To the Editor,
Tripeptidyl peptidase 2 (TPP2) deficiency is associated with a complex immunological phenotype including immunodeficiency and immune dysregulation. TPP2 is a large proteolytic complex in the cellular cytosol, catabolizing proteins to amino acids. A deficiency of TPP2 is thought to cause an imbalance in the cellular and lysosomal proteolytic pathways, allowing dominance of the lysosomal pathway. Lysosomes accumulate, reducing glycolysis and impairing both cellular metabolism and the production of effector cytokines [1]. To date, only 6 patients from 3 different families have been described [1, 2]. Here, we present an additional seventh case (P7) with milder clinical phenotype, resulting from a novel homozygous splice site mutation in TPP2 c.1913 + 5G > A, and summarize all published cases of TPP2 deficiency to date.
P7 is a 15-year-old girl of consanguineous Pakistani background. She was born by normal vaginal delivery at 42 weeks gestation weighing 2.2 kg, following a pregnancy complicated by hyperemesis. She has 3 older siblings of both sexes, who are all well, but mother had previously lost twins at 5 months gestation. She had seizures in the neonatal period and a further seizure at the age of 11 years in Pakistan, at which time a CT scan revealed periventricular calcification. Focused genetic testing for causes of intracranial calcification did not identify an abnormality. P7’s development was delayed from an early age (talking at 2 and walking at 3) and is currently in mainstream education requiring1:1 support. She completed the normal vaccination schedule including BCG without complication. She has mild asthma and eczema, digital and peri-oral warts, a history of chronic suppurative otitis media with grommet insertions, and recurrent paronychia leading to incision and drainage on two occasions. P7 developed immune thrombocytopenia (ITP) in 2015, requiring treatment with intravenous immunoglobulin (IVIG) for epistaxis with platelet count <5 × 109/L (normal range 150–400).
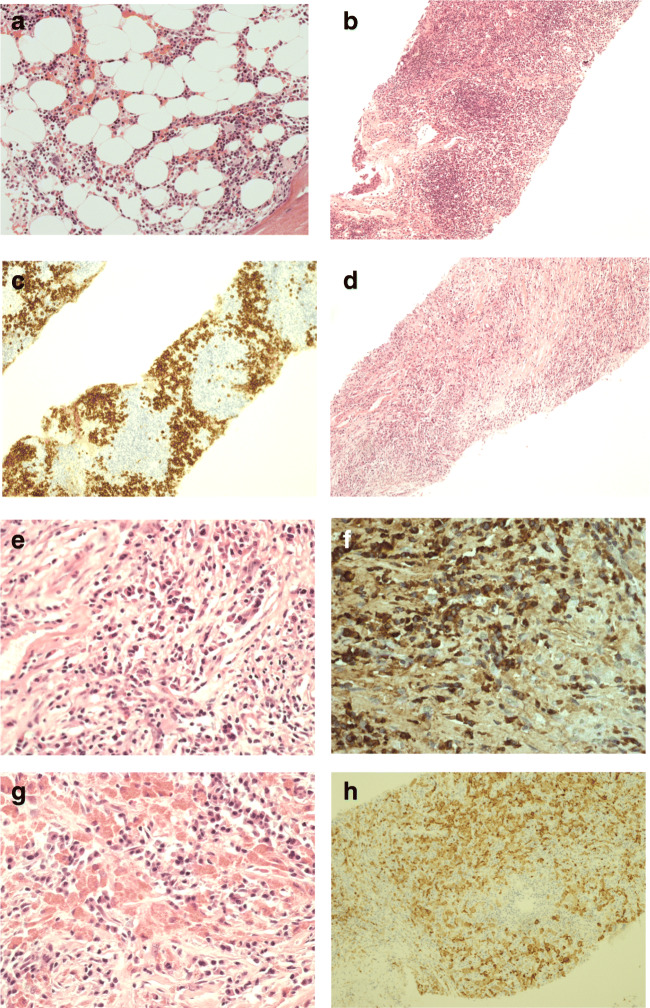
In 2016, the patient presented to her local hospital with chest pain following a push in the playground. Chest X-ray showed a mediastinal mass, revealed on CT to be a 7.4 cm × 4.8-cm anterior superior mediastinal mixed cystic and solid lesion. Suspicious abdominal lymph nodes were identified, but there was no hepatosplenomegaly. Biopsies of the mediastinal mass on two occasions and a groin lymph node biopsy were taken. The histology showed advanced fibrosis with unusual histiocyte response with prominent eosinophilic inclusion and increased IgG4-positive cells (Fig. 1, and Supplementary Fig. 1). Evaluation by the hematological malignancy diagnostic service excluded lymphoproliferative disease. Bone marrow trephine histology reveals normal trilineage hematopoiesis and no plasma cell excess or macrophage infiltrate (Fig. 1). Cultures for Mycobacterium tuberculosis and extended infection screens were negative. Given the finding of IgG4-positive plasma cells, but no features typical of IgG4-related disease (IgG4RD), the patient was referred to pediatric immunology for further evaluation.
