Abstract
Osteoarthritis (OA), one of the most common motor system disorders, is a degenerative disease involving progressive joint destruction caused by a variety of factors. At present, OA has become the fourth most common cause of disability in the world. However, the pathogenesis of OA is complex and has not yet been clarified. Long non-coding RNA (lncRNA) refers to a group of RNAs more than 200 nucleotides in length with limited protein-coding potential, which have a wide range of biological functions including regulating transcriptional patterns and protein activity, as well as binding to form endogenous small interference RNAs (siRNAs) and natural microRNA (miRNA) molecular sponges. In recent years, a large number of lncRNAs have been found to be differentially expressed in a variety of pathological processes of OA, including extracellular matrix (ECM) degradation, synovial inflammation, chondrocyte apoptosis, and angiogenesis. Obviously, lncRNAs play important roles in regulating gene expression, maintaining the phenotype of cartilage and synovial cells, and the stability of the intra-articular environment. This article reviews the results of the latest research into the role of lncRNAs in a variety of pathological processes of OA, in order to provide a new direction for the study of OA pathogenesis and a new target for prevention and treatment.
Cite this article: Bone Joint Res 2021;10(2):122–133.
Keywords: Long non-coding RNA, Osteoarthritis, Chondrocyte apoptosis, Extracellular matrix
Article focus
A large number of differentially expressed long non-coding RNAs (lncRNAs) are involved in various pathological changes of osteoarthritis (OA), including extracellular matrix (ECM) degradation, synovial inflammation, chondrocyte apoptosis, and angiogenesis.
The detailed mechanism of how lncRNA acts in the development of OA remains to be elucidated.
Key messages
High-throughput sequencing technology has been used to screen and identify key lncRNAs associated with OA.
LncRNA can regulate the key factors and signalling pathways in the pathogenesis of OA in various ways. Competitive endogenous RNA (ceRNA) is particularly prominent in recent research.
Strengths and limitations
This systematic review summarizes the role and molecular mechanisms of lncRNAs related to OA in recent years, with a view to providing new directions for the study of the pathogenesis of OA.
Interference or overexpression of specific lncRNAs can slow the occurrence and development of OA, but may cause adverse effects in other aspects of the body.
Introduction
Osteoarthritis (OA) is a degenerative joint disease caused by the degradation of cartilage matrix, the death of chondrocytes, and the formation of osteophytes.1 The main manifestation is progressive joint destruction, leading to joint pain, deformity, dysfunction, joint apraxia, and sometimes even disability. In 1999, the World Health Organization listed OA, cardiovascular disease, and cancer as the three major killers threatening human health.2 At present, OA is the fourth-largest cause of disability in the world. A variety of treatments are available to alleviate the symptoms of patients with OA. These include corticosteroids and non-steroidal anti-inflammatory drugs.3-5 Experimental stem cell therapy has been applied to treat specific forms of OA and biological agents are used to block inflammatory mediators such as cytokines, but there is still no specific cure for OA.
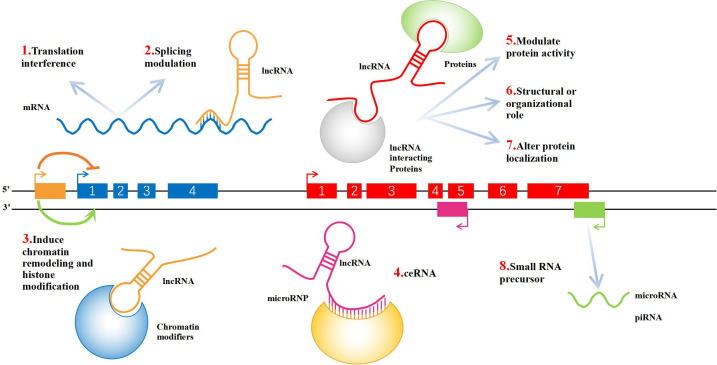
It is estimated that only 2% of the RNA in the human genome encodes proteins, while the vast majority (approximately 98%) is non-coding RNA.6 According to its size, non-coding RNA can be divided into two categories: non-coding small RNA molecules, such as microRNA (miRNA), small interfering RNA (siRNA), PIWI-interacting RNA (piRNA), and small nucleolar RNA (snoRNA); and long non-coding RNA (lncRNA).7 LncRNA is a type of non-coding RNA with a length greater than 200 nt, which lacks an obvious open reading frame and does not have the function of translating into protein.8 According to the relative position of the lncRNA and the coding gene on the chromosome, lncRNAs can be divided into five types: sense; antisense; bidirectional; intronic; and intergenic. They regulate gene expression by folding into a unique conformation and interacting with DNA, RNA, or protein (Figure 1). Gene regulation mainly occurs at three levels: pre-transcriptional; transcriptional; and post-transcriptional. Pre-transcriptional regulation includes lncRNA-mediated histone modification, DNA methylation, and chromosome remodelling,9 while transcriptional regulation includes lncRNA regulation of insulator function, interference with gene transcription, and control of transcription factors.10 Meanwhile, post-transcriptional regulation involves variable splicing of genes and subcellular localization of RNA,11 as well as binding to specific proteins to regulate protein activity12 as a structural component13 or by changing protein localization.14 As a precursor of small RNA, lncRNA can be processed into miRNA and piRNA by ribonucleases (RNases).15 Salmena et al16 found that lncRNA has a miRNA action site and can also compete with miRNA; that is, it acts as a competitive endogenous RNA (ceRNA).17,18 A large number of studies have shown that lncRNA plays important roles in growth and development and in the occurrence of many diseases, and is related to embryonic development, apoptosis, cell differentiation and maturation, immune system diseases, tumorigenesis, invasion, and distant metastasis.19-23 Some lncRNAs are defined as key regulatory factors in the pathogenesis and development of OA.24-27 In this article we review the role of lncRNA in the occurrence and development of OA, hoping to provide a new target and direction for the treatment of OA.
Fig. 1.
Schematic diagram of long non-coding RNA (lncRNA) function. 1) LncRNA can be transcribed with the upstream promoter region of a protein-coding gene to interfere with the expression of downstream genes. 2) LncRNA can form complementary double strands with the transcript of a protein-coding gene, interfering with the splicing of messenger RNA (mRNA) and forming different forms of splicing. 3) LncRNA can mediate chromatin remodelling and histone modification, affecting the expression of downstream genes. 4) LncRNA has microRNA (miRNA) action sites, which can be competitively combined with miRNA. RNAs that act this way are known as miRNA sponges (competitive endogenous RNAs (ceRNAs)). 5) In combination with specific proteins, lncRNA transcripts can regulate the activity of corresponding proteins. 6) As a structural component, it forms a nucleic acid–protein complex with protein. 7) LncRNA can bind to a specific protein, changing its cellular location. 8) LncRNA can form the precursor molecule of small RNAs (such as miRNA, PIWI-interacting RNA (piRNA)).
Non-coding RNAs and osteoarthritis
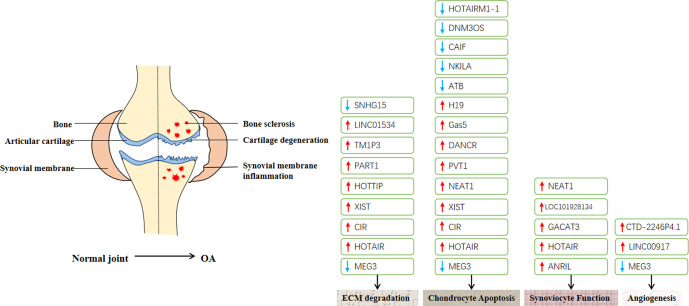
In the past, there have been many in-depth studies on the mechanism of miRNA in OA. miRNA is widely involved in the regulation of chondrogenesis, cartilage differentiation, chondrocyte proliferation, chondrocyte hypertrophy, endochondral osteogenesis, and proteolytic enzyme hydrolyze protein, chondrocyte apoptosis, and other biological processes.28-30 Compared with miRNA, lncRNA has longer transcripts and lower homology among species, but has higher tissue specificity and more conserved promoter sequences, which may indicate that the function of lncRNA is more conservative.31 With the development of bioinformatics and high-throughput sequencing, more and more studies have reported that lncRNA can affect biological processes such as cell proliferation, apoptosis, and differentiation, and affect the occurrence and prognosis of diseases.32 Fu et al33 identified 4,714 differentially expressed lncRNAs in knee cartilage of OA and non-OA patients using gene chip and bioinformatics techniques. Liu et al24 identified 153 lncRNAs differentially expressed in OA patients using gene chip technology, and considered that lncRNA-cartilage injury-related (CIR) is the key to matrix degradation of chondrocytes. Zhang et al34 also identified 2,042 differentially expressed lncRNAs in OA patients using gene chips. Thus, it can be seen that the incidence of OA is closely related to lncRNAs.35 Previous studies have found that: the lncRNA FOXD2-AS1 can regulate the expression of CCND1 through miR-206, regulating the proliferation of chondrocytes and promoting the occurrence of OA;36 the lncRNA-CIR promotes the degradation of cartilage extracellular matrix (ECM) by regulating miR-27b, which aggravates the progress of OA;37 and the lncRNA-PVT1 and miR-448-3p form a ceRNA, to promote the apoptosis of chondrocytes and aggravate the progress of OA.38 At present, research into the molecular mechanism of lncRNA in OA focuses on the binding of lncRNA and miRNA to form molecular sponges (ceRNAs). Many studies have shown that lncRNA plays an important role in regulating the synthesis and metabolism of ECM, synovitis, neovascularization, autophagy, and apoptosis of chondrocytes and other factors related to the occurrence and development of OA (Table I, Figure 2).
