to which an affective state is positive or negative) and activation/arousal (i.e., the extent to which one is feeling engaged or energized; Russell, 1980; Ekkekakis, 2013). While Russell’s (1980) circumplex model posits affective states vary across each dimension, the related two-dimensional model developed by Watson and Tellegan specifically focuses on negative and positive activated affective states (e.g., joy, fear) that reflect higher arousal (Watson & Tellegan, 1985; Watson, Wiese, Vaidya, & Tellegan, 1999).
Consistent with the recently developed Affect and Health Behavior Framework (Williams & Evans, 2014), incidental affect and affect response are key affective components underlying health behaviors. That is, incidental affect refers to affective states that predict health behaviors (which may in turn serve an affect regulatory function). Affect response refers to affective states during and following health behaviors, which in turn influences the likelihood of engaging in future behaviors via automatic and reflective processes (Williams & Evans, 2014). In line with principles of reinforcement and operant conditioning, positive outcomes (e.g., pleasurable affective responses) during and following physical activity may increase future engagement in physical activity via learned associations. In addition, affect responses are thought to influence cognitive appraisals and attitudes about physical activity, thereby contributing to self-efficacy about such behavior (Rhodes & Kate, 2015). Thus, affect response is thought to be a key factor that shapes longer-term physical activity habits via both automatic and conscious processes (Rhodes & Kate, 2015). In sum, the momentary relationships between affect and physical activity have important implications for the adoption and maintenance of physical activity, insofar as momentary incidental affect predicts engagement in physical activity, and in turn, physical activity has a positive or regulating effect on affect.
The role of incidental affect and affect response in relation to physical activity has been the focus of a considerable amount of research using ecological momentary assessment (EMA). A recent review of EMA studies concluded that higher positive affect reliably predicted physical activity over the next few hours and vice versa, though findings regarding negative affect were more mixed (Liao et al., 2015). Such inconsistencies may be due in part to the nature of affect (i.e., valence vs. arousal) and activity (i.e., intensity, duration, frequency) assessments, timing of intervals surrounding activity, and sample characteristics (e.g., Kim, Conroy, & Smyth, 2019).
Affect and Physical Activity in Youth
In youth without overweight or obesity, there also seems to be a bi-directional association between affect and physical activity. For instance, children are less likely to engage in physical activity and more likely to engage in sedentary behavior following psychological stress, especially among those who generally report more sedentary behavior and those who are more reactive to stress (Balantekin & Roemmich, 2012; Roemmich et al., 2003). In addition, intense exercise, compared to sedentary behavior, has been found to dampen physiological responses to interpersonal stressors in children (Roemmich et al., 2009). At the momentary level, naturalistic EMA research in children without overweight or obesity has found that feeling more energetic and less tired was associated with increased subsequent moderate-to-vigorous activity (MVPA), and greater MVPA was predictive higher subsequent positive affect and lower negative affect (Dunton et al., 2014; Wen et al., 2018). While momentary correlates of sedentary behavior remain largely understudied in children, recent EMA findings indicated that greater time spent in sedentary behavior predicted decreases in positive affect (Wen et al., 2018).
However, there remains little research examining device-based measures (accelerometers) of activity in youth with overweight or obesity (e.g., Hughes et al., 2006), and there is a dearth of research integrating accelerometer-based activity measures and EMA-measured affect. The lack of device-based measures of activity in youth research is especially problematic because self-report measures of activity are inherently subject to bias and do not adequately capture stationary behaviors and overall activity levels (Adamo et al., 2009; Norton et al., 2010; Warren et al., 2010). In addition, given that research in adults demonstrates that individuals with overweight or obesity have more variable responses to physical activity compared to normal weight counterparts (Ekkekakis et al., 2010; Unick et al., 2012, 2015), the momentary affective antecedents and consequences of physical activity in youth with obesity or overweight may not be consistent with the broader literature in children and adolescents, which could have important clinical implications. For instance, negative affective states, which may be more frequent or intense in this population for a variety of reasons (e.g., weight stigma and weight-related teasing, reduced health-related quality of life; Cornette, 2011; Pinhas-Hamiel et al., 2005; Pont et al., 2017), may impede engagement in physical activity. Moreover, children with overweight or obesity may find exercise less enjoyable, perhaps due to concerns about appearance, criticism regarding athletic competency, and/or physical discomfort (e.g., Gayes & Steele, 2014). As a result, the attenuated affective benefits from physical activity in this population could impede both the adoption and maintenance of physical activity over time.
Physical Activity and Eating Behavior
Lastly, while there is growing interest in examining proximal antecedents and consequences of physical activity to understand obesity risk in youth, it is important to consider how health behaviors cluster together, especially given that physical inactivity and sedentary behaviors tend to be associated with less healthy eating (Leech, McNaughton, & Timperio, 2015; Poortinga, 2007). This has led to increased focus on understanding temporal relationships between associated health behaviors and the processes by which they may directly impact each other (Schwarzer, 2008). Although higher levels of physical activity are related to regulation of appetite, lower food cue responsivity, and reduced binge eating (Andrade et al., 2010; Blanchet et al., 2018; Elfhag & Rossner, 2005; Luo et al., 2018), little research has examined the extent to which physical activity and eating behaviors may modify each other.
Associations between physical activity and eating behaviors are particularly important to explore in youth because contrary to EMA research in adults, incidental affect does not consistently predict dysregulated eating (Goldschmidt et al., 2018; Hilbert et al., 2009; Ranzenhofer et al., 2014). Furthermore, these relationships are especially relevant for youth with overweight or obesity given that child weight status is associated with physical inactivity (Prentice-Dunn & Prentice-Dunn, 2012), and binge eating symptoms (i.e., loss of control eating and overeating) are generally more prevalent in this group compared to peers of lower weight (Harriger & Thompson, 2012; He et al., 2017). It is therefore imperative to identify the momentary determinants of maladaptive eating in youth and better understand how physical activity and eating behaviors may interact in relation to pediatric obesity.
For example, physical activity could have a buffering effect on risk factors (e.g., affect, dysregulated eating) that predict future weight gain and/or onset of full-syndrome eating disorders. Consistent with this possibility, prior naturalistic research among adults with obesity found that dietary lapses and temptations were less likely to occur after exercising (Carels et al., 2004). Several mechanisms have been posited to account for relationships between physical activity and eating behaviors, including changes in food-related reward processes, appetite control, affect regulation, and neurocognitive functioning (Blanchet et al., 2018; Joseph et al., 2011). Thus, physical activity levels may indirectly affect multiple pathways leading to and maintaining pediatric obesity. However, to our knowledge, no research in children or adolescents with overweight or obesity has examined associations between physical activity and eating-related behaviors in the natural environment.
The Present Study
Taken together, it is yet unknown the extent to which physical activity and sedentary behavior are related to momentary incidental affect and affect response among youth with overweight and obesity, and how physical activity patterns also relate to disordered eating symptomatology and physiological eating cues at the momentary level. Therefore, this study aimed to better understand physical activity patterns in this population by elucidating micro-temporal associations with affect (both incidental affect and affect response) as well as potential interrelationships with eating-related behaviors (Figure 1). This model is informed by a several lines of research. First, cross-sectional and momentary research generally shows positive relationships between emotional well-being (e.g., higher positive and lower negative affect) and physical activity intensity levels (Costigan et al., 2019; Kim et al, 2019; Wen et al., 2018), yet negative relationships with disordered eating behaviors (e.g., Haedt-Matt & Keel, 2011; Lavender et al., 2015). Higher positive affect and lower negative affective states are therefore likely be followed by more adaptive weight-related behaviors, including (1) higher physical activity intensity levels (i.e., lower stationary behavior and higher light, moderate, and vigorous activity; Kim et al., 2019; Wen et al., 2018), and (2) less dysregulated eating (Engel et al., 2016). Second, evidence suggests it is possible that such health behaviors can modify each other. In particular, higher physical activity may modulate food-related reward responsivity (e.g., Evero et al., 2012), executive functioning (e.g., Joseph et al., 2011), and emotion regulation processes (e.g., Bernstein & McNally, 2017), each of which are key constructs implicated in the development of loss of control eating in youth (Tanofsky-Kraff, Schvey, & Grilo, 2020). Third, affective responses to health behaviors (both automatic reactions and cognitive appraisals of affective states) are thought to play a key role in shaping future weight-related behaviors via operant conditioning and influences on self-efficacy (Rhodes & Kate, 2015).
Figure 1.
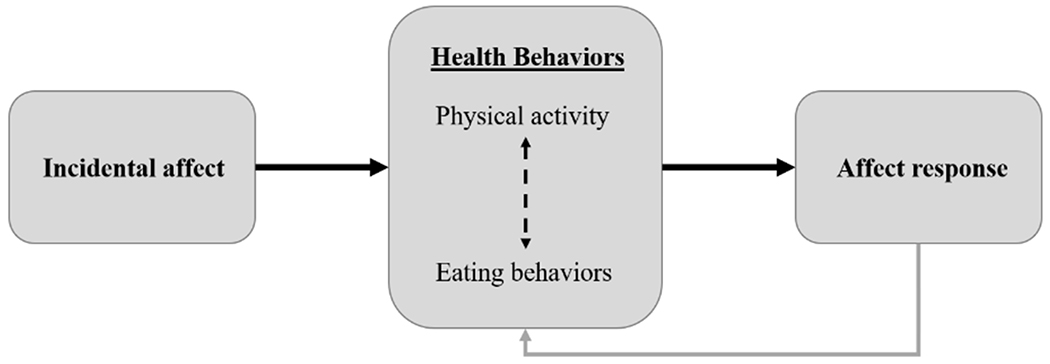
Conceptual model of theoretical relationships among constructs. (Dashed arrow reflects exploratory analysis; gray arrow indicates theoretical association not empirically examined in the present study.)
