Abstract
Fabry disease (FD) (Anderson-Fabry disease, OMIM 301500) is a genetic disorder caused by a pathogenic variant in the GLA gene on chromosome Xq22 that produces a deficiency in the lysosomal enzyme alpha-galactosidase A.
It is transmitted as an X-linked trait, although de novo mutations have been described. The objective of this report is to describe the clinical characteristics of a patient with FD who is a carrier of a mutation not previously studied, in order to provide information on the genotype-phenotype correlation in this pathology.
38-year-old patient who consulted Neurology for positional vertigo. He also reported acroparesthesia, anhidrosis, heat intolerance and episodes of abdominal pain, with postprandial discomfort from 10 years of age. Physical examination showed horizonto-rotatory nystagmus in both looks, the rest of the neurological evaluation did not present abnormalities. The presence of umbilical and thighs angiokeratomas was identified. Determination of Alpha-Galactosidase in blood was requested: 0.34 μmol/l/h (2.10–10.51 μmol/l/h). Genetic analysis detected a deletion of a guanine at position 448, in exon 3 of the GLA gene (c.448delG). This mutation was considered to be pathogenic, confirming the diagnosis of FD, although it is not described in the data bases. Genetic counseling and a family pedifree study were performed without finding relatives with this variant of the GLA gene or a family history of FD, which suggests a de novo mutation.
Keywords: Fabry disease, Alpha-galactosidase A enzyme, GLA gene, Novo mutation, Cardiac strain, Myocardial hypertrophy
1. Introduction
Fabry disease (FD) (Anderson-Fabry disease, OMIM 301500) is a genetic disorder caused by a pathogenic variant in the GLA gene on chromosome Xq22 that results in a deficiency in the lysosomal enzyme alpha-galactosidase. FD is transmitted as an X-linked trait, although de novo mutations have been described. Recently, there have been very important advances in molecular assessment for FD. More than 900 mutations have been identified in the GLA gene (databases www.hgmd.org and www.dbfgp.org/dbFgp/fabry), including nonsense mutations, small deletions/insertions, splice mutations, and large rearrangements of genes. Most of the pathogenic variants of the GLA gene involved are nonsense exon mutations and there are few descriptions of intronic mutations. These variants are usually inherited and cases of de novo appearance, that is, spontaneously arising, occur rarely [1,2].
The alpha-galactosidase A enzyme is widely expressed in different tissues and its deficiency results in the deposition of glycosphingolipids, particularly globotriaosylceramide (Gb3), in different organs and tissues (central and peripheral nervous system, skin, eyes, heart and kidneys). FD is a relatively rare condition with an annual incidence ranging from 1: 40,000 to 1: 117,000, but in view of its heterogeneous phenotype it is probably underdiagnosed [3]. The time elapsed from the first signs and symptoms to the diagnosis of FD is 15 years [4], because it is not suspected due to its low frequency and the diversity and non-specificity of its signs and symptoms [5].
Hemizygous male patients develop a severe phenotype with early symptoms called classical phenotype, and women heterozygous for the disease exhibit phenotypes that range from asymptomatic to those with vital organ involvement [6,7]. More recently, phenotypes called late variants have been described, in which manifestations are restricted to a single target organ, appear in adulthood, and lack the typical childhood symptoms of the classic forms.
Cardiovascular complications are the leading cause of death [8], 40% of patients die from cardiovascular complications versus <10% as a result of neurological and renal complications [9].
More than a half of the patients present cardiac compromise, the most prevalent alteration being hypertrophy of the left ventricle (LVH) [10]. Although hypertrophy usually precedes myocardial fibrosis, this may be the first manifestation of cardiac involvement, especially in women [10,11].
The most frequent alterations reported are neurological manifestations (84% men, 79% women). The most common symptom is neuropathic pain that begins in childhood in men and in adolescence in women, typically acroparesthesias [13]. Auditory symptoms, such as hearing loss and tinnitus, are evident in approximately half of patients [13].
Central nervous system (CNS) involvement manifests as vascular abnormalities demonstrated by brain magnetic resonance imaging (MRI) (hyperintense lesions of the white matter), transient ischemic attack (TIA) and cerebrovascular accident (CVA) particularly in patients under 55 years of age.
The progressive deterioration of renal function is observed in half of the patients. The need for transplantation or dialysis was reported in 17% of men and 1% of women. The presence of proteinuria is characteristic of FD nephropathy [13].
