Abstract
The global outbreak of coronavirus disease 2019 (COVID-19) is still threatening human health, economy, and social life worldwide. As a counteraction for this devastating disease, a number of vaccines are being developed with unprecedented speed combined with new technologies. As COVID-19 vaccines are being developed in the absence of a licensed human coronavirus vaccine, there remain further questions regarding the long-term efficacy and safety of the vaccines, as well as immunological mechanisms in depth. This review article discusses the current status of COVID-19 vaccine development, mainly focusing on antigen design, clinical trials in later stages, and immunological considerations for further study.
Keywords: COVID-19, Vaccines, Prefusion-stabilized, VAERD, Antibody-dependent enhancement, Pre-existing immunological memory
INTRODUCTION
Since its first reported case in winter 2019, coronavirus disease 2019 (COVID-19) has been spreading at an alarming rate worldwide. As of February 16, 2021, more than 100 million confirmed cases and 2.4 million deaths were reported worldwide. In addition to health problems, COVID-19 poses a significant threat to the global economy and social life. Although there has been no licensed human coronavirus (HCoV) vaccine to date, numerous vaccine candidates for pathogenic human viruses have been investigated in animal models as well as in clinical trials, including the vaccines against respiratory syncytial virus (RSV), influenza virus, HIV and Ebola virus. Information and new technologies accumulated from these previous studies have been accelerating the development of current COVID-19 vaccines. As of December 2020, 61 and 172 candidates based on diverse vaccine platform technologies are being tested in clinical and preclinical stages, respectively (1).
SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-CoV-2)
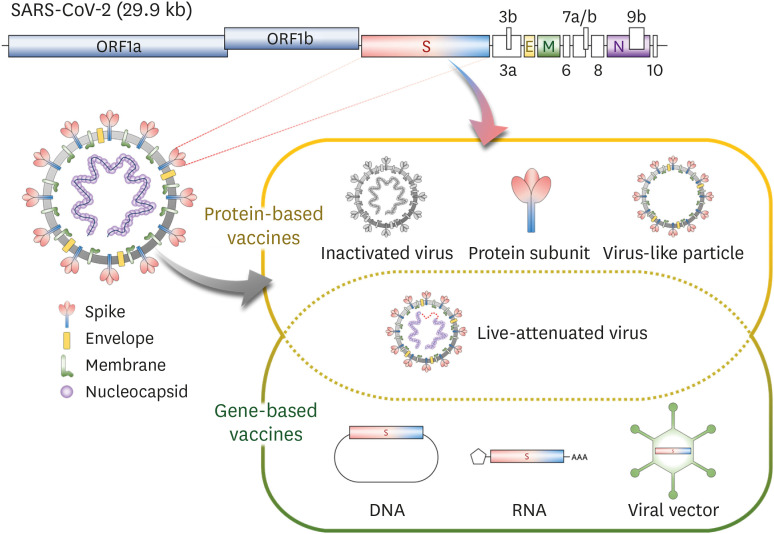
SARS-CoV-2, a causative agent of COVID-19, is a single-stranded positive-sense RNA virus belonging to the genus Betacoronavirus. The genome is composed of replicase genes encoded within the 5' end and structural protein genes in the 3' end. The structural proteins include spike (S), membrane (M), and envelope (E) proteins that are displayed on the envelop of SARS-CoV-2 virion, and the nucleocapsid (N) protein that form a helical ribonucleocapsid structure by binding to genomic RNA inside the virion. The S protein protrudes on the viral surfaces, forming trimeric structures (Fig. 1) (2).
Figure 1. Genome structure of SARS-CoV-2 and the general classification of the vaccine platforms platforms. Modified from Lee et al. (2).
ORF, open-reading frame; S, spike; E, envelope; M, membrane; N, Nucleocapsid.
SPIKE: A MAJOR TARGET ANTIGEN FOR COVID-19 VACCINES
SARS-CoV-2 gains entry into target cells by binding its S to angiotensin-converting enzyme 2 (ACE2) on host cells (3,4). ACE2 is expressed in various human organs including oral and nasal epithelium, nasopharynx, lung, small intestine, kidney, spleen, liver, colon and brain (5). SARS-CoV-2 primarily infects respiratory airway, despite its relatively low levels of ACE2 expression compared to other organs. Since SARS-CoV-2 enters target cells through the interaction between S and ACE2, S is considered as a primary target antigen for COVID-19 vaccine development.
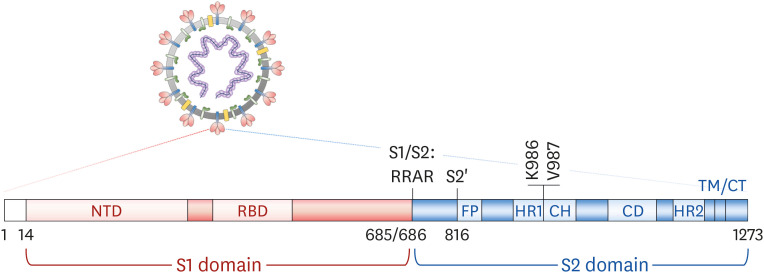
The S protein is composed of a S1 domain containing the N-terminal domain and receptor binding domain (RBD), and a S2 domain containing a fusion peptide (FP) and the transmembrane and cytoplasmic domains (4) (Fig. 2). Various forms of S protein, including full-length, ectodomain, S1, and RBD, have been investigated as target antigens, as shown in the SARS and Middle East respiratory syndrome (MERS) vaccine studies (2). Full-length S is one of the most frequently used antigens in COVID-19 vaccine development, especially for gene-based vaccines. The final candidates for mRNA vaccines of Moderna/National Institutes of Health (6) and Pfizer/BioNTech (7), a DNA vaccine of Inovio (8), and adenoviral-vectored vaccines of AstraZeneca/Oxford University (9), Janssen (10) and Gamaleya Research Institute (11) contain full-length S as an antigenic component. In these vaccines, the S protein is expressed in a M-bound form on the surface of transfected or infected cells. It is relatively easy to handle antigens containing hydrophobic transmembrane domains in genetic vaccines compared to recombinant protein vaccines. Novavax is investigating its full-length S recombinant protein-based COVID-19 vaccine in a phase 3 clinical trial (12).
Figure 2. Schematic diagram of a SARS-CoV-2 S protein.
CD, connector domain; CH, central helix; CT, cytoplasmic domain; HR1, heptad repeat 1; HR2, heptad repeat 2; NTD, N-terminal domain; S1/S2, S1/S2 protease cleavage site; S2', S2 protease cleavage site; TM, transmembrane domain.