However, key questions remain: (1) The posited pathways have yet to be fully examined in youth with overweight or obesity, who may have different affective experiences surrounding physical activity, which may ultimately impact the adoption and maintenance of such behaviors across their lifespan. (2) Momentary affect has been less consistently linked to eating-related constructs in youth, leaving a critical gap in the understanding of the development and maintenance of disordered eating (e.g., overeating, loss of control eating) in this population. (3) Despite theoretical relevance of physical activity for the development of loss of control eating, direct associations between activity levels and eating have not been explored in this population. Understanding such associations is necessary to design more focused interventions to target behavioral factors contributing to and maintaining pediatric obesity, especially if promotion of physical activity could exert a transfer effect on eating regulation.
Integrated, multimethod ambulatory assessment techniques (i.e., EMA combined with accelerometer-based physical activity assessment) provide an ideal method to examine these temporal relationships in real-time, as well as disentangle momentary (i.e., within-person) and trait-level (i.e., between-person) associations between these variables. Therefore, as a first step to explore these relationships, the present pilot study sought to examine (1) momentary affect preceding and following physical activity (i.e., duration of light activity and MVPA) and stationary behavior; and (2) dysregulated eating behavior (i.e., loss of control eating and overeating) and physiological hunger preceding and following physical activity and stationary behavior. Stationary behavior refers to any waking behavior done while lying, reclining, sitting, or standing, with no ambulation, irrespective of energy expenditure, and is preferred over the term “sedentary behavior” when using physical activity monitors that cannot accurately detect shifts in posture (Tremblay et al., 2017). Based on prior EMA work, it was hypothesized that higher positive and lower negative affect would predict increases in physical activity and decreases in stationary behavior within the next hour, and that higher physical activity and lower stationary behavior would predict increases in positive affect and decreases in negative affect in the next hour. Specifically, positive and negative activated affect were assessed by items from the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegan, 1988), which is consistent with prior EMA studies showing associations between physical activity and affect using this measure (Liao et al., 2015). Due to the lack of prior EMA research investigating associations between physical activity and eating-related constructs (e.g., disordered eating and appetite) among youth, the second aim was treated as exploratory.
Method
Participants
Participants (N=17; Mage=10.59±1.54 years; MzBMI=2.11±.47; 58.8% female; 65.8% African-American) were a subsample drawn from a larger study of 40 youth with overweight or obesity (BMI > 85th percentile for age and sex; Kuczmarski et al., 2000), which has been previously described (Goldschmidt et al., 2018a, 2019). Youth in the current sample had ambulatory actigraphy assessment data available as part of their participation. Participants who had Actiwatch data available in addition to completing the EMA protocol did not differ significantly in age, gender, z-BMI, ChEDE global scores, or number of completed EMA recordings compared to those who did not (ps>.07). Participants were recruited from two academic institutions (University of Chicago and Illinois Institute of Technology) via community flyers, pediatrician referrals, and from previous studies in which families consented to be contacted for future research (Goldschmidt et al., 2018b). Participants were excluded for meeting criteria for an eating disorder other than binge eating disorder, having a medical condition known to influence appetite or weight, taking medications known to influence appetite or weight, or receiving concurrent treatment for overweight or obesity.
Procedure
Study procedures received Institutional Review Board approval at The University of Chicago and Illinois Institute of Technology. After providing written informed assent/consent, participants completed a series of baseline assessments including measurement of height and weight. Participants and their parent or guardian then received training on how to complete the EMA protocol using the Real Time Assessment In the Natural Environment (ReTAINE) system on smartphones. Participants were also instructed to wear wrist-worn actigraphy monitors (Actiwatch 2, Respironics/Phillips, Bend, OR) continuously for the duration of the 14-day protocol and trained on its use. The Actiwatch and smartphone clocks were synchronized prior to data collection. During the EMA protocol, participants were asked to complete EMA recordings after eating (event-contingent); before bedtime (interval-contingent); and at 3-5 semi-random times throughout the day (signal-contingent). On weekends, semi-random prompts were delivered every 2-3 hours between 8:00am to 9:00pm, whereas on weekdays these prompts were delivered between 7:00-8:00am, 3:00-4:00pm, and 6:00-7:00pm so as not to interfere with school schedules. The first day of the EMA protocol was a practice day, during which ≥70% compliance to signals was required to initiate the 14-day EMA protocol; these data were not used in statistical analyses. Study team members contacted participants after the first day and then again every 2-3 days to answer any participant questions that arose about study procedures and give feedback about compliance, including trouble-shooting barriers to completing EMA recordings, assessing any difficulties with study devices, and reminding participants of the incentive structure. Upon completing the 14-day protocol, participants returned for a second in-person assessment and received payment for participating in the study.
Measures
Baseline assessments.
Height and weight were measured at baseline to calculate body mass index z-scores (z-BMI) using CDC growth charts (Kuczmarski et al., 2000). Demographic information was also collected from children and parents. The Child Eating Disorder Examination 12.0 (ChEDE; Bryant-Waugh, Cooper, Taylor, & Lask, 1996) was administered to assess disordered eating symptoms and rule out eating disorders other than binge eating disorder.
Physical activity and stationary behavior.
Physical activity and stationary time were measured using wrist-worn Actiwatch 2 accelerometers during all non-sleep time except during non-compatible activities such as swimming. Activity counts were recorded in continuous 30-second epochs, and thresholds for activity were consistent with national surveillance data (Belcher et al., 2010; Troiano et al., 2008). Age-specific thresholds for children’s activity levels were adjusted by applying the Freedson prediction equation (Freedson, Pober, & Janz, 2005; Harrell et al., 2005). Accumulated minutes of MVPA, light activity, and stationary time were derived from each 30-, 60-, and 120-minute time windows before and after all EMA prompts; only windows with at least two-thirds valid wear time (20, 40, or 80 minutes, respectively) were used in analyses. Activity count data downloaded by the Actiwatch software were further processed using R to create all different time windows of activity counts before and after EMA prompts. Non-wear time (>60 continuous minutes of zero activity counts) and non-valid days (<10 hours of wear time) were identified; all non-wear time and non-valid days were removed from analyses.
EMA measures.
At all EMA signals, participants rated their degree of hunger levels (“Please rate how much you agree with the following statement: I am hungry”), which were rated on a 1- to 5-point Likert-type scale (1=“disagree strongly,” and 5=“agree strongly”). Momentary negative and positive activated affect were assessed at every EMA recording using the PANAS (Watson et al., 1998). Each affect item (e.g., afraid, upset) was rated on a 5-point scale (“1”=“Not at all”; “5”=“Extremely”) and summed to form composite negative and positive activated affect scales (α=.69 and α=.95, respectively).
When participants reported the occurrence of eating episodes, items were administered that assessed their degree of overeating (“To what extent do you feel that you overate?”) and loss of control eating (“While you were eating, did you feel a sense of loss of control?”; “While you were eating, did you feel that you could not stop eating once you had started?”; “While you were eating, did you feel like you could not resist eating?”; “While you were eating, did you feel like a car without brakes, you just kept eating and eating?”). Responses were rated on a 1- to 5-point Likert-type scale (1=“no, not at all,” and 5=“yes, extremely”). The four items assessing loss of control eating were summed to create a composite score (α=.91). These items were modeled after conceptualizations of loss of control eating in previous EMA studies of children and adolescents (Hilbert et al., 2009; Ranzenhofer et al., 2014).
Statistical Analyses
EMA responses were time-matched to Actiwatch-assessed duration of stationary behavior (minutes) and total physical activity (i.e., total minutes of light activity and MVPA) in the 30, 60, and 120 minutes before and after each EMA survey. EMA surveys were time-stamped at the time of completion; thus, total physical activity and stationary behavior time in the 30, 60, and 120 minutes prior to EMA surveys included time spent responding to the EMA prompt. Separate generalized estimating equations (GEEs) were used to assess (1) positive and negative activated affect, loss of control eating, overeating, and hunger as predictors of the duration of total physical activity and stationary behavior in the 30, 60, and 120 minutes following EMA recordings; and (2) the duration of total physical activity and stationary behavior (measured within the 30, 60, and 120 minutes prior to EMA recordings) as predictors of positive and negative activated affect, loss of control eating, overeating, and hunger.
In each GEE, effects of independent variables were separated into within-person (i.e., person-mean centered) and between-person (i.e., grand-mean centered) components. That is, within-person associations indicate the degree to which changes in the independent variable, relative to an individual’s own mean, are related to the dependent variable, whereas between-person associations reflect the degree to which an individual’s average level of an independent variable across EMA ratings, relative to other individuals, is associated with the dependent variable. Given that EMA signals were separated by more than one hour, participants may have engaged in an eating episode earlier than or during the window before the EMA signal when the eating episode was reported. Therefore, GEEs assessing activity levels as predictors of loss of control eating and overeating included the lagged effect of within-person activity time (i.e., measured during the time before the prior EMA signal) to ensure that there was a temporal association between these variables.
