Abstract
Lung hernia following minimally invasive cardiac surgery is rare with few reported cases in the literature. Surgical repair is debated, and several methods have been described including a variety of synthetic and biological materials. We report a case of a 36-year-old woman who developed lung hernia and a strong retraction of the pectoralis major muscle after minithoracotomy that was performed for mitral valve surgery. The herniated lung was reduced and the chest wall defect was repaired with a non-cross linked acellular dermal matrix (ADM) anchored to the thoracic wall. At a 6-year follow-up, she was asymptomatic and without recurrence of the hernia. Our experience suggests that ADMs are a safe and reliable surgical technique for lung hernia repair due to their biological and mechanical properties, even in those secondary hernias to minithoracotomy where a complete muscle coverage of the matrix could not be provided.
Keywords: Lung hernia, Minimally invasive valve surgery, Minithoracotomy, Chest wall reconstruction, Acellular dermal matrix, Strattice
Introduction
The use of minithoracotomy in cardiothoracic surgery is steadily increasing due to a reduction in postoperative bleeding and pain, lower incidence of deep wound infection, improved cosmesis and decreased intensive care and hospital length of stay.1 Nevertheless, it seems to be more frequently associated with lung herniation than open thoracotomy. Some authors suggested as risk factors the extent of the operative trauma due to less ‘meticulous closure’ of the chest wall, and they recommend reapproximation of the pectoralis muscle to its original costal insertions; however, no specific guidelines are available.2
Latrogenic pulmonary hernia is rare and it is typically not life threatening, and surgical treatment is generally proposed for symptomatic hernias.3 Nevertheless, the presence of a pulmonary hernia may represent a problem in patients requiring further chest wall and breast surgery. Surgical alternatives include wire sutures, ribs approximation with non-resorbable sutures, autologous tissues, and a variety of synthetic and biological materials.2,4 We describe the use of a non-linked acellular dermal matrix (ADM) for lung hernia repair after minithoracotomy.
Case report
In 2014, a 36-year-old woman was admitted to our institution for a chest protrusion, which worsened with coughing and caused significant discomfort. Two-months before, she underwent minimally invasive mitral valve surgery through minithoracotomy. She also expressed the desire to undergo breast augmentation in the future. Chest computed tomography (CT) confirmed lung hernia between the III and IV right intercostal spaces with retraction of the pectoralis major muscle (Fig. 1). Operative repair of the hernia was performed by a team of thoracic and plastic surgeons. The skin incision was made in the inframammary fold at the same site of the previous minithoracotomy. The pectoralis major muscle appeared detached and strongly retracted; it was then isolated and raised along with the breast until the affected intercostal space was exposed. A chest wall defect measuring 4 x 8.5 cm between ribs 3 and 4 was identified (Fig. 2A). The lung was freed from adhesions and pulled into the pleural cavity, and the intercostal space diastasis was reduced with non-resorbable sutures (Ti-Cron 2). Due to the size of the defect, an ADM [Strattice™ (LifeCell Corp., Branchburg, N.J.), 16 x 20 cm] was used. The matrix was tightly anchored to the chest-wall securing it to the surrounding rib edges with interrupted non-resorbable sutures (Ethibond Excel 0), which were placed circumferentially around the defect in a concentric fashion, extending to one intercostal space above and below the defect. The pectoralis major muscle was released from the surrounding scar adhesions and it was stretched as much as possible in order to obtain a partial coverage of the matrix, and it was distally anchored to the ribs (Fig. 2B). A chest drain and one subcutaneous drain were finally placed, and they were removed after 4 and 15 days, respectively. The postoperative course was uneventful, and the patient was discharged on the sixth postoperative day. One year postoperatively, follow-up chest CT confirmed the correction of the pulmonary hernia (Fig. 3). At a 6-year follow-up, she was asymptomatic and without evidence of hernia recurrence as shown in the animation (Online Resource).