Table I.
Abnormally expressed long non-coding RNAs described in the text and their functions.
| lncRNA gene name |
Expression* | Related factor | Function† | Tissue/cell |
|---|---|---|---|---|
| TM1P3 | up | miR-22/TGF-β/MMP1339 | ECM degradation (+) | Human primary chondrocytes |
| CIR | up | miR-27b/MMP1337 | ECM degradation (+) | Human primary chondrocytes |
| miR-130a/BIM36 | Chondrocyte apoptosis (+) | Human primary chondrocytes | ||
| HOTAIR | up | miR-17-5p/FUT2/Wnt/β-catenin40 | ECM degradation (+); Chondrocyte apoptosis (+) |
Human primary chondrocytes |
| miR-130a-3p41 | Chondrocyte apoptosis (+) | Human primary chondrocytes | ||
| Wnt/β-catenin42 | Synovial cells proliferation (+) Synovial cells apoptosis (-) |
Rat synoviocytes | ||
| MEG3 | down | miR-361-5p/FOXO143 | ECM degradation (-) Chondrocyte apoptosis (-) |
Human primary chondrocytes |
| miR-93/TGFBR244 | ECM degradation (-) Chondrocyte apoptosis (-) |
Rat chondrocytes | ||
| VEGF25 | Vascular invasion (-) | Human primary chondrocytes | ||
| HOTTIP | up | miR-455-3p/CCL345 | ECM degradation (+) | Human primary chondrocytes |
| XIST | up | miR-1277-5p46 | ECM degradation (+) | Human primary chondrocytes |
| TIMP-347,48 | ECM degradation (+) | Human primary chondrocytes | ||
| miR-211/CXCR449 | Chondrocyte apoptosis (+) | Primary chondrocytes | ||
| miR-142-5p/SGTB50 | Chondrocyte apoptosis (+) | SW1353 (human osteosarcoma cells) | ||
| PART1 | up | miR-373-3p/SOX451 | ECM degradation (+) | Human primary chondrocytes |
| down | miR-590-3p/ TGFBR2/SMAD352 | ECM degradation (-) Chondrocyte apoptosis (-) |
Human primary chondrocytes | |
| SNHG15 | down | miR-7/KLF4 | ECM degradation (-) | Human primary chondrocytes |
| LINC01534 | up | miR140-5p53 | ECM degradation (+) | Human primary chondrocytes |
| GAS5 | up | miR-34a/Bcl-254 | Chondrocyte apoptosis (+) | Human primary chondrocytes |
| H19 | down | miR-61555 | ECM degradation (-) | Human primary chondrocytes |
| up | miR-130a[72] | Chondrocyte apoptosis (+) | Human primary chondrocytes | |
| up | miR-106a-5p56 | Chondrocyte apoptosis (+) | Human primary chondrocytes | |
| up | miR-140-5p57 | Chondrocyte apoptosis (+) | Human primary chondrocytes | |
| PVT1 | up | miR-27b-3p/TRAF358 | Chondrocyte apoptosis (+) | Human primary chondrocytes |
| miR-14959 | Inflammatory response (+) | Human primary chondrocytes | ||
| DANCR | up | miR-216a-5p/JAK2/STAT360 | Cartilage regeneration (+) Chondrocyte apoptosis (-) |
Human primary chondrocytes |
| miR-577/SphK261 | Chondrocyte apoptosis (-) | Human primary chondrocytes | ||
| NEAT1 | up | miR-193a-3p/SOX562 | Inflammatory response (+) Chondrocyte apoptosis (+) | Human primary chondrocytes |
| miR-181c/OPN63 | Synovial cells proliferation (+) | Human synoviocytes | ||
| CHRF | up | miR-146a/JAK1/STAT364 | Inflammatory response (+) Chondrocyte apoptosis (+) |
ATDC5 cells (mouse embryonic tumour cells) |
| ATB | down | miR-223/MyD88/NF-κB65 | Inflammatory response (-) Chondrocyte apoptosis (-) |
ATDC5 cells (mouse embryonic tumour cells) |
| NKILA | down | miR-145/SP1/NFκB66 | Cartilage regeneration (+) Chondrocyte apoptosis (-) |
Human primary chondrocytes |
| CAIF | down | miR-124667 | Chondrocyte apoptosis (-) | CHON-001 cells (fibroblast immortalized with hTERT) |
| DNM3OS | down | miR-126/IGF168 | Chondrocyte apoptosis (-) | CHON-001 cells (fibroblast immortalized with hTERT) |
| HOTAIRM1-1 | down | miR-125b/BMPR269 | Chondrocyte apoptosis (-) | Human primary chondrocytes |
| GACAT3 | up | IL-6/STAT370 | Synovial cells proliferation (+) | Human synoviocytes |
| ANRIL | up | miR-122-5p/DUSP471 | Synovial cells proliferation (+) Synovial cells apoptosis (-) |
Human synoviocytes |
| LOC101928134 | up | IFNA1/JAK/STAT72 | Synovial cells proliferation (+) | Rat synoviocytes |
| LINC00917 | up | SPHK173 | Vascular invasion (-) | Human chondrocytes |
| CTD-2246P4.1 | up | SPHK173 | Vascular invasion (-) | Human chondrocytes |
Long non-coding RNA expression during osteoarthritis.
(+) means promotion, (-) means inhibition.
ANRIL, antisense noncoding RNA in the INK4 locus; ATB, activated by TGF-β; Bcl-2, B‐cell lymphoma 2; BIM, B-cell lymphoma 2 interacting mediator of cell death; BMPR2, bone morphogenetic protein type II receptor; CAIF, cardiac autophagy inhibitory factor; CCL3, C-C Motif Chemokine Ligand 3; CHRF, cardiac hypertrophy related factor; CIR, cartilage injury–related; CXCR4, C-X-C motif chemokine receptor 4; DANCR, differentiation antagonizing non-protein coding RNA; DNM3OS, dynamin 3 opposite strand; DUSP4, Dual Specificity Phosphatase 4; ECM, extracellular matrix; FOXO1, forkhead box protein 1; FUT2, fucosyltransferase 2; GACAT3, gastric cancer associated transcript 3; GAS5, growth arrest-special transcript 5; H19, H19 imprinted maternally expressed transcript; HOTAIR, HOX transcript antisense intergenic RNA; HOTAIRM1-1, HOXA transcript antisense RNA myeloid-specific 1-1; HOTTIP, HOXA transcript at the distal tip; IFNA1, Interferon Alpha 1; IGF, insulin-like growth factor; IL-6, interleukin- 6; JAK, Janus Kinase; KLF4, Kruppel Like Factor 4; LINC00917, Long Intergenic Non-Protein Coding RNA 917; LINC01534, Long Intergenic Non-Protein Coding RNA 1534; lncRNA, long non-coding RNA; MEG3, maternally-expressed gene 3; miR, microRNA; MMP, matrix metalloproteinase; MyD88, myeloid differentiation factor 88; NEAT1, nuclear paraspeckle assembly transcript 1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NKILA, NF-KappaB Interacting LncRNA; OPN, osteopontin; PART1, prostate androgen regulated transcript 1; PVT1, plasmacytoma variant translocation 1; SGTB, Small Glutamine Rich Tetratricopeptide Repeat Containing Beta; SMAD3, SMAD Family Member 3; SNHG15, small nucleolar RNA host gene 15; SOX, SRY-associated high mobility protein; SP1, Sp1 Transcription Factor; SPHK1, Sphingosine Kinase 1; SphK2, Sphingosine Kinase 2; STAT3, signal transducer and transcriptional activator-3; TGF-β, transforming growth factor beta; TGFBR2, transforming growth factor β receptor 2; TIMP-3, tissue inhibitors of metalloproteinases-3; TRAF3, TNF Receptor-Associated Factor 3; VEGF, vascular endothelial growth factor; Wnt/β-catenin, Wnt/β-catenin pathway; XIST, X inactive specific transcript.
Fig. 2.
Abnormally expressed long non-coding RNAs (lncRNAs) described in the text. It is known that extracellular matrix (ECM) degradation, chondrocyte apoptosis, synovitis, and angiogenesis play important roles in the occurrence and development of osteoarthritis (OA). The red arrow indicates upward adjustment, and the blue arrow indicates downward adjustment.
