Abstract
Bone loss associated with microgravity exposure poses a significant barrier to long-duration spaceflight. Osteoprotegerin-Fc (OPG-Fc) is a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor that causes sustained inhibition of bone resorption after a single subcutaneous injection. We tested the ability of OPG-Fc to preserve bone mass during 12 days of spaceflight (SF). 64-day-old female C57BL/6J mice (n=12/group) were injected subcutaneously with OPG-Fc (20 mg/kg) or an inert vehicle (VEH), 24 hours prior to launch. Ground control (GC) mice (VEH or OPG-Fc) were maintained under environmental conditions that mimicked those in the space shuttle middeck. Age-matched baseline (BL) controls were sacrificed at launch. GC/VEH, but not SF/VEH mice, gained tibia BMD and trabecular volume fraction (BV/TV) during the mission (p<0.05 vs. BL). SF/VEH mice had lower BV/TV vs. GC/VEH mice, while SF/OPG-Fc mice had greater BV/TV than SF/VEH or GC/VEH. SF reduced femur elastic and maximum strength in VEH mice, with OPG-Fc increasing elastic strength in SF mice. Serum TRAP5b was elevated in SF/VEH mice vs. GC/VEH mice. Conversely, SF/OPG-Fc mice had lower TRAP5b levels, suggesting that OPG-Fc preserved bone during spaceflight via inhibition of osteoclast-mediated bone resorption. Decreased bone formation also contributed to the observed osteopenia, based on the reduced femur periosteal bone formation rate and serum osteocalcin level. Overall, these observations suggest that the beneficial effects of OPG-Fc during SF are primarily due to dramatic and sustained suppression of bone resorption. In growing mice, this effect appears to compensate for the SF-related inhibition of bone formation, while preventing any SF-related increase in bone resorption. We have demonstrated that the young mouse is an appropriate new model for SF-induced osteopenia, and that a single pre-flight treatment with OPG-Fc can effectively prevent the deleterious effects of SF on mouse bone.
Keywords: spaceflight, osteoprotegerin, mice, bone loss, countermeasure
1. INTRODUCTION
With the National Aeronautics and Space Administration (NASA) defining a goal of human missions to an asteroid and then Mars within the next 20 years, the prospect of long duration spaceflight beyond Earth’s orbit is quickly becoming a reality. In order to safely and efficiently undertake these endeavors, it is important to first understand the long-term complications of exposure to the spaceflight environment. Constant exposure to microgravity (i.e., “weightlessness”) results in a number of deleterious effects on human physiology – effects that could compromise the safety of astronauts both during their mission and following their return to Earth. Aside from the well-documented development of muscle atrophy [1, 2], spaceflight is also known to produce deleterious changes in immune function [3] and cause metabolic disruptions [4]. Microgravity also results in the mechanical unloading of the skeletal system, inducing an osteopenic state similar to that found in patients confined to bed for long periods of time [5]. In the case of a Mars mission, the development of significant bone loss could leave astronauts at increased risk for fracture during mission activities on the planet’s surface. This scenario represents a major obstacle for the future of long-duration spaceflight [6, 7].
In the microgravity environment, skeletal unloading leads to increased bone resorption and the demineralization of load-bearing bones [6]. Studies have demonstrated an average net loss of approximately 230 mg of calcium per day [8]. For astronauts that spent four to six months on the International Space Station (ISS), Lang and colleagues documented substantial trabecular and cortical bone loss in the hip and spine [9]. Bone loss in these individuals was found to occur at a rapid pace, with bone mass decreasing 0.5–2.0% per month. Skeletal deterioration on this scale could increase the risk of fractures. Other factors also contribute to fracture risk, including bone quality, neuromuscular strength and coordination, and the specific physical activities being conducted. Regardless, it is clear that reduced bone integrity is a modifiable risk factor that warrants attention. Indeed, comparisons of pre- and post-flight finite element modeling demonstrated that some astronauts may lose as much femoral strength as would be expected due to a lifetime of aging on Earth [10]. With potential exploratory missions lasting from six to thirty months, the possibility of in-mission fractures should not be considered trivial. Also a concern is the fact that post-flight recovery of bone mass takes two to three times longer than the period of actual microgravity exposure [11]. A year after prolonged ISS missions, it was found that astronauts’ femoral bone mineral density (BMD) had made only a partial recovery [12]. To minimize these deleterious effects, and ensure the long-term health of the relatively young astronaut population, it will be necessary to develop safe and effective countermeasures.
Spaceflight-induced bone loss occurs primarily as a result of the activation of osteoclast-mediated bone resorption [13] with a concomitant decline in bone formation [6]. This is different from more familiar forms of pathological bone loss, such as estrogen-deficiency osteoporosis, where both bone resorption and formation increase, although the former to a greater degree. In the case of pharmacological options for bone loss, the bisphosphonates are first line drugs prescribed for the treatment of post-menopausal osteoporosis. Bisphosphonates (e.g., alendronate, risedronate, ibandronate, etc.) help maintain bone mass, inhibit osteoclast-mediated bone resorption, and reduce the risk of both vertebral and non-vertebral fractures [14]. While bisphosphonates can inhibit loss of bone mass and strength in ground-based disuse models, examination of their utility during spaceflight is limited to a single human study. Leblanc and colleagues examined the combination of alendronate and exercise in astronauts and found that it was able to ameliorate nearly all deleterious changes in bone physiology during spaceflight [15].
Osteoprotegerin-Fc (OPG-Fc) is a monoclonal antibody with activity analogous to endogenous OPG, a secreted tumor necrosis factor receptor (TNFR)-related protein that acts as a decoy receptor for receptor activator of nuclear factor kappa-B ligand (RANKL) [16] and is an essential mediator of osteoclast activity [17]. RANKL binds to its receptor RANK on the surface of osteoclasts, which leads to osteoclast activation, differentiation, and induction of bone resorption [17–20]. OPG-Fc has been shown to prevent loss of bone mass and strength in ground-based models of disuse [21], but spaceflight has the potential to have negative impacts on bone beyond those directly related to skeletal unloading. Before contemplating the use of RANKL inhibitors in astronauts, it could be important to determine whether RANKL inhibition can mitigate bone loss resulting from the panoply of factors, including microgravity, that are present in the unique setting of spaceflight. Considering this, it would be desirable to pursue various avenues for the prevention of spaceflight-induced bone loss, both in regards to mechanism of action and route/frequency of administration.
In the present study, we sought to determine the utility of OPG-Fc administration as a surrogate for human use of the drug denosumab to prevent spaceflight-induced bone loss. Denosumab is a human monoclonal antibody that, like OPG-Fc in mice, acts as an analogue to endogenous OPG. Denosumab itself could not be used in this study because it is specific to human RANKL. OPG-Fc and denosumab have been shown to have similar pharmacodynamic effects, with the main difference being the significantly longer half-life of denosumab [22]. To accomplish this objective, mice were flown for a twelve-day mission on the Space Shuttle Endeavour (STS-108) with and without prophylactic OPG-Fc treatment. A single dose of OPG-Fc at 5 mg/kg was shown to markedly reduce osteoclast numbers for 20 days in normal rats [23] and it was expected that a single dose of OPG-Fc at 20 mg/kg would adequately inhibit osteoclasts throughout a 12-day space mission. At the time of this study, all previous animal studies on the space shuttle had utilized rats. Regardless, to date only three papers have been published where spaceflight animals were subjected to therapeutic intervention [24–26]. Thus, this paper documents not only the first space shuttle flight experiment with mice, but also the first in-flight animal testing of an antiresorptive agent to prevent spaceflight-induced bone loss. Given its well-defined role in skeletal homeostasis, it was hypothesized that OPG-Fc would be able to mitigate the effects of microgravity unloading on bone. Furthermore, information regarding the activity of OPG-Fc in mice during spaceflight could provide insight into the potential utility of denosumab as countermeasure to bone loss in astronauts.