Gastrointestinal manifestations (abdominal pain and diarrhea) are identified in approximately 50% of cases [13].
The diagnosis of FD is essential because, unlike other pathologies with which it is essential to make a differential diagnosis, it is possible to perform enzyme replacement therapy (ERT) with Agalsidase Alpha or Beta. ERT has been shown to improve quality of life and reduce the progression of cardiovascular and renal compromise in patients with FD [14].
The aim of this report is to describe the clinical characteristics of a patient with FD who is a carrier of a mutation not previously studied, in order to provide information on the genotype-phenotype correlation in this disease.
2. Case report
38-year-old patient who consulted Neurology because of objective positional vertigo associated with nausea. He also reported acroparesthesia and dysesthesia in hands and feet from the age of 10, in treatment with carbamezepine 400 mg/day. He also manifested anhidrosis and heat intolerance at the same time of evolution and episodes of abdominal pain, with postprandial discomfort.
2.1. He denied personal and family medical history as well as cardiovascular risk factors
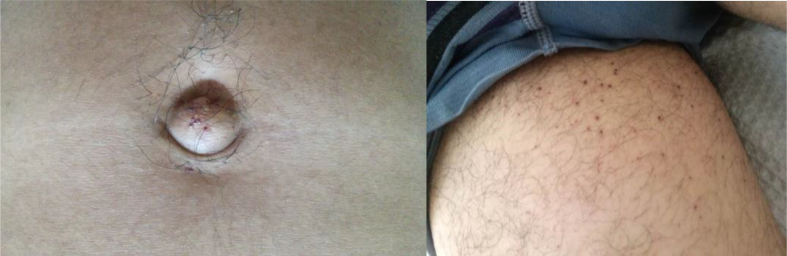
Physical examination showed horizonto-rotatory nystagmus in both looks, the rest of the neurological evaluation did not present abnormalities. The presence of umbilical and thighs angiokeratomas was identified (Fig. 1).
Fig. 1.
Umbilical and thigh angiokeratomas.
2.2. Complementary exams
2.2.1. Laboratory
The most relevant data from the general laboratory were: Hematocrit: 34.5%, Hb: 11.1 g/dl, Leukocytes: 9120/mm3, Platelets: 509000/mm3, Creat: 0.83 mg/dl, Uremia: 17 mg/dl, Total Col: 132 mg/dl, LDL Col: 93.4 mg/dl, HDL Col: 37.1 mg/dl, TGL: 62 mg/dl and Glyc: 104 mg/dl. The presence of anemia stands out, the rest of the parameters did not show alterations.
The evaluation of renal function reported values within the normal range: [24–]hour microalbuminuria: 11.8 mg and Creat Cl: 113.4 ml/min.
Given the suspicion of FD, the determination of Alpha-Galactosidase in blood was requested: Alpha-Galactosidase enzymatic activity: 0.34 μmol/l/h (2.10–10.51 μmol/l/h). Once the diagnosis was confirmed, a sample was sent for genetic analysis.
Genetic analysis was performed by DNA isolation from blood, PCR amplification, purification and subsequent sequencing of the 7 exons of the GLA gene. A deletion of a guanine at position 448 was detected in exon 3 of the GLA gene (c.448delG), which produces a change in the reading frame of the protein. This mutation was considered pathogenic, confirming the diagnosis of FD.
2.3. Subsequently, tests were requested for cardiological, CNS, ophthalmological and renal evaluation
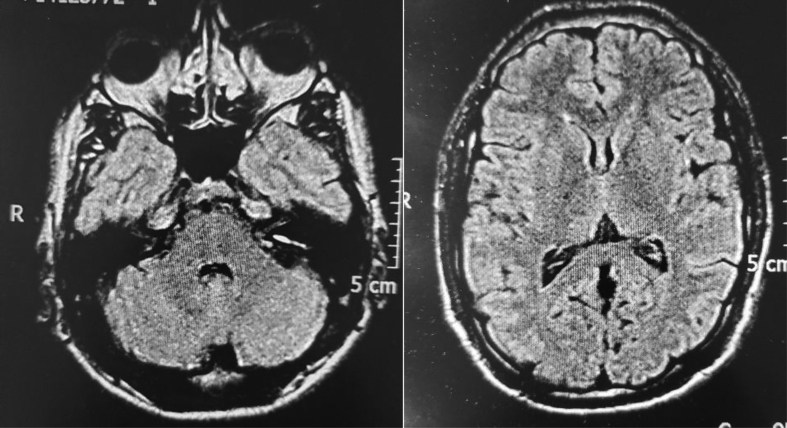
Chest X-ray, renal ultrasound, ECG and brain MRI did not report alterations (Fig. 2).