Preliminary analyses indicated total wear time did not significantly differ between boys and girls (t[15]=.43, p=.674). There were medium, albeit non-significant, associations between total wear time and z-BMI (r=−.33, p=.194) and age (r=.35, p=.166), indicating participants who had lower z-BMI and were older tended to have higher wear times. z-BMI was associated with stationary and total physical activity time before (r= .59 and −.46, respectively) and after (r= .61 and −.48, respectively) EMA prompts.1 Age was also associated with stationary and total physical activity time before (r= .18 and −.23, respectively) and after (r= .35 and −.44, respectively) EMA prompts. Activity duration surrounding EMA prompts did not significantly differ across genders (ps>.288). Therefore, age and z-BMI were also included as covariates (grand-mean centered). In addition, total valid wear time within each specified time window was included as a covariate in all models. All GEEs specified an AR1 serial autocorrelation to account for dependence within the nested data. A negative binomial function was used for GEEs predicting duration of stationary behavior, and gamma link functions were used for GEEs predicting affect and eating variables to account for skewed distributions of these variables; GEEs predicting duration of physical activity employed a linear function. Given that this was a pilot study and analyses of disordered eating and hunger were exploratory in nature to inform research questions in future studies, power analyses are not presented, and it was concluded that adjusting for multiple comparisons would limit discoveries from these exploratory data. Therefore, alpha was set at .05 for all analyses. GEEs were conducted using SPSS version 25.
Results
Descriptive Characteristics
The 17 participants completed a total of 680 EMA recordings (68.1% semi-random signal recordings, 22.4% bedtime recordings, 9.6% event-contingent eating episode recordings), with an average of 40.0±18.17 signals completed per person. Participants wore the Actiwatches for an average of 13.08±9.54 hours per day, with an average of 8.59±6.59 days during which the Actiwatch was worn ≥10 hours per day. Table 1 displays descriptive information for overall physical activity and stationary time measured by the Actiwatch, and Table 2 displays descriptive information for Actiwatch wear time and activity time prior to and following EMA prompts (60 minute windows), as well as mean levels of EMA-reported positive and negative activated affect, hunger, loss of control eating, and overeating. Of the 17 participants, 3 met physical activity guidelines (i.e., ≥60 minutes of MVPA/day; Centers for Disease Control and Prevention, 2019) on at least one of the valid wear days (range: 1-4 days). To examine between- and within-person variability in EMA measures, we also calculated the intra-class coefficient (ICC), which reflects the variation in an outcome that can be attributed to a clustering variable (i.e., person). With the exception of a somewhat higher ICC for positive affect (.75), ICCs were moderate in magnitude, reflecting substantial variability within persons with respect to hunger (.47), overeating (.35), loss of control eating (.61), and negative affect (.44).
Table 1.
Overall physical activity levels per day measured via Actiwatch1
| M | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Stationary time (minutes) | 610.90 | 141.53 | 100.00 | 974.00 |
| Stationary time (%) | 47.29 | 10.64 | 14.80 | 91.19 |
| Light time (minutes) | 668.84 | 148.96 | 56.00 | 961.50 |
| Light time (%) | 51.59 | 10.40 | 8.36 | 84.90 |
| MVPA time (minutes) | 14.31 | 16.73 | 0.00 | 116.00 |
| MVPA time (%) | 1.11 | 1.35 | 0.00 | 9.31 |
Note. MVPA=moderate-to-vigorous physical activity.
Descriptive information is based on days during which participants wore the Actiwatch for ≥10 hours.
Table 2.
Descriptive statistics for EMA variables and activity time (minutes) in the 60 minutes prior to and following EMA recordings.
| Observations | M | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Valid wear time (before) | 664 | 52.45 | 19.32 | 0.00 | 60.00 |
| Valid wear time (after) | 658 | 52.44 | 19.09 | 0.00 | 60.00 |
| Stationary (before) | 576 | 15.18 | 15.38 | 0.00 | 59.00 |
| Light activity (before) | 576 | 33.00 | 15.81 | 0.00 | 59.00 |
| MVPA (before) | 576 | 0.25 | 1.20 | 0.00 | 14.00 |
| Total physical activity (before) | 576 | 33.25 | 15.89 | 0.00 | 59.00 |
| Stationary (after) | 567 | 14.98 | 15.38 | 0.00 | 59.00 |
| Light activity (after) | 567 | 33.03 | 15.99 | 0.00 | 60.00 |
| MVPA (after) | 567 | 0.14 | 0.61 | 0.00 | 8.00 |
| Total physical activity (after) | 567 | 33.17 | 16.09 | 0.00 | 60.00 |
| Negative affect | 680 | 12.22 | 2.42 | 10.00 | 28.00 |
| Positive affect | 680 | 28.32 | 14.92 | 10.00 | 50.00 |
| Hunger | 680 | 1.89 | 1.40 | 1.00 | 5.00 |
| Overeating severity | 317 | 1.27 | 0.73 | 1.00 | 5.00 |
| LOC eating severity | 317 | 4.75 | 2.15 | 4.00 | 16.00 |
Note. EMA=ecological momentary assessment; “before” indicates the 60-minute time window prior to EMA prompts; “after” indicates the 60-minute time window following EMA prompts; MVPA=moderate-to-vigorous physical activity; Total physical activity =sum of light activity and MVPA minutes; LOC=loss of control. Activity times were based on 60-minute time windows with ≥ 40 minute valid wear time.
Affect Predicting Activity Patterns
With respect to antecedents of stationary behavior (Table 3) and total physical activity time (Table 4), GEE results showed significant within-person effects of negative activated affect predicting stationary minutes across all time windows, in that higher momentary negative activated affect (relative to participants’ own average levels) was related to greater subsequent stationary time over the next 30, 60, and 120 minutes. In addition, there was a within-person effect of negative activated affect predicting total physical activity 120 minutes after EMA prompts, such that higher negative activated affect was related to less subsequent activity time. There were also significant between-person effects of negative activated affect predicting stationary time (60- and 120-minute models) and total activity (120-minute model), such that participants with higher overall negative activated affect exhibited higher stationary time and less total physical activity time over the EMA protocol.
Table 3.
Generalized estimating equations examining predictors of stationary behavior following EMA recordings (N=17)
| 30 minutes after EMA | 60 minutes after EMA | 120 minutes after EMA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | |
| Intercept | 1.90 | 0.07 | 0.000 | 7.00 | 2.12 | 0.001 | 4.08 | 1.15 | 0.000 |
| Wear time | 0.06 | 0.12 | 0.000 | −0.07 | 0.04 | 0.040 | −0.01 | 0.01 | 0.584 |
| zBMI | 0.60 | 0.12 | 0.000 | 0.88 | 0.12 | 0.000 | 0.69 | 0.13 | 0.000 |
| Age | 0.10 | 0.04 | 0.005 | 0.07 | 0.04 | 0.079 | 0.03 | 0.06 | 0.563 |
| LOC eating (between) | 0.07 | 0.03 | 0.028 | 0.06 | 0.03 | 0.064 | 0.03 | 0.04 | 0.479 |
| LOC eating (within) | 0.08 | 0.04 | 0.023 | 0.08 | 0.02 | 0.001 | 0.08 | 0.03 | 0.004 |
| Intercept | 1.91 | 0.07 | 0.000 | 7.07 | 1.95 | 0.000 | 4.11 | 1.15 | 0.000 |
| Wear time | 0.06 | 0.00 | 0.000 | −0.07 | 0.03 | 0.024 | −0.01 | 0.01 | 0.570 |
| zBMI | 0.59 | 0.12 | 0.000 | 0.87 | 0.12 | 0.000 | 0.68 | 0.13 | 0.000 |
| Age | 0.10 | 0.04 | 0.012 | 0.07 | 0.04 | 0.124 | 0.02 | 0.06 | 0.662 |
| Overeating (between) | 0.18 | 0.12 | 0.119 | 0.15 | 0.12 | 0.202 | 0.00 | 0.12 | 0.976 |
| Overeating (within) | 0.16 | 0.16 | 0.338 | 0.13 | 0.14 | 0.343 | 0.12 | 0.11 | 0.269 |
| Intercept | 3.75 | 0.76 | 0.000 | 8.37 | 0.99 | 0.000 | 5.37 | 0.94 | 0.000 |
| Wear time | −0.06 | 0.03 | 0.017 | −0.10 | 0.02 | 0.000 | −0.02 | 0.01 | 0.030 |
| zBMI | 0.45 | 0.12 | 0.000 | 0.59 | 0.10 | 0.000 | 0.52 | 0.07 | 0.000 |
| Age | 0.09 | 0.04 | 0.026 | 0.09 | 0.03 | 0.007 | 0.10 | 0.02 | 0.000 |
| Hunger (between) | 0.13 | 0.08 | 0.096 | 0.18 | 0.08 | 0.028 | 0.22 | 0.07 | 0.003 |
| Hunger (within) | −0.05 | 0.06 | 0.405 | −0.08 | 0.07 | 0.288 | −0.13 | 0.07 | 0.052 |
| Intercept | 3.15 | 0.60 | 0.000 | 8.70 | 1.10 | 0.000 | 5.43 | 0.89 | 0.000 |
| Wear time | −0.04 | 0.02 | 0.063 | −0.10 | 0.02 | 0.000 | −0.02 | 0.01 | 0.025 |
| zBMI | 0.53 | 0.10 | 0.000 | 0.71 | 0.09 | 0.000 | 0.65 | 0.10 | 0.000 |
| Age | 0.07 | 0.05 | 0.118 | 0.07 | 0.04 | 0.085 | 0.07 | 0.03 | 0.029 |
| NA (between) | 0.06 | 0.05 | 0.219 | 0.10 | 0.05 | 0.027 | 0.12 | 0.04 | 0.001 |
| NA (within) | 0.05 | 0.03 | 0.038 | 0.07 | 0.02 | 0.003 | 0.08 | 0.02 | 0.000 |
| Intercept | 3.56 | 0.57 | 0.000 | 8.70 | 1.08 | 0.000 | 5.66 | 0.97 | 0.000 |
| Wear time | −0.05 | 0.02 | 0.009 | −0.10 | 0.02 | 0.000 | −0.02 | 0.01 | 0.024 |
| zBMI | 0.45 | 0.13 | 0.001 | 0.60 | 0.13 | 0.000 | 0.54 | 0.08 | 0.000 |
| Age | 0.01 | 0.06 | 0.915 | −0.03 | 0.05 | 0.610 | −0.05 | 0.03 | 0.091 |
| PA (between) | −0.01 | 0.01 | 0.055 | −0.02 | 0.01 | 0.002 | −0.02 | 0.00 | 0.000 |
| PA (within) | −0.03 | 0.01 | 0.000 | −0.04 | 0.01 | 0.000 | −0.03 | 0.01 | 0.000 |
Note. EMA=ecological momentary assessment; zBMI= body mass index z-score; PA=positive affect; NA=negative affect; LOC=loss of control; between=grand-mean centered variable; within=person-mean centered variable. Stationary behavior was assessed in the 30/60/120 minutes following ratings of LOC, overeating, hunger, PA and NA. Wear time was the total valid wear time within each specified time window (30/60/120 minutes).