Fig. 1.
Histology of bone marrow, lymph node, and mediastinal mass biopsy. a Bone marrow trephine histology normal trilineage hematopoiesis, no plasma cell excess or macrophage infiltrate. b Lymph node biopsy showing retained architecture with B cell follicles and extensive plasmacytosis as shown in c lymph node biopsy CD138 IHC. Mediastinal mass biopsy (d) showed extensive fibrosis and mixed inflammatory infiltrate rich in plasma cells (e), with excess of IgG4 expressing plasma cells (f). In several areas, plasma cells were intermingled between macrophages with prominent eosinophilic cytoplasmic inclusions (g) and CD68 (h)
Investigations showed mild thrombocytopenia (platelet count 109 × 109/L, normal range 150–400) with otherwise normal full blood count and immunoglobulin levels and adequate levels of functional antibodies to haemophilus, tetanus, and pneumococcus. Complement C3 and C4 levels were within normal limits and functional studies showed adequate activation of classical and alternate complement pathways. Basic lymphocyte profile revealed T, B, and NK cell numbers to be within the normal range. There were relatively low levels of naïve (CD45RA+/CD27+) CD4 and CD8 T cells (27.6% (normal range 49–90 and 23.76% (normal range 49–88)), respectively. HLA-DR was expressed on the surface of CD4+ (52.05%) and CD8+ T cells (59.37%). B cell were largely naïve (CD27−/IgD+) with low levels of non-switched (CD19+ CD27+ IgD+ 1.21%) and class-switched (CD19+ CD27+ IgD− 2.81%) memory B cells. There was no expansion of double negative T cell population and vitamin B12 levels were normal. Further tests showed TCR distribution comparable to control, apart from absent Vb7.2 (typically 1.47% in HC); functional T cell studies showed partial reduction in lymphocyte proliferation in response to PHA (Supplementary Table 1, and Supplementary Table 2). Additional autoimmune screen showed low level positive anti-La antibody in isolation. Anti-GAD, thyroid peroxidase, adrenal cortex, islet cell, and gastric parietal, mitochondrial, smooth muscle, and LKM antibodies were all negative. She had evidence of past infection with EBV, CMV, VZV, and HSV with no obvious sequelae and negative blood EBV and CMV PCRs.
The working diagnosis at this stage lies between autoimmune lymphoproliferative syndrome, IgG4RD, and complex autoimmunity with lymphoproliferation. P7 was suffering from dysphagia and palpitations, and although neither was felt clinically to be related to her mediastinal mass, she was commenced on a course of corticosteroids for 6 weeks, and the family consented for genetic studies to elucidate the diagnosis. There was minimal improvement in the mass on subsequent imaging following the course of corticosteroids, and it has remained stable since, with resolution of the other lymph nodes.
