Abstract
Adenovirus was originally used as a vector for gene therapy. In recent years, with the development of the next-generation vectors with increased safety and high immunogenicity to transgene products, its utility as a vaccine vector has continued to increase. Adenovirus-based vaccines are currently being tested not only to prevent various infectious diseases but also to be applied as cancer vaccines. In this review, I discuss the innate and adaptive aspects of the immunological characteristics of adenovirus vectors and further examine the current status of advanced adenovirus-based vaccine development. Various methods that can overcome the limitations of currently used adenoviruses as vaccine vehicles are also discussed. Through this study, I hope that vaccine development using adenovirus vectors will be expedited and more successful.
Keywords: Adenovirus, Genetic vector, Adenovector, Vaccine
INTRODUCTION
Traditionally, vaccines have been made in inactivated or attenuated forms, but advances in molecular biology have revealed the molecular mechanisms of action of some specific viruses, allowing them to be used as vaccine vectors. Among them, adenovirus was initially mainly used as a delivery vector for specific genes, but as its highly immunogenic properties were elucidated, it was recognized as a vaccine vector capable of inducing various immune responses. In this review, I discuss how recombinant adenoviruses stand out as vaccine vectors and highlight the status of recombinant adenovirus-based vaccines that have been developed or are currently in development.
Adenoviruses are nonenveloped virus with a double-stranded DNA genome. They infect a broad range of vertebrate hosts, and in humans, more than 50 serotypes have been found to cause illnesses, from mild respiratory infections to life-threatening multi-organ diseases. Most infections with adenoviruses in humans occur in the upper respiratory tract, causing symptoms of the common cold, conjunctivitis, and tonsillitis. Human adenoviruses are classified into serotypes depending on whether they are cross-neutralized by Abs. To date, approximately 50 serotypes have been discovered and are divided into 6 subgroups from A to F. Among them, human adenovirus serotypes 1, 2 and 5 (HAd1, HAd2, and HAd5, respectively) mainly cause mild respiratory diseases, and human adenovirus serotypes 4 and 7 (HAd4 and HAd7, respectively) may cause more severe pneumonia. In particular, when adenoviruses are used as vaccine vectors, their prevalence in humans is important because pre-existing immunity can reduce the effectiveness of the vaccine. HAd2 and HAd5 infections are the most common, and in some cases, it has been reported that ≤80% of the population has neutralizing Abs against HAd5 (1). Therefore, there are increasing attempts to develop adenovirus vectors derived from other serotypes of adenoviruses with a low Ab prevalence or from adenoviruses of other species such as chimpanzees.
Adenovirus has been studied for a long time since its discovery and has been mainly used as a vector for gene transfer due to its highly efficient infectious properties (2,3,4,5). Considering the advantages of adenovirus as a vector, first, the structure of the genome is well understood, and it can be easily manipulated. Thus, it is relatively easy to insert foreign genes that are unable to replicate. Through this, the desired gene transfer or Ag expression can be easily achieved. Second, adenovirus has a very broad spectrum of host cell tropism, so it can infect host cells regardless of cell division, and this is an excellent characteristic of a delivery vector. Furthermore, it has been shown that the recombinant genome is stably maintained through continuous passages, and it can be quickly and massively produced. In addition to these advantages, when a recombinant adenovirus-based vaccine is used via a mucosal route, high transduction efficiency can be expected because mucosal infection is an inherent characteristic of adenoviruses. Thus, adenoviral vectors have emerged as very promising platforms for vaccines due to their documented immunogenicity and ability to induce host protection in multiple species, including humans.