An important feature introduced to full-length S-based vaccines is prefusion-stabilizing mutations. S protein is firstly expressed as a single polypeptide and then is readily cleaved by furin-like protease into S1 and S2 fragments in the host cells (13,14). These 2 fragments exist in a metastable prefusion conformation on the viral M. Once S1 binds to hACE2, transmembrane protease serine subtype 2, a serine protease on the host cells, cleaves the S2' site (15). This additional proteolytic cleavage triggers a conformational change in the S2 domain, leading to the dissociation of the S1 fragment. Finally, the S2 undergoes an irreversible ‘jack-knife transition’, resulting in a stable postfusion structure (Fig. 3) (14,16). Previous studies have reported that proline substitutions in the loop between the first heptad repeat and the central helix stabilize the prefusion structure of M fusion proteins such as HIV-1 gp160, RSV fusion, and influenza virus hemagglutinin proteins (17,18,19). Similarly, 2 consecutive mutations in MERS-CoV S (V1060P and L1061P) resulted in a stable prefusion form of S, increasing the immunogenicity and efficacy of the recombinant protein antigen (20). Based on these previous studies, the efficacy of COVID-19 vaccine candidates that harbor 2 proline substitutions (2P) in the S2 loop (K986P and V987P) or mutations in the S1/S2 furin cleavage site have been extensively evaluated. In many preclinical studies, prefusion-stabilized S provided increased neutralizing Ab responses and protective efficacy against SARS-CoV-2 and MERS-CoV, compared to wild-type S (6,10,20). Currently, the final products or candidates of Moderna, Pfizer/BioNTech, Janssen CureVac, and Novavax include this prefusion-stabilized S protein as a target antigen (Fig. 3).
Figure 3. Proteolytic activation of S and prefusion-stabilized S antigens. S protein is expressed as a single polypeptide and cleaved by a furin-like protease into S1 and S2 (①). The two fragments exist in a metastable prefusion conformation on the viral membrane (②). Upon binding of S1 to hACE2, a TMPRSS2 cleaves the S2' site. The proteolytic cleavage triggers a conformational change in S2 and then S1 dissociates from S2 (③). Finally, the S2 undergoes an irreversible ‘jack-knife transition’ into a stable postfusion structure (④). Substitution of K986 and V987 into two prolines and/or mutation in S1/S2 cleavage site prevent the S protein from changing into a postfusion conformation, resulting in enhanced immunogenicity and efficacy of COVID-19 vaccines (⑤).
TMPRSS2, transmembrane protease serine subtype 2; S1/S2, S1/S2 protease cleavage site; S2', S2 protease cleavage site.
RBD is another promising vaccine target as the most antibodies with neutralizing activities bind to RBD (21). Moreover, RBD is glycosylated at a relatively low level within S protein and therefore potentially immunogenic (22). However, RBD exhibits lower immunogenicity than S, possibly due to its smaller molecular weight and lower stability in vivo. There have been several approaches to overcome such limitation, including Fc fusion (3) or multimerization of RBD (23). In particular, compared to RBD monomer, RBD dimer or RBD protein nanoparticles significantly increased neutralizing Ab responses and improved protective efficacy in a murine model (23,24). These results may be attributed to the increase in the molecular weight of RBD as well as the induction of efficient B cell receptor cross-linking by the repeated structure of a multivalent antigen (25,26).
Besides the S protein, SARS-CoV-2 has other structural proteins such as M, E and N. As the sera immunized with SARS-CoV-2 M and E failed to neutralize the virus (27), these 2 proteins are currently not considered as target antigens for COVID-19 vaccines. On the other hand, N is highly immunogenic and induces robust humoral and cellular immune responses (28). Since the amino acid sequence of N is highly conserved among HCoVs (29,30), N-specific immunity can induce cross-reactive responses. A prior study has shown that N-specific cellular immune responses in the respiratory mucosa could provide partial cross-protective immunity between SARS-CoV and MERS-CoV. Additionally, vaccination with MERS-CoV N induced cross-reactive cellular immune responses against various coronaviruses, including SARS-CoV (31). However, another study has reported that the immunization with recombinant vaccinia virus expressing SARS-CoV N caused severe pneumonia accompanied by infiltration of eosinophils, neutrophils, and lymphocytes into the lungs upon subsequent viral infection in mice (32). It remains unclear whether the pneumonia was caused by SARS-CoV N and SARS-CoV-2 N has a potential of inducing such a side effect. Accordingly, the utilization of N as a COVID-19 vaccine antigen requires careful consideration. Currently, several candidates containing the N antigen are being evaluated in the preclinical development of the COVID-19 vaccine (1).
COVID-19 VACCINES IN LATER STAGES OF DEVELOPMENT
As of December 2020, over 200 COVID-19 vaccine candidates are in development based on several different platforms: inactivated virus, live-attenuated virus, protein subunit, virus-like particle (VLP), DNA, RNA, and viral vectored vaccines (Fig. 1). Among them, 13 candidates are being assessed in phase 3 clinical trials, and a few of them have been approved for human use in several countries as of December 2020. Tables 1 and 2 summarize the features of each vaccine platform and information about COVID-19 vaccines in phase 3 trials and beyond, respectively (Tables 1 and 2).
Table 1. Characteristics of each vaccine platform.
| Vaccine platform | Advantages | Limitations | Human-approved vaccines (except COVID-19) |
|---|---|---|---|
| Inactivated virus | Stable and no risk of reversion | Biosafety issue | Influenza (injection), polio (injection), hepatitis A |
| Strong antibody response | Usually requires adjuvants | ||
| Cost-effective | Weak cellular immune response | ||
| Live attenuated virus | Strong immune responses | Biosafety issue | Influenza (nasal), polio (oral), measles |
| No adjuvant required | Risk of reversion to virulence | ||
| Cost-effective | Time-consuming development | ||
| Recombinant protein subunit | No risk of infection and reversion | Low immunogenicity | Hepatitis B, influenza (injection) |
| Fewer side effects | Requires adjuvants | ||
| Easy antigen modification | High cost | ||
| VLPs | No risk of infection and reversion | Complicated manufacturing process | Cervical cancer by human papillomavirus |
| Fewer side effects | Requires adjuvants | ||
| Good antibody response | High cost | ||
| DNA | Rapid development and production | Low immunogenicity | - |
| Stable in room temperature | Requires a delivery device (electroporator or jet-injector) | ||
| High producibility | |||
| mRNA | Cell-free | Unstable | - |
| Rapid development and production | High cost | ||
| Good immunogenicity | Requires low temperature storage | ||
| Viral-vectored | Strong immune responses | Pre-existing immunity against the vector | Ebola |
| Various viral vectors | |||
| Large-scalable |
VLP, virus-like particle.