LncRNAs regulate ECM degradation
The essence of OA is that under the action of mechanical and biological factors, the normal synthesis and degradation of articular chondrocytes, ECM, and subchondral bone are out of balance, and its pathophysiological process is due to the catabolism of articular cartilage being significantly greater than its synthetic metabolism.74 Studies have shown that the occurrence of OA is mainly manifested in the changes of articular cartilage, which is largely composed of chondrocytes and ECM, in which type Ⅱ collagen (Col II) and aggrecan composed of hyaluronic acid (HA) and chondroitin sulfate (CS) constitute the ECM. When joint OA occurs, the expression and content of various matrix metalloproteinases (MMPs) increase, which enhances the hydrolysis and metabolism of Col II, destroys the ECM, and leads to cartilage wear and degeneration. Because the degradation products of cartilage matrix molecules can promote further degradation of the ECM, a persistent vicious circle develops. Recent studies have found that the expression of the lncRNA-TM1P3 is significantly increased in OA chondrocytes. Li et al39 revealed that lncRNA-TM1P3 promotes the expression of activin receptor-like kinase 1 (ALK1) by acting as a miR-22 ceRNA, further causing increased phosphorylation of SMAD, thereby upregulating the expression of MMP-13 and causing ECM degradation. ALK1 is a binding receptor for the transforming growth factor beta (TGF-β) signalling pathway. Activated ALK1 promotes the upregulation of phosphorylated SMAD and MMP13, suggesting that lncRNA-TM1P3 promotes ECM degradation through the miR-22/ALK1/MMP13 axis in OA. In another study by Li et al,37 lncRNA-CIR was found to participate in the degradation of ECM in OA chondrocytes through the miR-27b/MMP13 axis. In addition, inhibition of lncRNA-CIR expression in OA cartilage by small interference RNA (siRNA) can inhibit the expression of MMP-13 and a disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS-5), thus promoting the anabolism of Col II, type Ⅰ collagen, and aggrecan in OA cartilage.24
HOX transcript antisense intergenic RNA (lncRNA-HOTAIR) is a trans-acting lncRNA, proposed by Rinn et al75 and reported to bind to polycomb repressive complex 2 (PRC2).76 HOTAIR promotes cell proliferation by inhibiting miR-148b-3p in glioma cells and leads to tumour cell invasion and malignant tumour development.77 Liu et al78 reported that HOTAIR acts as the ceRNA of miR-331-3p to regulate the expression of human epidermal growth factor receptor-2 (HER2) in gastric cancer. Hu et al40 found that the expression of HOTAIR and fucosyltransferase 2 (FUT2) was negatively correlated with miR-17-5p in OA cartilage. HOTAIR indirectly regulates FUT2 in chondrocytes by acting as ceRNA of miR-17-5p to increase activity of the wnt/β-catenin pathway. The FUT family is a group of fucosyltransferases that catalyze the conversion of fucose to oligosaccharides, glycoproteins, or glycolipids on the substrate. Studies have reported that FUT1, FUT2, and FUT4 are abnormally upregulated in OA cartilage tissue, and FUT plays an important role in the activation of the wnt/β-catenin signalling pathway in various diseases.79 Yang et al80 found that FUT8 promotes epithelial-mesenchymal transformation of breast cancer stem cells by activating the wnt/β-catenin signalling pathway. Zhang et al81 proved that FUT4 promotes embryo adhesion and implantation through the wnt/β-catenin signalling pathway. These results indicate that HOTAIR promotes degradation of the ECM through the miR-17-5p/FUT2/wnt/β-catenin axis.
LncRNA-maternally-expressed gene 3 (MEG3) has proven to be an important factor in tumour development.82 In addition to its role in the development of a variety of cancers,83 including lung cancer, breast cancer, and oesophageal cancer, studies have also found that MEG3 is a potential therapeutic target for OA. Chen et al44 found that MEG3, as a ceRNA of miR-93, can promote the expression of transforming growth factor β receptor 2 (TGFBR2), and then activate the TGF-β signalling pathway to reduce ECM degradation. In another study also aimed at MEG3, Wang et al43 found that MEG3 inhibits ECM degradation through the miR-361-5p/FOXO1 axis. The expression of MEG3 in human OA chondrocytes was downregulated, while overexpression of MEG3 significantly downregulated the expression of miR-93 and miR-361-5p, inhibiting the expression of MMP-13 and ADAMTS-5 which thus reduced degradation of the ECM.43
The lncRNA HOTTIP is a functional lncRNA transcribed from the 5' end of the HOXA gene.84 With downregulation of the HOXA13 gene, its expression increases significantly, and the level of integrin-α-1(ITGα1) decreases significantly after HOXA-13 siRNA is introduced into human OA chondrocytes.85 Overexpression of ITGα1 promotes cartilage formation, while mice lacking ITGα1 develop degenerated cartilage at a younger age and show an increase in (ITGα2) synthesis. HOTTIP may promote ECM degradation in chondrocytes by inhibiting the HOXA-13/ITGα1/MMP2 signalling pathway.85 Mao et al45 found that HOTTIP can also act as a molecular sponge of miR-455-3p to indirectly regulate the expression of the chemokine CCL3, leading to cartilage degradation.
The lncRNA X inactive specific transcript (XIST) has been extensively studied in many types of cancer, including colorectal cancer, pancreatic cancer, osteosarcoma, non-small cell lung cancer, and bladder cancer.86,87 Wang et al46 recently found that XIST promotes the degradation of ECM by acting as the ceRNA of miR-1277-5p in OA. XIST is upregulated in OA, while miR-1277-5p is downregulated. The detection of MMP-13 and ADAMTS5 showed that overexpression of miR-1277-5p could effectively reverse the degradation of ECM, and XIST could act as a molecular sponge of miR-1277-5p to competitively inhibit its function, resulting in increased expression of MMP-13 and ADAMTS5. In addition to the above-mentioned lncRNA, Zhu and Jiang51 found increased expression of lncRNA PART1 in cartilage of patients with OA and verified the interaction between the PART1, miR-373-3p, and SRY-associated high mobility protein 4 (SOX4) by double luciferase reporter assay and RNA immunoprecipitation (RIP). Sun et al88 found that SOX4 led to the degradation of ECM. Takahata et al89 believe that SOX4 induces cartilage degradation of OA by upregulating ADAMTS4 and ADAMTS5. In OA patients, the high expression of PART1 upregulates the expression of SOX4 through the ceRNA miR-373-3p, thus promoting the increased expression of MMP-13, ADAMTS4, and ADAMTS5, resulting in ECM degradation.51 Interestingly, Lu et al52 studied chondrocytes in OA patients and found that the expression of PART1 in OA decreased. Silencing PART1 in primary chondrocytes induced by interleukin (IL)-1β can reduce cell viability and induce apoptosis. It has been proven that PART1, as the ceRNA of miR-590-3p, regulates the TGFBR2/SMAD3 signalling pathway to inhibit the degradation and apoptosis of the ECM of OA chondrocytes. The results of these two studies on cartilage in patients with OA are thus diametrically opposed, and more experiments are needed to prove the role of PART1 in OA.
LncRNAs regulate chondrocyte apoptosis
Apoptosis, also known as programmed cell death, is a programmed self-destruction process initiated by cysteinyl aspartate specific proteases (caspases) in disabled or damaged cells.90 Disorders of apoptosis can lead to pathological conditions such as cancer, developmental abnormalities, and degenerative diseases.91 In the process of apoptosis, cells exhibit distinct morphological characteristics such as cell contraction, plasma membrane blistering, chromatin condensation, DNA fragmentation, and apoptotic body formation.92 Chondrocytes resist mechanical load by synthesizing ECM components and play a vital role in maintaining joint integrity and physiology.93 It is well known that the decrease in the number of chondrocytes due to apoptosis is an important cause of cartilage degeneration in the development of OA.94 In recent years, a large number of studies have found that there are a variety of abnormally expressed lncRNAs in chondrocytes during the pathogenesis of OA. Functional and mechanistic studies have shown that these lncRNAs regulate chondrocyte apoptosis at epigenetic, transcriptional, and post-transcriptional levels. LncRNA-growth arrest-special transcript 5 (GAS5) was originally identified from the subtracted complementary DNA (cDNA) library and its expression level was found to be increased with growth arrest in mammalian cells.95 It is located at 1q25 and contains 11 introns and 12 exons. The exons are alternately spliced to produce two mature lncRNAs (GAS5a and GAS5b). The intron encodes a snoRNA of 10boxC/D. Because of the role of GAS5 in cell growth inhibition and apoptosis, its abnormal expression has been found in many diseases.96 Ji et al54 found that the expression of GAS5 was increased in OA chondrocytes, while silencing GAS5 led to a decrease in the expression of tumour necrosis factor-α (TNF-α) and IL-6. Overexpression of GAS5 inhibited the expression of miR-34a and promoted chondrocyte apoptosis.