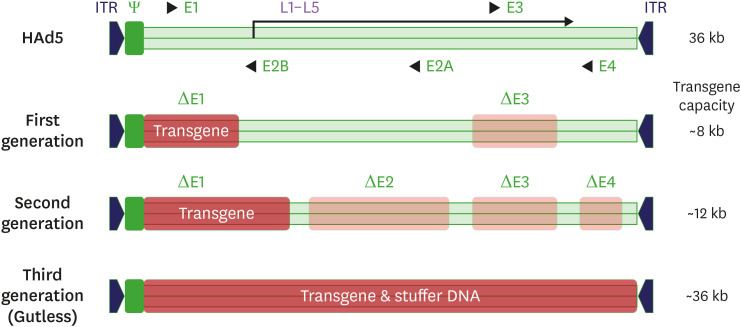
To use adenovirus vectors safely and effectively in humans, the adenovirus genome has been improved through genetic engineering. First-generation adenoviral vectors are developed by E1/E3 deletion for transgene insertion, replication-defective properties, and improvement of immunogenicity (6) (Fig. 1). However, in this generation, replication-competent adenovirus (RCA) is likely to occur due to the homology of the vector genome and the E1 part inserted into the packaging cell line HEK293. To resolve this shortcoming, cell lines with minimal homology, such as PERC.6, can be used in production (7). In second-generation adenoviral vectors, the transgene capacity is further increased by additionally deleting the E2/E4 site, and the likelihood of RCA formation might be decreased. However, the overall production yield is lower than that of first-generation adenoviral vectors due to decreased replication ability in producer cell lines (8). Third-generation adenoviral vectors are also called helper-dependent or gutless adenovirus vectors because they are created by deleting almost all genomic sequences except for sequences that are essential for packaging, such as inverted terminal repeat sequences (9,10). In addition, it boasts high capacity as it can insert multiple transgene expression cassettes, but it is more difficult to manufacture, its immunogenicity is lower than that of previous generations, and there is still the possibility of helper virus contamination.
Figure 1. Schematic representation of the adenovirus genome and adenovirus-based vectors.
ITR, inverted terminal repeat.
INTERACTIONS BETWEEN ADENOVECTORS AND THE IMMUNE SYSTEM
Innate immunity
How the virus enters the cell and which cells it binds to are essential for the interaction between adenoviral vectors and innate immunity. Adenovirus mainly binds to different receptors according to its serotypes. Except for the B serotype, which binds to CD46, the others bind to the coxsackie-adenovirus receptor (CAR) and initiate the entry process. First, the binding of the HAd5 fiber and CAR induces strong proinflammatory activation in epithelial respiratory cells, including the induction of ERK1/2 and p38MAPK signaling and the expression of proinflammatory genes, such as and IL-6, IL-8, RANTES, and IP-10 (11).
Interaction between the adenoviral penton base protein and cellular integrin occurs during internalization after initial attachment. The interaction of the RGD motif of the penton protein and integrin triggers two signaling pathways, namely, the PI3K/p38 MAPK and Raf-1/ERK1/2 pathways, which eventually trigger the expression of proinflammatory cytokine and chemokine genes through NF-κB (12). Thus, various membrane-associated kinase signals occurring in the process of adenovirus binding and entry play important roles in determining early innate immune responses. In addition to the binding and entry processes, adenoviruses stimulate innate immunity via pathogen-associated molecular patterns, such as viral capsid proteins, cytosolic DNA, and subsequently, IFNs and proinflammatory cytokines such as IL-6, IL-12, and TNF-α, which promote innate immunity (13,14).
Mucosal-associated-invariant T (MAIT) cells are innate immune cells that are primarily activated by bacterial metabolites, such as vitamin B2 in the mucosa, and are known as important components of antimicrobial immunity (15). According to a recent report, MAIT cells were activated by a chimpanzee-derived ChAdOx-1 adenovirus vaccine in vitro and in vivo, and this MAIT cell activation induced by the vaccine was required for Ag-specific CD8 T-cell response (16). This report implies that innate immune environments stimulated by adenovirus vectors may also contain innate T cells such as MAIT cells.