Table 2. COVID-19 vaccines in phase 3 clinical trials and beyond (as of December 2020).
| Platform | Developer (product name) | Target antigen | Comments |
|---|---|---|---|
| mRNA | Moderna (mRNA-1273) | S protein with 2P (K986P and V987P) | LNP-encapsulated |
| Pfizer/BioNTech (BNT-162b2) | S protein with 2P (K986P and V987P) | LNP-encapsulated | |
| CureVac AG (CVnCoV) | S protein | LNP-encapsulated | |
| Viral-vectored | CanSino Biological Inc vaccine (Ad5-nCoV) | S protein | Human Ad5 |
| Oxford/AstraZenaca (AZD-1222) | S protein | Chimpanzee adenovirus | |
| Gamaleya Research Institutes (Gam-COVID-Vac) | S protein | rAd5 and rAd26 prime-boost | |
| Janssen Pharmaceutical Companies (Ad26.COV2.5) | S protein with 2P (K986P and V987P) and 2 mutations at furin cleavage site (R682S and R685G) | Ad26 | |
| Inactivated virus | Wuhan Institute of Biological Products/Sinopharm (NA) | Whole pathogen | Alum adjuvant |
| Beijing Institute of Biological Products/Sinopharm (BBIBP-CorV) | Whole pathogen | Alum adjuvant | |
| Sinovac Life Sciences (CoronaVac) | Whole pathogen | Alum adjuvant | |
| Recombinant protein subunit | Novavax (NVX-CoV2373) | S protein with 2P (K986P and V987P) and 3 mutations at furin cleavage site (R682Q, R683Q and R685Q) | Protein nanoparticle, matrix-M™ adjuvant |
| Anhui Zhifei Longcom Biopharmaceutical (NA) | RBD | RBD-dimer, alum adjuvant | |
| DNA | Inovio (INO-4800) | S protein | Electroporation, intradermal injection |
| Osaka University/AnGes/Takara Bio (AG0301-COVID19) | S protein | Alum adjuvant, intramuscular injection |
LNP, lipid nanoparticle; NA, not available.
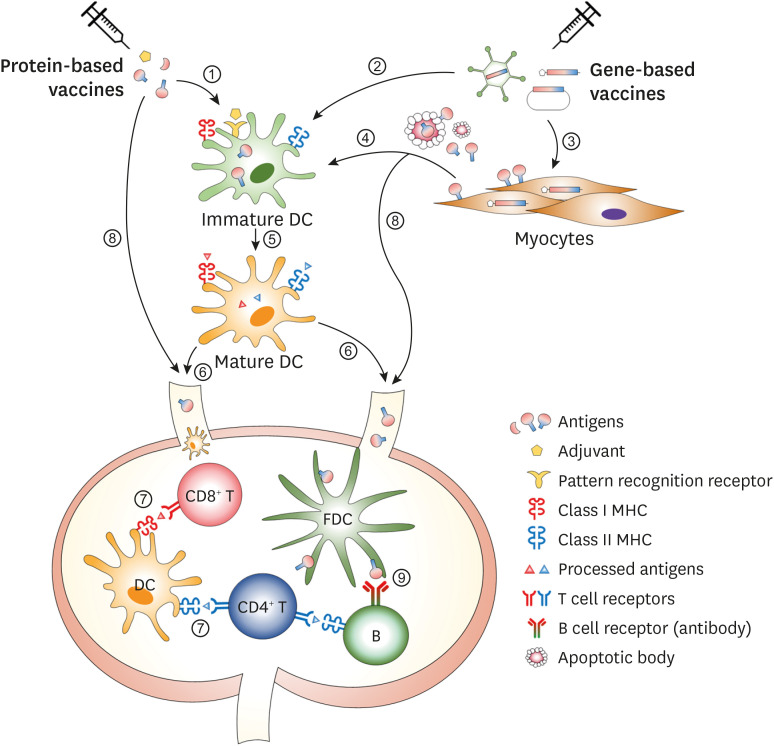
As summarized in Table 1, the magnitude and quality of the immune responses following vaccination varies depending on the type and composition of each vaccine. However, the immune system utilizes multiple types of cells and molecules in common for inducing efficient immune responses. Among them, dendritic cells (DCs) play an indispensable role in the recognition of danger signal provided by adjuvants, the processing of ingested antigens and the activation of T cells. Follicular DCs, which originate from stromal cells, are also critical in the induction of Ab responses to conformational epitopes. Once antigen-specific T and B cells experience the cognate vaccine antigen(s), they are activated and differentiated into the specialized subset for the optimal function. The general cellular mechanisms of immune induction following vaccination are shown in Fig. 4.
Figure 4. Cellular mechanisms of the induction of vaccine-specific immune responses. DCs can uptake protein vaccine antigen(s) (①) or be transfected with gene-based vaccines to express the vaccine antigen inside the cells (②). Gene-based vaccines can be also transfected to or infected into myocytes (③). The expressed antigens in the myocytes are either secreted or released from the cells and taken up by DCs (④). DCs then process the antigen into the antigenic peptides and present them on the MHC I or II molecules (⑤). Then, DCs migrate into the draining LNs (⑥) where the mature DCs prime antigen-specific CD4+ or CD8+ T cells (⑦). Vaccine antigens also can be directly drained into LNs through the lymphatic vessels (⑧). In the draining LNs, FDCs trap the soluble antigens and present them to antigen-specific B cells, leading to an antibody response to conformational epitopes (⑨).