Table 4.
Generalized estimating equations examining predictors of total activity time following EMA recordings (N=17)
| 30 minutes after EMA | 60 minutes after EMA | 120 minutes after EMA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | |
| Intercept | 16.83 | 0.46 | 0.000 | −72.75 | 24.76 | 0.003 | −47.90 | 26.76 | 0.073 |
| Wear time | 0.56 | 0.01 | 0.000 | 1.77 | 0.42 | 0.000 | 0.94 | 0.23 | 0.000 |
| zBMI | −3.65 | 0.75 | 0.000 | −9.40 | 1.62 | 0.000 | −18.10 | 4.68 | 0.000 |
| Age | −1.35 | 0.21 | 0.000 | −2.10 | 0.58 | 0.000 | −2.64 | 1.99 | 0.186 |
| LOC eating (between) | −0.78 | 0.21 | 0.000 | −1.48 | 0.39 | 0.000 | −1.63 | 1.29 | 0.207 |
| LOC eating (within) | −0.59 | 0.38 | 0.124 | −1.36 | 0.46 | 0.003 | −2.83 | 0.82 | 0.001 |
| Intercept | 16.77 | 0.46 | 0.000 | −76.65 | 22.39 | 0.001 | −49.13 | 28.59 | 0.086 |
| Wear time | 0.56 | 0.72 | 0.000 | 1.84 | 0.37 | 0.000 | 0.95 | 0.25 | 0.000 |
| zBMI | −3.79 | 0.72 | 0.000 | −9.67 | 1.66 | 0.000 | −18.59 | 4.78 | 0.000 |
| Age | −1.34 | 0.24 | 0.000 | −2.08 | 0.65 | 0.001 | −2.27 | 2.02 | 0.261 |
| Overeating (between) | −2.37 | 0.71 | 0.001 | −4.39 | 1.40 | 0.002 | −2.91 | 3.92 | 0.457 |
| Overeating (within) | −0.74 | 1.41 | 0.601 | −2.38 | 2.65 | 0.370 | −4.81 | 4.65 | 0.301 |
| Intercept | −29.06 | 6.42 | 0.000 | −75.46 | 15.26 | 0.000 | −69.66 | 28.86 | 0.016 |
| Wear time | 1.53 | 0.21 | 0.000 | 1.82 | 0.26 | 0.000 | 1.14 | 0.24 | 0.000 |
| zBMI | −3.36 | 1.07 | 0.002 | −7.58 | 1.98 | 0.000 | −14.05 | 3.02 | 0.000 |
| Age | −1.08 | 0.31 | 0.001 | −2.19 | 0.45 | 0.000 | −4.16 | 0.56 | 0.000 |
| Hunger (between) | −1.48 | 0.67 | 0.026 | −3.68 | 1.25 | 0.003 | −7.83 | 2.13 | 0.000 |
| Hunger (within) | 0.42 | 0.35 | 0.240 | 1.19 | 0.89 | 0.185 | 3.27 | 1.81 | 0.072 |
| Intercept | −26.32 | 4.72 | 0.000 | −78.89 | 14.93 | 0.000 | −78.56 | 25.95 | 0.002 |
| Wear time | 1.42 | 0.17 | 0.000 | 1.86 | 0.25 | 0.000 | 1.20 | 0.22 | 0.000 |
| zBMI | −3.94 | 0.91 | 0.000 | −9.05 | 1.60 | 0.000 | −17.41 | 3.02 | 0.000 |
| Age | −0.89 | 0.39 | 0.022 | −1.73 | 0.68 | 0.012 | −3.27 | 1.00 | 0.001 |
| NA (between) | −0.51 | 0.48 | 0.287 | −1.45 | 0.89 | 0.105 | −3.67 | 1.43 | 0.010 |
| NA (within) | −0.34 | 0.23 | 0.127 | −0.71 | 0.39 | 0.069 | −1.66 | 0.58 | 0.004 |
| Intercept | −28.29 | 4.97 | 0.000 | −76.65 | 18.28 | 0.000 | −85.89 | 28.29 | 0.002 |
| Wear time | 1.48 | 0.17 | 0.000 | 1.81 | 0.31 | 0.000 | 1.24 | 0.24 | 0.000 |
| zBMI | −3.53 | 1.07 | 0.001 | −7.88 | 2.04 | 0.000 | −14.37 | 2.78 | 0.000 |
| Age | −0.42 | 0.55 | 0.447 | −0.50 | 0.95 | 0.598 | −0.17 | 1.12 | 0.878 |
| PA (between) | 0.09 | 0.05 | 0.057 | 0.23 | 0.08 | 0.006 | 0.59 | 0.10 | 0.000 |
| PA (within) | 0.24 | 0.08 | 0.002 | 0.51 | 0.16 | 0.001 | 0.87 | 0.25 | 0.001 |
Note. EMA=ecological momentary assessment; zBMI= body mass index z-score; PA=positive affect; NA=negative affect; LOC=loss of control; between=grand-mean centered variable; within=person-mean centered variable. Physical activity was assessed in the 30/60/120 minutes following ratings of LOC, overeating, hunger, PA and NA. Wear time was the total valid wear time within each specified time window (30/60/120 minutes).
Within-person effects of positive activated affect emerged across all models, such that moments of lower positive activated affect (relative to participants’ own average levels) were associated with less stationary minutes and more total activity minutes in the 30, 60, and 120-minutes following EMA prompts. There were also significant effects of between--person positive activated affect (60- and 120-minute models) predicting stationary minutes and total physical activity minutes; that is, participants with lower overall positive activated affect exhibited higher stationary time and less total physical activity time over the EMA protocol.
Eating-Related Variables Predicting Activity Patterns
Regarding eating-related variables, there were significant between-person effects of hunger predicting stationary time (60- and 120-minute models) and total physical activity minutes (all models) (, such that participants who reported higher overall hunger exhibited higher stationary time and less total physical activity time throughout the EMA protocol. Similarly, there were between-person effects of loss of control eating and overeating predicting total physical activity minutes (30- and 60-minute models), in that participants who had higher overall loss of control eating and overeating behavior evidenced less total physical activity time during the EMA protocol. The between-person effect of loss of control predicting stationary time was also significant (30-minute model), indicating that those with higher loss of control eating tended to engage in more stationary behavior. In addition, within-person effects emerged for loss of control eating predicting stationary behaviors in the 30, 60, and 120 minutes following EMA prompts, as well as and total physical activity minutes in the 60 and 120 minutes following prompts, such that when participants reported higher loss of control eating ratings (relative to their own average levels) were associated with greater stationary time and lower total physical activity time.
Activity Patterns Predicting Affect
GEEs examining consequences of stationary and total physical activity time are shown in Table 5. Across all models, significant within-person effects emerged for stationary minutes and total physical activity minutes predicting positive activated affect, such that decreases in stationary time and increases in total physical activity time in the 30, 60, and 120 minutes to prior to EMA prompts (relative to participants’ own average levels) were associated with higher positive activated affect ratings. In addition, there were between-person effects for stationary time and total activity time predicting positive affect in the 120-minute model; similar to the previously described effects, participants with higher activity levels and lower stationary behavior tended to have higher overall positive affect.
Table 5.