The innate immune environment activated by the signaling and inflammation caused by adenovirus may induce trained immunity, which is considered an innate memory. Mucosal delivery of recombinant adenovirus vectors has been shown to induce long-lasting trained innate immunity in the lungs, suggesting that adenoviral vector-based vaccines could be applied as novel vaccine strategies for respiratory pathogens (17). Interestingly, this report showed that T-cell help was necessary to induce a long-lasting memory alveolar macrophage response after intranasal delivery of an adenoviral vector-based vaccine. Unlike traditional vaccines, this characteristic of adenoviral vector-based vaccines are not Ag-specific, so they can be used for more general purposes. For example, if it takes a long time to develop a vaccine in a pandemic situation, adenoviral vector-based vaccines can be used to provide nonspecific defenses by inducing trained immunity. Recently, a company named Altimmune started a phase I/II clinical trial for the treatment of early coronavirus disease 2019 (COVID-19) with a HAd5-based investigational agent which showed immunomodulatory effects on the innate immune responses and conferred protection to lethal challenge with respiratory virus in preclinical settings (https://www.globenewswire.com/news-release/2020/06/01/2041361/0/en/Altimmune-Launches-Clinical-Trial-of-T-COVIDTM-an-Investigational-Intranasal-Immune-Modulator-for-the-Treatment-of-Patients-with-Early-COVID-19.html).
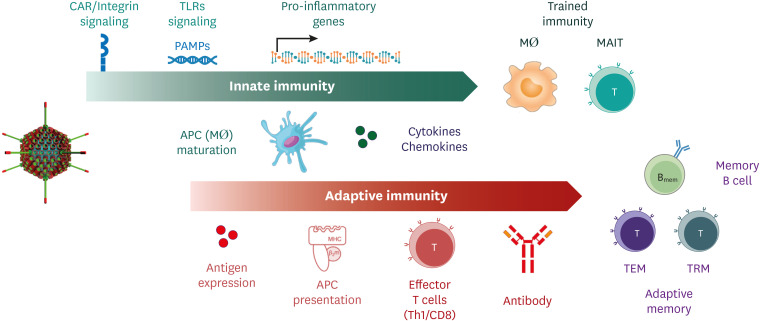
As mentioned above, adenovirus actively interacts with the innate immune system through various pathways and mechanisms to change the immune environment. Thus, the innate immune environment induced by the adenovirus vector itself is a condition that strengthens the Ag-specific adaptive immunity that develops later. In addition, nonspecific defenses by the stimulated innate immune system may act in a favorable direction for the host against infection (Fig. 2).
Figure 2. The interaction between adenovirus and the innate and adaptive immune environment.
Adaptive immunity
As mentioned above, adenovirus stimulates innate immune cells by activating several innate immune signaling pathways and induces secretion of various proinflammatory cytokines and chemokines. This altered innate immune environment effectively induces robust adaptive humoral and cellular immune responses (Fig. 2). In mice, intramuscular immunization with adenoviral vectors leads to efficient and sustained Ag expression in muscle tissues (18). Under these conditions, direct and cross-presentation of Ags to CD8 T cells occurs with high efficiency. To remove intracellular pathogens such as viruses, CD8 CTL responses are essential. Since transgene Ags derived from adenovectors are effectively presented to T cells by MHC class I molecules, efficient and robust CTL responses can be induced by adenovector vaccines. In particular, because of the diverse tropism of adenovirus, transgene Ags can be expressed in various cell types, which is advantageous for Ag presentation through MHC molecules. Although CTLs cannot provide sterilizing protection, their potential to limit the viral replication and reduce disease severity is another advantage.
Adenovector vaccines have been also shown to induce strong and durable humoral responses in animal models and humans (13). For example, the single injection of replication-defective adenovirus serotype 5 vectors elicit durable Ab responses in nonhuman primates (19) and in humans (20). These properties allow efficient induction of robust and durable cell-mediated and humoral immunity and make adenoviral vectors promising vaccine vectors.