FDC, follicular dendritic cell; LN, lymph node.
mRNA vaccines
mRNA vaccines have induced optimal protective immunity against infectious pathogens in various animal models (33,34,35). Before COVID-19 outbreak, mRNA vaccines targeting infectious viruses, including influenza virus, Zika virus and HIV, have been investigated in clinical stages (36,37). Moderna performed a phase 3 clinical trial (NCT04470427) of COVID-19 vaccine using the mRNA encoding full-length S with 2P substitutions (mRNA-1273) and showed 94.1% vaccine efficacy compared to the placebo group (38). In the preclinical stage, mRNA-1273 induced neutralizing antibodies against pseudovirus expressing SARS-CoV-2 S protein in BALB/c, C57BL/6, B6C3 mouse models and rhesus macaques (6,39). After vaccination with mRNA-1273, participants in phase 1 clinical trial showed neutralizing Ab titers similar to that of convalescent serum (40). Currently, the emergency use of mRNA-1273 has been authorized in several countries, including the US and Canada. Pfizer/BioNTech is investigating the mRNA vaccine BNT162 in clinical trials. BNT162 is divided into 4 mRNA types: 1) BNT162a1, unmodified mRNA encoding RBD; 2) BNT162b1, nucleoside-modified mRNA (modRNA) encoding trimeric RBD; 3) BNT162b2, modRNA encoding full-length S with 2P or prefusion-stabilized S; 4) BNT162c2, self-amplifying mRNA encoding full-length S. Among them, BNT162b1 and BNT162b2 have entered into phase 2/3 clinical trials (NCT04368728), based on safety and immunogenicity tests in preclinical and clinical phase 1 studies (41). Preclinical results showed that BNT162b2 effectively induced neutralizing antibodies against SARS-CoV-2 pseudovirus and authentic SARS-CoV-2 in BALB/c mice and rhesus macaques, respectively. T cell responses were also induced in both animal models. More importantly, the induction of immune responses by BNT162b2 resulted in potent protection against SARS-CoV-2 in rhesus macaques (42). In clinical results, although both BNT162b1 and BNT162b2 induced similar levels of neutralizing antibodies, BNT162b2 showed lower incidence and severity of adverse events, especially in the elderly (41). Neutralizing Ab levels induced by BNT162b2 in the participants were similar to the convalescent serum samples (41). The results of a phase 3 trial of BNT162b2 showed 94.8% efficacy compared to that of the placebo group (43). Based on its outstanding safety and efficacy, BNT162b2 has been authorized in the EU and US and recently been pre-qualified by World Health Organization (WHO). CureVac AG, another company developing mRNA vaccine, is currently investigating a vaccine encoding full-length S protein called CVnCoV in a phase 2/3 trial (NCT04652102) after completing a phase 1 study (NCT04449276), where potent SARS-CoV-2-binding antibodies and neutralizing Ab responses were observed in immunized participants. Based on these results, the immunization dose was determined for a phase 3 trials, which is currently being conducted (44).
Adenoviral-vectored vaccines
The safety and efficacy of an adenovirus (Ad), a non-replicating viral vector, has been already investigated in phase 3 trials and beyond. CanSino Biological Inc. developed the human Ad5-vectored vaccine expressing full-length S. No severe adverse effects were observed and neutralizing antibodies were effectively induced in the vaccinated group. However, as the titer of pre-existing neutralizing Ab against Ad5 was higher, seroconversion and T cell immune responses showed decreasing tendency (45). Despite these phenomena, the vaccine induced a higher titer of neutralizing antibodies than did the placebo control. CanSino Biological Inc. is further investigating a mucosal vaccine using the Ad5 vector expressing full-length S in a phase 1 clinical trial (NCT04552366). The University of Oxford/AstraZenaca has developed a chimpanzee Ad-vectored vaccine expressing full-length S (AZD1222, formerly named ChAdOx1 nCoV-19) that can bypass pre-existing vector-specific immunity. In preclinical stages, AZD1222 induced neutralizing antibodies and T cell responses in BALB/c mice and rhesus macaques and protected rhesus macaques from SARS-CoV-2 infection (9). In phase 1/2 trials, the participants immunized with ADZ1222 exhibited robust T cell responses as well as neutralizing antibodies, similar to convalescent plasma (46). Although the neutralizing Ab titer was increased by booster immunization, there were no changes in the T cell responses, likely due to the immune responses toward homologous viral vector vaccine. In a phase 3 trial, participants administered with half dose at the first vaccination showed 90% vaccine efficacy, while those receiving the full dose at the first vaccination showed 62.1% vaccine efficacy (70.4% efficacy on average) (NCT04400838, ISRCTN89951424) (47). AZD1222 has been approved in several countries including the UK, India, Argentina, El Salvador, and South Korea. Gamaleya Research Institutes tested the safety of rAd5 or rAd26 expressing full-length S in a phase 1 trial and investigated their immunogenicity after prime-boost vaccination in a phase 2 study (NCT04436471, NCT04437875). Neutralizing antibodies and T cell responses were detected in all participants (11). Participants receiving the heterologous vaccination elicited a similar titer of neutralizing antibodies compared to convalescent individuals. Gamaleya Research Institutes is currently conducting a phase 3 clinical trial with rAd5/rAd26 prime-boost immunization (NCT04530396). Interim results are showing 91.4% efficacy in the vaccinated group compared to placebo group. Janssen Pharmaceutical Companies completed preclinical and phase 1/2 clinical tests using the rAd26 vectored vaccine expressing full-length S with 2P and mutations in furin cleavage site (NCT04436276) and entered a phase 3 study (NCT04505722). In a preclinical study comparing the immunogenicity of various S mutants in mice, rAd26.S.PP with a furin cleavage site mutation and 2P in the S2 hinge region was selected and named rAd26.CoV.S (48). A single immunization with rAd26.CoV.S was sufficient to inhibit viral replication in the lungs and nasal region of non-human primates (NHPs), and this was well-correlated with the increased neutralizing Ab titer (10). In line with a preclinical study, the results from a phase 1/2a trial showed that a single immunization induced high levels of neutralizing antibodies similar to those in patients recovered from SARS-CoV-2 infection (49). After vaccination with rAd26.CoV.S, the Th1/Th2 ratio in participants was 28.9, indicating Th1-skewed responses, and potent CD8+ T cell responses were also induced.