Fig. 2.
Brain MRI axial, flair effect normal.
Audiometry: bilateral hearing loss at high tones (4000 to 8000 Hz) of mild to moderate degree.
Slit lamp ophthalmological study: presence of spiral bands in the cornea (cornea verticilata).
2.4. Fine fiber study (QST) commitment of fibers to delta and C
2.4.1. Echocardiogram
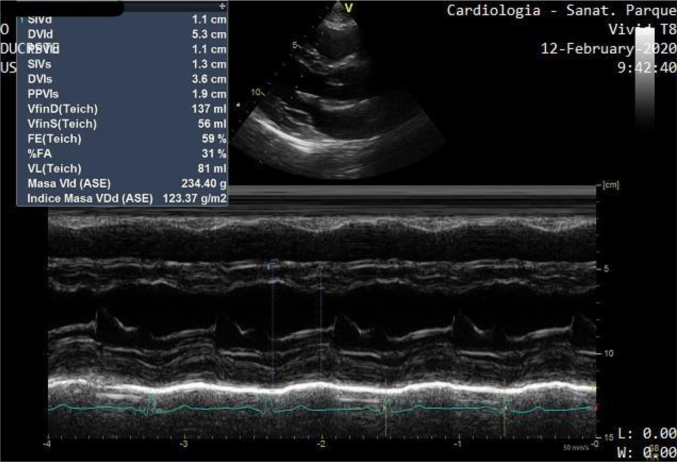
Left ventricular end diastolic diameter (LVEDD): 53 mm, left ventricular end diastolic volume (LVEDV): 137 ml, left ventricular end sistolic diameter (LVESD): 36 mm, left ventricular end sistolic volume (LVESV): 56 ml, interventricular septum diameter (diastolic) (IVSD): 11 mm, posterior wall diameter (diastolic) (PWD): 11 mm, normal wall motility, ejection fraction (EF): 59%, left ventricular mass index (LVMI): 123 g/m2, left atrium (LA): 38 ml/m2, E/e': 6.
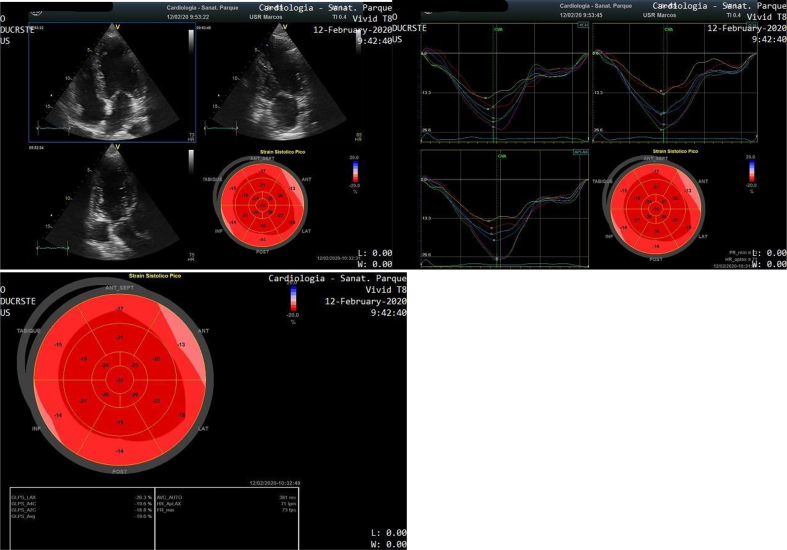
Speckle Tracking Echocardiography (STE) assessment was performed reporting normal Global Longitudinal Strain (GLS): - 19.6%. Segmental Longitudinal Strain analysis showed the presence of 3 segments (inferobasal, posterobasal and anterobasal) with values > −15% (Fig. 3, Fig. 4).
Fig. 3.
M-mode echocardiogram reporting LVH (LVMI: 123 g/m2).
Fig. 4.
Speckle Tracking Echocardiography showing normal GLS - 19.6%. Inferobasal, posterobasal and anterobasal segments (yellow, red and blue curves) reported values > − 15%, suggesting deterioration of the Longitudinal Strain.