Effects on tissue-resident memory T (TRM) cells
The memory T-cell population elicited by adenoviral vectors in mice seems to be composed primarily of effector memory (TEM; CD127+CD62L−) T cells (21). Similarly, the Ag-specific CD8 T cells induced by chimpanzee-derived rAd in macaques mainly display TEM phenotypes (22). Many studies have demonstrated that TEM cells provide better protection against certain pathogens, especially those that infect peripheral tissues, than central memory T cells (23). However, in local pathogen infections, it is now believed that TRM cells play an essential role in protective immunity. In particular, an important role of TRM cells in antiviral immunity has recently emerged. Considerable evidence suggests that the induction of lung tissue-resident TRM cells depends on the route of vaccination. For example, respiratory mucosal vaccination induces strong lung TRM cell responses, whereas parenteral vaccination fails to do so (24,25,26). In fact, in vaccines that induce T-cell responses to the internal proteins of influenza viruses, such as nucleoproteins (NPs), it has been reported that the vaccination route is important for lung TRM cell induction (27,28). Use of the parenteral route of vaccination is unable to effectively induce mucosal IgA Abs or TRM cells in the lungs. By comparison, the respiratory mucosal route of vaccination is adept at inducing Abs and TRM cells in the respiratory mucosa. With NP-expressing recombinant adenovirus vector-based vaccines, nasal vaccination has been found to induce lung TRM cell responses more effectively than nasal infection with influenza virus vector-based vaccines (29). Therefore, when a recombinant adenovirus-based vaccine is administered via the mucosal route, the protective efficacy of the vaccine may be increased due to activation of mucosal innate immunity and tissue-resident T-cell memory responses, which is important for antiviral defense.
Memory inflation
According to a recent report, typical memory inflation was observed for specific epitopes in the CD8 T-cell immune responses to Ags expressed with nonreplicating adenovirus vectors (30). Memory inflation is described as a mirror image of an exhaustion phenomenon that mainly occurs in T cells in response to chronic infections (31). Unlike typical memory T-cell responses, with memory inflation, memory T-cell contraction does not occur over time; in contrast, the functional number of T cells increases. This kind of CD8 T-cell memory inflation, which increases only the memory response to a specific epitope among several epitopes in a single protein, has mainly been observed in chronic viral infections, such as murine cytomegalovirus infections (32). CD8 T-cell memory inflation was also recently observed in immune responses induced by adenovirus vectors, and it occurred independent of the replication ability of the virus (5,33). This is useful for improving CD8 T-cell memory when using nonreplicating recombinant adenoviruses as vaccine vectors. However, for Ag-specific T-cell memory inflation associated with nonreplicating adenoviruses to occur, it is necessary to determine whether any specific immune environment such as persistent Ag expression, epitope-processing proteasomes, or a specific subset of Ag-presenting-cells and cytokines are required.
CURRENT STATUS OF ADENOVIRUS-BASED VACCINE DEVELOPMENT
Ebola/COVID-19 vaccine
The development of adenovirus-based vaccines has a long history. The first adenovirus-based vaccine approved for use in humans for preventive purposes was the Ebola vaccine named Ad26.ZEBOV. For fatal diseases such as Ebola, research using adenovector technology has been intensively conducted because of the urgent need for a vaccine. Of the 2 components of the new Ebola vaccine approved in Europe in 2020, Ad26.ZEBOV is used in the first dose.
Regarding a vaccine for COVID-19, which is currently being evaluated with large-scale clinical trials, a significant proportion of the lead candidates uses recombinant adenovector platform accounts (34). For example, vaccines using platforms such as HAd5 (nonreplicating), HAd26 (nonreplicating), and ChAdOx-1 (nonreplicating) are being developed through large-scale clinical trials. Most of these vaccine candidates are designed to express the S protein or RBD of SARS-CoV-2. Adenovirus-based vaccines can induce both strong cellular and humoral immunity, and the natural tropism of adenoviruses for the respiratory mucosa is an advantage of adenoviral vector-based vaccines for COVID-19-associated respiratory infections. However, if there is any pre-existing immunity in humans to the backbone of the vaccine vector itself, the efficacy of the vaccine may be reduced. Indeed, the HAd5-based vaccine efficacy was reduced by pre-existing immunity to HAd5, while the vaccine induced both Ab and T-cell responses (35,36). To overcome this shortcoming, adenoviruses from other species, such as ChAdOx-1 derived from chimpanzees, have been employed. Recently reported phase 2/3 trial studies show its safety and immunogenicity inducing both Ab and T-cell responses following a single parenteral injection or homologous booster injection (37,38). Another human adenovirus-based COVID-19 vaccine, known as Ad26.COV2.S, also offered protection in a non-human primate model with a single intramuscular injection (39).