Inactivated virus vaccines
The Wuhan Institute of Biological Products/Sinopharm tested the COVID-19 vaccine using inactivated SARS-CoV-2. In a phase 1/2 trial, participants immunized with inactivated SARS-CoV-2 with alum adjuvant showed higher titers of neutralizing antibodies and increased T cell responses compared to those of the placebo group. Moreover, the SARS-CoV-2-specific Ab titer was increased with the number of vaccinations (ChiCTR2000031809) (50). The Beijing Institute of Biological Products/Sinopharm has also investigated inactivated SARS-CoV-2 (BBIBP-CorV) with alum adjuvant. In a phase 1/2 clinical trial (ChiCTR2000032459), BBIBP-CorV generated high titers of antibodies in immunized participants (51). BBIBP-CorV achieved 79.3% efficacy in preventing SARS-CoV-2 infection, and participants immunized with this vaccine showed 99.5% seroconversion after 2 doses as shown by interim clinical results. BBIBP-CorV received conditional approval in China for emergency use. Also, Sinovac Life Sciences has investigated CoronaVac, an inactivated SARS-CoV-2. In preclinical studies, CoronaVac effectively induced neutralizing antibodies and T cell responses in BALB/c mice and rhesus macaques (52). Subsequently, they tested the safety and immunogenicity of CoronaVac in multiple phase 1/2 clinical trials (NCT04383574, NCT04352608, NCT04551547). Participants immunized with CoronaVac with alum adjuvant showed seroconversion without serious adverse events (53). They are currently conducting phase 3 clinical trials (NCT04456595, NCT04508075, NCT04582344, NCT04617483, NCT04617483). CoronaVac has been approved in China for emergency use in high-risk groups. It has been reported that vaccination with formalin-inactivated SARS-CoV increases, rather than decreases, lesions induced by SARS-CoV challenge (52). This result raised concerns that inactivated virus-based COVID-19 vaccines could cause Ab-dependent enhancement (ADE) or vaccine-associated enhanced respiratory disease (VAERD), but such phenomenon has not been reported to date.
Recombinant protein subunit vaccines
Novavax is investigating a protein nanoparticle vaccine consisting of prefusion-stabilized full-length S in combination with Matrix-MTM (NVX-CoV2373). Preclinical results using cynomolgus macaques showed that NVX-CoV2373 effectively induced S-specific neutralizing Ab responses (12). This vaccine also effectively induced neutralizing antibodies that exceeded the levels of convalescent individuals and predominantly induced Th1 responses with mild or no side effects in phase 1/2 trials (NCT04368988, NCT04533399) (54). Multiple phase 3 studies are being conducted in the US, Mexico, and Peru (NCT04611802, NCT04583995, EUCTR2020-004123-16). Anhui Zhifei Longcom Biopharmaceutical designed a disulfide bonded RBD-dimer vaccine purified from mammalian cells. In preclinical studies, immunization with RBD-dimer with alum adjuvant effectively induced Ab responses with neutralizing activity against pseudovirus or live SARS-CoV-2, but did not induce T cell responses, in a BALB/c mouse model (23). Although they have completed phase 1/2 trials, the results have not been reported yet (NCT04445194, NCT04466085, ChiCTR2000035691, NCT04550351). Currently, they are recruiting volunteers for a phase 3 trial (ChiCTR2000040153).
An adjuvant is one of the most critical factors affecting the efficacy of protein-based vaccines. In most prophylactic vaccines, neutralizing antibodies has been considered critical, but the importance of the cellular immune response is also increasingly being emphasized. Recently, many novel adjuvants have been developed that can simultaneously enhance humoral and cellular immune responses. Therefore, in addition to alum, various adjuvants such as MF-59, Matrix-MTM, AS03, and GpG1018, are also combined with the COVID-19 vaccine (1). Adjuvants mainly stimulate pattern recognition receptors directly or indirectly to provide an “infection-like signal” in the host. Thus, innate immune responses induced by adjuvants significantly affect the quality, intensity and persistence of antigen-specific immune responses. When two cervical cancer vaccines with similar antigenic preparation and different adjuvants were tested, a significant difference was observed between those two vaccines in the long-term immune response (55). Considering the possibility of COVID-19 recurrence after the current global pandemic, an adjuvant inducing long-term immunity could be a critical determinant for the efficacy of the protein-based vaccines.
DNA vaccines
DNA vaccines have been applied to various diseases, such as cancer, autoimmune diseases, allergies and infectious diseases. Several DNA vaccine candidates against influenza, hepatitis B virus and HIV-1 have been tested in clinical trials. In the context of SARS-CoV-2, 2 DNA vaccine candidates are being tested in phase 3 trials. Inovio tested the immunogenicity of a DNA vaccine construct encoding a full-length S (INO-4800) in mice and guinea pigs in preclinical studies (8). Intramuscular injection of INO-4800 effectively induced SARS-CoV-2-specific Ab and T cell responses. Sera from BALB/c and C57BL/6 mice immunized with INO-4800 effectively neutralized the pseudovirus expressing SARS-CoV-2 S protein and live SARS-CoV-2. Intradermal immunization of INO-4800 induced robust SARS-CoV-2 S-specific Ab and T cell responses in NHPs and the immunized sera effectively neutralized both wild-type and D614G variant SARS-CoV-2 (56). INO-4800 also elicited neutralizing antibodies and T cells that are comparable with those of convalescent samples in phase 1/2 trials (NCT04336410) (57). Inovio is currently conducting a phase 3 trial (NCT04642638). Osaka University/AnGes/Takara Bio developed a DNA vaccine expressing a full-length S (AG0301-COVID19) and tested its safety and efficacy in a phase 1/2 trial (NCT04463472), but the results have not been reported. They have recently initiated a phase 3 clinical study (NCT04655625). In a preclinical study, AG0301-COVID19 with an alum adjuvant effectively induced neutralizing antibodies and T cell responses in the rats, with the complete absence of toxic reactions to various organs (58). To date, no DNA vaccine has completed phase 3 trials or has been approved.
EFFICACY AND SAFETY OF COVID-19 VACCINES
Immune correlates of protection (ICP)
ICP or correlates of protective immunity is a specific immune marker or response that is associated with protection against infection (59). As neutralizing antibodies are the most critical ICP in many infectious diseases such as influenza and hepatitis A and B, the primary objective of most prophylactic vaccines is inducing potent neutralizing Ab responses (60,61,62). In the case of COVID-19, neutralizing antibodies have been considered as the primary ICP as well. Passive immunization of convalescent sera or antibodies purified from convalescent patients and S-specific monoclonal antibodies effectively inhibited SARS-CoV-2 infection or alleviated disease symptoms in preclinical or clinical studies (63,64,65). It has also been shown that vaccine candidates whose efficacy has been validated in phase 3 trials exhibited higher neutralizing Ab titers than convalescent patient sera (49). Recently, beyond neutralizing potential, polyfunctionality of the antibodies is considered as a substantial factor affecting protective immunity. It has been reported that polyfunctional antibodies are closely associated with disease outcomes in patients infected with human immunodeficiency, influenza and Ebola viruses (66,67,68). Several COVID-19 vaccine studies have also demonstrated Ab functions such as Ab-dependent cellular phagocytosis, Ab-dependent neutrophil phagocytosis, Ab-dependent NK cell degranulation and Ab-dependent complement deposition (69,70). These Ab features are differently presented in convalescent and deceased individuals infected with SARS-CoV-2 (71). However, little is known about the direct correlation of polyfunctional antibodies with protection against infectious agents including SARS-CoV-2, raising the need for further investigation.