A modified exome sequencing approach was performed using Agilent SureSelectXT with All Exon v5 capture library and sequenced on Illumina HiSeq 3000 for 2 × 150-bp paired-end sequencing. Genetic testing (for further details, please see supplementary materials) identified a potentially damaging splice site mutation in TPP2, c.1913 + 5G > A. This mutation was homozygous in P7 and showed segregation with disease (Fig. 2). P7 mRNA analysis showed mis-splicing, with skipping of exon 15 (Fig. 3a). The translation reading frame is shifted in the mis-spliced transcript, which now contains a premature stop codon and would produce a truncated polypeptide of 614 amino acids (Fig. 3a). Subsequent Western blot of PBMC protein lysate from the patient showed that the full-length protein is not detectable (Fig. 3b). We also did not detect predicted truncated TPP2 fragment; however, this might be due to the fact that the anti-TPP2 antibody we used does not recognize the N-terminus. Nevertheless, even if the truncated peptide fragment was present, it would be missing the central domain and the C-terminus, where TPP2 monomers interact to form dimers and large homo oligomeric “spindle” complexes, which are essential for peptidase function of TPP2.
Fig. 2.
TPP2 gene sequences were amplified by PCR and Sanger sequenced using genomic DNA from blood samples. Pedigree showing affected proband (black circle), silent carriers (half-filled symbols), and wild-type (WT) family members clearly demonstrates segregation of the c.1913 + 5G > A mutation with disease
Fig. 3.
a cDNA was made from P7 mRNA, amplified by PCR and Sanger sequenced. Skipping of 77 nucleotides, equating to the whole of exon 15, is clearly visible. The translated amino acid sequence of the mis-spliced transcript is shown above the nucleotide sequence. b Western blot of PBMC protein lysate from the TPP2 patient (P7) and a control individual, staining for TPP2 (138KDa) and GAPDH (36KDa). Full length TPP2 is present in the control but absent in the patient confirming TPP2 deficiency. GAPDH intensity is similar in both patient and control confirming similar loading of protein. A non-specific band is present at approximately 115 KDa in both the patient and control
P7 suffered a relapse of ITP during 2016, with platelet count dropping to 36 × 109/L. She was treated with a further course of steroids (prednisolone 40 mg od). Despite initial response, she became steroid dependent and was converted to eltrombopag with good effect.
On the basis of the persistence of her mediastinal mass, thrombocytopenia requiring eltrombopag, and the impact of TPP2 deficiency on mTOR dynamics [1], P7 was commenced on sirolimus, allowing withdrawal of the eltrombopag, but there was no further improvement in the mediastinal mass. Since commencing sirolimus, P7 required several courses of antibiotics for infected nares, now resolved, likely related to her facial filiform warts, an episode of herpetic whitlow, and a period of prolonged fever on return from Pakistan. The latter episode was associated with a return of thrombocytopenia, treated as influenza and otitis media initially, then as enterococcus UTI, neither of which improved her fever, which finally settled. She also suffered from microbiologically proven COVID-19, with a mild clinical course, and documented viral clearance. She has not developed any new autoimmune phenomena in 5 years of follow-up, although her thyroid has now been noted to be multicystic, with normal thyroid function tests.
Recently repeated immunological investigations, while on treatment with sirolimus, showed normal basic lymphocyte profile, but still reduced naïve CD4 T cells (22.5%) and reduced levels of non-switched and class-switched memory B cells (Supplementary Table 1). Interestingly, repeat functional T cell studies showed proliferative responses to PHA and anti-CD3, which are now comparable to healthy controls (Supplementary Tables 1 and 2). The recovery of T cell function in this case might be a result of sirolimus therapy, since a similar improvement has been observed in a case of activated phosphoinositide 3-kinase δ syndrome where sirolimus treatment resulted in restoring T cell proliferation and IL-2 secretion [3].
Previous studies of TPP2-deficient patients (P1) showed that TPP2 deficiency was associated with premature T cell senescence and increased expression of CD57 on the CD8 T cells. In the case of P7, these investigations were not done at the time of her initial presentation, but were performed later after she had been treated with sirolimus. The recent investigations showed an increased expression of CD57 on CD8 T cells (50%) compared to HC (average 5.4%) (Supplementary Fig. 2). The expression of PD-1 on the CD4 and CD8 T cells was comparable to healthy controls. Considering that TPP2 deficiency is linked with immunodysregulation and autoimmunity, we analyzed the distribution of CD4 T helper (Th) cells Th1, Th2, and Th17. Compared to healthy controls, the patient showed a higher proportion of Th1, reduced Th2, and similar percentage of Th17 cells (Supplementary Fig. 3).