2. MATERIALS AND METHODS
2.1. Animals
Sixty, 64-day-old, female C57BL/6J mice (Jackson Laboratory; Bar Harbor, ME) were utilized for this study. Mice were assigned to one of three groups: Baseline (n = 12), Spaceflight (SF; n = 24), and Ground Control (GC; n = 24). Half of the animals in the SF and GC groups (n=12/group) received a subcutaneous injection of OPG-Fc (20 mg/kg) and half received vehicle (VEH; phosphate-buffered saline, PBS) of equal volume (0.2 mL); baseline controls received no treatment. Mice then received an intraperitoneal injection of calcein (20 mg/kg) and a subcutaneous injection of PBS (180 μL). Injections occurred approximately 22 hours prior launch. At this time, the animals were handed over to NASA personnel. The animals were 64 days old at loading and 65-days old at the time of launch. The Animal Care and Use Committees of the University of Colorado, Amgen Inc., NASA Ames Research Center, and NASA Kennedy Space Center reviewed and approved the animal protocols for this study.
Mice were flown on the Space Shuttle Endeavour (STS-108/UF-1), which lifted off from the Kennedy Space Center (Cape Canaveral, FL) on December 5th, 2001, returning December 17th, 2001. The total mission time was 11 days and 20 hours, of which >11 days and 19 hours was spent in the microgravity environment of low Earth orbit.
2.2. Flight Hardware & Housing
Both the SF and GC mice were housed in Animal Enclosure Module (AEM) hardware provided by NASA Ames Research Center (Moffett Field, CA). The AEMs provide rodent food (NASA Rodent Diet TD97071, Harlan-Teklad, Wisconsin, USA) [27] and water ad libitum. Because urine and feces produced by the animals are free to float around, the AEMs have a constant airflow mechanism designed to move waste towards an exhaust filter. Housing density was within National Institutes of Health (NIH) guidelines with >45 cm2 floor area per mouse in ground configuration. Eight mice were housed per AEM, with a divider separating half the mice. Ground control AEMs were housed in the Orbiter Environmental Simulator (OES) at NASA’s Life Science Support Facility (“Hanger L”) at Cape Canaveral Air Force Station (Cape Canaveral, FL). The OES creates an environment that is able to mimic the temperature, humidity, and CO2 levels within the cabin of the space shuttle. Parameters of concern on the space shuttle included atmospheric CO2, which averaged more than 3000 ppm during the STS-108 flight, and a cabin temperature above normal room temperature. This level is normal in spacecraft and is approximately ten times that of a well-ventilated room on Earth. All procedures for ground control mice were identical to those of the flight group, but on a 48 hours delay in order to allow for adjustment of environmental conditions matching the flight animals.
2.3. Necropsy
Approximately 3.5 hours after landing, the now 77-day-old mice were weighed, anaesthetized with isoflurane, and killed by exsanguination via cardiac puncture. Hind limbs were removed and both tibiae and femora were cleaned of all nonosseous tissue. The left femur of each animal, required for mechanical testing and compositional analysis, was allowed to air-dry. The right femur and tibia were fixed in a 10% neutral buffered formalin solution for 48 hours, rinsed with distilled water, and stored in 70% ethanol. Dissections were completed within five hours of landing.
2.4. Mechanical, Material, and Structural Assays
In order simulate in vivo properties, the air-dried left femora were rehydrated in PBS for 90 minutes prior to evaluation [28]. Three-point bending tests were performed using an Instron 5582 (Instron Corp.; Norwood, MA). Femurs were tested to failure with a 9 mm span length and a deflection rate of 5 mm/min. All bones were tested in the same orientation: the single-point load was applied mid-diaphysis on the anterior surface. The maximal force (Pm; N) and deflection at Pm (δm; mm) were measured for all mechanically tested bones. These two properties were also determined at the elastic limit (Pe, δe) and the failure point. Stiffness (N/mm) was calculated from elastic force/elastic deflection (Pe/δe).
Two-dimensional, cross-sectional moments of inertia of the femur mid-diaphysis (IX and IY; mm4) were also calculated. These values were calculated using the assumption that the periosteal and endocortical surfaces were in the approximate shape of concentric ellipses [29].
Femurs prepared for histomorphometric analysis were also utilized for testing the material properties of the femur diaphysis by microhardness indentation. Three microhardness indents were placed in extant bone within each sectioned and polished femur cross-section using a pyramid-shaped Vicker’s diamond indenter (Fischer Scope-H1100 and WINHCU 1.3 software, Fischer Technology; Windsor, CT) with a 50 g load for 10 sec. In order to minimize edge effects, one indent length was maintained between the indent site, sample edges, and visible lacunae [30]. Pyramid diagonal lengths were measured (250×), and the Vicker’s hardness number (VHN; kgf/mm2) was calculated using the formula: VHN=(2Fsin(x/2))/d2, where F=applied load, x=pyramid angle (136°), and d=average measure of the two diagonal lengths.
2.5. Femur Cortical Quantitative Histomorphometry
Left femurs were placed in 10% neutral-buffered formalin for 48 hours, rinsed with distilled water and stored in 70% ethanol. All bones were allowed to air-dry and were then embedded with non-infiltrating EpoKwick epoxy according to the manufacturers directions (Buehler; Lake Bluff, IL) [30]. The epoxy disks were sectioned with a low-speed saw (Buehler; 12.7 cm × 0.5 mm diamond blade) at the mid-diaphysis of the femur. The sections were wheel-polished to a flat, smooth surface with 600-, 800-, and 1200-grit carbide paper, followed by a cloth impregnated with 6 μm diamond paste. This allowed micrographs at 50× magnification to be taken of bone cross-sections under a blue light (400 nm). Green calcein labels were visualized, indicating the bone formation sites present during the period of the study. Quantitative histomorphometric analysis was performed using these photographs and SigmaScan Pro software (SPSS; Chicago, IL).
Measurements of femur cortical bone morphology [31] included total bone volume (TV) enclosed by periosteal bone surface (Ps.BS) and volume of the medullary cavity (Med.V) enclosed by the endocortical bone surface (Ec.BS). Bone volume (BV) was calculated as TV – Med.V. Cortical thickness was measured at the medial, lateral, posterior and anterior location of the femur diaphysis and averaged (Av.Ct.Th). The area between the calcein label and the cortical perimeter was measured as bone formation volume and divided by 12 days to provide bone formation volume (BFR) values. BFR was normalized to BV for the Ps, Ec and total (T) surfaces. The linear content of the labeled perimeter was defined as active mineralizing surface (MS; the single labeled surface of calcein administered 22 hours before launch). Mineral apposition rate (MAR=BFR/MS) was calculated and normalized to BS for the periosteal (Ps.MAR/BS) and endocortical (Ec.MAR/BS) surfaces.
2.6. Trabecular Histomorphometry and Histology
The right proximal tibiae and fifth lumbar vertebra (L5) were embedded in a methyl methacrylate resin (Osteo-Bed; Polysciences, Warrington, PA, USA) and cut into sagittal sections with a thickness of 5 μm using a tungsten carbide blade. Slides were unstained and coverslipped. Quantitative histomorphometric analysis was performed using SigmaScan Pro software on the micrographs captured at 10× magnification under a UV light (400 nm). Histomorphometric evaluation was performed throughout the metaphysis, starting approximately 0.25 mm distal from the growth plate (excluding the primary spongiosa in L5) and extending a further 0.5 mm. The following trabecular parameters were measured or calculated: BFR/BV, MS/BS; and MAR). The presence of osteoclasts was determined by tartrate-resistant acid phosphatase (TRAP) staining of the slides using a commercial kit (Sigma, St. Louis, MO) and then counterstaining with hematoxylin (Sigma) to allow identification of osteoblasts. Surface measurements were quantified relative to total bone surface (BS). These measurements included osteoblast surface (Ob.S/BS; %), osteoclast surface (Oc.S/BS; %).
2.7. Bone Mineral Composition
Mineral-content analysis was performed on the femora fractured for mechanical testing. Prior to analysis, the ends of the femora were separated where distal and proximal metaphyses join the diaphysis. Mineral content data was obtained separately from these bone ends and the diaphysis itself. A properly calibrated analytical scale (Mettler Toledo UMT2; Columbus, OH) was used for all measurements. Dry mass (Dry-M) was measured after heating the bones to 105°C for 24 hours. Mineral mass (Min-M) was measured after the bones had been ashed at 800°C for an additional 24 hours. Organic mass was calculated as the difference between the two (Org-M = Dry-M – Min-M). Percent mineralization was calculated as: %Min=(Min-M)/(Dry-M)*100.