Generalized estimating equations examining physical activity and stationary behavior prior to EMA recordings as predictors of affect, eating behaviors, and hunger (N=17)
| 30 minutes before EMA | 60 minutes before EMA | 120 minutes before EMA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DV: Loss of control eating | B | SE | p | B | SE | p | B | SE | p |
| Intercept | 1.04 | 0.33 | 0.002 | 1.93 | 0.42 | 0.000 | 1.60 | 0.07 | 0.000 |
| Wear time | 0.02 | 0.01 | 0.166 | −0.01 | 0.01 | 0.320 | 0.00 | 0.00 | 0.342 |
| zBMI | 0.00 | 0.09 | 0.962 | −0.04 | 0.12 | 0.750 | −0.05 | 0.12 | 0.682 |
| Age | −0.10 | 0.03 | 0.002 | −0.10 | 0.03 | 0.004 | −0.10 | 0.03 | 0.001 |
| Stationary (between) | 0.05 | 0.02 | 0.026 | 0.02 | 0.01 | 0.054 | 0.01 | 0.00 | 0.016 |
| Stationary (within) 1 | −0.01 | 0.00 | 0.011 | 0.00 | 0.00 | 0.071 | 0.00 | 0.00 | 0.067 |
| Intercept | 1.16 | 0.31 | 0.000 | 1.97 | 0.43 | 0.000 | 1.63 | 0.08 | 0.000 |
| Wear time | 0.01 | 0.01 | 0.248 | −0.01 | 0.01 | 0.305 | 0.00 | 0.00 | 0.268 |
| zBMI | 0.03 | 0.07 | 0.640 | −0.01 | 0.10 | 0.910 | −0.03 | 0.10 | 0.804 |
| Age | −0.10 | 0.03 | 0.000 | −0.10 | 0.03 | 0.002 | −0.11 | 0.03 | 0.000 |
| Activity (between) | −0.04 | 0.02 | 0.027 | −0.02 | 0.01 | 0.026 | −0.01 | 0.00 | 0.005 |
| Activity (within) 1 | 0.01 | 0.00 | 0.025 | 0.00 | 0.00 | 0.071 | 0.00 | 0.00 | 0.185 |
| DV: Overeating | B | SE | p | B | SE | p | B | SE | p |
| Intercept | −0.60 | 0.52 | 0.254 | 0.86 | 0.56 | 0.121 | 0.35 | 0.07 | 0.000 |
| Wear time | 0.03 | 0.02 | 0.104 | −0.01 | 0.01 | 0.289 | 0.00 | 0.00 | 0.438 |
| zBMI | −0.09 | 0.11 | 0.401 | −0.14 | 0.20 | 0.475 | −0.12 | 0.19 | 0.546 |
| Age | −0.15 | 0.02 | 0.000 | −0.15 | 0.03 | 0.000 | −0.16 | 0.03 | 0.000 |
| Stationary (between) | 0.08 | 0.02 | 0.000 | 0.03 | 0.01 | 0.022 | 0.01 | 0.01 | 0.025 |
| Stationary (within) 1 | −0.01 | 0.01 | 0.021 | −0.01 | 0.00 | 0.065 | 0.00 | 0.00 | 0.139 |
| Intercept | −0.46 | 0.54 | 0.398 | 0.88 | 0.57 | 0.122 | 0.37 | 0.06 | 0.000 |
| Wear time | 0.02 | 0.02 | 0.184 | −0.01 | 0.01 | 0.290 | 0.00 | 0.00 | 0.188 |
| zBMI | −0.03 | 0.09 | 0.783 | −0.07 | 0.17 | 0.668 | −0.07 | 0.16 | 0.652 |
| Age | −0.15 | 0.02 | 0.000 | −0.15 | 0.03 | 0.000 | −0.16 | 0.03 | 0.000 |
| Activity (between) | −0.06 | 0.02 | 0.001 | −0.02 | 0.01 | 0.029 | −0.01 | 0.00 | 0.017 |
| Activity (within)1 | 0.01 | 0.01 | 0.022 | 0.01 | 0.00 | 0.086 | 0.00 | 0.00 | 0.301 |
| DV: Hunger | B | SE | p | B | SE | p | B | SE | p |
| Intercept | −1.39 | 0.64 | 0.030 | −3.14 | 0.65 | 0.000 | 1.02 | 0.86 | 0.232 |
| Wear time | 0.07 | 0.02 | 0.001 | 0.06 | 0.01 | 0.000 | 0.00 | 0.01 | 0.616 |
| zBMI | −0.14 | 0.20 | 0.495 | −0.37 | 0.17 | 0.034 | −0.38 | 0.15 | 0.009 |
| Age | −0.09 | 0.05 | 0.084 | −0.09 | 0.05 | 0.055 | −0.09 | 0.04 | 0.023 |
| Stationary (between) | 0.09 | 0.04 | 0.010 | 0.06 | 0.02 | 0.000 | 0.03 | 0.01 | 0.000 |
| Stationary (within) | 0.00 | 0.00 | 0.408 | 0.00 | 0.00 | 0.214 | 0.00 | 0.00 | 0.168 |
| Intercept | −1.31 | 0.68 | 0.055 | −3.15 | 0.67 | 0.000 | 0.78 | 0.88 | 0.372 |
| Wear time | 0.06 | 0.02 | 0.003 | 0.06 | 0.01 | 0.000 | 0.00 | 0.01 | 0.834 |
| zBMI | −0.07 | 0.19 | 0.718 | −0.22 | 0.16 | 0.157 | −0.26 | 0.14 | 0.056 |
| Age | −0.08 | 0.05 | 0.094 | −0.09 | 0.04 | 0.040 | −0.10 | 0.04 | 0.011 |
| Activity (between) | −0.07 | 0.03 | 0.016 | −0.05 | 0.01 | 0.000 | −0.03 | 0.00 | 0.000 |
| Activity (within) | 0.00 | 0.00 | 0.520 | 0.00 | 0.00 | 0.233 | 0.00 | 0.00 | 0.130 |
| DV: Negative affect | B | SE | p | B | SE | p | B | SE | p |
| Intercept | 2.39 | 0.18 | 0.000 | 2.66 | 0.15 | 0.000 | 2.35 | 0.15 | 0.000 |
| Wear time | 0.00 | 0.01 | 0.571 | 0.00 | 0.00 | 0.300 | 0.00 | 0.00 | 0.334 |
| zBMI | −0.06 | 0.07 | 0.395 | −0.07 | 0.08 | 0.357 | −0.08 | 0.08 | 0.305 |
| Age | −0.01 | 0.01 | 0.478 | −0.01 | 0.01 | 0.496 | −0.01 | 0.01 | 0.425 |
| Stationary (between) | 0.01 | 0.01 | 0.402 | 0.00 | 0.01 | 0.485 | 0.00 | 0.00 | 0.383 |
| Stationary (within) | 0.00 | 0.00 | 0.139 | 0.00 | 0.00 | 0.389 | 0.00 | 0.00 | 0.853 |
| Intercept | 2.34 | 0.19 | 0.000 | 2.60 | 0.16 | 0.000 | 2.28 | 0.16 | 0.000 |
| Wear time | 0.01 | 0.01 | 0.412 | 0.00 | 0.00 | 0.512 | 0.00 | 0.00 | 0.193 |
| zBMI | −0.07 | 0.07 | 0.315 | −0.08 | 0.07 | 0.246 | −0.09 | 0.07 | 0.219 |
| Age | −0.01 | 0.01 | 0.412 | −0.01 | 0.01 | 0.413 | −0.01 | 0.01 | 0.334 |
| Activity (between) | −0.01 | 0.01 | 0.238 | −0.01 | 0.01 | 0.253 | 0.00 | 0.00 | 0.191 |
| Activity (within) | 0.00 | 0.00 | 0.097 | 0.00 | 0.00 | 0.221 | 0.00 | 0.00 | 0.458 |
| DV: Positive affect | B | SE | p | B | SE | p | B | SE | p |
| Intercept | 3.21 | 0.43 | 0.000 | 3.00 | 0.57 | 0.000 | 3.59 | 0.33 | 0.000 |
| Wear time | 0.00 | 0.01 | 0.799 | 0.01 | 0.01 | 0.558 | 0.00 | 0.00 | 0.334 |
| zBMI | 0.19 | 0.29 | 0.521 | 0.28 | 0.33 | 0.397 | 0.40 | 0.28 | 0.159 |
| Age | −0.19 | 0.06 | 0.001 | −0.19 | 0.06 | 0.001 | −0.18 | 0.06 | 0.001 |
| Stationary (between) | −0.08 | 0.05 | 0.080 | −0.04 | 0.03 | 0.150 | −0.02 | 0.01 | 0.013 |
| Stationary (within) | −0.01 | 0.00 | 0.010 | 0.00 | 0.00 | 0.016 | 0.00 | 0.00 | 0.021 |
| Intercept | 3.25 | 0.40 | 0.000 | 3.10 | 0.60 | 0.000 | 3.70 | 0.35 | 0.000 |
| Wear time | 0.00 | 0.01 | 0.863 | 0.00 | 0.01 | 0.715 | 0.00 | 0.00 | 0.206 |
| zBMI | 0.15 | 0.27 | 0.585 | 0.20 | 0.28 | 0.470 | 0.30 | 0.26 | 0.250 |
| Age | −0.19 | 0.05 | 0.000 | −0.18 | 0.06 | 0.001 | −0.18 | 0.06 | 0.002 |
| Activity (between) | 0.06 | 0.04 | 0.104 | 0.03 | 0.02 | 0.112 | 0.02 | 0.01 | 0.014 |
| Activity (within) | 0.01 | 0.00 | 0.006 | 0.00 | 0.00 | 0.009 | 0.00 | 0.00 | 0.011 |
Note. DV=dependent variable; EMA=ecological momentary assessment; zBMI= body mass index z-score; stationary=minutes of stationary activity; Total physical activity =minutes of light and moderate-to-vigorous physical activity; LOC=loss of control; between=grand-mean centered variable; within=person-mean centered variable. Physical activity and stationary behavior were assessed within 30/60/120 minutes before EMA ratings. Wear time was the total valid wear time within each specified time window (30/60/120 minutes).