Influenza vaccine
Studies of influenza vaccines based on adenovectors are actively being conducted because there is a great need for new vaccine development due to the various shortcomings of the currently used trivalent inactivated vaccine. In particular, as adenovectors can stimulate humoral immunity and induce broadly cross-reactive T-cell immunity against conserved Ags, many studies have employed the adenovector platform for universal influenza vaccines (40). As a typical example of universal influenza vaccines that are being developed, Abs can be induced by targeting the HA stalk region or the ectodomain of the M2 ion channel with relatively high conservancy, or broadly cross-reactive T cells can be induced to the NP or M1 as the target. Several human clinical trials have been conducted with HAd5-based influenza vaccines expressing HA, and their safety and immunogenicity were well proven in most cases (41). A dose-escalating phase II trial is currently being conducted to develop an intranasal spray-type influenza vaccine with HAd5-based vaccines expressing H1HA (https://clinicaltrials.gov/ct2/show/results/NCT03232567; Single-Ascending-Dose Study of the Safety and Immunogenicity of NasoVAX—Study Results. Available online). An optimal influenza vaccine should induce both broad cross-reactive T-cell immunity and neutralizing Abs, and the protective immunity should be sustained for a long period of time. In this regard, influenza vaccines using adenovector platform are attractive choices.
HIV-1 vaccine
The high immunogenicity of adenovirus vectors has resulted in excellent efficacy of HIV-1 vaccines. Indeed, experiments conducted on nonhuman primates have shown very good efficacy in preventing infection (42). However, the subsequent large-scale STEP trial with a HAd5-based vaccine in humans did not show the expected efficacy in the vaccination group. Rather, HIV-1 infections tended to increase in HAd5-seropositive volunteers (43,44). Further research is needed to elucidate the mechanism in detail, but it is likely that vaccination-induced activation of CD4 T cells contributed to increased HIV-1 infection. Research on adenovector-based HIV-1 vaccines with a new strategy considering this hypothesis is currently being actively conducted (45). For example, to avoid pre-existing immunity and take advantage of the high immunogenicity of adenovectors, one clinical trial adopted a strategy using adenovectors of different serotypes for priming and boosting (46).
CHALLENGES OF AND SOLUTIONS FOR ADENOVIRAL VECTORS
Adenoviruses are highly immunogenic, and the neutralizing Ab responses against the hexon, penton base, and fiber Ag on the viral surface are strongly induced by infection (47,48). In addition, Th1 CD4 T-cell responses, which are cross-reactive to several serotypes, are also induced (49). Since such vector immunity can reduce the efficacy of HAd5-based vaccines, several strategies have been applied: use of an HAd5 vector with a chimeric hexon protein created through genetic engineering (50); coating the vector itself with a polymer (51); and immunization through oral or nasal routes to avoid systemic vector immunity (52,53,54). HAd26 and HAd35 showing lower seroprevalence in humans have been developed (55), and simian adenoviruses, such as AdC68, can also be used to induce immune responses similar to those induced by human adenovectors (1,56). Furthermore, if the prime-boost immunization regimen is adopted using adenovectors derived from different serotypes or different species, better efficacy than that achieved with single vaccination or homologous prime-boost immunizations can be expected.
CONCLUSION
New infectious diseases that have emerged in recent years and concerns about bioterrorism increase needs for vaccine development. In this situation, the versatility and potency of adenovirus vectors make them useful as a platform for vaccines against emerging pathogens or for vaccines that need to be upgraded. For this, more in-depth molecular biological and immunological studies on adenoviruses are needed to resolve their current shortcomings. In conclusion, adenovirus vectors for vaccine development will be improved through continuous research and will become indispensable tools for vaccinologists.