2.8. Microcomputed Tomography
Trabecular bone architecture was analyzed using microcomputed tomography (μCT20; Scanco Medical AG; Brüttisellen Switzerland) with an isotropic voxel size of 9 μm with scan settings of 55 KVp, 145 mA, and 200 ms integration time. Trabecular microarchitecture was scanned immediately distal to the growth plate in the proximal tibiae and proximal humerus. Analysis of trabecular bone was performed on 100 slices (0.9 mm total) for the tibia and 70 slices (0.6 mm total) for the humerus, producing images for analysis. Bone morphometric parameters were then quantified using Scanco software. Trabecular bone parameters included: trabecular bone volume fraction (BV/TV); connectivity density (Conn.D); trabecular number (Tb.N); trabecular separation (Tb.Sp); and structural model index (SMI).
2.9. Serum Chemistry
At sacrifice, samples of whole blood were collected by cardiac puncture and serum was separated. The concentration of various bone turnover markers was determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits. ELISAs were performed for tartrate-resistant acid phosphatase 5b (TRAP-5b; ImmunoDiagnostic Systems Inc., Fountain Hills, AZ), and osteocalcin (Biomedical Technologies Inc, Stoughton, MA). All ELISA procedures were performed according to the manufacturers’ protocols. In addition, serum levels of alkaline phosphatase (also a bone formation marker), calcium levels were determined using a chemical auto analyzer (Hitachi 717 Automatic Chemistry Analyzer, Roche Diagnostics, Indianapolis, IN).
2.10. Statistics
Data are presented at mean ± SEM. In all cases, 2-way-ANOVA differences are presented. Significant differences within Loading (GC vs. SF) or Drug (VEH vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between GC and SF within VEH or OPG-Fc; Drug (D) indicates a significant difference between VEH and OPG-Fc within GC or SF.
3. RESULTS
3.1. Mechanical, Material, and Structural Assays
3.1.1. Effect of Spaceflight
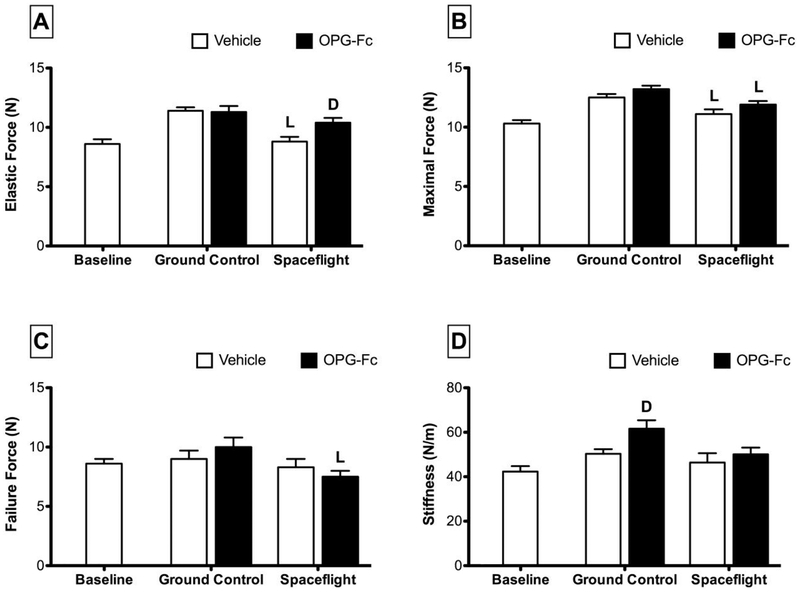
Spaceflight was found to have an effect on the mechanical properties of mouse femora (Figure 1). The stiffness of femora isolated from vehicle-treated spaceflight (SF/VEH) mice was not significantly different from vehicle-treated ground controls (GC/VEH). Elastic force of SF/VEH mice was 23% lower than GC/VEH. The effect of spaceflight on maximal force was more modest, with SF/VEH mice having values 11% lower than GC/VEH and osteoprotegerin-treated spaceflight (SF/OPG-Fc) mice having values 10% lower than OPG-Fc-treated ground controls (GC/OPG-Fc). The only significant effect of spaceflight on failure force was found for mice treated with OPG-Fc: the femora of SF/OPG-Fc mice had a failure force that was approximately 25% lower than OPG-Fc-treated ground control mice.
Figure 1.
Mechanical properties as assessed by three-point bending of femora collected from female C57BL/6J mice (77-days-old at euthanasia). Mice were assigned to one of five groups (n=12/group): Baseline, Ground Control with Vehicle (phosphate-buffered saline), Ground Control with Osteoprotegerin (OPG-Fc; 20 mg/kg), Spaceflight with Vehicle, and Spaceflight with OPG-Fc. The duration of microgravity exposure during spaceflight was 12 days. Data are presented at mean ± SEM. 2-way-ANOVA differences are presented. Significant differences within Loading (Ground Control vs. Spaceflight) or Drug (Vehicle vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between Ground Control and Spaceflight within Vehicle or OPG-Fc; Drug (D) indicates a significant difference between Vehicle and OPG-Fc within Ground Control or Spaceflight.
There was no significant effect of spaceflight on femur microhardness (Table 1). Imax of SF/VEH mice was found to be 18% lower than GC/VEH mice. Imin was not significantly different between SF/VEH and GC/VEH mice.
Table 1.
Summary of material and structural assays properties of femora collected from female C57BL/6J mice (77-days-old at euthanasia). Mice were assigned to one of five groups (n=12/group): Baseline, Ground Control (GC) with Vehicle (VEH; phosphate-buffered saline), GC with Osteoprotegerin (OPG-Fc; 20 mg/kg), Spaceflight (SF) with VEH, and SF with OPG-Fc. The duration of microgravity exposure during SF was 12 days.
| 2-Way ANOVA | ||||
|---|---|---|---|---|
| Micro-Hardness (kgf/mm2) | 67.6 ± 1.7 | 69.4 ± 2.4 | 71.7 ± 1.2 | - |
| Imax | 184 ± 9 | 229 ± 9 | 210 ± 6 | SF<GC |
| Imin | 97 ± 4 | 115 ± 2 | 110 ± 3D | - |
Data are presented at mean ± SEM. 2-way-ANOVA differences are presented in the right-most column. Significant differences within Loading (GV vs. SF) or Drug (VEH vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between GC and SF within VEH or OPG-Fc; Drug (D) indicates a significant difference between VEH and OPG-FC within GC or SF.
3.1.2. Effect of OPG-Fc
Femur stiffness in GC/OPG-Fc was 22% greater than that of GC/VEH mice, whereas OPG-Fc did not significantly increase stiffness in the SF setting (Figure 1). The SF/OPG-Fc femurs exhibited significantly greater elastic force (+18%) relative to SF/VEH controls, whereas the modest increase in elastic force in the GC/OPG-Fc group did not reach significance relative to GC/VEH controls. There was no effect of OPG-Fc on either maximum force or failure force in GC or SF groups.
There was no effect of OPG-Fc on microhardness values for either ground control or spaceflight mice (Table 1). No significant changes in Imax were observed for femora from GC/OPG-Fc or SF/OPG-Fc when compared to their ground controls. However, when compared to SF/VEH, Imin was 10% greater in SF/OPG-Fc mice.
3.2. Femur Cortical Histology and Dynamic Histomorphometry
3.2.1. Effect of Spaceflight
Spaceflight had modest effects on femur cortical BV and TV (cortical plus medullary volume), decreasing these parameters 7% and 6%, respectively, for SF/VEH and SF/OPG-Fc mice when compared to their ground controls (Table 2). Med.V was not affected by spaceflight for either VEH or OPG-Fc-treated groups; however, Av.Ct.Th values for SF/VEH were 11% lower than GC/VEH mice.
Table 2.