Lagged activity/stationary time (i.e., occurring before the prior EMA signal) were used as predictors of loss of control eating and overeating reported at the next EMA signal to ensure that there was a temporal association between these variables.
Activity Patterns Predicting Eating-Related Variables
Consistent with the prior models, there were significant between-person effects of stationary minutes and total activity time predicting loss of control eating, overeating, and hunger in all models, with the exception of one in which this effect was marginally significant (i.e., loss of control 60-minute model). That is, participants who evidenced greater overall stationary time and less physical activity reported greater hunger, loss of control eating, and overeating. At the momentary level, within-person effects emerged within the 30-minute windows for loss of control eating and overeating. That is, less stationary time and greater total physical activity time in the 30 minutes prior to an EMA prompt (time t), relative to participants’ average levels, predicted higher subsequent ratings of loss of control eating and overeating (i.e., measured at the following EMA prompt, time t+1).
Discussion
The present pilot study represents the first research to our knowledge to examine momentary affective correlates (i.e., incidental affect, affect response) of device-based measures of physical activity and stationary behavior specifically among youth with overweight and obesity in the natural environment, as well as associations between physical activity and eating-related behaviors. Consistent with expectations, results indicated that youth with higher levels of positive activated affect and lower levels of negative activated affect generally evidenced lower stationary behavior and higher total physical activity over the course of EMA. At the momentary level, higher positive and lower negative activated affect predicted increases in total physical activity and decreases in stationary time up to two hours after EMA prompts, though the negative association between negative activated affect and total activity only emerged at a longer interval (120 minutes). Conversely, higher total physical activity and lower stationary behaviors up to two hours before EMA prompts were related to higher positive activated affect, though activity levels were not related to subsequent negative activated affect.
With respect to eating behaviors and hunger, between-person effects indicated that participants who exhibited lower total physical activity time and more stationary behavior evidenced greater loss of control eating, overeating, and physiological hunger over the course of the study. Within-person effects indicated eating episodes characterized by higher loss of control eating were related to subsequent increases in stationary behavior time and decreases in total physical activity time up to two hours later. However, higher physical activity and lower stationary behavior in the 30 minutes prior to EMA prompts were related to momentary increases in loss of control eating and overeating. Collectively, these results highlight the importance of assessing not only trait-level correlates of physical activity and stationary behaviors, but also their momentary associations with psychological and behavioral domains that are highly relevant to weight regulation and eating disorder prevention in this population.
The findings pertaining to momentary incidental affect and affect response are generally consistent with the broader literature in youth and adults, though important caveats also emerged. While the finding that lower positive activated affect and higher negative affect predicted increased subsequent stationary behavior and decreased physical activity is supported by behavioral theories that posit individuals who experience low mood often engage in avoidance and withdrawal behaviors, these results are also particularly notable given that other EMA research in children has not found affect to be predictive of subsequent activity (Dunton et al., 2014). In line with prior work in adults, it may be that for youth with overweight or obesity, who generally engage in low levels of habitual physical activity, initiation of activity and engagement in stationary behavior is especially susceptible to fluctuations in mood (Carels et al., 2007).
Concerning momentary affective responses, the decreases in positive activated affect following relative increases in stationary behavior time or decreases in total physical activity time replicates prior findings in children across the weight spectrum (Dunton et al., 2014; Wen et al., 2018). However, contrary to these studies, duration of stationary behavior and physical activity did not have an effect on subsequent negative activated affect. It may be that stationary time promotes feelings of boredom and fatigue, but not necessarily negative activated affect; conversely, more intense activity (i.e., light activity/MVPA) may reduce feelings of boredom and fatigue via increasing arousal. It is also possible that longer or more intense bouts of activity are needed to regulate negative affective states. Nevertheless, these findings are also encouraging in that even modest increases in activity/decreases in stationary behavior could have a meaningful influence on positive affective states, which may influence future engagement in non-stationary activity (Rhodes & Kate, 2015).
With respect to between-person affective correlates, the associations between negative activated affect and lower physical activity/higher stationary behavior are supported by prior evidence showing higher physical activity and lower sedentary behavior are related to decreased negative emotionality in youth and adults (e.g., Biddle & Asare, 2011; Teychenne, Ball, & Salmon, 2010). In addition, the negative association between overall positive activated affect and stationary behavior time, as well as the positive association between overall positive activated affect and total physical activity time, are compatible with cross-sectional research showing higher physical activity and lower stationary behavior are independently associated with higher positive affect (Hogan et al., 2015). While mechanisms underlying associations between positive affect and stationary behavior are not yet clear, these findings are broadly consistent with theoretical frameworks that explain associations between physical activity levels and emotional functioning through a variety of psychosocial, behavioral, and biological pathways, such as improvements in self-perception, self-efficacy, and cognitive functioning in youth (e.g., Lubans et al., 2016; Tomporowski et al., 2011). Given that stationary behavior is related to adverse mental and physical health consequences independent of physical activity (Lewis et al., 2017), examining the role of stationary behavior in these frameworks will be important questions for future research.
As an exploratory aim, the present study also examined the relationships between physical activity and stationary behavior time and eating-related constructs. The between-person associations between stationary behavior, total physical activity, and hunger, loss of control eating, and overeating, are consistent with prior research suggesting that physical activity may have regulating effects on eating behaviors and appetitive processes (e.g., Blanchet et al., 2018; Joseph et al., 2011). However, directionality cannot be inferred from these associations. Thus, future research is needed to explore the potential moderators (e.g., weight status) and mediators (e.g., affect) of such associations using larger samples and longitudinal designs. While the sample size of the present study precluded examination of such questions, one possibility is that physical activity improves affect for some individuals, which in turn reduces likelihood of dysregulated eating behavior. In turn, it is also possible that dysregulated eating behavior negatively impacts affect, which in turn influences activity levels. Importantly, findings from such investigations may ultimately be used to optimize the combination and sequencing of obesity prevention and intervention components if eating behaviors and physical activity patterns are shown to directly influence each other.
In line with this possibility, the within-person associations indicated loss of control eating may have detrimental acute effects on subsequent activity levels, while this association was not statistically significant for overeating. Episodes of loss of control eating may be broader indicators of states of reduced self-regulation; consequently, youth may be more likely to engage in avoidance or withdrawal behaviors and less likely to engage in physical activity at these times. It is also intriguing that short-term increases in overeating and loss of control eating occurred after 30 (but not 60 or 120) minutes of increased activity time and decreased stationary time (relative to individual’s average levels). Thus, it may be that youth experience temporary increases in eating motivation after engaging in more physical exertion than usual. However, a prior meta-analysis showed that acute exercise suppressed ghrelin levels (an appetite-stimulating hormone; Schubert et al., 2014), and another meta-analytic review showed that while energy intake increased following acute exercise, there was not a meaningful change in absolute energy intake -i.e., energy intake did not increase to match deficits from exercise bouts (Schubert et al., 2013). In light of the between-person associations in the present study suggesting a potential buffering effect of activity on dysregulated eating and hunger, further research in larger samples is needed to clarify these momentary effects, particularly by examining (1) the objective quantity and types of foods consumed at eating episodes after acute increases in activity, (2) the threshold of physical activity intensity and duration needed to yield beneficial momentary effects on eating, and (3) contextual factors that could influence associations between physical activity and eating (e.g., external food cues, social environments).
While the low levels of MVPA precluded separate analyses in the present study, it is also important to note that MVPA time was lower in this sample (i.e., maximum of 8-12 minutes of MVPA in the hour before or after EMA prompts) compared to similar EMA studies of youth without overweight or obesity (Dunton et al., 2014; Wen et al., 2018). Furthermore, less than one-fifth of participants (3 out of 17) met recommended guidelines for MVPA on any day during the EMA protocol. Given the importance of MVPA for long-term health and weight regulation, these findings indicate a clear need to examine the contexts that impede and promote higher intensities of physical activity, as well as factors that may improve affective responses to more intense levels of activity. In other studies, social and environmental factors (e.g., parental stress, people present, location) have been shown to influence children’s momentary affect during physical activity (Dunton et al., 2011, 2015; Wen et al., 2018), which would be useful to explore among youth with overweight or obesity as well. Furthermore, research is needed to address the degree to which affective responses (or lack thereof) to varying intensities, types, and durations of activity predict longer-term emotional and physical outcomes in youth with and without overweight or obesity.