Acknowledgments
This study was supported by a grant of the National Research Foundation (grant No. NRF-2018R1A2B6002388) and Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant No. HV20C0049).
Footnotes
Conflict of Interest: The author declares no potential conflicts of interest.
References
- 1.Farina SF, Gao GP, Xiang ZQ, Rux JJ, Burnett RM, Alvira MR, Marsh J, Ertl HC, Wilson JM. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afkhami S, Yao Y, Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev. 2016;3:16030. doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majhen D, Calderon H, Chandra N, Fajardo CA, Rajan A, Alemany R, Custers J. Adenovirus-based vaccines for fighting infectious diseases and cancer: progress in the field. Hum Gene Ther. 2014;25:301–317. doi: 10.1089/hum.2013.235. [DOI] [PubMed] [Google Scholar]
- 5.Bolinger B, Sims S, Swadling L, O'Hara G, de Lara C, Baban D, Saghal N, Lee LN, Marchi E, Davis M, et al. Adenoviral vector vaccination induces a conserved program of CD8(+) T cell memory differentiation in mouse and man. Cell Reports. 2015;13:1578–1588. doi: 10.1016/j.celrep.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovesdi I, Hedley SJ. Adenoviral producer cells. Viruses. 2010;2:1681–1703. doi: 10.3390/v2081681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Q, Finer MH. Second-generation adenovirus vectors. Nat Med. 1996;2:714–716. doi: 10.1038/nm0696-714. [DOI] [PubMed] [Google Scholar]
- 9.Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci U S A. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamanini A, Nicolis E, Bonizzato A, Bezzerri V, Melotti P, Assael BM, Cabrini G. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol. 2006;80:11241–11254. doi: 10.1128/JVI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 13.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 15.Napier RJ, Adams EJ, Gold MC, Lewinsohn DM. The role of mucosal associated invariant T cells in antimicrobial immunity. Front Immunol. 2015;6:344. doi: 10.3389/fimmu.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Provine NM, Amini A, Garner LC, Dold C, Hutchings C, FitzPatrick MEB, Reyes LS, Chinnakannan S, Oguti B, Raymond M, et al. Activation of mait cells plays a critical role in viral vector vaccine immunogenicity. bioRxiv. 2019 [Google Scholar]
- 17.Yao Y, Jeyanathan M, Haddadi S, Barra NG, Vaseghi-Shanjani M, Damjanovic D, Lai R, Afkhami S, Chen Y, Dvorkin-Gheva A, et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell. 2018;175:1634–1650.e17. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santra S, Seaman MS, Xu L, Barouch DH, Lord CI, Lifton MA, Gorgone DA, Beaudry KR, Svehla K, Welcher B, et al. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005;79:6516–6522. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JX, Hou LH, Meng FY, Wu SP, Hu YM, Liang Q, Chu K, Zhang Z, Xu JJ, Tang R, et al. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob Health. 2017;5:e324–e334. doi: 10.1016/S2214-109X(16)30367-9. [DOI] [PubMed] [Google Scholar]
- 21.Yang TC, Millar J, Groves T, Grinshtein N, Parsons R, Takenaka S, Wan Y, Bramson JL. The CD8+ T cell population elicited by recombinant adenovirus displays a novel partially exhausted phenotype associated with prolonged antigen presentation that nonetheless provides long-term immunity. J Immunol. 2006;176:200–210. doi: 10.4049/jimmunol.176.1.200. [DOI] [PubMed] [Google Scholar]
- 22.Lasaro MO, Haut LH, Zhou X, Xiang Z, Zhou D, Li Y, Giles-Davis W, Li H, Engram JC, Dimenna LJ, et al. Vaccine-induced T cells provide partial protection against high-dose rectal SIVmac239 challenge of rhesus macaques. Mol Ther. 2011;19:417–426. doi: 10.1038/mt.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 24.Haddadi S, Vaseghi-Shanjani M, Yao Y, Afkhami S, D’Agostino MR, Zganiacz A, Jeyanathan M, Xing Z. Mucosal-pull induction of lung-resident memory CD8 T cells in parenteral tb vaccine-primed hosts requires cognate antigens and CD4 T cells. Front Immunol. 