Summary of cortical histomorphometric structural parameters from femur mid-diaphysis cross-sections collected from female C57BL/6J mice (77-days-old at euthanasia). Mice were assigned to one of five groups (n=12/group): Baseline, Ground Control (GC) with Vehicle (VEH; phosphate-buffered saline), GC with Osteoprotegerin (OPG-Fc; 20 mg/kg), Spaceflight (SF) with VEH, and SF with OPG-Fc. The duration of microgravity exposure during SF was 12 days. Mice received an injection of the bone label calcein (20 mg/kg) approximately 22 hours prior to the start of the study.
| 2-Way ANOVA | ||||
|---|---|---|---|---|
| BV (mm3) | 0.65 ± 0.01 | 0.71 ± 0.01 | 0.67 ± 0.01L | SF<GC |
| TV (mm3) | 1.54 ± 0.02 | 1.61 ± 0.01 | 1.56 ± 0.02L | SF<GC |
| Med.V (mm3) | 0.90 ± 0.01 | 0.90 ± 0.01 | 0.88 ± 0.01 | - |
| Av.Ct.Th (μm) | 170 ± 6 | 189 ± 6 | 188 ±6D | SF<GC |
| Ec.ES/BS (%) | - | 14.6 ± 3.3D | 17.1 ± 2.3D | OPG-Fc<VEH |
Data are presented at mean ± SEM. 2-way-ANOVA differences are presented in the right-most column. Significant differences within Loading (GC vs. SF) or Drug (VEH vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between SF and GC within VEH or OPG-Fc; Drug (D) indicates a significant difference between OPG-Fc and VEH within GC or SF.
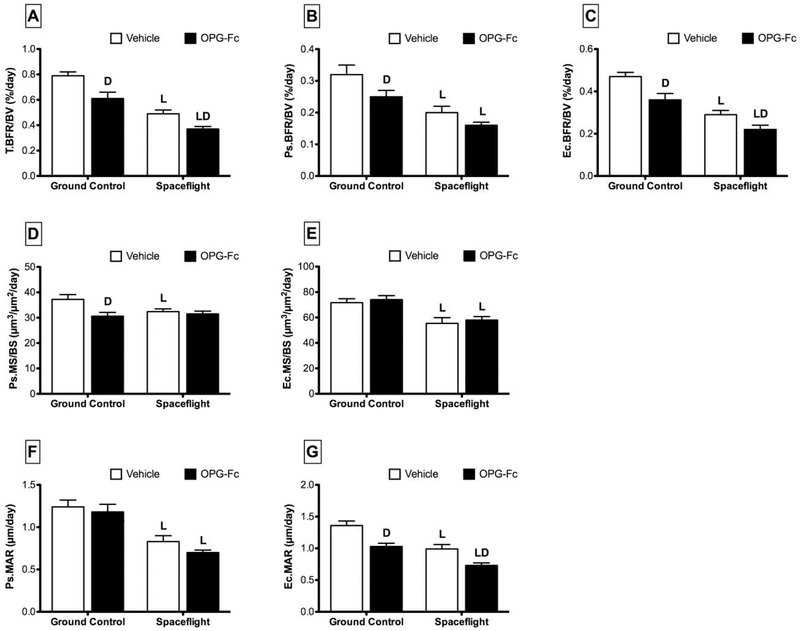
T.BFR/BV for SF/VEH mice was 38% lower than GC/VEH mice (Figure 2). In addition, SF/VEH mice experienced a 38% decline in both Ps.BFR/BV and Ec.BFR/BV when compared to GC/VEH. For SF/OPG-Fc mice, T.BFR/BV declined 39% versus GC/OPG-Fc, while Ps.BFR/BV and Ec.BFR/BV declined by 36% and 39%, respectively. The pattern and magnitude of response was similar when BFR was normalized to BS (data not shown).
Figure 2.
Cortical quantitative histomorphometric parameters from femur mid-diaphysis cross-sections collected from female C57BL/6J mice (77-days-old at euthanasia). Mice were assigned to one of five groups (n=12/group): Baseline, Ground Control with Vehicle (phosphate-buffered saline), Ground Control with Osteoprotegerin (OPG-Fc; 20 mg/kg), Spaceflight with Vehicle, and Spaceflight with OPG-Fc. The duration of microgravity exposure during spaceflight was 12 days. Data are presented as mean ± SEM. 2-way-ANOVA differences are presented. Significant differences within Loading (Ground vs. Spaceflight) or Drug (Vehicle vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between Ground and Spaceflight within Vehicle or OPG-Fc; Drug (D) indicates a significant difference between Vehicle and OPG-Fc within Ground Control or Spaceflight.
Spaceflight caused a 13% decline in Ps.MS/BS for SF/VEH mice compared to GC/VEH (Figure 2). At the endocortical surface, Ec.MS/BS was 23% lower than GC/VEH. Ps.MAR for SF/VEH mice was 33% lower than GC/VEH mice. A similar response was seen for Ec.MAR. There was no effect of spaceflight on Ec.ES/BS for vehicle-treated mice (Table 2). There was no effect of spaceflight on Ps.MS/BS for SF/OPG-Fc mice, however Ec.MS/BS did decrease by 22% when SF/OPG-Fc was compared to GC/OPG-Fc (Figure 2). Ps.MAR and Ec.MAR decreased by 41% and 29%, respectively, for SF/OPG-Fc vs. GC/OPG-Fc. There was no effect of spaceflight on Ec.ES/BS for SF/OPG-Fc mice (Table 2).
3.2.2. Effect of OPG-Fc
OPG-Fc treatment had no effect on BV, TV, or Med.V in either ground control or spaceflight conditions (Table 2). Av.Ct.Th was significantly greater for SF/OPG-Fc mice (+9%) compared to SF/VEH. OPG-Fc had no effect on Av.Ct.Th in ground control mice.
Compared to vehicle, OPG-Fc-treated mice had a T.BFR/BV that was approximately 22–23% lower than vehicle in both ground control and spaceflight conditions (Figure 2). This response was mainly the result of changes at the endocortical surface. For SF/OPG-Fc mice, Ec.BFR/BV decreased by 24%, while there was no significant change in Ps.BFR/BV. For GC/OPG-Fc mice, Ec.BFR/BV decreased by 23%, while Ps.BFR/BV decreased by 22% relative to GC/VEH mice.
Compared to GC/VEH, Ps.MS/BS was 18% lower in GC/OPG-Fc (Figure 2). There was no effect of OPG-Fc treatment on Ecs.MS/BS during spaceflight, and no significant effect of OPG-Fc on Ec.Ms/BS in mice on the ground or in space. While MAR at the periosteal surface was not affected by OPG-Fc treatment, Ec.MAR was significantly decreased in both GC/OPG-Fc (−24%) and SF/OPG-Fc (−26%) conditions when compared to their vehicle controls. OPG-Fc treatment was also found to have an effect on Ec.ES/BS for both ground control (−40%) and spaceflight (−31%) mice (Table 2).
3.3. Mineral Composition
3.3.1. Effect of Spaceflight
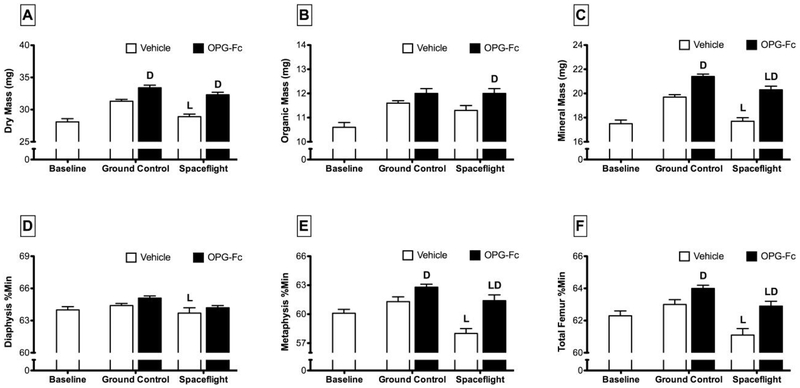
Dry mass of whole femurs from SF/VEH mice was 7% less than GC/VEH mice (Figure 3). While organic mass was not greatly affected by spaceflight, mineral mass was approximately 10% lower in SF/VEH mice compared to GC/VEH controls. The only observed effect of spaceflight on the femoral mass of OPG-Fc-treated mice was a 5% lower mineral mass in SF/OPG-Fc mice compared to GC/OPG-Fc. The total femur percent mineral content of SF/VEH was significantly lower than GC/VEH mice (−3%). Percent mineralization in the diaphysis following spaceflight was not different from ground control. However, in the metaphysis, percent mineralization in SF/VEH mice was 5% lower than GC/VEH mice. The pattern of response for OPG-Fc-treated mice was similar.
Figure 3.