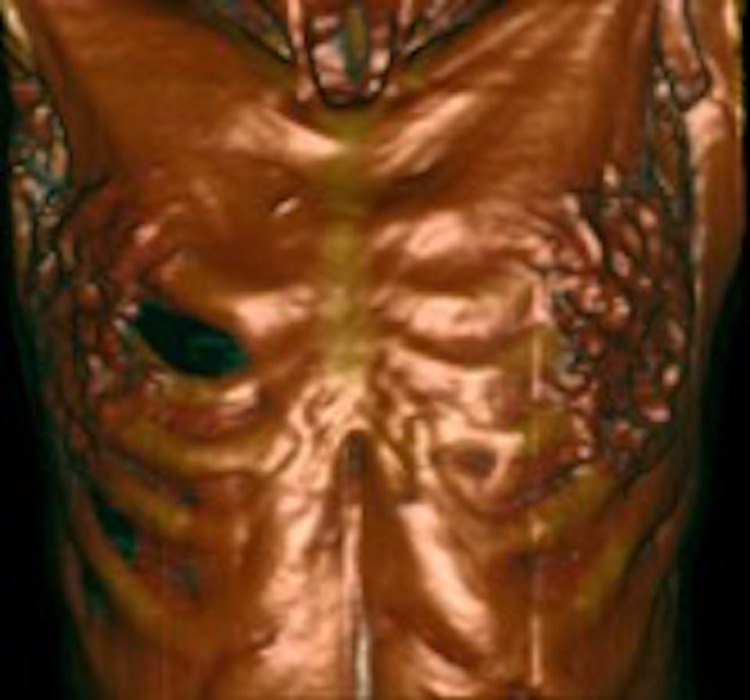
Fig. 1.
Preoperative chest CT scan showing a right-sided intercostal lung hernia with retraction of the pectoralis major muscle (coronal view with 3D reconstruction).
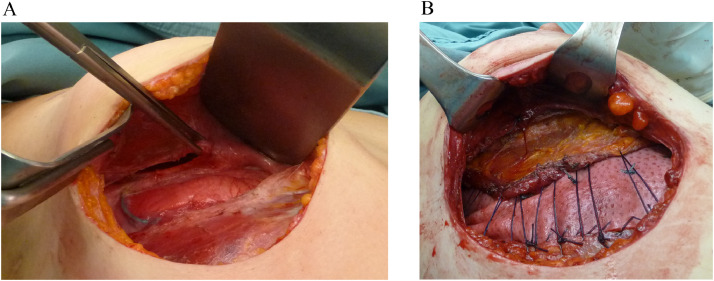
Fig. 2.
Intraoperative image of the hernia and pectoralis major retracted (A). The intercostal space diastasis was reduced with interrupted not-resorbable sutures, and a patch of StratticeTM was placed. The matrix was partially covered with the stretched pectoralis major muscle which was distally anchored to the ribs (B).
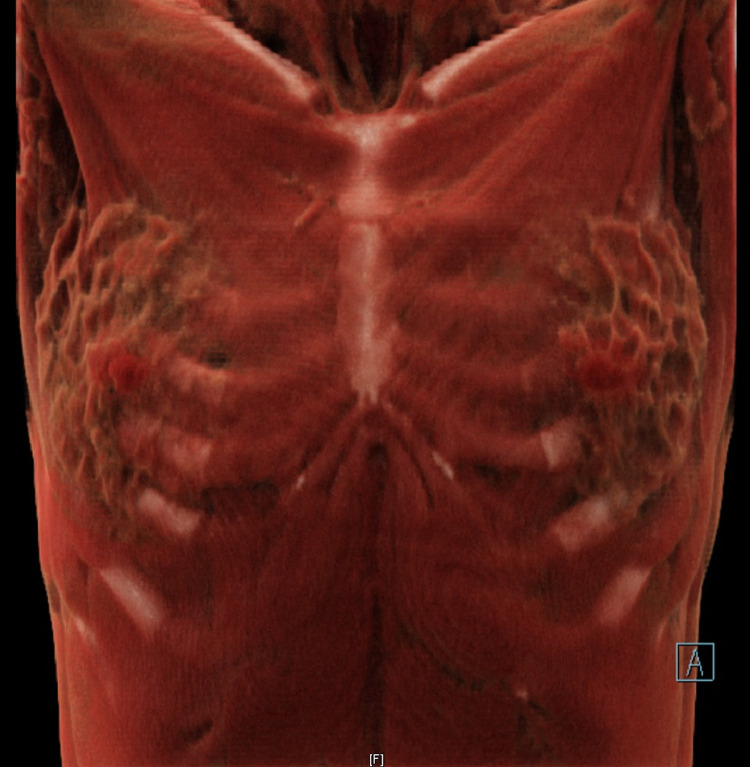
Fig. 3.