Despite several strengths of this research, including the use of multimethod ambulatory assessment in a heterogeneous community-based sample, the current study is not without limitations. The small sample size warrants replication of our findings in larger samples. Eating behaviors were assessed via self-report EMA questions, and therefore it is not clear whether overeating reflects objectively large quantities of food consumed. The study was limited to children and younger adolescents, and thus it is unknown whether associations would be similar in older adolescents. Further, all participants had overweight or obesity, so future studies are needed to examine the relative influence of weight status on the observed relationships. The Actiwatch was a wrist-worn device that does not have validated cutoffs, and may be less precise in measuring stationary behavior compared to hip-worn accelerometers (e.g., Marcotte et al., 2020). The measurement of affect was limited to positive and negative activated affect as measured by the PANAS, which excludes low activation states (e.g., fatigue, calmness). As such, it would be informative for future studies in this population to distinguish affective valence from arousal given their potential differential associations with activity levels (Kim et al., 2019). In addition, there was substantial variability in Actiwatch wear time, which warrants more systematic assessment of factors related to wear time (e.g., wear logs) to enhance compliance in future studies. It would also be important to consider time-varying moderators (e.g., people present, location, type of activity) that may influence relationships between affect, eating, and physical activity levels. While this study focused on direct associations between physical activity and eating, further research is warranted to examine variables that mediate these associations (e.g., appetite regulation, neurocognitive functioning). Although the EMA surveys were designed to be brief (e.g., 3-4 minutes), the time participants spent responding to EMA prompts was not timed, and therefore this may have impacted activity levels in the windows prior to EMA signals (which were time stamped at the time of completion). Future EMA work in this area should therefore time stamp signals at the time of delivery and completion to mitigate this confound. Lastly, although ambulatory assessments allowed for examination of temporal associations, the study was nevertheless cross-sectional and observational in nature and cannot speak to causal relationships. Future experimental designs may identify potential causal mechanisms, and research using multiple EMA waves would be helpful to examine directionality of associations over extended time periods, including predictive associations with weight outcomes and progression of eating disorder symptomatology.
Taken together, this pilot study represents an important first step in elucidating momentary as well as trait-level associations between physical activity, stationary behavior, and eating-related processes in youth with overweight or obesity. Certainly, further research is needed to replicate and extend these findings, though initial results indicate that that compared to children and adolescents of normal weight, engagement in physical activity among youth with overweight/obesity may be more strongly linked to affective processes. Thus, it could be useful for intervention strategies to further examine and target factors contributing to low moods that impede physical activity in this population. Further, disordered eating (i.e., loss of control eating) is associated with acute changes in physical activity levels. Continued research is warranted to identify and target the affective states that most reliably predict and sustain physical activity in youth with overweight or obesity, as well as examine the mechanisms by which physical activity promotion may or may not buffer risk for disordered eating symptomatology.
Acknowledgments
Funding: This research was funded by grants from the National Center for Advancing Translational Sciences (UL1-TR000430) and the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK105234).
Footnotes
Activity time variables were aggregated within person prior to conducting correlation analyses.
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review boards (Illinois Institute of Technology IRB2015-048; University of Chicago IRB14-1212-CR004) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Adamo KB, Prince SA, Tricco AC, Connor-Gorber S, & Tremblay M (2009). A comparison of indirect versus direct measures for assessing physical activity in the pediatric population: a systematic review. International Journal of Pediatric Obesity, 4(1), 2–27. [DOI] [PubMed] [Google Scholar]
- Andrade AM, Coutinho SR, Silva MN, Mata J, Vieira PN, Minderico CS, … & Teixeira PJ (2010). The effect of physical activity on weight loss is mediated by eating self-regulation. Patient Education and Counseling, 79(3), 320–326. [DOI] [PubMed] [Google Scholar]
- Balantekin KN, & Roemmich JN (2012). Children’s coping after psychological stress. Choices among food, physical activity, and television. Appetite, 59(2), 298–304. [DOI] [PubMed] [Google Scholar]
- Belcher BR, Berrigan D, Dodd KW, Emken BA, Chou CP, & Spruijt-Metz D (2010). Physical activity in U.S. youth: Effect of race/ethnicity, age, gender, and weight status. Medicine and Science in Sports and Exercise, 42, 2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein EE, & McNally RJ (2017). Acute aerobic exercise helps overcome emotion regulation deficits. Cognition and Emotion, 31(4), 834–843. [DOI] [PubMed] [Google Scholar]
- Biddle SJ, & Asare M (2011). Physical activity and mental health in children and adolescents: a review of reviews. British Journal of Sports Medicine, 45(11), 886–895. [DOI] [PubMed] [Google Scholar]
- Biddle SJ, Ciaccioni S, Thomas G, & Vergeer I (2018). Physical activity and mental health in children and adolescents: An updated review of reviews and an analysis of causality. Psychology of Sport and Exercise. [Google Scholar]
- Blanchet C, Mathieu MÈ, St-Laurent A, Fecteau S, St-Amour N, & Drapeau V (2018). A systematic review of physical activity interventions in individuals with binge eating disorders. Current Obesity Reports, 7(1), 76–88. [DOI] [PubMed] [Google Scholar]
- Bossmann T, Kanning M, Koudela S, Hey S, & Ebner-Priemer UW (2013). The association between short periods of everyday life activities and affective states: a replication study using ambulatory assessment. Frontiers in Psychology, 4, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant Waugh RJ, Cooper PJ, Taylor CL, & Lask BD (1996). The use of the eating disorder examination with children: A pilot study. International Journal of Eating Disorders, 19(4), 391–397. [DOI] [PubMed] [Google Scholar]
- Carels RA, Coit C, Young K, & Berger B (2007). Exercise makes you feel good, but does feeling good make you exercise?: An examination of obese dieters. Journal of Sport and Exercise Psychology, 29(6), 706–722. [DOI] [PubMed] [Google Scholar]
- Carels RA, Douglass OM, Cacciapaglia HM, & O’Brien WH (2004). An ecological momentary assessment of relapse crises in dieting. Journal of Consulting and Clinical Psychology, 72(2), 341. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019). Physical activity guidelines for school-aged children and adolescents. Retrieved from https://www.cdc.gov/healthyschools/physicalactivity/guidelines.htm
- Conner M, McEachan R, Taylor N, O’Hara J, & Lawton R (2015). Role of affective attitudes and anticipated affective reactions in predicting health behaviors. Health Psychology, 34(6), 642. [DOI] [PubMed] [Google Scholar]
- Cornette RE (2011). The emotional impact of obesity on children. Global Perspectives on Childhood Obesity, 257–264. [Google Scholar]
- Costigan SA, Lubans DR, Lonsdale C, Sanders T, & del Pozo Cruz B (2019). Associations between physical activity intensity and well-being in adolescents. Preventive Medicine, 125, 55–61. [DOI] [PubMed] [Google Scholar]
- Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH, & Chekroud AM (2018). Association between physical exercise and mental health in 1· 2 million individuals in the USA between 2011 and 2015: a cross-sectional study. The Lancet Psychiatry, 5(9), 739–746. [DOI] [PubMed] [Google Scholar]
- Dunton GF, Huh J, Leventhal AM, Riggs N, Hedeker D, Spruijt-Metz D, & Pentz MA (2014). Momentary assessment of affect, physical feeling states, and physical activity in children. Health Psychology, 33(3), 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MK, Addoh O, Herod SM, Rhodes RE, & Loprinzi PD (2017). A conceptual neurocognitive affect-related model for the promotion of exercise among obese adults. Current Obesity Reports, 6(1), 86–92. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P (2013). The measurement of affect, mood, and emotion: A guide for health-behavioral research. Cambridge University Press: New York. [Google Scholar]
- Ekkekakis P, Lind E, & Vazou S (2010). Affective responses to increasing levels of exercise intensity in normal-weight, overweight, and obese middle-aged women. Obesity, 18(1), 79–85. [DOI] [PubMed] [Google Scholar]
- Elfhag K, & Rössner S (2005). Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obesity Reviews, 6(1), 67–85. [DOI] [PubMed] [Google Scholar]
- Engel SG, Crosby RD, Thomas G, Bond D, Lavender JM, Mason T, … & Wonderlich SA (2016). Ecological momentary assessment in eating disorder and obesity research: a review of the recent literature. Current Psychiatry Reports, 18(4), 37. [DOI] [PubMed] [Google Scholar]
- Evero N, Hackett LC, Clark RD, Phelan S, & Hagobian TA (2012). Aerobic exercise reduces neuronal responses in food reward brain regions. Journal of Applied Physiology, 112(9), 1612–1619. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. American Psychologist, 56(3), 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson P, Pober D, & Janz KF (2005). Calibration of accelerometer output for children. Medicine and Science in Sports and Exercise, 37(Suppl.), S523–S530. [DOI] [PubMed] [Google Scholar]
- Gayes LA, & Steele RG (2014). Comparison of two measures of weight criticism in youth: Associations with physical activity engagement and attitudes, weight status, and health-related quality of life. Journal of Pediatric Psychology, 40(2), 228–237. [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Smith KE, Crosby RD, Boyd HK, Dougherty E, Engel SG, & Haedt-Matt A (2018a). Ecological momentary assessment of maladaptive eating in children and adolescents with overweight or obesity. International Journal of Eating Disorders, 51(6), 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, O’Brien S, Lavender JM, Pearson CM, Le Grange D, & Hunter SJ (2018b). Executive functioning in a racially diverse sample of children who are overweight and at risk for eating disorders. Appetite, 124, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Smith KE, Lavender JM, Engel SG, & Haedt-Matt A (2019). Trait-level facets of impulsivity and momentary, naturalistic eating behavior in children and adolescents with overweight/obesity. Journal of Psychiatric Research, 110, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, & Keel PK (2011). Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychological Bulletin, 137(4), 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell JS, McMurray RG, Baggett CD, Pennell ML, Pearce PF, & Bangdiwala SI (2005). Energy costs of physical activities in children and adolescents. Medicine and Science in Sports and Exercise, 37, 329–336. [DOI] [PubMed] [Google Scholar]