Mineralization properties of femurs collected from female C57BL/6J mice (77-days-old at euthanasia). Mice were assigned to one of five groups (n=12/group): Baseline, Ground Control with Vehicle (phosphate-buffered saline), Ground Control with Osteoprotegerin (OPG-Fc; 20 mg/kg), Spaceflight with Vehicle, and Spaceflight with OPG-Fc. The duration of microgravity exposure during spaceflight was 12 days. Data are presented as mean ± SEM. 2-way-ANOVA differences are presented. Significant differences within Loading (Ground Control vs. Spaceflight) or Drug (Vehicle vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between Ground Control and Spaceflight within Vehicle or OPG-Fc; Drug (D) indicates a significant difference between Vehicle and OPG-Fc within Ground Control or Spaceflight.
3.3.2. Effect of OPG-Fc
OPG-Fc treatment led to significant increases in dry, organic, and mineral mass for both ground control and spaceflight animals (Figure 3). OPG-Fc treatment lead to small increases in total femur percent mineralization for both GC/OPG-Fc (+2%) and SF/OPG-Fc (+3%) mice when compared to their ground controls. These overall changes were primarily related to changes in the metaphysis, as there was no significant effect of OPG-Fc on diaphyseal mineral content. Percent mineral content of the metaphysis in SF/OPG-Fc mice was 2% greater than SF/VEH mice. The effect of OPG-Fc on metaphyseal mineral content in spaceflight mice was much larger, with SF/OPG-Fc having a percent mineral content at this site 6% greater than SF/VEH.
3.4. Trabecular Microarchitecture
3.4.1. Effect of Spaceflight
The effects of spaceflight on trabecular microarchitecture were complicated. In the tibia, trabecular BV/TV of SF/VEH was 26% lower than GC/VEH, while Conn.D was 27% lower (although not significantly), Tb.Th was 16% lower, and SMI was 6% greater (Table 3). There was no effect of spaceflight on any of these parameters when SF/OPG-Fc mice were compared to GC/OPG-Fc.
Table 3.
Summary of proximal tibia and humerus trabecular microarchitecture parameters and tibia and fifth lumbar vertebrae volumetric bone mineral density (BMD) collected from female C57BL/6J mice (77-days-old at euthanasia). Mice were assigned to one of five groups (n=12/group): Baseline, Ground Control (GC) with Vehicle (VEH; phosphate-buffered saline), GC with Osteoprotegerin (OPG-Fc; 20 mg/kg), Spaceflight (SF) with VEH, and SF with OPG-Fc. The duration of microgravity exposure during SF was 12 days.
| 2-Way ANOVA | ||||
|---|---|---|---|---|
| Proximal Tibia μCT | ||||
| BV/TV | 5.7 ± 0.4 | 10.5 ± 0.6D | 10.6 ± 0.3 D | OPG-Fc>VEH |
| Conn.D | 30 ± 7 | 102 ± 10 | 113 ± 7 D | OPG-Fc>VEH |
| SMI | 3.08 ± 0.06 | 2.52 ± 0.06 | 2.46 ± 0.03D | OPG-Fc<VEH |
| Tb.N | 4.07 ± 0.07 | 4.88 ± 0.07D | 4.80 ± 0.08 | OPG-Fc>VEH |
| Tb.Th | 35.9 ± 0.7 | 40.2 ± 0.9 | 40.1 ± 0.5D | SF<GC; OPG-Fc>VEH |
| Tb.Sp | 247 ± 5 | 203 ± 4D | 206 ± 4 | OPG-Fc<VEH |
| Proximal Humerus μCT | ||||
| BV/TV | 10.4 ± 0.5 | 13.4 ± 0.5D | 12.5 ± 0.04D | SF<GC; OPG-Fc>VEH |
| Conn.D | 80 ± 7 | 111 ± 7 D | 109 ± 7D | OPG-Fc>VEH |
| Proximal Tibia pQCT | ||||
| Total vBMD | 382 ± 5 | 528 ± 6LD | 507 ± 5D | SF<GC; OPG-Fc>VEH |
| L5 Vertebra pQCT | ||||
| Total vBMD | 298 ± 6 | 345 ± 4D | 331 ± 6LLD | SF<GC; OPG-Fc>VEH |
Data are presented at mean ± SEM. 2-way-ANOVA differences are presented in the right-most column. Significant differences within Loading (GC vs. SF) or Drug (VEH vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between SF and GC within VEH or OPG-Fc; Drug (D) indicates a significant difference between OPG-Fc and VEH within GC or SF.
At the proximal humeri, both BV/TV and Conn.D were not changed by spaceflight (Table 3).
pQCT analysis of the proximal tibiae showed that spaceflight resulted in significantly lower total vBMD in SF/VEH mice when compared to GC/VEH (−11%) (Table 3). For SF/OPG-Fc mice, the total proximal tibia vBMD was 4% lower versus GC/OPG-Fc. At the L5 vertebrae, vBMD of SF/VEH was 6% lower than GC/VEH mice, while vBMD of SF/OPG-Fc was 4% lower than GC/OPG-Fc.
3.4.2. Effect of OPG-Fc
Proximal tibia BV/TV for OPG-Fc-treated mice was greater than vehicle in all cases, with increases of 14% and 56% for ground control and spaceflight conditions, respectively (Table 3). Conn.D was 77% greater for SF/OPG-Fc mice versus SF/VEH mice. Conn.D was not changed by OPG-Fc treatment in ground control mice. Proximal tibia SMI was decreased with OPG-Fc treatment under spaceflight (−12%) conditions, but not ground control. Conversely, increases in Tb.N were seen for GC/OPG-Fc mice (+10%), but not SF/OPG-Fc when compared to their vehicle-treated counterparts. A similar pattern of response was seen for Tb.Sp. Changes in Tb.Th were modest in ground control mice, while SF/OPG-Fc mice had a Tb.Th that was 18% greater than SF/VEH.
At the proximal humeri, OPG-Fc treatment resulted in significantly increased BV/TV for ground control (+34%) and spaceflight (+40%) animals (Table 3). Conn.D was also significantly increased for both ground control (+52%) and spaceflight (+40%) animals.
vBMD at the proximal tibia of OPG-Fc-treated mice was greater than vehicle-treated for both ground control (+22%) and spaceflight (32%) (Table 3). vBMD at mid-L5 vertebrae was also increased approximately 13% by OPG-Fc treatment for both ground control and spaceflight mice.
3.5. Trabecular Histology and Dynamic Histomorphometry
3.5.1. Effect of Spaceflight
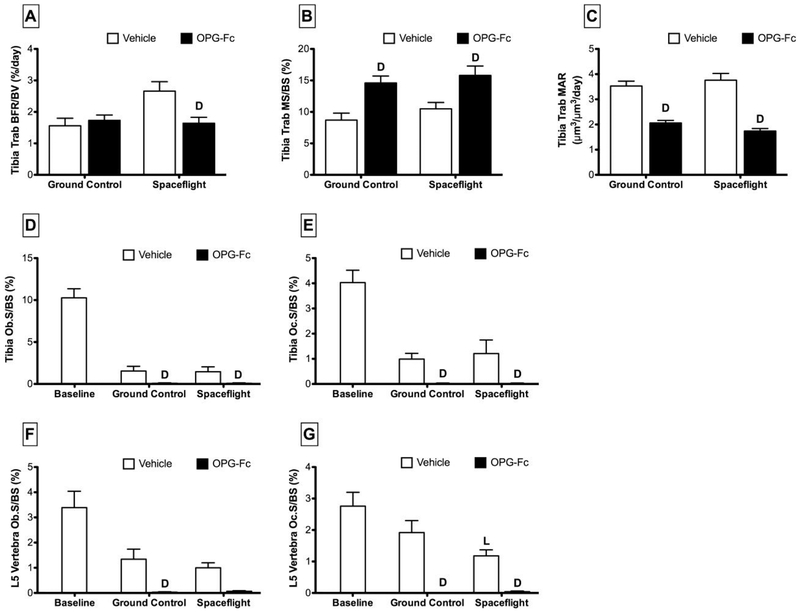
At the proximal tibia, trabecular BFR/BV at the proximal tibia of SF/VEH mice was substantially greater than in GC/VEH mice (+71%) (Figure 4). Spaceflight had no significant effect on MS/BS or MAR for SF/VEH vs. GC/VEH.