H19 was the first lncRNA to be discovered.97 It is located on chromosome 11p15.5 in the human genome, which is very close to the insulin-like growth factor 2 (IGF2) gene.98 It is transcribed by RNA polymerase II into a non-coding RNA transcript of 2.3 kb and spliced to five exons. The H19 sequence may contain a miRNA (miR-675), and can be used as the precursor of miR-675 transfection to produce miR-675. Steck et al55 found that miR-675 regulates the expression of Col II, while proinflammatory cytokines IL-1β and TNF-α significantly downregulate the expression of H19 and miR-675. Steck et al55 believe that increasing the expression of H19 can increase cartilage synthesis, reduce ECM degradation, and improve cartilage tissue regeneration. However, in several recent studies on H19, it was found that the expression of H19 increased in chondrocytes treated with IL-1β and lipopolysaccharide (LPS). Zhang et al99 found that H19, as the ceRNA of miR-106a-5p, could promote chondrocyte apoptosis, while Hu et al56 found that H19 could also promote chondrocyte apoptosis by acting as the ceRNA of miR-130a. Yang et al57 found that H19 can also be used as the ceRNA of miR-140-5p to promote chondrocyte apoptosis. LncRNAs can regulate the expression of multiple miRNAs through the ceRNA network, and these miRNAs can work together to promote or inhibit the progression of a disease. Two distinct results have been reported so far concerning the function and mechanism of H19. Further research is needed to explore the role of H19 in the occurrence and development of OA.
Some studies have found that the lncRNA-plasmacytoma variant translocation 1 (PVT1) plays a key role in the occurrence and development of malignant tumours.100 PVT1 can act as a ceRNA, a variety of miRNA.101,102 Lu et al58 found that after stimulation of human chondrocytes with IL-1β, the expression of PVT1 increased. Silencing PVT1 enhanced the survival rate and autophagy of cells treated with IL-1β, but inhibited apoptosis and inflammation. Silencing PVT1 also antagonized the production of inflammatory factors including nitric oxide (NO) and cytokines such as prostaglandin E2 (PGE2), IL-6, IL-8, and TNF-α.59 Overexpression of miR-27b-3p can reverse apoptosis and inflammation induced by PVT1, while TNF receptor-associated factor 3 (TRAF3) can weaken the inhibitory effect of miR-27b-3p on PVT1. Previous studies have suggested that miR-27b-3p and TRAF3 can regulate the adenosine-monophosphate-activated protein kinase (AMPK) signalling pathway.103 PVT1 regulates chondrocyte apoptosis and inflammation through the miR-27b-3p/TRAF3/AMPK axis and participates in the occurrence of OA.
LncRNA differentiation-antagonizing non-protein-coding RNA (DANCR), formerly known as anti-differentiation non-coding RNA (ANCR), is located on human chromosome 4q12. It is reported to play an important role in a variety of cellular biological processes. Yuan et al104 found that DANCR enhances the stemness features of hepatocellular carcinoma by reducing the expression of β-catenin (CTNNB1), promoting tumour formation and extrahepatic tumour colonization. In the cartilage of patients with OA, Zhang et al60 found that the expression of DANCR was significantly increased, while silencing DANCR could significantly inhibit the expression of IL-6 and IL-8 in OA chondrocytes. DANCR also plays a role in promoting inflammation, cell proliferation, and anti-apoptosis as a ceRNA regulatory JAK2/signal transducer and transcriptional activator-3 (STAT3) signalling pathway of miR-216a-5p. Fan et al61 also confirmed that DANCR can promote the proliferation of OA chondrocytes and reduce apoptosis through the miR-577/SphK2 axis. In mouse ATDC5 cells, Yu et al64 found that lncRNA cardiac hypertrophy related factor (CHRF) can also promote the apoptosis of OA chondrocytes through the JAK2/STAT3 signalling pathway.
LncRNA activated by TGF-β (ATB) is the first lncRNA that can be activated by TGF and has been found to be abnormal in breast cancer,105 colon cancer,106 and pancreatic cancer.107 The imbalance of ATB can promote the growth, migration, and invasion of cancer cells in differentiated cancer.108 Ying et al65 found that the expression of ATB was downregulated in LPS-treated ATDC5 cells, while overexpression of ATB significantly reduced LPS-induced inflammatory damage in ATDC5 cells. Studies have further shown that lncRNA ATB inhibits the myeloid differentiation factor 88 (MyD88)/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and p38MAPK signalling pathways by downregulating miR-223 in cells, thereby reducing cell inflammation and apoptosis.109 Other lncRNAs that can inhibit apoptosis in OA are NF-KappaB Interacting LncRNA (NKILA),66 cardiac autophagy inhibitory factor (CAIF),67 dynamin 3 opposite strand (DNM3OS),68 and HOXA transcript antisense RNA myeloid-specific 1-1 (HOTAIRM1-1).69 NKILA regulates cell proliferation and apoptosis through the miR-145/SP1/NF-κB axis. CAIF can downregulate miR-1246 to inhibit the occurrence and development of OA. Overexpression of DNM3OS can upregulate the expression of insulin-like growth factor-1 (IGF1) to promote the proliferation of chondrocytes and inhibit apoptosis. HOTAIRM1-1 can activate the janus kinase/mitogen-activated protein kinase/extracellular regulated protein kinases (JNK/MAPK/ERK) signalling pathway to inhibit apoptosis, and promote mesenchymal stem cells (MSC) activity and chondrogenic differentiation.
CIR is a lncRNA highly expressed in OA, discovered by Liu et al24 using gene chip analysis. In addition, CIR can promote ECM degradation, promote chondrocyte apoptosis, and reduce chondrocyte autophagy. Lu et al36 found that overexpression of CIR can inhibit the expression of miR-130a and promote the expression of B‐cell lymphoma 2 (Bcl-2) interacting mediators of cell death (BIM) in chondrocytes stimulated by IL-1β or TNF-α, accompanied by increased levels of reactive oxygen species, release of inflammatory mediators, and apoptosis. Wang et al110 found that silencing CIR increases expression of the autophagy-related proteins LC3BI/II and BECLIN-1 in cartilage of patients with OA. The above studies show that CIR is closely related to the occurrence and development of OA and can be used as a potential target for the treatment of OA. HOTAIR,40 nuclear paraspeckle assembly transcript 1 (NEAT1),62 XIST,50 and MEG3 43,44 also regulate not only the metabolism of ECM but also the apoptosis of chondrocytes. Several studies have shown that XIST can promote apoptosis in OA chondrocytes. Li et al49 found that XIST inhibits the proliferation of OA chondrocytes and promotes their apoptosis. In IL-1β-induced chondrocytes, XIST, as the ceRNA of miR-211, regulates the downstream MAPK signalling pathway by promoting the expression of CXCR4, which leads to reduced proliferation and increased apoptosis of chondrocytes. MAPK/ERK play an important role in inflammation and immune response.47 Activation of the CXCR4/CXCL12 axis increases the expression of MAPK/ERK.111 In addition, inhibition of the p38-MARK signalling pathway inhibits apoptosis of OA chondrocytes.112 Also in IL-1β-induced primary chondrocytes, Sun et al50 found that the increase of XIST was related to the decrease of Col2A1 and Bcl-2 and the increase of MMP13 and Bax. It is speculated that XIST may regulate the proliferation and apoptosis of chondrocytes through the miR-142-5p/small glutamine rich tetratricopeptide repeat containing beta (SGTB) axis.
LncRNAs regulate synoviocyte function
Synovitis is one of the most important pathological features of OA. Its histological features include synovial cell hypertrophy, proliferation, lining cell proliferation, and inflammatory cell infiltration. The stimulated synovial cells also secrete a large number of cytokines, chemokines, reactive oxygen species, lipids, lipid mediators, complement pathway components, and MMPs, which are all significantly increased in the synovial fluid of patients;113 thus stimulating synovial tissue proliferation, causing cartilage tissue erosion, and leading to cartilage matrix destruction, dissolution, and fibrosis. In contrast to chondrocytes, the increase in the number of synovial cells promotes the development of OA. Gastric cancer-associated transcript 3 (GACAT3) is a newly discovered lncRNA. Li et al70 found that the expression of GACAT3 was increased in osteoarthritis synovial cells (OAS), and the proliferation of OAS cells transfected with siRNA was significantly inhibited. In their experiment, the OAS cell cycle was blocked in G0/G1 phase, and the apoptosis rate increased. GACAT3 affects the proliferation of OAS through the IL-6/STAT3 signalling pathway. In the synovium of the knee joint of OA rats, Yang et al72 found that high expression of lncRNA LOC101928134 regulates expression of the IFNA1 gene and inhibits the JAK/STAT signalling pathway. Silencing LOC101928134 inhibits the expression of IL-1β and TNF-α, which leads to the relief of knee synovitis, inflammatory injury, and knee cartilage injury in OA rats. In addition, silencing LOC101928134 promotes the apoptosis of synovial cells and inhibits the apoptosis of chondrocytes in OA rats.
The antisense noncoding RNA in the INK4 locus (lncRNA ANRIL) is located in a full-length 3.8 kb sequence in the 9p21.3 region of the chromosome.114 ANRIL is expressed in a variety of normal human tissues, with the highest expression in ovary and the lowest in muscle.115 Genome-wide association studies (GWAS) have identified ANRIL as a risk site for a variety of cancers, including breast cancer, nasopharyngeal carcinoma, glioma, and others.116 Li et al71 found that the expression of ANRIL is increased in OAS cells, and ANRIL can act as a ceRNA of miR-122-5p to regulate the expression of dual specificity phosphatase 4 (DUSP4). Silencing ANRIL can block synovial cell proliferation and reduce apoptosis, while overexpression of miR-122-5p can have the same effect.
The lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) is transcribed from the transcriptional site of multiple endocrine neoplasia (MEN) type Ⅰ located on human chromosome 11, and is involved in the occurrence and development of a variety of tumours including tumour cell proliferation, invasion, and metastasis.117 NEAT1 promotes the inflammation and apoptosis of chondrocytes in OA.62 Wang et al63 found that the expression of NEAT1 and osteopontin (OPN) increased in OAS cells. OPN is reported to regulate the expression of a variety of inflammatory factors related to the pathogenesis of OA, including MMP13, IL-6, and IL-8. After NEAT1 gene knockout, the expression of MMP13, IL-6, and IL-8 in synovial cells decreased, cell proliferation was inhibited, and the level of OPN protein decreased. NEAT1, which can inhibit synovial cell proliferation and promote synovial cell apoptosis, has a negative correlation with miR-181c. Other lncRNAs such as HOTAIR, MEG3, and prostate cancer gene expression marker 1 (PCGEM1) have also been reported to be involved in regulating the proliferation, apoptosis, and differentiation of synovial cells.42,118 These lncRNAs are potential biomarkers and targets for the treatment of OA and synovitis.
LncRNAs regulate angiogenesis
Angiogenesis is very important for physiological processes such as tissue growth, development, regenerative circulation, and repair, but it also plays an important role in the pathological changes of some diseases. One study has suggested that OA is actually the activation of secondary ossification centres, resulting in repeated endochondral ossification.119 Angiogenesis is an important link in the process of endochondral osteogenesis, which can lead to subchondral bone reconstruction, synovial hyperplasia, and osteophyte formation. There are no blood vessels in normal articular cartilage, but a large number of blood vessels can be found in OA cartilage. Other studies have pointed out that the invasion of blood vessels into cartilage destroys the barrier between articular bone and cartilage and aggravates the inflammatory reaction, which is an important factor leading to clinical symptoms and disease progression.120 When normal articular cartilage was implanted into the chorioallantoic villi of chicken embryos, it retained its blood vessel-free character,121 while cartilage derived from OA patients showed obvious vascular growth.120 This indicates that normal cartilage has the ability to suppress angiogenesis, while this ability is significantly weakened in OA cartilage. Vascular endothelial growth factor (VEGF) is considered to be the key factor in angiogenesis. Inflammatory factors (IL-1β, TNF-α), hypoxia, and mechanical stress upregulate the expression of VEGF in OA joints through multiple signalling pathways.122 The expression of VEGF in the surface, middle, and deep layers of OA cartilage has been shown to be upregulated, while angiogenesis mainly occurs in the deep cartilage.120 The lncRNA-MEG3 is a type of imprinted gene, which is located on chromosome 14q32.3. It is a human homologue of mouse maternally imprinted gene trap locus 2 (Glt2), which was first discovered by Miyoshi et al123 in 2000. MEG3 has been reported in previous studies to reduce ECM degradation in OA chondrocytes,43,44 and the interaction between MEG3 and SRY-associated high mobility protein-2 (SOX2) induces the expression of BMP4 to promote osteogenic differentiation of bone marrow mesenchymal stem cells.124 In addition, other studies have pointed out that overexpression of MEG3 leads to downregulation of the serine/threonine-specific protein kinase (known as protein kinase B (AKT)) signalling pathway in breast cancer, and the AKT signalling pathway plays a key role in the growth, invasion, and angiogenesis of breast cancer cells.125 By comparing chondrocytes between patients with OA and normal controls, Su et al25 found that the expression of MEG3 in articular cartilage of OA was significantly downregulated and the expression of VEGF was significantly upregulated. Other studies have found that MEG3 can stimulate the transcription of p53,126 and p53 negatively regulates the transcription of VEGF by binding to the transcription factor Sp1 site on the VEGF promoter.127 Therefore, downregulation of MEG3 in OA cartilage may promote the transcription of VEGF by reducing the activity of p53, which leads to angiogenesis in OA cartilage. Sphingosine kinase 1 (SPHK1), a member of the sphingosine kinase (SPHK) family, has been shown to play a vital role in cell migration.128 Some studies have shown that SPHK1 is involved in angiogenesis. In the absence of ECM, the overexpression of SPHK1 promotes the survival of endothelial cells and plays an important role in angiogenesis.129 Minashima et al130 found that the interaction between ankylosis protein/MYB binding protein 1a (ANK/MYBBP1a) and SPHK1 can affect catabolism in the process of cartilage degradation mediated by IL-1β. Studies have shown that SPHK1 can promote the development of OA. Chen et al73 found that the lncRNAs LINC00917 and CTD-2246P4.1 regulate angiogenesis by affecting SPHK1 and play an important role in the progression of OA.
In conclusion, the pathogenesis of OA is complex and has not been elucidated so far. Although many studies have partially revealed the regulatory mechanism of OA and explored the treatment of OA-related diseases, the results are still not satisfactory. As a new hot topic in the regulation of gene expression, lncRNA may play a key role in the pathogenesis of OA by regulating extrachondral matrix metabolism, chondrocyte apoptosis, synovial hyperplasia, and peripheral neovascularization. Through continuous research, it has been found that thousands of lncRNAs are differentially expressed in OA, and some of the maladjusted lncRNAs have potential as valuable diagnostic biomarkers and therapeutic targets. Once a new lncRNA is found, its function should be clarified and verified in vivo and in vitro. However, in the process of verifying the function of a lncRNA, because lncRNAs are not conserved among species there are often no homologous genes in animals. It is therefore not easy to find an in vivo model to test the function and mechanism of lncRNA in detail. Consequently, many animal models of lncRNA knockouts are constructed on the basis of gene disruption, targeted promoter deletions, and premature termination strategies.131,132 The use of lncRNAs as an approach to treat cartilage-related disease is in its infancy. In the near future, lncRNA targeted therapy may become a new hope for the cure of OA. Through advanced technology, knockout or overexpression of key lncRNAs may become a feasible method for the treatment of cartilage-related diseases in future.133 For example, since 2010 several new delivery strategies have been developed to reduce off-target effects, especially using nanoparticles which have the characteristics of improved stability, minimal size, biocompatibility, and self-assembly.134 This also allows nanoparticles to improve the stability and targeting of lncRNA. However, silencing of MEG3 aggravates LPS-stimulated human lung cell injury,135 while silencing NEAT1 can inhibit immunity136 and silencing digeorge syndrome critical region gene 5 (DGCR5) can enhance the growth, migration, and invasion of cervical cancer.136 If targeted knockout or overexpression of OA-related lncRNA is planned as a treatment for OA, it will first be necessary to pay attention to the side effects of the knockout or overexpression of the lncRNA. The specific mechanisms and functions of these OA-related lncRNAs need to be further studied and investigated, taking in vitro chondrocytes, OA animal models, and OA patients as the research objects. This is in order to further discover and verify the influence of lncRNA on the pathogenesis and pathological changes of OA, and lay the foundation for its diagnosis, prognosis, prevention, and treatment.
Author contributions
C. P. He: Collected, assembled, analyzed, and interpreted the data, Critically revised the article for important intellectual content.
X. C. Jiang: Collected, assembled, analyzed, and interpreted the data, Critically revised the article for important intellectual content.
C. Chen: Collected, assembled, analyzed, and interpreted the data, Critically revised the article for important intellectual content.
H. B. Zhang: Collected, assembled, analyzed, and interpreted the data, Critically revised the article for important intellectual content.
W. D. Cao: Collected, assembled, analyzed, and interpreted the data, Critically revised the article for important intellectual content.
Q. Wu: Collected, assembled, analyzed, and interpreted the data, Critically revised the article for important intellectual content.
C. Ma: Created the illustrations, Checked and modified the references.
Funding statement
This research was supported by grants from the Chinese People's Liberation Army (PLA) youth training program (NO.19QNP014), Natural Science Foundation of Hunan Province (NO.2018JJ6033), Hunan Provincial Innovation Foundation for Postgraduate (NO.CX20200548), Scientific Research Project of Hunan Health Commission (NO. B2019149, C2019141 and B2016210), Hunan Provincial Department of Education Project (NO.15A115), and the Natural Science Foundation of Jishou University (NO. JDlc1904).
ICMJE COI statement
The authors declare that they have no conflict of interest.