One-year follow-up chest CT confirmed restoration of the hernia (coronal view with 3D reconstruction).
Discussion
Lung hernia following minithoracotomy is rare with few reports, even if its real incidence could be potentially underestimated.2,3,5 Autologous musculofascial flaps for chest wall hernia repair have been proposed. They have the advantage of cost savings and a theoretical benefit of reducing the risk of infection. However, mobilizing intercostal and periosteal tissue is often challenging because the surrounding musculature can be attenuated and of poor quality. In those cases, the use of synthetic meshes such as Vicryl, PTFE, Dacron, Marlex, and Goretex or biological prosthesis is advisable. A bio-prosthetic implant confers a relatively reduced risk of infection whilst ensuring sustained durability. Therefore, biological mesh may represent an alternative to permanent materials, potentially acting as a matrix for cellular ingrowth over time.4,6 In the last decade ADMs have been proposed for chest wall reconstruction, even in patients at increased risk for synthetic mesh-related complications.7,8 Some ADMs have been chemically cross-linked to increase strength and durability.9 Nevertheless, several studies demonstrated that non-cross linked grafts are more bio-compatible and allow a more rapid and higher degree of cellular penetration and vascularization, resulting in stronger attachment to the tissue, and showed a lower rate of infection in a contaminated field compared with cross-linked mesh.10 Currently, ADM have been mainly used for chest wall reconstruction in case of postoncological defects,8, 9, 10, 11 whereas their use for lung hernia repair have been rarely reported. Mirza et al. described the use of Permacol for a lung herniation following a spontaneous rib fracture,4 and Humpries et al. reported the preventive use of Strattice for an intercostal damage secondary to multiple rib fractures.12
To our knowledge, the present seems to be the first report of lung hernia repair with ADM following a minithoracotomy, and it poses some considerations.
First, in the case of lung hernia a retracted pectoralis major muscle could cover only a portion of the mesh used for hernia repair, whereas the remaining surface would be covered only by skin and subcutaneous tissue. It is well known, as any other synthetic prosthesis, that the tissue thickness has a certain importance in limiting the incidence of complications. The vascular supply ensured by a muscle, in fact, is much greater than fat and skin alone, thus limiting the incidence of infections, which is the main risk of all synthetic durable implants.
Secondary, the risk of developing a pulmonary hernia is of particular interest in women who may require subsequent reconstructive or esthetic breast surgery. In fact, if the pectoralis muscle is no longer in its original location and a breast implant is needed, alternative techniques such as the latissimus dorsi flap, or an ADM would be necessary for an adequate coverage of the implant, making the operation more difficult, with a higher incidence of complications, more surgical scars and higher costs.
Finally, a synthetic mesh could cause a friction on the implant surface with a consequent theoretical injury of the breast prosthesis.