Figure 4.
Tibia trabecular histomorphometric parameters and tibia and fifth lumbar vertebra osteoblast/osteoclast indices collected from female C57BL/6J mice (77-days-old at euthanasia). Mice were assigned to one of five groups (n=12/group): Baseline, Ground Control with Vehicle (phosphate-buffered saline), Ground Control with Osteoprotegerin (OPG-Fc; 20 mg/kg), Spaceflight with Vehicle, and Spaceflight with OPG-Fc. The duration of microgravity exposure during spaceflight was 12 days. Data are presented as mean ± SEM. 2-way-ANOVA differences are presented. Significant differences within Loading (Ground Control vs. Spaceflight) or Drug (Vehicle vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between Ground Control and Spaceflight within Vehicle or OPG-Fc; Drug (D) indicates a significant difference between Vehicle and OPG-Fc within Ground Control or Spaceflight.
Proximal tibia, Ob.S/BS and Oc.S/BS of SF/VEH was not different from GC/VEH (Figure 4). At the fifth lumbar vertebrae, Ob.S/BS of SF/VEH mice was not different from GC/VEH, while Oc.S/BS decreased by 39%. There were no changes in any of these parameters when SF/OPG-Fc were compared to GC/OPG-Fc.
3.5.2. Effect of OPG-Fc
Proximal tibia trabecular BFR/BV of OPG-Fc-treated mice was 38% less than vehicle under spaceflight conditions (Figure 4). There was no effect of OPG-Fc on bone formation for ground control mice. OPG-Fc-treated mice experienced a significant increase in MS/BS when compared to vehicle in both ground control (+68%) and spaceflight (+50%) conditions. Conversely, MAR was decreased with OPG-Fc-treatment under ground control (−42%) and spaceflight (−54%) conditions.
At the proximal tibia, OPG-Fc treatment essentially obliterated both osteoblast and osteoclast surface (Figure 4). Treatment with OPG-Fc resulted in lower Ob.S/BS under both ground control (−95%) and spaceflight (−100%) conditions. Similarly, Oc.S/BS of OPG-Fc-treated mice was lower than vehicle under both ground control (−100%) and spaceflight (−98%) conditions. At the fifth lumbar vertebrae, Ob.S/BS of OPG-Fc-treated mice was 98% lower than vehicle-treated ground controls. There was no effect of OPG-Fc on Ob.S/BS in spaceflight conditions. Oc.S/BS at L5 was obliterated by OPG-Fc treatment, decreasing 100% and 97% for ground control and spaceflight conditions, respectively.
3.6. Serum Chemistry
3.6.1. Effect of Spaceflight
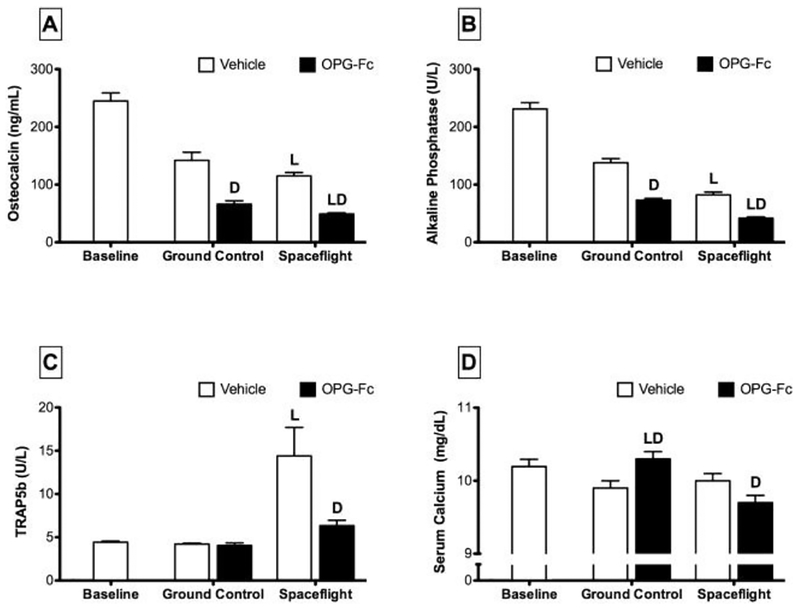
Serum chemistry analyses were conducted on blood collected from SF and GC animals at the study endpoint. Serum osteocalcin and alkaline phosphatase are both systemic markers of osteoblast-mediated bone formation, and spaceflight resulted in a significant decrease of both of these factors (Figure 5). Osteocalcin levels in SF/VEH animals were 19% lower than GC/VEH mice. Similarly, alkaline phosphatase levels of SF/VEH mice were 41% lower than GC/VEH mice. Serum TRACP-5b is a systemic marker of osteoclast-mediated bone resorption. TRACP-5b levels in SF/VEH mice were 241% greater than that found in GC/VEH mice. For SF/OPG-Fc mice, osteocalcin and alkaline phosphatase decreased by 26% and 42%, respectively, compared to GC/OPG-Fc. There was no change in the levels of serum TRACP-5b for these animals.
Figure 5.
Levels of bone formation (osteocalcin and alkaline phosphatase) and bone resorption (TRAP5b) markers in serum collected from female C57BL/6J mice (77-days-old at euthanasia). Mice were assigned to one of five groups (n=12/group): Baseline, Ground Control with Vehicle (phosphate-buffered saline), Ground Control with Osteoprotegerin (OPG-Fc; 20 mg/kg), Spaceflight with Vehicle, and Spaceflight with OPG-Fc. The duration of microgravity exposure during spaceflight was 12 days. Data are presented at mean ± SEM. 2-way-ANOVA differences are presented. Significant differences within Loading (Ground Control vs. Spaceflight) or Drug (Vehicle vs. OPG-Fc) were tested by a Tukey follow-up test (significance set at P<0.05). Loading (L) indicates a significant difference between Ground Control and Spaceflight within Vehicle or OPG-Fc; Drug (D) indicates a significant difference between Vehicle and OPG-Fc within Ground Control or Spaceflight.
3.6.2. Effect of OPG-Fc
Treatment with OPG-Fc generally halved the levels of serum markers of bone formation for all loading conditions, including animals with spaceflight-related reductions in bone formation markers (Figure 5). Serum osteocalcin levels in SF/OPG-Fc were 57% lower than SF/VEH mice. Osteocalcin levels were decreased a similar 54% for GC/OPG-Fc mice. Decreases in alkaline phosphatase level for OPG-Fc treated mice were similar to that of osteocalcin, with these mice seeing approximately 48% lower serum concentrations versus vehicle-treated.
4. DISCUSSION
Increased bone resorption, leading to deleterious effects on bone strength, fracture risk, and propensity for renal stone formation due to elevated calcium excretion, represents a significant obstacle for the future of long duration spaceflight. In the present study, we have demonstrated for the first time that mice experience significant, deleterious effects of spaceflight on skeletal mass, strength and structure and that many of these effects can be ameliorated with a potent antiresorptive agent. The antiresorptive agent, OPG-Fc, is a RANKL inhibitor that was administered once pre-flight by subcutaneous injection. OPG-Fc was used as a surrogate for denosumab, a fully human monoclonal antibody that inhibits RANKL. Denosumab is approved to reduce the risk of fractures in men and women with sex hormone deficiency and those at high risk of fracture [32]. The subcutaneous route of administration, and infrequent (twice-yearly) administration schedule, suggest potential utility for denosumab in male and female astronauts that are expected to lose significant bone mass during long-duration spaceflight. However, it is important to note that the safety and efficacy of denosumab, or other osteoporosis agents, has yet to be established in this unique population and would represent “off-label” use.