© 2021 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21 Suppl 1:S1–S6. [DOI] [PubMed] [Google Scholar]
- 2. March L, Smith EU, Hoy DG, et al, et al. . Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol. 2014;28(3):353–366. [DOI] [PubMed] [Google Scholar]
- 3. Bohensky J, Terkhorn SP, Freeman TA, et al. . Regulation of autophagy in human and murine cartilage: hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009;60(5):1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Busse P, Vater C, Stiehler M, et al. . Cytotoxicity of drugs injected into joints in orthopaedics. Bone Joint Res. 2019;8(2):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishida K, Matsushita T, Takayama K, et al. . Intraperitoneal injection of the SIRT1 activator SRT1720 attenuates the progression of experimental osteoarthritis in mice. Bone Joint Res. 2018;7(3):252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H, Yang HH, Sun ZG, Tang HB, Min JK. Whole-transcriptome sequencing of knee joint cartilage from osteoarthritis patients. Bone Joint Res. 2019;8(7):290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song J, Ahn C, Chun C-H, Jin E-J. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J Orthop Res. 2014;32(12):1628–1635. [DOI] [PubMed] [Google Scholar]
- 8. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. [DOI] [PubMed] [Google Scholar]
- 9. Almeida M, Pintacuda G, Masui O, et al. . PCGF3/5-PRC1 initiates polycomb recruitment in X chromosome inactivation. Science. 2017;356(6342):1081–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Y, Zhang X, Hu X, et al. . The effects of lncRNA MALAT1 on proliferation, invasion and migration in colorectal cancer through regulating SOX9. Mol Med. 2018;24(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malakar P, Shilo A, Mogilevsky A, et al. . Long noncoding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Res. 2017;77(5):1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng J, Bi C, Clark BS, et al. . The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20(11):1470–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao Y, Li J, Wang L. Large intervening non-coding RNA HOTAIR is an indicator of poor prognosis and a therapeutic target in human cancers. Int J Mol Sci. 2014;15(10):18985–18999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prasanth KV, Prasanth SG, Xuan Z, et al. . Regulating gene expression through RNA nuclear retention. Cell. 2005;123(2):249–263. [DOI] [PubMed] [Google Scholar]
- 15. Rashid F, Shah A, Shan G. Long non-coding RNAs in the cytoplasm. Genomics Proteomics Bioinformatics. 2016;14(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen H, Chen L. An integrated analysis of the competing endogenous RNA network and co-expression network revealed seven hub long non-coding RNAs in osteoarthritis. Bone Joint Res. 2020;9(3):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81(1):145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cesana M, Cacchiarelli D, Legnini I, et al. . A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339(2):159–166. [DOI] [PubMed] [Google Scholar]
- 22. Xie X, Liu M, Meng Q. Angelica polysaccharide promotes proliferation and osteoblast differentiation of mesenchymal stem cells by regulation of long non-coding RNA H19: an animal study. Bone Joint Res. 2019;8(7):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang J, Hao X, Yin M, Xu T, Guo F. Long non-coding RNA in osteogenesis: a new world to be explored. Bone Joint Res. 2019;8(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Q, Zhang X, Dai L, et al. . Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 2014;66(4):969–978. [DOI] [PubMed] [Google Scholar]
- 25. Su W, Xie W, Shang Q, Su B, et al. . The long noncoding RNA MEG3 is downregulated and inversely associated with VEGF levels in osteoarthritis. Biomed Res Int. 2015;2015(4):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang M, Lu Q, Budden T, Wang J. Nfat1 protects articular cartilage against osteoarthritic degradation by directly regulating transcription of specific anabolic and catabolic genes. Bone Joint Res. 2019;8(2):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Y, Xing D, Wang Y, et al. . A long non-coding RNA, HOTAIR, promotes cartilage degradation in osteoarthritis by inhibiting WIF-1 expression and activating Wnt pathway. BMC Mol Cell Biol. 2020;21(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu CD, Miao WH, Zhang YY, Zou MJ, Yan XF. Inhibition of miR-126 protects chondrocytes from IL-1β induced inflammation via upregulation of Bcl-2. Bone Joint Res. 2018;7(6):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mirzamohammadi F, Papaioannou G, Kobayashi T. Micrornas in cartilage development, homeostasis, and disease. Curr Osteoporos Rep. 2014;12(4):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu C, Tian B, Qu X, et al. . Micrornas play a role in chondrogenesis and osteoarthritis (review). Int J Mol Med. 2014;34(1):13–23. [DOI] [PubMed] [Google Scholar]
- 31. Peng S, Cao L, He S, et al. . An overview of long noncoding RNAs involved in bone regeneration from mesenchymal stem cells. Stem Cells Int. 2018;2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J-B, Jin Y, Wu P, et al. . Tumor suppressor PLZF regulated by lncRNA ANRIL suppresses proliferation and epithelial mesenchymal transformation of gastric cancer cells. Oncol Rep. 2019;41(2):1007–1018. [DOI] [PubMed] [Google Scholar]
- 33. Fu M, Huang G, Zhang Z, et al. . Expression profile of long noncoding RNAs in cartilage from knee osteoarthritis patients. Osteoarthritis Cartilage. 2015;23(3):423–432. [DOI] [PubMed] [Google Scholar]
- 34. Zhang H, Chen C, Cui Y, et al. . lnc-SAMD14-4 can regulate expression of the COL1A1 and COL1A2 in human chondrocytes. PeerJ. 2019;7(5):e7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen K, Fang H, Xu N. Lncrna LOXL1-AS1 is transcriptionally activated by JunD and contributes to osteoarthritis progression via targeting the miR-423-5p/KDM5C axis. Life Sci. 2020;258:118095. [DOI] [PubMed] [Google Scholar]
- 36. Lu Z, Luo M, Huang Y. lncRNA‐CIR regulates cell apoptosis of chondrocytes in osteoarthritis. J Cell Biochem. 2019;120(5):7229–7237. [DOI] [PubMed] [Google Scholar]
- 37. Li Y-F, Li S-H, Liu Y, Luo Y-T. Long noncoding RNA CIR promotes chondrocyte extracellular matrix degradation in osteoarthritis by acting as a sponge for miR-27b. Cell Physiol Biochem. 2017;43(2):602–610. [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Li S, Luo Y, Liu Y, Yu N. Lncrna PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-488-3p. DNA Cell Biol. 2017;36(7):571–580. [DOI] [PubMed] [Google Scholar]
- 39. Li Y, Li Z, Li C, Zeng Y, Liu Y. Long noncoding RNA TM1P3 is involved in osteoarthritis by mediating chondrocyte extracellular matrix degradation. J Cell Biochem. 2019;120(8):12702–12712. [DOI] [PubMed] [Google Scholar]
- 40. Hu J, Wang Z, Shan Y, et al. . Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/β-catenin axis. Cell Death Dis. 2018;9(7):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He B, Jiang D. HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol Int. 2020;44(2):524–535. [DOI] [PubMed] [Google Scholar]
- 42. Mao T, He C, Wu H, Yang B, Li X. Silencing lncRNA HOTAIR declines synovial inflammation and synoviocyte proliferation and promotes synoviocyte apoptosis in osteoarthritis rats by inhibiting Wnt/β-catenin signaling pathway. Cell Cycle. 2019;18(22):3189–3205. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Wang A, Hu N, Zhang Y, et al. . Meg3 promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-361-5p/FOXO1 axis. BMC Med Genomics. 2019;12(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen K, Zhu H, Zheng M-Q, Dong Q-R. LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage. 2019;15(4):194760351985575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mao G, Kang Y, Lin R, et al. . Long non-coding RNA HOTTIP promotes CCL3 expression and induces cartilage degradation by sponging miR-455-3p. Front Cell Dev Biol. 2019;7:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang T, Liu Y, Wang Y, et al. . Long non-coding RNA Xist promotes extracellular matrix degradation by functioning as a competing endogenous RNA of miR-1277-5p in osteoarthritis. Int J Mol Med. 2019;44(2):630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu Y, Liu J-P, Zhu Y, Lu N-H. The importance of Toll-like receptors in NF-κB signaling pathway activation by Helicobacter pylori infection and the regulators of this response. Helicobacter. 2016;21(5):428–440. [DOI] [PubMed] [Google Scholar]
- 48. Chen H, Yang S, Shao R. Long non-coding Xist raises methylation of TIMP-3 promoter to regulate collagen degradation in osteoarthritic chondrocytes after tibial plateau fracture. Arthritis Res Ther. 2019;21(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li L, Lv G, Wang B, Kuang L. The role of lncRNA XIST/miR-211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK signaling. Biochem Biophys Res Commun. 2018;503(4):2555–2562. [DOI] [PubMed] [Google Scholar]