Here, we describe a novel TPP2 mutation that is associated with a milder phenotype compared to the cases previously described. The reasons for milder clinical phenotype in this case, despite the novel mutation leading to likely complete loss of protein function, remain uncertain. The published cases of TPP2 deficiency have so far illustrated severe autoimmune and immunodeficiency phenotypes. Of the 7 patients described so far, 6 had ITP, 5 had autoimmune hemolytic anemia, 3 had neutropenia, 5 had recurrent or persistent viral infections, and 4 had recurrent lower respiratory tract infections. Moreover, 6 of the 7 patients had combined autoimmunity and infection symptoms. Only one patient had autoimmunity without the presence of infection and died at the age of 37 months from acute hemolytic crisis. Three of these patients have had hematological stem cell transplant with one patient death following transplant. Another patient had an orthotopic liver transplant and subsequently died [1, 2]. A summary of these cases is given in Table 1.
Table 1.
A summary of cases
Blank space = not affected
Colored space = affected
F female, M male, ITP immune thrombocytopenia, AIHA autoimmune hemolytic anemia, HSCT hematopoietic stem cell transplantation
The patient described here has overall a milder phenotype compared to earlier reported cases and several unique features. The finding of a cystic mass appears to be novel. In addition, the histological description of histiocytes within the mediastinal mass has not been reported previously in this context. The histiocytes have similar appearance to the cells found in crystal-storing histiocytosis (CSH). This is a rare condition in which crystalline material accumulates in the cytoplasm of histiocytes [4]. CSH is usually associated with hematological disorders that express monoclonal immunoglobulins, such as lymphoplasmacytic lymphoma (LPL), multiple myeloma (MM), and monoclonal gammopathy of undetermined significance (MGUS) [4]. Typically, the crystalline material is predominantly made up of kappa light chains. In these conditions, the accumulation of the crystalline material is usually due to excessive production of immunoglobulins. In contrast, in TPP2 deficiency, this occurrence might be due to impaired lysosomal function, since the lysosome-mediated degradation is the usual route for removing antibody-antigen complexes. However, there are other variants of CSH in which the crystalline material is not an immunoglobulin [5]. Furthermore, there are cases where CSH is not obviously associated with hematological disorder. Whether such rare, unexplained cases might be caused by a milder form of TPP2 deficiency remains to be determined.
Supplementary Information
Higher magnification of macrophages (panel G in Fig. 1) to emphasize the dense eosinophilic cytoplasmic inclusions. Arrows identify several examples where these inclusions have distinct sharp borders and include rhomboid and elongated shapes. The morphological pattern is distinctive and consistent with the diagnosis of a crystal-storing histiocytosis. (PNG 1528 kb)
T-helper (Th) 1, Th2 and Th17 distribution in peripheral blood. Whole EDTA blood was stained with a combination of CD3-V500, CD4-BV421, CCR6-Pe and CXCR3-Alexa-Fluoro 647 A) representative dot plot shows the gating strategy B) The results from 5 healthy controls (HC)-dark circles and the patient (P7)-dark squares. All investigations were done whilst the patients was on treatment with sirolimus. (PNG 484 kb)
CD57 expression on T cells. Whole EDTA blood was stained with a combination of CD3-V500, CD4-BV421 and CD57 Alexa fluoro 488 (all antibodies from Becton Dickinson, UK) at room temperature protected from light. Erythrocytes were subsequently lysed using BD red cell lysis solution and following a further 10 min incubation the samples were washed with PBS/1% FBS using centrifugation (1500rmp for 6 mins). Following the final wash, cells were resuspended in 400ul of PBS +0.5% formaldehye and cells analyzed using FACSCanto II flow cytomter (BD) using FACSDIVA software. A) The percentage of CD57+ cells from gated CD3+ CD4+ and B) The percentage of CD57 CD8+ T cells identified from CD3+ CD4− T gate. HC-healthy control, P7 (TPP2 deficient patient). All investigations were done whilst the patients was on treatment with sirolimus. (PNG 265 kb)
(DOCX 19 kb)
(DOCX 19 kb)
Author Contribution
CS, SO, and SP collected clinical information; LR, CC, IB, and JP performed experiments, RT obtained and analyzed histology data, CS, LR, and SS wrote the first draft of the manuscript. RA, RT, and SS analyzed the data. All authors read, edited, and approved the manuscript. SS design and funded the study.