A unique attribute of OPG-Fc and other RANKL inhibitors is their ability to markedly reduce osteoclast numbers, which makes it relatively easy to understand the extent to which bone resorption was inhibited at the tissue-level [33]. Osteoclast numbers were markedly reduced by OPG-Fc in spaceflight animals, indicating that inhibition of bone resorption explained the positive changes in bone mass, geometry, architecture and strength with OPG-Fc in the spaceflight animals. In these relatively young growing mice, the effects of exogenous OPG-Fc administration appear to compensate for the spaceflight-related inhibition of bone formation, while also preventing any direct increases in bone resorption due to skeletal unloading in microgravity. This study represents the first flight of mice on the space shuttle, an important development in the use of animal models in microgravity. As such, we have demonstrated that the young mouse can be considered an applicable model for spaceflight-induced osteopenia [34]. Previous studies of pre- and post-flight finite element models of astronaut’s proximal femur following 4.3–6.5 month stays on the International Space Station (ISS) have demonstrated a rapid decline in estimated strength of the proximal femur, on the order of 2.0–2.6% per month [10]. In fact, the rate of decline in strength is more rapid than that of bone mineral density (0.4–1.8% per month) as determined by DXA [7, 9, 12]. These results complement the findings of the present study, which demonstrate a loss of strength and stiffness at the mid-diaphysis of the femur with spaceflight. There were also significant decreases in the rate of cortical bone formation and in the measured moments of inertia. We did not observe any changes in material properties of bones that were assayed, suggesting that the aforementioned structural properties contribute more to the observed changes in bone strength with spaceflight compared to material properties.
The specific location of bone loss is also an important consideration. In the present study, there was significant spaceflight-related loss of both trabecular and cortical bone volume and density, but cortical bone appeared to be affected to a greater degree. Possible mechanisms for bone loss in these two compartments could be important to understand, to the extent that OPG-Fc has direct effects on osteoclasts but not on osteoblasts. For cortical bone, endocortical and periosteal bone formation were significantly reduced with spaceflight and these reductions were further increased by OPG-Fc. Nonetheless, OPG-Fc prevented decreases in cortical thickness and strength in spaceflight mice, indicating that osteoclast inhibition with OPG-Fc fully compensated for the reductions in bone formation. The salutary effects of OPG-Fc on cortical bone could be attributed to reductions in endocortical eroded surfaces. In apparent contrast, trabecular bone formation in the proximal tibia was increased with spaceflight, suggesting that the observed relative reduction in trabecular bone volume with spaceflight was related to increased bone resorption. This is not consistent, however, with the known uncoupled nature of bone remodeling during disuse. It may represent a unique phenomenon of the loading pattern of the group-housed mice in the AEM or an artifact of the single label histomorphometry. This phenomenon has been reported in other models of disuse osteopenia [35] and is also very notable in the physiological state of lactational bone loss [36]. There are limited data on trabecular bone remodeling in rodent disuse osteopenia models; Tian and colleagues showed a significant reduction in trabecular bone formation rate at the proximal tibia after four weeks of hindlimb immobilization, but there was no reduction in bone volume fraction, so it is not clear that this model provides a catalyst for trabecular bone loss [37].
Cortical histomorphometry revealed declines in bone formation rate on the order of 20–40%, depending on surface, during spaceflight. These findings stand in contrast to the lack of significant changes observed in trabecular bone volume fraction (BV/TV) observed when spaceflight mice were compared to ground controls. In addition, composition data revealed declines in total percent mineralization of the femur in spaceflight mice, with similar losses on the order of 3–5% at both the metaphysis and diaphysis. This compares to previous studies by Lang and colleagues which suggests that bone loss tended to occur in the most non-load bearing compartments of the bone (i.e., trabecular versus cortical bone) [9].
Traditional forms of pathological bone loss, such as that which occurs in post-menopausal osteoporosis, occur as a result of an increase in osteoclast-mediated bone resorption with a concomitant increase in osteoblast-mediated bone formation. Although coupled, the net effect of these processes is clearly in favor of bone loss. In contrast, current data in human models suggests that spaceflight-induced bone loss is mainly due to a rapid increase in bone resorption uncoupled from bone formation, which remains stable or actually declines slightly [6, 8, 38]. These findings are primarily based upon assays conducted on astronaut serum samples. Our quantitative histomorphometry data and serum markers indicate spaceflight mice experienced an uncoupling of bone formation and bone resorption, but balanced towards a greater inhibition of bone formation at cortical sites. This is consistent with other experiments from rats and hindlimb suspension studies [39–41]. The decision to fly 10-week old animals was made after considering previous shuttle flights with rats and the need to strike a balance between mice that are not growing at an excessive rate and the logistical need to observe changes in a relatively short twelve day period. The young age of the animals represents a potential limitation to interpretation of the results. In particular, it is ultimately necessary to model an astronaut population that is generally skeletally mature. Spaceflight animal studies currently in progress are utilizing skeletally mature (16-week-old or greater) mice in order to address these concerns. A positive of using young animals is the ability to examine changes in periosteal bone formation in a model where periosteal formation is active, particularly when the duration of spaceflight was limited to 12 days,
The twelve-day duration of the study is also an important consideration in interpreting these results, especially considering the mechanism of action of the osteoprotegerin administered and the time to maximal efficacy. Although this study represents a space shuttle flight of typical duration, there are some limitations in extrapolation to longer-term missions (such as extended stays on the ISS or during a prolonged flight from the Earth to Mars). It was also important for us to consider the development of an immunogenic response to human OPG-Fc in mice and previous data from two-week shuttle missions. Despite this, both the consistency and significance of the results throughout this study make it an important foundation for our knowledge of pharmacological prevention of bone loss in space.
The development of an effective therapeutic countermeasure to spaceflight-induced osteoporosis should not be undertaken without regard for the long-term health of the astronaut population. Excessive antiresorptive treatment and severe inhibition of bone resorption may not be necessary to protect an astronaut’s skeletal system from the temporary stress of the space environment. Previous studies by our laboratory have looked at the use of more conventional bisphosphonate antiresorptives compared to OPG-Fc [21]. Over the course of a series of iterative, proof-of-concept studies we were able to demonstrate profound inhibition of bone formation at so-called supramaximal doses. Through a process of dose refinement we were able to establish preliminary guidelines and a minimally efficacious dosing regimen that would be able to balance the need for inhibition of bone resorption with the deleterious inhibition of bone formation already compromised by microgravity unloading. Combined with the results of the present study, we can see how the practical implications of this ground-based study can be put in to effect. A single dose of OPG-Fc was able to mitigate most of the deleterious effects of spaceflight on the skeletal system of mice. The next step would involve the refinement of a dosing regimen using ground-based animal and human data from denosumab clinical trials to maximize protection of bone, while limiting the long-term effects of the therapy following return to Earth.
In summary, osteoprotegerin is a protein that causes sustained inhibition of bone resorption after a single injection. In the present study, we sought to test the ability of OPG-Fc to preserve bone during spaceflight. After landing, spaceflight mice treated with OPG-Fc had greater BMD than spaceflight or ground control mice treated with inert vehicle. In addition, OPG-Fc was found to increase tibial trabecular bone volume and vBMD in all groups. The effect of OPG-Fc provides a plausible mechanism for the preservation of bone during spaceflight; however, inhibition of bone formation appeared to be a more significant mechanism for spaceflight-induced osteopenia. OPG-Fc did not result in any reversal of spaceflight-related changes in bone formation parameters. These observations suggest that the beneficial effects of OPG-Fc on mouse bone during spaceflight are due to dramatic and sustained suppression of bone resorption. Taken together, we have demonstrated that the young mouse is an appropriate model for spaceflight-induced osteopenia and that a single pre-flight treatment with OPG-Fc effectively ameliorates the deleterious effects of spaceflight on mouse bone.
Highlights.
Bone loss associated with microgravity exposure poses a significant barrier to long-duration spaceflight
We tested the ability of recombinant osteoprotegerin, a RANKL inhibitor, to preserve bone mass during twelve days of spaceflight
Osteoprotegerin preserved bone mass in mice subjected to spaceflight primarily through inhibition of osteoclast-mediated bone resorption.