The importance of the cellular immune response in protective immunity has also been discussed. Virus-specific T cells are crucial for the clearance of SARS-CoV or MERS-CoV (72,73,74). In a recent study, CD4+ and CD8+ T cells played an important role in protection against SARS-CoV-2 infection in a murine model (27). Moreover, strong virus-specific T cell responses were observed in asymptomatic or mild COVID-19 patients, suggesting the potential of cellular immune responses in the protection or clearance of SARS-CoV-2 (75,76). Therefore, a vaccine that can simultaneously induce neutralizing antibodies and cellular immune responses is thought to be ideal. Currently, most COVID-19 vaccine candidates are validating both parameters.
Recently, a WHO international standard serum for SARS-CoV-2 has been developed by the National Institute for Biological Standards and Control, UK (77). However, in the aspect of cellular immunity, it is relatively difficult to prepare a standard sample or quantify the immune response compared to the Ab response. The role of antigen-specific T cells in the protection from SARS-CoV-2 is also controversial (78). Further studies are required to develop a standard assay or establish surrogate markers to quantify the cellular immune response and understand the correlation between cellular immunity and protective immunity.
VAERD and ADE of disease
In some cases, virus-specific immune responses generated by prior infection or vaccination can increase viral pathogenicity when subsequent infection occurs. There are 2 different mechanisms; VAERD and ADE of the disease. VAERD is allergic inflammation in the respiratory tract caused by excessive Th2-biased immune responses induced by prior vaccination. In a clinical trial of the RSV vaccine in the 1960s, a significant portion (16 out of 20) of children administered with a formalin-inactivated RSV vaccine developed severe symptoms following natural infection with RSV, whereas only one out of 21 children in the placebo group was hospitalized (79). Subsequently, it was revealed that the inactivated RSV vaccine induced strong Th2-biased immune responses and caused hyper-production of IL-4, IL-5, and IL-13, and excessive lung inflammation by eosinophils (80). ADE is an event in which a suboptimal concentration of neutralizing antibodies or cross-reactive non-neutralizing antibodies increases viral infection through interaction with Fc receptors (81,82). ADE is well known in flaviviruses such as dengue virus and Zika virus (83,84), but inactivated SARS-CoV or recombinant viral vectored-SARS vaccines also increased liver or respiratory lesions during subsequent SARS-CoV infection (85,86). ADE was also observed in cats immunized with a feline infectious peritonitis virus (FIPV) vaccine or passively administered with FIPV-specific antibodies (87,88). The possibility of ADE may generate serious concerns in the development of the COVID-19 vaccine. However, no severe side effects have been reported in any of the following cases: passive transfer of convalescent plasma to COVID-19 patients (89), infection of vaccinated animals with SARS-CoV-2, and large-scale phase 3 trials. It is required to investigate the potential adverse effects of COVID-19 vaccines depending on the antigen design and vaccine formulation.
Pre-existing memory response cross-reactive to SARS-CoV-2
Recently, T cell and Ab responses reactive to SARS-CoV-2 have been observed in people who have not been exposed to SARS-CoV-2 (collected before the COVID-19 outbreak or seronegative for SARS-CoV-2). This cross-reactivity was presumed to be induced by infection with seasonal HCoVs. In many cases, the cross-reactive responses recognize epitopes in the S2 domain (90,91). Meanwhile, pre-existing cross-reactive immunity may affect the immune response following vaccination as well as viral infection. Influenza virus-specific pre-existing memory CD4+ T cells increased the influenza vaccine-induced Ab response in clinical trials (92). Pre-existing memory B cells also affect the outcome of a quadrivalent influenza vaccine (QIV); when pre-existing B cell memory exhibits a dominant response to a particular subtype (subtype immunodominance), Ab response to QIV was positively correlated with the preexisting memory (93). Also, it was recently reported that the kinetics and magnitude of Ab response to a hepatitis B vaccine were significantly increased in the presence of hepatitis B vaccine-specific memory CD4+ T cells (94). Ab response to HCoV can be observed in most adults (95) and the S2 domain of SARS-CoV-2 S exhibits relatively high amino acid sequence homology with those of seasonal HCoVs (up to 42%) (90). In particular, epitopes in the FP are highly conserved among various coronaviruses (96). Therefore, seasonal HCoV-induced pre-existing immunological memory that is cross-reactive to SARS-CoV-2 may affect the immune responses induced by a COVID-19 vaccine, especially those containing the S2 domain. Considering that most COVID-19 vaccines in later stages of development have the S2 domain, it is necessary to study the influence of cross-reactive pre-existing immunity on the efficacy of the vaccine and the diversity of immune responses.
CONCLUDING REMARKS
Paradoxically, COVID-19, a serious threat to human health and the economy, is accelerating the advancement of the vaccine field. Technologies that have been utilized in preclinical studies and clinical trials are being integrated into the development of COVID-19 vaccines. mRNA and viral vectored vaccines have been approved and are now being used in several countries. Some of these vaccines harbor a prefusion-stabilized antigen that has never been observed in conventional licensed vaccines. Various types of vehicles such as Ad5, Ad26, chimpanzee Ad, and other replication-competent viruses are being widely studied for viral vectored vaccines. Recombinant protein vaccines have also made significant advancements combined with self-assembling nanoparticle technology, which is also considered promising. Safety is the most crucial factor to be considered in vaccine development. Considering that a substantial portion of the population needs to be COVID-19-vaccinated, it needs to be further investigated the long-term study on the potential adverse effects, such as VAERD and ADE, and the immunological correlation with seasonal HCoVs.
ACKNOWLEDGEMENTS
This work was supported by grants of the Bio & Medical Technology Development Program (2016M3A9B6918675 and 2018M3A9H4077992) of the National Research Foundation of Korea (NRF) and the KRIBB Initiative program (KGM9942112), funded by the Korean government (Ministry of Science & ICT). We would like to thank Dr. SW Kim and Editage (www.editage.co.kr) for language editing.