- 50. Sun P, Wu Y, Li X, Jia Y. miR-142-5p protects against osteoarthritis through competing with lncRNA Xist. J Gene Med. 2020;22(4):e3158. [DOI] [PubMed] [Google Scholar]
- 51. Zhu Y-J, Jiang D-M. LncRNA PART1 modulates chondrocyte proliferation, apoptosis, and extracellular matrix degradation in osteoarthritis via regulating miR-373-3p/SOX4 axis. Eur Rev Med Pharmacol Sci. 2019;23(19):8175–8185. [DOI] [PubMed] [Google Scholar]
- 52. Lu C, Li Z, Hu S, Cai Y, Peng K. LncRNA PART-1 targets TGFBR2/Smad3 to regulate cell viability and apoptosis of chondrocytes via acting as miR-590-3p sponge in osteoarthritis. J Cell Mol Med. 2019;23(12):8196–8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wei W, He S, Wang Z, et al. . LINC01534 Promotes the Aberrant Metabolic Dysfunction and Inflammation in IL-1β-Simulated Osteoarthritic Chondrocytes by Targeting miR-140-5p. Cartilage. 2019. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ji Q, Qiao X, Liu Y, Wang D, Yan J. Silencing of long‑chain non‑coding RNA GAS5 in osteoarthritic chondrocytes is mediated by targeting the miR‑34a/Bcl‑2 axis. Mol Med Rep. 2020;21(3):1310–1319. [DOI] [PubMed] [Google Scholar]
- 55. Steck E, Boeuf S, Gabler J, et al. . Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med. 2012;90(10):1185–1195. [DOI] [PubMed] [Google Scholar]
- 56. Hu Y, Li S, Zou Y. Knockdown of lncRNA H19 relieves LPS-induced damage by modulating miR-130a in osteoarthritis. Yonsei Med J. 2019;60(4):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang B, Xu L, Wang S. Regulation of lncRNA-H19/miR-140-5p in cartilage matrix degradation and calcification in osteoarthritis. Ann Palliat Med. 2020;9(4):1896–1904. [DOI] [PubMed] [Google Scholar]
- 58. Lu X, Yu Y, Yin F, et al. . Knockdown of PVT1 inhibits IL-1β-induced injury in chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int Immunopharmacol. 2020;79:106052. [DOI] [PubMed] [Google Scholar]
- 59. Zhao Y, Zhao J, Guo X, She J, Liu Y. Long non-coding RNA PVT1, a molecular sponge for miR-149, contributes aberrant metabolic dysfunction and inflammation in IL-1β-simulated osteoarthritic chondrocytes. Biosci Rep. 2018;38(5):BSR20180576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang L, Zhang P, Sun X, Zhou L, Zhao J. Long non-coding RNA DANCR regulates proliferation and apoptosis of chondrocytes in osteoarthritis via miR-216a-5p-JAK2-STAT3 axis. Biosci Rep. 2018;38(6):BSR20181228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fan X, Yuan J, Xie J, et al. . Long non-protein coding RNA DANCR functions as a competing endogenous RNA to regulate osteoarthritis progression via miR-577/SphK2 axis. Biochem Biophys Res Commun. 2018;500(3):658–664. [DOI] [PubMed] [Google Scholar]
- 62. Liu F, Liu X, Yang Y, et al. . NEAT1/miR-193a-3p/SOX5 axis regulates cartilage matrix degradation in human osteoarthritis. Cell Biol Int. 2020;44(4):947–957. [DOI] [PubMed] [Google Scholar]
- 63. Wang Q, Wang W, Zhang F, Deng Y, Long Z. NEAT1/miR-181c Regulates Osteopontin (OPN)-Mediated Synoviocyte Proliferation in Osteoarthritis. J Cell Biochem. 2017;118(11):3775–3784. [DOI] [PubMed] [Google Scholar]
- 64. Yu C, Shi D, Li Z, Wan G, Shi X. Long noncoding RNA CHRF exacerbates IL-6-induced inflammatory damages by downregulating microRNA-146a in ATDC5 cells. J Cell Physiol. 2019;234(12):21851–21859. [DOI] [PubMed] [Google Scholar]
- 65. Ying H, Wang Y, Gao Z, Zhang Q. Long non-coding RNA activated by transforming growth factor beta alleviates lipopolysaccharide-induced inflammatory injury via regulating microRNA-223 in ATDC5 cells. Int Immunopharmacol. 2019;69:313–320. [DOI] [PubMed] [Google Scholar]
- 66. Xue H, Yu P, Wang W-Z, Niu Y-Y, Li X. The reduced lncRNA NKILA inhibited proliferation and promoted apoptosis of chondrocytes via miR-145/SP1/NF-κB signaling in human osteoarthritis. Eur Rev Med Pharmacol Sci. 2020;24(2):535–548. [DOI] [PubMed] [Google Scholar]
- 67. Qi K, Lin R, Xue C, et al. . Long non-coding RNA (lncRNA) CAIF is downregulated in osteoarthritis and inhibits LPS-induced interleukin 6 (IL-6) upregulation by downregulation of miR-1246. Med Sci Monit. 2019;25:8019–8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ai D, Yu F. LncRNA DNM3OS promotes proliferation and inhibits apoptosis through modulating IGF1 expression by sponging MiR-126 in CHON-001 cells. Diagn Pathol. 2019;14(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiao Y, Yan X, Yang Y, Ma X. Downregulation of long noncoding RNA HOTAIRM1 variant 1 contributes to osteoarthritis via regulating miR-125b/BMPR2 axis and activating JNK/MAPK/ERK pathway. Biomed Pharmacother. 2019;109:1569–1577. [DOI] [PubMed] [Google Scholar]
- 70. Li X, Ren W, Xiao Z-Y, et al. . GACAT3 promoted proliferation of osteoarthritis synoviocytes by IL-6/STAT3 signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(16):5114–5120. [DOI] [PubMed] [Google Scholar]
- 71. Li X, Huang T-L, Zhang G-D, Jiang J-T, Guo P-Y. Lncrna ANRIL impacts the progress of osteoarthritis via regulating proliferation and apoptosis of osteoarthritis synoviocytes. Eur Rev Med Pharmacol Sci. 2019;23(22):9729–9737. [DOI] [PubMed] [Google Scholar]
- 72. Yang D-W, Zhang X, Qian G-B, et al. . Downregulation of long noncoding RNA LOC101928134 inhibits the synovial hyperplasia and cartilage destruction of osteoarthritis rats through the activation of the Janus kinase/signal transducers and activators of transcription signaling pathway by upregulating IFNA1. J Cell Physiol. 2019;234(7):10523–10534. [DOI] [PubMed] [Google Scholar]
- 73. Chen Y, Ni H, Zhao Y, et al. . Potential role of lncRNAs in contributing to pathogenesis of intervertebral disc degeneration based on microarray data. Med Sci Monit. 2015;21:3449–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hayami T, Pickarski M, Zhuo Y, et al. . Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234–243. [DOI] [PubMed] [Google Scholar]
- 75. Rinn JL, Kertesz M, Wang JK, et al. . Functional demarcation of active and silent chromatin domains in human Hox loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li L, Dang Q, Xie H, et al. . Correction: Infiltrating mast cells enhance prostate cancer invasion via altering LncRNA-HOTAIR/PRC2-androgen receptor (AR)-MMP9 signals and increased stem/progenitor cell population. Oncotarget. 2016;7(50):83828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang G, Li Z, Tian N, et al. . miR-148b-3p inhibits malignant biological behaviors of human glioma cells induced by high HOTAIR expression. Oncol Lett. 2016;12(2):879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu X-H, Sun M, Nie F-Q, et al. . Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hu J, Wang Z, Pan Y, et al. . Mir-26A and miR-26b mediate osteoarthritis progression by targeting FUT4 via NF-κB signaling pathway. Int J Biochem Cell Biol. 2018;94:79–88. [DOI] [PubMed] [Google Scholar]
- 80. Yang H-F, Yu M, Jin H-D, et al. . Fentanyl Promotes Breast Cancer Cell Stemness and Epithelial-Mesenchymal Transition by Upregulating α1, 6-Fucosylation via Wnt/β-Catenin Signaling Pathway. Front Physiol. 2017;8:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang Y-M, Zhang Y-Y, Bulbul A, et al. . Baicalin promotes embryo adhesion and implantation by upregulating fucosyltransferase IV (FUT4) via Wnt/beta-catenin signaling pathway. FEBS Lett. 2015;589(11):1225–1233. [DOI] [PubMed] [Google Scholar]
- 82. Yan-Hua L, Xiang-Lei L, Hong L, Jian-Jun W. Long noncoding ribonucleic acids maternally expressed gene 3 inhibits lung cancer tumor progression through downregulation of Myc. Indian J Cancer. 2015;52 Suppl 3:E190–193. [DOI] [PubMed] [Google Scholar]
- 83. Dong Z, Zhang A, Liu S, et al. . Aberrant methylation-mediated silencing of lncRNA MEG3 functions as a ceRNA in esophageal cancer. Mol Cancer Res. 2017;15(7):800–810. [DOI] [PubMed] [Google Scholar]
- 84. Chen Z, He A, Wang D, Liu Y, Huang W. -Long noncoding RNA HOTTIP as a novel predictor of lymph node metastasis and survival in human cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(8):14126–14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim D, Song J, Han J, et al. . Two non-coding RNAs, MicroRNA-101 and HOTTIP contribute cartilage integrity by epigenetic and homeotic regulation of integrin-α1. Cell Signal. 2013;25(12):2878–2887. [DOI] [PubMed] [Google Scholar]
- 86. Li C, Wan L, Liu Z, et al. . Long non-coding RNA Xist promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018;418:185–195. [DOI] [PubMed] [Google Scholar]
- 87. Sun Z, Zhang B, Cui T. Long non-coding RNA Xist exerts oncogenic functions in pancreatic cancer via miR-34a-5p. Oncol Rep. 2018;39(4):1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sun J-C, Zheng B, Sun R-X, et al. . MiR-499a-5p suppresses apoptosis of human nucleus pulposus cells and degradation of their extracellular matrix by targeting SOX4. Biomed Pharmacother. 2019;113:108652. [DOI] [PubMed] [Google Scholar]