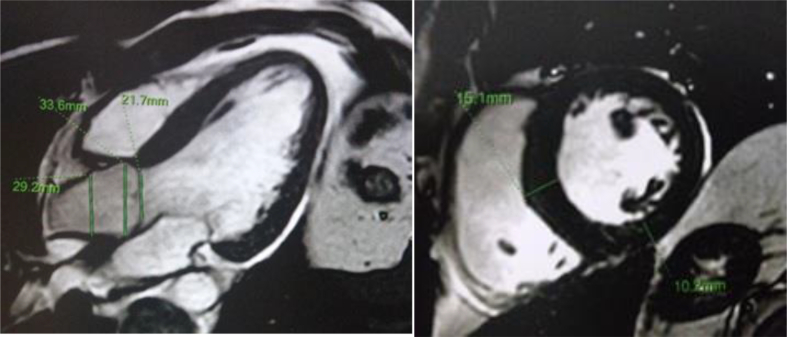
Cardiac Magnetic Resonance Imaging (MRI): left ventricular end diastolic volume (LVEDV): 144 ml, left ventricular end sistolic volume (LVESV): 64 ml, interventricular septum diameter (diastolic) (IVSD): 14 mm, posterior wall diameter (diastolic) (PWD): 12 mm, ejection fraction (EF): 52%. No evidence of Gadolinium Late Enhancement (Fig. 5).
Fig. 5.
MRI reporting LVH. No evidence of Gadolinium late enhancement.
Finally, genetic counseling and a family pedifree study were carried out. Father and mother deceased, without significant antecedents, sister and son were negative. Two children of a mother's sister were also studied with negative results. No relatives with this variant of the GLA gene or family history of Fabry disease were found. These data suggest that the present case would be a de novo mutation.
2.5. Therapeutic behavior
ERT (Agalsidase Alfa) was started due to the presence of LVH, LA dilation and segmental deterioration of the myocardial Longitudinal Systolic Strain, accompanied by acroparesthesia, hearing loss, anhidrosis, vertigo and abdominal pain.
3. Discussion
FD is a rare disease in which significant advances in diagnosis and therapy have been made in recent years. The heterogeneity of its manifestations causes a high degree of variation in its clinical symptoms, both in hemizygous and heterozygous patients. Phenotypic presentations are even more variable in heterozygous patients due both to the nature and type of the GLA variant and to the X chromosome inactivation profiles in the different organs. Due to the wide heterogeneity in the natural history of patients with FD, there has been a growing interest among experts in the scientific community in establishing potential associations between genotypes and phenotypic presentations of GLA (for example, international database of genotypes and phenotypes Fabry disease - hgmg.org - dbfgp.org/dbFgp/fabry/ - fabry-database.org) to optimize clinical management. However, many GLA variants remain absent or unclassified in these specific gene databases [1].
In the present report, the clinical compromise of a patient with FD with a c.448delG mutation was described that we interpret as pathogenic since it is a deletion that generates displacement in the reading frame resulting in a reduced activity of alpha galactosidase and a characteristic phenotype of the desease. The frequency of de novo mutations in FD is unknown, Rodriguez-Mari et al. studied 22 families Spanish with FD and detected a de novo mutation was 4,5% (1 of 22 families). In other studies, Kobayashi et al. studied 74 families Japanese and detected a de novo mutation 6,8% (5 of 74 families). Patients with Fabry have a relatively low frequency of de novo mutations of the GLA gene in relation to the frequency de novo widely reported in other X-linked disorders such as Duchenne muscular dystrophy and hemophilia where approximately one-third of mutations of these two diseases are expeted to arise de novo [15].
This patient presents multisystemic involvement, including acroparesthesia, hearing loss (in pure tone audiometry evaluation), anhidosis, vertigo, abdominal pain, cornea verticilata, angiokeratomas and heart disease (LVH and segmental alterations of the myocardial Strain). There was no evidence of involvement of the kidneys. This variant is considered pathogenic although it is not described in the data banks.
In the bibliographic review, it was found that this mutation was previously named but the description of its clinical manifestations or the evolution of the organic involvement of the disease over time was never made. We believe that it is very important to characterize its clinical features, and to assess its severity and prognosis. In prior presentations this mutation was included, focusing the study of possible nephrological compromise, and in the case of this patient, no renal function alteration was demonstrated, highlighting the cardiological and neurological involvement [[16], [17], [18], [19]].
Hearing loss in EF is a common symptom, and has been reported in both children and adults. As in the case described, many people are unaware of their decreased hearing as evidenced by different reports of hearing loss in FD, where 41% of patients refer it but when performing a pure tonal audiometry the alteration is demonstrated in 78% of the FD patients [20].