RANKL inhibition may be a plausible means to prevent the deleterious effects of long duration spaceflight on astronaut bone health
5. ACKNOWLEDGEMENTS
The author’s would like to acknowledge grant support from the National Space Biomedical Research Institute (BL01302 through NASA NCC 9–58) and NIH 5 R01 AR059221–02. The authors would like to thank and acknowledge Mark Rupert from BioServe Space Technologies at the University of Colorado and Dr. Beverly Girten and the NASA Ames Research Center team. In addition, we thank Ramona Bober for coordinating all animal activities at the Kennedy Space Center. Thanks to the astronauts aboard STS-108 for their critical role in helping to manage this experimental payload and bring it home safely to Earth. Thanks to Zhaopo Geng for histomorphometry support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA, Peters JR, Romatowski JG, Bain JL, Riley DA. Prolonged Space Flight-Induced Alterations in the Structure and Function of Human Skeletal Muscle Fibres. J Physiol 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol 2005;93: 463–8. [DOI] [PubMed] [Google Scholar]
- [3].Zayzafoon M, Meyers VE, McDonald JM. Microgravity: the immune response and bone. Immunol Rev 2005;208: 267–80. [DOI] [PubMed] [Google Scholar]
- [4].Markin AA, Zhuravleva OA, Morukov BV, Vostrikova LV, Zabolotskaia IV, Poluektova VP. [Characteristics of cosmonauts’ metabolism after extended missions on the international space station]. Aviakosm Ekolog Med 2005;39: 36–41. [PubMed] [Google Scholar]
- [5].Spector ER, Smith SM, Sibonga JD. Skeletal effects of long-duration head-down bed rest. Aviat Space Environ Med 2009;80: A23–8. [DOI] [PubMed] [Google Scholar]
- [6].Smith SM, Wastney ME, O’Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J Bone Miner Res 2005;20: 208–18. [DOI] [PubMed] [Google Scholar]
- [7].Tilton FE, Degioanni JJ, Schneider VS. Long-term follow-up of Skylab bone demineralization. Aviat Space Environ Med 1980;51: 1209–13. [PubMed] [Google Scholar]
- [8].Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol 1999;277: R1–10. [DOI] [PubMed] [Google Scholar]
- [9].Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 2004;19: 1006–12. [DOI] [PubMed] [Google Scholar]
- [10].Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF. Reduction in proximal femoral strength due to long-duration spaceflight. Bone 2009;44: 449–53. [DOI] [PubMed] [Google Scholar]
- [11].Lane HW, Smith SM. Physiological adaptations to space flight. Life Support Biosph Sci 1999;6: 13–8. [PubMed] [Google Scholar]
- [12].Lang TF, Leblanc AD, Evans HJ, Lu Y. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res 2006;21: 1224–30. [DOI] [PubMed] [Google Scholar]
- [13].Tamma R, Colaianni G, Camerino C, Di Benedetto A, Greco G, Strippoli M, Vergari R, Grano A, Mancini L, Mori G, Colucci S, Grano M, Zallone A. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J 2009. [DOI] [PubMed] [Google Scholar]
- [14].Fleisch HA. Bisphosphonates: preclinical aspects and use in osteoporosis. Ann Med 1997;29: 55–62. [DOI] [PubMed] [Google Scholar]
- [15].Leblanc A, Matsumoto T, Jones J, Shapiro J, Lang T, Shackelford L, Smith SM, Evans H, Spector E, Ploutz-Snyder R, Sibonga J, Keyak J, Nakamura T, Kohri K, Ohshima H. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos Int 2013;24: 2105–14. [DOI] [PubMed] [Google Scholar]
- [16].Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423: 337–42. [DOI] [PubMed] [Google Scholar]
- [17].Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93: 165–76. [DOI] [PubMed] [Google Scholar]
- [18].Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T, Kelley M, Burgess TL, Boyle WJ, Polverino AJ. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 2000;157: 435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 1999;20: 345–57. [DOI] [PubMed] [Google Scholar]
- [20].Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun 1999;256: 449–55. [DOI] [PubMed] [Google Scholar]
- [21].Lloyd SA, Travis ND, Lu T, Bateman TA. Development of a low-dose anti-resorptive drug regimen reveals synergistic suppression of bone formation when coupled with disuse. J Appl Physiol 2008;104: 729–38. [DOI] [PubMed] [Google Scholar]
- [22].Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, Chen C, Li L, Cattley RC, Van G, Scully S, Elliott R, Grisanti M, Morony S, Tan HL, Asuncion F, Li X, Ominsky MS, Stolina M, Dwyer D, Dougall WC, Hawkins N, Boyle WJ, Simonet WS, Sullivan JK. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res 2009;24: 182–95. [DOI] [PubMed] [Google Scholar]
- [23].Capparelli C, Morony S, Warmington K, Adamu S, Lacey D, Dunstan CR, Stouch B, Martin S, Kostenuik PJ. Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): a pharmacodynamic and pharmacokinetic analysis in rats. J Bone Miner Res 2003;18: 852–8. [DOI] [PubMed] [Google Scholar]
- [24].Turner RT. Effects of short-term spaceflight and recombinant human growth hormone (rhGH) on bone growth in young rats. Aviat Space Environ Med 1995;66: 763–9. [PubMed] [Google Scholar]
- [25].Chapes SK, Simske SJ, Sonnenfeld G, Miller ES, Zimmerman RJ. Effects of spaceflight and PEG-IL-2 on rat physiological and immunological responses. J Appl Physiol 1999;86: 2065–76. [DOI] [PubMed] [Google Scholar]
- [26].Bateman TA, Zimmerman RJ, Ayers RA, Ferguson VL, Chapes SK, Simske SJ. Histomorphometric, physical, and mechanical effects of spaceflight and insulin-like growth factor-I on rat long bones. Bone 1998;23: 527–35. [DOI] [PubMed] [Google Scholar]
- [27].Zerath E, Holy X, Andre C, Renault S. Effects of space food bar feeding on bone mass and metabolism in normal and unloaded rats. Nutr Res 2002;22: 1309–18. [DOI] [PubMed] [Google Scholar]
- [28].Broz JJ, Simske SJ, Greenberg AR, Luttges MW. Effects of rehydration state on the flexural properties of whole mouse long bones. J Biomech Eng 1993;115: 447–9. [DOI] [PubMed] [Google Scholar]
- [29].Simske SJ, Guerra KM, Greenberg AR, Luttges MW. The physical and mechanical effects of suspension-induced osteopenia on mouse long bones. J Biomech 1992;25: 489–99. [DOI] [PubMed] [Google Scholar]
- [30].Li C, Risnes S. A comparison of resins for embedding teeth, with special emphasis on adaptation to enamel surface as evaluated by scanning electron microscopy. Arch Oral Biol 2004;49: 77–83. [DOI] [PubMed] [Google Scholar]
- [31].Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 1987;2: 595–610. [DOI] [PubMed] [Google Scholar]
- [32].Suresh E, Abrahamsen B. Denosumab: A novel antiresorptive drug for osteoporosis. Cleve Clin J Med 2015;82: 105–114. [DOI] [PubMed] [Google Scholar]
- [33].Morony S, Warmington K, Adamu S, Asuncion F, Geng Z, Grisanti M, Tan HL, Capparelli C, Starnes C, Weimann B, Dunstan CR, Kostenuik PJ. The inhibition of RANKL causes greater suppression of bone resorption and hypercalcemia compared with bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology 2005;146: 3235–43. [DOI] [PubMed] [Google Scholar]
- [34].Dalton P, Gould M, Girten B, Stodieck LS, Bateman TA. Preventing annoyance from odors in spaceflight: a method for evaluating the sensory impact of rodent housing. J Appl Physiol 2003;95: 2113–21. [DOI] [PubMed] [Google Scholar]
- [35].Bloomfield SA. Disuse osteopenia. Curr Osteoporos Rep 2010;8: 91–7. [DOI] [PubMed] [Google Scholar]
- [36].Salari P, Abdollahi M. The influence of pregnancy and lactation on maternal bone health: a systematic review. J Family Reprod Health 2014;8: 135–48. [PMC free article] [PubMed] [Google Scholar]
- [37].Tian X, Jee WS, Li X, Paszty C, Ke HZ. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone 2011;48: 197–201. [DOI] [PubMed] [Google Scholar]
- [38].Morey ER, Baylink DJ. Inhibition of bone formation during space flight. Science 1978;201: 1138–41. [DOI] [PubMed] [Google Scholar]
- [39].Bikle DD, Halloran BP. The response of bone to unloading. J Bone Miner Metab 1999;17: 233–44. [DOI] [PubMed] [Google Scholar]
- [40].Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravit Space Biol Bull 2003;16: 45–54. [PubMed] [Google Scholar]
- [41].Vico L, Novikov VE, Very JM, Alexandre C. Bone histomorphometric comparison of rat tibial metaphysis after 7-day tail suspension vs. 7-day spaceflight. Aviat Space Environ Med 1991;62: 26–31. [PubMed] [Google Scholar]