- 89. Takahata Y, Nakamura E, Hata K, et al. . Sox4 is involved in osteoarthritic cartilage deterioration through induction of ADAMTS4 and ADAMTS5. Faseb J. 2019;33(1):619–630. [DOI] [PubMed] [Google Scholar]
- 90. Ouyang L, Shi Z, Zhao S, et al. . Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Szklarczyk R, Nooteboom M, Osiewacz HD. Control of mitochondrial integrity in ageing and disease. Philos Trans R Soc Lond B Biol Sci. 2014;369(1646):20130439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vanden Berghe T, Kaiser WJ, Bertrand MJ, Vandenabeele P. Molecular crosstalk between apoptosis, necroptosis, and survival signaling. Mol Cell Oncol. 2015;2(4):e975093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qi K, Lin R, Xue C. Long non-coding RNA (lncRNA) CAIF is downregulated in osteoarthritis and inhibits LPS-induced interleukin 6 (IL-6) upregulation by downregulation of miR-1246. Med Sci Monit. 2019;25:8019–8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Musumeci G, Castrogiovanni P, Trovato FM, et al. . Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16(9):20560–20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54(6):787–793. [DOI] [PubMed] [Google Scholar]
- 96. Gasic V, Stankovic B, Zukic B, et al. . Expression pattern of long non-coding RNA growth arrest-specific 5 in the remission induction therapy in childhood acute lymphoblastic leukemia. J Med Biochem. 2019;38(3):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pachnis V, Belayew A, Tilghman SM. Locus unlinked to alpha-fetoprotein under the control of the murine Raf and Rif genes. Proc Natl Acad Sci U S A. 1984;81(17):5523–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Matouk IJ, Halle D, Gilon M, Hochberg A. The non-coding RNAs of the H19-IGF2 imprinted loci: a focus on biological roles and therapeutic potential in lung cancer. J Transl Med. 2015;13(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang X, Liu X, Ni X, Feng P, Wang YU. Long non-coding RNA H19 modulates proliferation and apoptosis in osteoarthritis via regulating miR-106a-5p. J Biosci. 2019;44(6):128. [PubMed] [Google Scholar]
- 100. Ghafouri-Fard S, Omrani MD, Taheri M. Long noncoding RNA PVT1: a highly dysregulated gene in malignancy. J Cell Physiol. 2020;235(2):818–835. [DOI] [PubMed] [Google Scholar]
- 101. Chen J, Yu Y, Li H, et al. . Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer. 2019;18(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mao Z, Xu B, He L, Zhang G. PVT1 Promotes Angiogenesis by Regulating miR-29c/Vascular Endothelial Growth Factor (VEGF) Signaling Pathway in Non-Small-Cell Lung Cancer (NSCLC). Med Sci Monit. 2019;25:5418–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee K-A, Cho K-C, Kim B, et al. . Inflammation-Modulated Metabolic Reprogramming Is Required for DUOX-Dependent Gut Immunity in Drosophila. Cell Host Microbe. 2018;23(3): 338–352.e5. [DOI] [PubMed] [Google Scholar]
- 104. Yuan SX, Wang J, Yang F, et al. . Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology. 2016;63(2):499–511. [DOI] [PubMed] [Google Scholar]
- 105. Shi S-J, Wang L-J, Yu B, et al. . LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget. 2015;6(13):11652–11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yue B, Qiu S, Zhao S, et al. . LncRNA-ATB mediated E-cadherin repression promotes the progression of colon cancer and predicts poor prognosis. J Gastroenterol Hepatol. 2016;31(3):595–603. [DOI] [PubMed] [Google Scholar]
- 107. Qu S, Yang X, Song W, et al. . Downregulation of lncRNA-ATB correlates with clinical progression and unfavorable prognosis in pancreatic cancer. Tumour Biol. 2016;37(3):3933–3938. [DOI] [PubMed] [Google Scholar]
- 108. Xiong J, Liu Y, Jiang L, Zeng Y, Tang W. High expression of long non-coding RNA lncRNA-ATB is correlated with metastases and promotes cell migration and invasion in renal cell carcinoma. Jpn J Clin Oncol. 2016;46(4):378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dang X, Lian L, Wu D. The diagnostic value and pathogenetic role of lncRNA-ATB in patients with osteoarthritis. Cell Mol Biol Lett. 2018;23(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang C-L, Peng J-P, Chen X-D. LncRNA-CIR promotes articular cartilage degeneration in osteoarthritis by regulating autophagy. Biochem Biophys Res Commun. 2018;505(3):692–698. [DOI] [PubMed] [Google Scholar]
- 111. Song Z-Y, Wang F, Cui S-X, Qu X-J. Knockdown of CXCR4 inhibits CXCL12-induced angiogenesis in HUVECs through downregulation of the MAPK/ERK and PI3K/Akt and the Wnt/β-catenin pathways. Cancer Invest. 2018;36(1):10–18. [DOI] [PubMed] [Google Scholar]
- 112. Sun H-Y, Hu K-Z, Yin Z-S. Inhibition of the p38-MAPK signaling pathway suppresses the apoptosis and expression of proinflammatory cytokines in human osteoarthritis chondrocytes. Cytokine. 2017;90:135–143. [DOI] [PubMed] [Google Scholar]
- 113. Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93-A(3):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kotake Y, Nakagawa T, Kitagawa K, et al. . Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30(16):1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pasmant E, Laurendeau I, Héron D, et al. . Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007;67(8):3963–3969. [DOI] [PubMed] [Google Scholar]
- 116. Tano K, Akimitsu N. Long non-coding RNAs in cancer progression. Front Genet. 2012;3:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ding N, Wu H, Tao T, Peng E. Neat1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. Onco Targets Ther. 2017;10:4905–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. You D, Yang C, Huang J, et al. . Long non-coding RNA MEG3 inhibits chondrogenic differentiation of synovium-derived mesenchymal stem cells by epigenetically inhibiting TRIB2 via methyltransferase EZH2. Cell Signal. 2019;63:109379. [DOI] [PubMed] [Google Scholar]
- 119. Zhen G, Wen C, Jia X, et al. . Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390–398. [DOI] [PubMed] [Google Scholar]
- 121. Blanke M, Carl HD, Klinger P, et al. . Transplanted chondrocytes inhibit endochondral ossification within cartilage repair tissue. Calcif Tissue Int. 2009;85(5):421–433. [DOI] [PubMed] [Google Scholar]
- 122. Ashraf S, Walsh DA. Angiogenesis in osteoarthritis. Curr Opin Rheumatol. 2008;20(5):573–580. [DOI] [PubMed] [Google Scholar]
- 123. Miyoshi N, Wagatsuma H, Wakana S, et al. . Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells. 2000;5(3):211–220. [DOI] [PubMed] [Google Scholar]
- 124. Zhuang W, Ge X, Yang S, et al. . Upregulation of lncRNA MEG3 promotes osteogenic differentiation of mesenchymal stem cells from multiple myeloma patients by targeting BMP4 transcription. Stem Cells. 2015;33(6):1985–1997. [DOI] [PubMed] [Google Scholar]
- 125. Zhang C-Y, Yu M-S, Li X, et al. . Overexpression of long non-coding RNA MEG3 suppresses breast cancer cell proliferation, invasion, and angiogenesis through Akt pathway. Tumour Biol. 2017;39(6):101042831770131. [DOI] [PubMed] [Google Scholar]
- 126. Zhou Y, Zhong Y, Wang Y, et al. . Activation of p53 by MEG3 non-coding RNA. J Biol Chem. 2007;282(34):24731–24742. [DOI] [PubMed] [Google Scholar]
- 127. Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61(18):6952–6957. [PubMed] [Google Scholar]
- 128. Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4(5):397–407. [DOI] [PubMed] [Google Scholar]
- 129. Limaye V, Li X, Hahn C, et al. . Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105(8):3169–3177. [DOI] [PubMed] [Google Scholar]
- 130. Minashima T, Campbell KA, Hadley SR, Zhang Y, Kirsch T. The role of ANK interactions with Mybbp1a and SphK1 in catabolic events of articular chondrocytes. Osteoarthritis Cartilage. 2014;22(6):852–861. [DOI] [PubMed] [Google Scholar]
- 131. Li L, Chang HY. Physiological roles of long noncoding RNAs: insight from knockout mice. Trends Cell Biol. 2014;24(10):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mancini-Dinardo D, Steele SJS, Levorse JM, Ingram RS, Tilghman SM. Elongation of the KCNQ1OT1 transcript is required for genomic imprinting of neighboring genes. Genes Dev. 2006;20(10):1268–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ichim TE, Li M, Qian H, et al. . RNA interference: a potent tool for gene-specific therapeutics. Am J Transplant. 2004;4(8):1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fekrazad R, Naghdi N, Nokhbatolfoghahaei H, Bagheri H. The combination of laser therapy and metal nanoparticles in cancer treatment originated from epithelial tissues: a literature review. J Lasers Med Sci. 2016;7(2):62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Li X, Zhang Q, Yang Z. Silence of MEG3 intensifies lipopolysaccharide-stimulated damage of human lung cells through modulating miR-4262. Artif Cells Nanomed Biotechnol. 2019;47(1):2369–2378. [DOI] [PubMed] [Google Scholar]
- 136. Chen J-X, Xu X, Zhang S. Silence of long noncoding RNA NEAT1 exerts suppressive effects on immunity during sepsis by promoting microRNA-125-dependent MCEMP1 downregulation. IUBMB Life. 2019;71(7):956–968. [DOI] [PubMed] [Google Scholar]