In our opinion ADM may represent a safe and reliable material for lung hernia repair following minithoracotomy. As a biological graft, ADMs are usually well integrated and incorporated with the host tissues, resulting in a soft, pliable and strong newly formed tissue. Among biological allografts, we choose a non-cross-linked ADM due to its physical and biological properties, which allow for more rapid vascularization and a reduced risk of infections. These features are even more important when a complete muscle coverage of the mesh could not be provided. Furthermore, as ADMs are fully integrated after a few months, there are no risks or contraindications for further chest wall or breast surgery. A drawback of ADM over synthetic meshes or autologous tissues is the higher cost. We also experienced a prolonged serum production which implies leaving drains in place for at least 10 to 15 days, in order to prevent seroma formation, which is a typical and well-known complication of ADMs in reconstructive surgery. Finally, as lung hernia following minithoracotomy remains unpredictable and probably underestimated, a preventive use of ADMs for a primary strengthening of intercostal space could be considered, especially when the local tissues have been injured by the surgical trauma.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
Ethical approval
For this type of article formal consent from a local ethic committee is not required.
Funding
None
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jpra.2021.01.012.
Appendix. Supplementary materials
References
- 1.Kastengren M., Svenarud P., Ahlsson A., Dalén M. Minimally invasive mitral valve surgery is associated with a low rate of complications. J. Intern. Med. 2019 doi: 10.1111/joim.12974. [DOI] [PubMed] [Google Scholar]
- 2.Athanassiadi K., Bagaev E., Simon A., Haverich A. Lung herniation: a rare complication in minimally invasive cardiothoracic surgery. Eur J Cardio-thoracic Surg. 2008 doi: 10.1016/j.ejcts.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Bhamidipati C.M., Iyalla K.I., Seymour K.A., Lutz C.J. Lung hernia following robotic-assisted mitral valve repair. J Card Surg. 2012 doi: 10.1111/j.1540-8191.2012.01463.x. [DOI] [PubMed] [Google Scholar]
- 4.Mirza A., Gogna R., Kumaran M., Malik M., Martin-Ucar A. The surgical management of intercostal lung herniation using bioprosthesis. J Surg Case Reports. 2011 doi: 10.1093/jscr/2011.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara R., Matsumoto K., Yamasaki N. Two cases of lung herniation treated by surgery or observation. Gen Thorac Cardiovasc Surg. 2016 doi: 10.1007/s11748-015-0556-5. [DOI] [PubMed] [Google Scholar]
- 6.Seder C.W., Allen M.S., Nichols F.C. Primary and prosthetic repair of acquired chest wall hernias: a 20-year experience. Ann Thorac Surg. 2014 doi: 10.1016/j.athoracsur.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Butler C.E., Langstein H.N., Kronowitz S.J. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg. 2005 doi: 10.1097/01.prs.0000181692.71901.bd. [DOI] [PubMed] [Google Scholar]
- 8.Giordano S., Garvey P.B., Clemens M.W. Synthetic mesh versus acellular dermal matrix for oncologic chest wall reconstruction: a comparative analysis. Ann Surg Oncol. 2020 doi: 10.1245/s10434-019-08168-z. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt J., Redwan B., Koesek V. Thoracic wall reconstruction with acellular porcine dermal collagen matrix. Thorac Cardiovasc Surg. 2016 doi: 10.1055/s-0034-1383512. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico G., Manfredi R., Nita G. Reconstruction of the thoracic wall with biologic mesh after resection for chest wall tumors: a presentation of a case series and original technique. Surg Innov. 2018 doi: 10.1177/1553350617745954. [DOI] [PubMed] [Google Scholar]
- 11.Khalil H.H., Kalkat M., Malahias M.N. Chest wall reconstruction with porcine acellular dermal matrix (strattice) and autologous tissue transfer for high risk patients with chest wall tumors. Plast Reconstr Surg - Glob Open. 2018 doi: 10.1097/GOX.0000000000001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries A.R., Lube M. Use of porcine dermal allograft to prevent pulmonary herniation in a patient with multiple displaced rib fractures requiring Surgical Fixation. Am. Surg. 2015 doi: 10.1177/000313481508100308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.