Funding
This research is supported by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre and grant from CSL Behring. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Declarations
Ethics Approval
Ethics approval was granted by the Leeds (East) Research Ethics Committee. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to Participate
Participants provided their consent to participate in this study.
Consent for Publication
Consent was sought and gained from the participants to publish the findings of this study.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Claire Stockdale and Laura Rice contributed equally to this work.
References
- 1.Lu W, Yu Z, McDonald DO, Hambleton S, Helen CS, et al. Dual proteolytic pathways govern glycolysis and immune competence. Cell. 2014;159:1578–1590. doi: 10.1016/j.cell.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stepensky P, Rensing-Ehl A, Gather R, Revel-Vilk S, Fischer U, Nabhani S, Beier F, Brümmendorf TH, Fuchs S, Zenke S, Firat E, Pessach VM, Borkhardt A, Rakhmanov M, Keller B, Warnatz K, Eibel H, Niedermann G, Elpeleg O, Ehl S. Early-onset Evans syndrome, immunodeficiency, and premature immunosenescence associated with tripeptidyl-peptidase II deficiency. Blood. 2015;125(5):753–761. doi: 10.1182/blood-2014-08-593202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, Stoddard J, Ouyang W, Frucht DM, Rao VK, Atkinson TP, Agharahimi A, Hussey AA, Folio LR, Olivier KN, Fleisher TA, Pittaluga S, Holland SM, Cohen JI, Oliveira JB, Tangye SG, Schwartzberg PL, Lenardo MJ, Uzel G. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dogan S, Barenes L, Cruz-Vetrano WP. Crystal-storing histiocytosis: report of a case, review of the literature (80 cases) and a proposed classification. Head Neck Pathol. 2012;6(1):111–120. doi: 10.1007/s12105-011-0326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebrail F, Knapp M, Perrotta G, Cualing H. Crystalline histiocytosis in hereditary cystinosis. Arch Pathol Lab Med. 2002;126(9):1135. doi: 10.5858/2002-126-1135-CHIHC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Higher magnification of macrophages (panel G in Fig. 1) to emphasize the dense eosinophilic cytoplasmic inclusions. Arrows identify several examples where these inclusions have distinct sharp borders and include rhomboid and elongated shapes. The morphological pattern is distinctive and consistent with the diagnosis of a crystal-storing histiocytosis. (PNG 1528 kb)
T-helper (Th) 1, Th2 and Th17 distribution in peripheral blood. Whole EDTA blood was stained with a combination of CD3-V500, CD4-BV421, CCR6-Pe and CXCR3-Alexa-Fluoro 647 A) representative dot plot shows the gating strategy B) The results from 5 healthy controls (HC)-dark circles and the patient (P7)-dark squares. All investigations were done whilst the patients was on treatment with sirolimus. (PNG 484 kb)
CD57 expression on T cells. Whole EDTA blood was stained with a combination of CD3-V500, CD4-BV421 and CD57 Alexa fluoro 488 (all antibodies from Becton Dickinson, UK) at room temperature protected from light. Erythrocytes were subsequently lysed using BD red cell lysis solution and following a further 10 min incubation the samples were washed with PBS/1% FBS using centrifugation (1500rmp for 6 mins). Following the final wash, cells were resuspended in 400ul of PBS +0.5% formaldehye and cells analyzed using FACSCanto II flow cytomter (BD) using FACSDIVA software. A) The percentage of CD57+ cells from gated CD3+ CD4+ and B) The percentage of CD57 CD8+ T cells identified from CD3+ CD4− T gate. HC-healthy control, P7 (TPP2 deficient patient). All investigations were done whilst the patients was on treatment with sirolimus. (PNG 265 kb)
(DOCX 19 kb)
(DOCX 19 kb)