Thus, the importance of studying the heart in these patients is fundamental, since cardiomyopathy can begin in early stages and, as mentioned, it is the cause of death in most cases.
The presence of unexplained LVH, especially if it is concentric, symmetric and non-obstructive, combined with the rest of the signs and symptoms described above should make the diagnosis of FD suspect [21].
STE evaluation is a relatively inexpensive method that allows the analysis of global systolic function and of each one of the myocardial segments, semi-automatically, minimizing angular dependence and intra- and inter-observer variability, Thus, it is an interesting option for the diagnosis, prognosis and follow-up of patients with FD [[22], [23], [24]].
There is growing evidence that the presence of segmental alterations of the Longitudinal Sistolic Strain (a cut-off point of −15% has been reported) is an early marker of myocardial involvement [25], whereas a value higher than - 12.5% is a strong indicator of replacement fibrosis [26].
Cardiac MRI frequently shows late Gadolinium enhancement in the inferolateral basal wall (fibrosis) [12]. Despite the fact that in most cases LVH precedes fibrosis, the latter may be the presenting form of cardiomyopathy in some patients, especially women [27,28].
The patient described presents a preserved GLS although 3 segments with myocardial strain > −15% but < −12.5 were identified. These findings suggest an early deterioration of myocardial deformation in the absence of significative fibrosis, as confirmed by cardiac MRI.
A very promising tool for the early diagnosis of cardiac involvement is MRI with T1 mapping. A low native T1 is specific for FD cardiomyopathy, being very rare in hypertrophic cardiomyopathies due to sarcomeric genetic mutations, amyloidosis, and hypertension [29].
In a 5-year longitudinal analysis ERT was shown to reduce LVH and significantly increase systolic function after 3 years of treatment [14].
4. Conclusions
Diagnosis of FD was made in a 38-year-old patient with the de novo mutation (c.448delG), whose phenotype was not previously described in the literature, who is undergoing follow-up. Clinically, vertigo, acroparesthesia, anhidrosis and episodes of postprandial abdominal pain were observed. Physical examination revealed horizonto-rotatory nystagmus in both gazes and hearing loss (in pure tone audiometry evaluation), as well as angiokeratomas at the time of diagnosis. Slit lamp study demonstrated the presence of cornea verticilata and cardiac images reported LVH, LA dilation, and segmental deterioration of longitudinal strain, without myocardial fibrosis. Cardiac involvement was decisive for the initiation of ERT with Agalsidase Alfa. It is to consider that the absence of a family history of symptoms compatible with FD does not invalidate the possible diagnosis of this entity in a patient with clinical suspicion.
The first genotype-phenotype correlation of the mutation (c.448delG) was made in the literature.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Germain D.P. Use of a rare disease registry for establishing phenotypic classification of previously unassigned GLA variants: a consensus classification system by a multispecialty Fabry disease genotype–phenotype workgroup. J. Med. Genet. Aug 2020;57(8):542–551. doi: 10.1136/jmedgenet-2019-106467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iemolo F. De novo mutation in male patient with Fabry disease: a case report. BMC Res. Notes. 2014;7:11. doi: 10.1186/1756-0500-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bersano A. Review Article Neurological features of Fabry disease: clinical, pathophysiological aspects and therapy. Acta Neurol. Scand. 2012;126:77–97. doi: 10.1111/j.1600-0404.2012.01661.x. [DOI] [PubMed] [Google Scholar]
- 4.Germain D.P. Fabry disease. Orphanet J. Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagege A.A., Germain D.P. Adult patients with Fabry disease: what does the cardiologist need to know? Heart. 2015;101:916–918. doi: 10.1136/heartjnl-2015-307472. [DOI] [PubMed] [Google Scholar]
- 6.Fellgiebel A. Diagnostic utility of different MRI and MR angiography measures in Fabry disease. Neurology. 2009;72:63–68. doi: 10.1212/01.wnl.0000338566.54190.8a. [DOI] [PubMed] [Google Scholar]
- 7.Kolodny E. Cerebrovascular involvement in Fabry disease. Stroke. 2015;46:302–313. doi: 10.1161/STROKEAHA.114.006283. [DOI] [PubMed] [Google Scholar]