- Harriger JA, & Thompson JK (2012). Psychological consequences of obesity: weight bias and body image in overweight and obese youth. International Review of Psychiatry, 24(3), 247–253. [DOI] [PubMed] [Google Scholar]
- He J, Cai Z, & Fan X (2017). Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: An exploratory meta-analysis. International Journal of Eating Disorders, 50(2), 91–103. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Rief W, Tuschen-Caffier B, de Zwaan M, & Czaja J (2009). Loss of control eating and psychological maintenance in children: An ecological momentary assessment study. Behavior Research and Therapy, 47(1), 26–33. [DOI] [PubMed] [Google Scholar]
- Hoare E, Milton K, Foster C, & Allender S (2016). The associations between stationary behaviour and mental health among adolescents: a systematic review. International Journal of Behavioral Nutrition and Physical Activity, 13(1), 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan CL, Catalino LI, Mata J, & Fredrickson BL (2015). Beyond emotional benefits: Physical activity and stationary behaviour affect psychosocial resources through emotions. Psychology & Health, 30(3), 354–369. [DOI] [PubMed] [Google Scholar]
- Hughes AR, Henderson A, Ortiz-Rodriguez V, Artinou ML, & Reilly JJ (2006). Habitual physical activity and stationary behaviour in a clinical sample of obese children. International Journal of Obesity, 30(10), 1494. [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Boyce WF, King MA, & Pickett W (2004). Overweight and obesity in Canadian adolescents and their associations with dietary habits and physical activity patterns. Journal of Adolescent Health, 35(5), 360–367. [DOI] [PubMed] [Google Scholar]
- Joseph RJ, Alonso-Alonso M, Bond DS, Pascual-Leone A, & Blackburn GL (2011). The neurocognitive connection between physical activity and eating behaviour. Obesity Reviews, 12(10), 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Conroy DE, & Smyth JM (2019). Bidirectional associations of momentary affect with physical activity and stationary behaviors in working adults. Annals of Behavioral Medicine. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Krebs NF, & Jacobson MS (2003). Prevention of pediatric overweight and obesity. Pediatrics, 112(2), 424–430. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ (2000). CDC growth charts; United States. [Google Scholar]
- Lavender JM, Wonderlich SA, Engel SG, Gordon KH, Kaye WH, & Mitchell JE (2015). Dimensions of emotion dysregulation in anorexia nervosa and bulimia nervosa: A conceptual review of the empirical literature. Clinical Psychology Review, 40, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech RM, McNaughton SA, & Timperio A (2015). Clustering of diet, physical activity and stationary behavior among Australian children: cross-sectional and longitudinal associations with overweight and obesity. International Journal of Obesity, 39(7), 1079. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Napolitano MA, Buman MP, Williams DM, & Nigg CR (2017). Future directions in physical activity intervention research: expanding our focus to stationary behaviors, technology, and dissemination. Journal of Behavioral Medicine, 40(1), 112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Shonkoff ET, & Dunton GF (2015). The acute relationships between affect, physical feeling states, and physical activity in daily life: a review of current evidence. Frontiers in Psychology, 6, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn A, Simmonds M, Owen CG, & Woolacott N (2016). Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obesity Reviews, 17(1), 56–67. [DOI] [PubMed] [Google Scholar]
- Lubans DR, Plotnikoff RC, & Lubans NJ (2012). A systematic review of the impact of physical activity programmes on social and emotional well-being in at-risk youth. Child and Adolescent Mental Health, 17(1), 2–13. [DOI] [PubMed] [Google Scholar]
- Luo S, O’Connor SG, Belcher BR, & Page KA (2018). Effects of Physical Activity and Stationary Behavior on Brain Response to High-Calorie Food Cues in Young Adults. Obesity, 26(3), 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte RT, Petrucci JG, Cox MF, Freedson PS, Staudenmayer JW, & Sirard JR (2019). Estimating stationary time from a hip-and wrist-worn accelerometer. Medicine and Science in Sports and Exercise. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton K, Norton L, & Sadgrove D (2010). Position statement on physical activity and exercise intensity terminology. Journal of Science and Medicine in Sport, 13(5), 496–502. [DOI] [PubMed] [Google Scholar]
- Pinhas-Hamiel O, Singer S, Pilpel N, Fradkin A, Modan D, & Reichman B (2006). Health-related quality of life among children and adolescents: associations with obesity. International Journal of Obesity, 30(2), 267. [DOI] [PubMed] [Google Scholar]
- Pont SJ, Puhl R, Cook SR, & Slusser W (2017). Stigma experienced by children and adolescents with obesity. Pediatrics, 140(6), e20173034. [DOI] [PubMed] [Google Scholar]
- Poortinga W (2007). The prevalence and clustering of four major lifestyle risk factors in an English adult population. Preventive Medicine, 44(2), 124–128. [DOI] [PubMed] [Google Scholar]
- Prentice-Dunn H, & Prentice-Dunn S (2012). Physical activity, stationary behavior, and childhood obesity: A review of cross-sectional studies. Psychology, Health & Medicine, 17(3), 255–273. [DOI] [PubMed] [Google Scholar]
- Ranzenhofer LM, Engel SG, Crosby RD, Anderson M, Vannucci A, Cohen LA, … & Tanofsky-Kraff M (2014). Using ecological momentary assessment to examine interpersonal and affective predictors of loss of control eating in adolescent girls. International Journal of Eating Disorders, 47(7), 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich JN, Gurgol CM, & Epstein LH (2003). Influence of an interpersonal laboratory stressor on youths’ choice to be physically active. Obesity Research, 11(9), 1080–1087. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Lambiase M, Salvy SJ, & Horvath PJ (2009). Protective effect of interval exercise on psychophysiological stress reactivity in children. Psychophysiology, 46(4), 852–861. [DOI] [PubMed] [Google Scholar]
- RStudio Team (2015). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. [Google Scholar]
- Russell JA (1980). A circumplex model of affect. Journal of Personality and Social Psychology, 39(6), 1161–1178. [Google Scholar]
- Schubert MM, Desbrow B, Sabapathy S, & Leveritt M (2013). Acute exercise and subsequent energy intake. A meta-analysis. Appetite, 63, 92–104. [DOI] [PubMed] [Google Scholar]
- Schubert MM, Sabapathy S, Leveritt M, & Desbrow B (2014). Acute exercise and hormones related to appetite regulation: a meta-analysis. Sports Medicine, 44(3), 387–403. [DOI] [PubMed] [Google Scholar]
- Schwarzer R (2008). Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Applied Psychology, 57(1), 1–29. [Google Scholar]
- Simmonds M, Llewellyn A, Owen CG, & Woolacott N (2016). Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obesity Reviews, 17(2), 95–107. [DOI] [PubMed] [Google Scholar]
- Sims J, Scarborough P, & Foster C (2015). The effectiveness of interventions on sustained childhood physical activity: a systematic review and meta-analysis of controlled studies. PLoS One, 10(7), e0132935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner AC, Perrin EM, & Skelton JA (2016). Prevalence of obesity and severe obesity in US children, 1999-2014. Obesity, 24(5), 1116–1123. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Schvey NA, & Grilo CM (2020). A developmental framework of binge-eating disorder based on pediatric loss of control eating. American Psychologist, 75(2), 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teychenne M, Ball K, & Salmon J (2010). Sedentary behavior and depression among adults: a review. International Journal of Behavioral Medicine, 17(4), 246–254. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD, Lambourne K, & Okumura MS (2011). Physical activity interventions and children’s mental function: an introduction and overview. Preventive Medicine, 52, S3–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, … & Chinapaw MJ (2017). Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. International Journal of Behavioral Nutrition and Physical Activity, 14(1), 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, & McDowell M (2008). Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise, 40, 181–188. [DOI] [PubMed] [Google Scholar]
- Trost SG, Kerr LM, Ward DS, & Pate RR (2001). Physical activity and determinants of physical activity in obese and non-obese children. International Journal of Obesity, 25(6), 822. [DOI] [PubMed] [Google Scholar]
- Unick JL, Michael JC, & Jakicic JM (2012). Affective responses to exercise in overweight women: Initial insight and possible influence on energy intake. Psychology of Sport and Exercise, 13(5), 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick JL, Strohacker K, Papandonatos GD, Williams D, O’Leary KC, Dorfman L, … & Wing RR (2015). Examination of the consistency in affective response to acute exercise in overweight and obese women. Journal of Sport and Exercise Psychology, 37(5), 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM, Ekelund U, Besson H, Mezzani A, Geladas N, & Vanhees L (2010). Assessment of physical activity–a review of methodologies with reference to epidemiological research: a report of the exercise physiology section of the European Association of Cardiovascular Prevention and Rehabilitation. European Journal of Cardiovascular Prevention & Rehabilitation, 17(2), 127–139. [DOI] [PubMed] [Google Scholar]
- Watson D, & Tellegen A (1985). Toward a consensual structure of mood. Psychological bulletin, 98(2), 219–235. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, & Tellegen A (1999). The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology, 76(5), 820–838. [Google Scholar]
- Wen CKF, Liao Y, Maher JP, Huh J, Belcher BR, Dzubur E, & Dunton GF (2018). Relationships among affective states, physical activity, and stationary behavior in children: Moderation by perceived stress. Health Psychology, 37(10), 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, & Evans DR (2014). Current emotion research in health behavior science. Emotion Review, 6(3), 277–287. [Google Scholar]