Abbreviations
- 2P
Two proline substitution
- ACE2
Angiotensin-converting enzyme 2
- Ad
Adenovirus
- ADE
antibody-dependent enhancement
- COVID-19
coronavirus disease 2019
- DC
dendritic cell
- E
envelope
- FIPV
feline infectious peritonitis virus
- FP
fusion peptide
- HCoV
human coronavirus
- ICP
immune correlates of protection
- M
membrane
- MERS
Middle East respiratory syndrome
- modRNA
nucleoside-modified mRNA
- N
nucleocapsid
- NHP
non-human primate
- QIV
quadrivalent influenza vaccine
- RBD
receptor binding domain
- RSV
respiratory syncytial virus
- S
spike
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- VAERD
vaccine-associated enhanced respiratory disease
- VLP
virus-like particle
- WHO
World Health Organization
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Kim DJ, Seo SH.
- Funding acquisition: Kim DJ.
- Supervision: Kim DJ.
- Visualization: Kim DJ.
- Writing - original draft: Lee P, Kim CU, Kim DJ.
- Writing - review & editing: Lee P, Seo SH, Kim DJ.
References
- 1.World Health Organization. Draft landscape of COVID-19 candidate vaccines [Internet] [accessed on 22 December 2020]. Available at https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 2.Lee P, Kim DJ. Newly emerging human coronaviruses: animal models and vaccine research for SARS, MERS, and COVID-19. Immune Netw. 2020;20:e28. doi: 10.4110/in.2020.20.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu H, Chen Q, Yang G, He L, Fan H, Deng YQ, Wang Y, Teng Y, Zhao Z, Cui Y, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369:1603–1607. doi: 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch S, Roth N, Schwendt K, Fotin-Mleczek M, Mueller SO, Petsch B. mRNA based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus neutralizing antibodies and mediates protection in rodents [Internet] [accessed on 10 January 2021]. Available at . [DOI] [PMC free article] [PubMed]
- 8.Smith TR, Patel A, Ramos S, Elwood D, Zhu X, Yan J, Gary EN, Walker SN, Schultheis K, Purwar M, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, Avanzato VA, Bushmaker T, Flaxman A, Ulaszewska M, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, Liu J, Peter L, McMahan K, Tostanoski LH, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Shcheblyakov DV, Dzharullaeva AS, Grousova DM, Erokhova AS, Kovyrshina AV, Botikov AG, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guebre-Xabier M, Patel N, Tian JH, Zhou B, Maciejewski S, Lam K, Portnoff AD, Massare MJ, Frieman MB, Piedra PA, et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine. 2020;38:7892–7896. doi: 10.1016/j.vaccine.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Y, Zhang J, Xiao T, Peng H, Sterling SM, Walsh RM, Jr, Rawson S, Rits-Volloch S, Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369:1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders RW, Vesanen M, Schuelke N, Master A, Schiffner L, Kalyanaraman R, Paluch M, Berkhout B, Maddon PJ, Olson WC, et al. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2002;76:8875–8889. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015;349:1301–1306. doi: 10.1126/science.aac7263. [DOI] [PubMed] [Google Scholar]
- 19.Kong L, He L, de Val N, Vora N, Morris CD, Azadnia P, Sok D, Zhou B, Burton DR, Ward AB, et al. Uncleaved prefusion-optimized gp140 trimers derived from analysis of HIV-1 envelope metastability. Nat Commun. 2016;7:12040. doi: 10.1038/ncomms12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, Cottrell CA, Becker MM, Wang L, Shi W, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi R, Shan C, Duan X, Chen Z, Liu P, Song J, Song T, Bi X, Han C, Wu L, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 22.Grant OC, Montgomery D, Ito K, Woods RJ. Analysis of the SARS-CoV-2 spike protein glycan shield reveals implications for immune recognition. Sci Rep. 2020;10:14991. doi: 10.1038/s41598-020-71748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai L, Zheng T, Xu K, Han Y, Xu L, Huang E, An Y, Cheng Y, Li S, Liu M, et al. A universal design of Betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182:722–733.e11. doi: 10.1016/j.cell.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walls AC, Fiala B, Schäfer A, Wrenn S, Pham MN, Murphy M, Tse LV, Shehata L, O'Connor MA, Chen C, et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell. 2020;183:1367–1382.e17. doi: 10.1016/j.cell.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett NR, Zwick DB, Courtney AH, Kiessling LL. Multivalent antigens for promoting b and t cell activation. ACS Chem Biol. 2015;10:1817–1824. doi: 10.1021/acschembio.5b00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Zhuang Z, Zheng J, Li K, Wong RL, Liu D, Huang J, He J, Zhu A, Zhao J, et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell. 2020;182:734–743.e5. doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sariol A, Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020;53:248–263. doi: 10.1016/j.immuni.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuwała K, Golda A, Kabala W, Burmistrz M, Zdzalik M, Nowak P, Kedracka-Krok S, Zarebski M, Dobrucki J, Florek D, et al. The nucleocapsid protein of human coronavirus NL63. PLoS One. 2015;10:e0117833. doi: 10.1371/journal.pone.0117833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–680.e2. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Zhao J, Mangalam AK, Channappanavar R, Fett C, Meyerholz DK, Agnihothram S, Baric RS, David CS, Perlman S. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasui F, Kai C, Kitabatake M, Inoue S, Yoneda M, Yokochi S, Kase R, Sekiguchi S, Morita K, Hishima T, et al. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 33.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnee M, Vogel AB, Voss D, Petsch B, Baumhof P, Kramps T, Stitz L. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl Trop Dis. 2016;10:e0004746. doi: 10.1371/journal.pntd.0004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson NA, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbett KS, Flynn B, Foulds KE, Francica JR, Boyoglu-Barnum S, Werner AP, Flach B, O'Connell S, Bock KW, Minai M, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, Kranz LM, Walzer KC, Hein S, Güler A, et al. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates [Internet] [accessed on 10 January 2021]. Available at . [DOI]
- 43.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kremsner P, Mann P, Bosch J, Fendel R, Gabor JJ, Kreidenweiss A, Kroidl A, Leroux-Roels I, Leroux-Roels G, Schindler C, et al. Phase 1 assessment of the safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2 in human volunteers [Internet] [accessed on 10 January 2021]. Available at . [DOI]
- 45.Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bos R, Rutten L, van der Lubbe JE, Bakkers MJ, Hardenberg G, Wegmann F, Zuijdgeest D, de Wilde AH, Koornneef A, Verwilligen A, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. doi: 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, et al. Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial [Internet] [accessed on 10 January 2021]. Available at . [DOI]