2019;10:2075. doi: 10.3389/fimmu.2019.02075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeyanathan M, Yao Y, Afkhami S, Smaill F, Xing Z. New tuberculosis vaccine strategies: Taking aim at un-natural immunity. Trends Immunol. 2018;39:419–433. doi: 10.1016/j.it.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uddback IE, Pedersen LM, Pedersen SR, Steffensen MA, Holst PJ, Thomsen AR, Christensen JP. Combined local and systemic immunization is essential for durable T-cell mediated heterosubtypic immunity against influenza A virus. Sci Rep. 2016;6:20137. doi: 10.1038/srep20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MH, Kang JO, Kim JY, Jung HE, Lee HK, Chang J. Single mucosal vaccination targeting nucleoprotein provides broad protection against two lineages of influenza B virus. Antiviral Res. 2019;163:19–28. doi: 10.1016/j.antiviral.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Uddbäck I, Cartwright EK, Schøller AS, Wein AN, Hayward SL, Lobby J, Takamura S, Thomsen AR, Kohlmeier JE, Christensen JP. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol. 2021;14:92–99. doi: 10.1038/s41385-020-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolinger B, Sims S, O'Hara G, de Lara C, Tchilian E, Firner S, Engeler D, Ludewig B, Klenerman P. A new model for CD8+ T cell memory inflation based upon a recombinant adenoviral vector. J Immunol. 2013;190:4162–4174. doi: 10.4049/jimmunol.1202665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klenerman P. The (gradual) rise of memory inflation. Immunol Rev. 2018;283:99–112. doi: 10.1111/imr.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee LN, Bolinger B, Banki Z, de Lara C, Highton AJ, Colston JM, Hutchings C, Klenerman P. Adenoviral vaccine induction of CD8+ T cell memory inflation: impact of co-infection and infection order. PLoS Pathog. 2017;13:e1006782. doi: 10.1371/journal.ppat.1006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, Li JX, Yang BF, Wang L, Wang WJ, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, Liu J, Peter L, McMahan K, Tostanoski LH, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coughlan L, Mullarkey C, Gilbert S. Adenoviral vectors as novel vaccines for influenza. J Pharm Pharmacol. 2015;67:382–399. doi: 10.1111/jphp.12350. [DOI] [PubMed] [Google Scholar]
- 41.Sayedahmed EE, Elkashif A, Alhashimi M, Sambhara S, Mittal SK. Adenoviral vector-based vaccine platforms for developing the next generation of influenza vaccines. Vaccines (Basel) 2020;8:574. doi: 10.3390/vaccines8040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 43.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5:386–390. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baden LR, Karita E, Mutua G, Bekker LG, Gray G, Page-Shipp L, Walsh SR, Nyombayire J, Anzala O, Roux S, et al. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: a randomized trial. Ann Intern Med. 2016;164:313–322. doi: 10.7326/M15-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62:2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong SS, Habib NA, Franqueville L, Jensen S, Boulanger PA. Identification of adenovirus (ad) penton base neutralizing epitopes by use of sera from patients who had received conditionally replicative ad (addl1520) for treatment of liver tumors. J Virol. 2003;77:10366–10375. doi: 10.1128/JVI.77.19.10366-10375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 50.Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 51.O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo . Hum Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 52.Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol. 2003;77:10780–10789. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, Feldmann H, Kobinger GP. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008;3:e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008;82:2350–2357. doi: 10.1128/JVI.02372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, Honko A, Johnson J, Mulangu S, Pau MG, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol. 2011;85:4222–4233. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, Wilson JM, Ertl HC. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]