- 50.Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2020;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicoli F, Mantelli B, Gallerani E, Telatin V, Bonazzi I, Marconi P, Gavioli R, Gabrielli L, Lazzarotto T, Barzon L, et al. HPV-specific systemic antibody responses and memory b cells are independently maintained up to 6 years and in a vaccine-specific manner following immunization with cervarix and gardasil in adolescent and young adult women in vaccination programs in Italy. Vaccines (Basel) 2020;8:26. doi: 10.3390/vaccines8010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel A, Walters J, Reuschel EL, Schultheis K, Parzych E, Gary EN, Maricic I, Purwar M, Eblimit Z, Walker SN, et al. Intradermal-delivered DNA vaccine provides anamnestic protection in a rhesus macaque SARS-CoV-2 challenge model [Internet] [accessed on 10 January 2021]. Available at . [DOI] [PMC free article] [PubMed]
- 57.Tebas P, Yang S, Boyer JD, Reuschel EL, Patel A, Christensen-Quick A, Andrade VM, Morrow MP, Kraynyak K, Agnes J, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, phase 1 clinical trial. EClinicalMedicine. 2020;31:100689. doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayashi H, Sun J, Yanagida Y, Otera T, Kubota-Kotetsu R, Shioda T, Ono C, Matsuura Y, Arase H, Yoshida S, et al. Preclinical study of DNA vaccines targeting SARS-CoV-2 [Internet] [accessed on 10 January 2021]. Available at . [DOI] [PMC free article] [PubMed]
- 59.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 60.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PK, Peng MY, Wan HL, Chen JH, Hu BS, Perng CL, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang W, Vassell R, Song HS, Chen Q, Keller PW, Verma S, Alvarado-Facundo E, Wan H, Schmeisser F, Meseda CA, et al. Generation of a protective murine monoclonal antibody against the stem of influenza hemagglutinins from group 1 viruses and identification of resistance mutations against it. PLoS One. 2019;14:e0222436. doi: 10.1371/journal.pone.0222436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, van Buskirk C, Grossman BJ, Joyner M, Henderson JP, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi: 10.3389/fimmu.2020.01660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr Opin HIV AIDS. 2009;4:373–379. doi: 10.1097/COH.0b013e32832f00c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cobey S, Hensley SE. Immune history and influenza virus susceptibility. Curr Opin Virol. 2017;22:105–111. doi: 10.1016/j.coviro.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saphire EO, Schendel SL, Gunn BM, Milligan JC, Alter G. Antibody-mediated protection against Ebola virus. Nat Immunol. 2018;19:1169–1178. doi: 10.1038/s41590-018-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, Halkerston R, Hill J, Jenkin D, Stockdale L, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 70.Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, Nkolola JP, Liu J, Li Z, Chandrashekar A, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atyeo C, Fischinger S, Zohar T, Slein MD, Burke J, Loos C, McCulloch DJ, Newman KL, Wolf C, Yu J, et al. Distinct early serological signatures track with SARS-CoV-2 survival. Immunity. 2020;53:524–532.e4. doi: 10.1016/j.immuni.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao J, Zhao J, Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol. 2010;84:9318–9325. doi: 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ, Jr, Baric RS, Enjuanes L, Gallagher T, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, Kuri-Cervantes L, Pampena MB, D'Andrea K, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, Llewellyn-Lacey S, Kamal H, Bogdanovic G, Muschiol S, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.World Health Organization. Establishment of the who international standard and reference panel for anti-SARS-CoV-2 antibody [Internet] [accessed on 11 January 2021]. Available at https://www.who.int/publications/m/item/WHO-BS-2020.2403.
- 78.Altmann DM, Boyton RJ. SARS-CoV-2 T cell immunity: specificity, function, durability, and role in protection. Sci Immunol. 2020;5:eabd6160. doi: 10.1126/sciimmunol.abd6160. [DOI] [PubMed] [Google Scholar]
- 79.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 80.De Swart RL, Kuiken T, Timmerman HH, van Amerongen G, Van Den Hoogen BG, Vos HW, Neijens HJ, Andeweg AC, Osterhaus AD. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol. 2002;76:11561–11569. doi: 10.1128/JVI.76.22.11561-11569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 84.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 85.Weingartl H, Czub M, Czub S, Neufeld J, Marszal P, Gren J, Smith G, Jones S, Proulx R, Deschambault Y, et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, Funkhouser W, Gralinski L, Totura A, Heise M, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takano T, Kawakami C, Yamada S, Satoh R, Hohdatsu T. Antibody-dependent enhancement occurs upon re-infection with the identical serotype virus in feline infectious peritonitis virus infection. J Vet Med Sci. 2008;70:1315–1321. doi: 10.1292/jvms.70.1315. [DOI] [PubMed] [Google Scholar]
- 88.Weiss RC, Scott FW. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp Immunol Microbiol Infect Dis. 1981;4:175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, Wiggins CC, Senefeld JW, Klompas AM, Hodge DO, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95:1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, Hippenstiel S, Dingeldey M, Kruse B, Fauchere F, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 91.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nienen M, Stervbo U, Mölder F, Kaliszczyk S, Kuchenbecker L, Gayova L, Schweiger B, Jürchott K, Hecht J, Neumann AU, et al. The role of pre-existing cross-reactive central memory CD4 T-cells in vaccination with previously unseen influenza strains. Front Immunol. 2019;10:593. doi: 10.3389/fimmu.2019.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abreu RB, Kirchenbaum GA, Clutter EF, Sautto GA, Ross TM. Preexisting subtype immunodominance shapes memory B cell recall response to influenza vaccination. JCI Insight. 2020;5:e132155. doi: 10.1172/jci.insight.132155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elias G, Meysman P, Bartholomeus E, Neuter ND, Keersmaekers N, Suls A, Jansens H, Souquette A, Reu HD, Smits E, et al. Preexisting memory CD4 T cells in naïve individuals confer robust immunity upon vaccination [Internet] [accessed on 10 January 2021]. Available at . [DOI] [PMC free article] [PubMed]
- 95.Gorse GJ, Patel GB, Vitale JN, O'Connor TZ. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin Vaccine Immunol. 2010;17:1875–1880. doi: 10.1128/CVI.00278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ng KW, Faulkner N, Cornish GH, Rosa A, Harvey R, Hussain S, Ulferts R, Earl C, Wrobel AG, Benton DJ, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370:1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]