- 8.Mehta A., Clarke J.T., Giugliani R. Natural course of Fabry disease: changing pattern of causes of death in FOS - Fabry outcome survey. J. Med. Genet. 2009;46:548–552. doi: 10.1136/jmg.2008.065904. [DOI] [PubMed] [Google Scholar]
- 9.Waldek S., Patel M.R., Banikazemi M., Lemay R., Lee P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry registry. Genet. Med. 2009;11:790–796. doi: 10.1097/GIM.0b013e3181bb05bb. [DOI] [PubMed] [Google Scholar]
- 10.Linhart A., Kampmann C., Zamorano J.L. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur. Heart J. 2007;28:1228–1235. doi: 10.1093/eurheartj/ehm153. [DOI] [PubMed] [Google Scholar]
- 11.Moon J.C. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur. Heart J. 2003;24:2151–2155. doi: 10.1016/j.ehj.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Kramer J., Niemann M., Liu D. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur. Heart J. 2013;34:1587e96. doi: 10.1093/eurheartj/eht098. [DOI] [PubMed] [Google Scholar]
- 13.Mehta A., Ricci R., Widmer U. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry outcome survey. Eur. J. Clin. Investig. 2004;34(3):236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 14.Giugliani R. A 15-year perspective of the Fabry outcome survey. J. Inborn Errors Metab. Screen. 2016;4:1–12. [Google Scholar]
- 15.Kobasyashi M. Frequency of de novo mutations in Japanese patients with Fabry disease. Mol. Genet. Metab. Rep. 2014;1:283–287. doi: 10.1016/j.ymgmr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perretta F. Revista Nefrologia Argentina Año, Vol 17 N2. July 2019. Enf de Fabry: Hiperfiltracion Glomerular y Variables Clínicas Asociadas. [Google Scholar]
- 17.Jaurretche S. Nefropatia por enfermedad de Fabry. Rol del Nefrologo y variables clínicas asociadas al diagnóstico. Nefrologia. 2019;39(3):294–300. doi: 10.1016/j.nefro.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Jaurretche S. Controversias en la evaluación de tratamiento de pacientes con enfermedad de Fabry en la Argentina. Rev. Arg. Med. 2018;6(3):167–172. [Google Scholar]
- 19.Jaurretche S. Dired correlation between age at diagnosis and severity of nefropathy in Fabry Disease patient. Ind. J. Nephrol. 2019;29:398–401. doi: 10.4103/ijn.IJN_167_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ries M. Neuropathic and cerebrovascular correlates of Hearing loss in Fabry disease. Brain. January 2007;130:143–150. doi: 10.1093/brain/awl310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson R.B., Chow K., Khan A. T (1) mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex. Circ. Cardiovasc. Imag. 2013;6:637–645. doi: 10.1161/CIRCIMAGING.113.000482. [DOI] [PubMed] [Google Scholar]
- 22.Yeung D.F., Sirrs S., Tsang M.Y.C. Echocardiographic assessment of patients with Fabry disease. J. Am. Soc. Echocardiogr. 2018;31:639–649. doi: 10.1016/j.echo.2018.01.016. e2. [DOI] [PubMed] [Google Scholar]
- 23.Monserrat L., Gimeno-Blanes J.R., Marin F. Prevalence of Fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2007;50:2399e403. doi: 10.1016/j.jacc.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 24.Lang Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. March 2007;20(3):234–243. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Perk G., Tunick P.A., Kronzon I. Non-doppler two-dimensional strain imaging by echocardiographyefrom technical considerations to clinical applications. J. Am. Soc. Echocardiogr. 2007;20:234e43. doi: 10.1016/j.echo.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Saccheri M. Two-dimensional speckle tracking echocardiography for early detection of myocardial damage in young patients with Fabry disease. Echocardiography. 2013;30:1069–1077. doi: 10.1111/echo.12216. [DOI] [PubMed] [Google Scholar]
- 27.Moon J.C., Sheppard M., Reed E., Lee P., Elliott P.M., Pennell D.J. The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J. Cardiovasc. Magn. Reson. 2006;8:479–482. doi: 10.1080/10976640600605002. [DOI] [PubMed] [Google Scholar]
- 28.Hsu T.R., Hung S.C., Chang F.P. Later onset Fabry disease, cardiac damage progress in silence: experience with a highly prevalent mutation. J. Am. Coll. Cardiol. 2016;68:2554–2563. doi: 10.1016/j.jacc.2016.09.943. [DOI] [PubMed] [Google Scholar]
- 29.Niemann M., Herrmann S., Hu K. Differences in Fabry cardiomyopathy between female and male patients: consequences for diagnostic assessment. JACC Cardiovasc. Imaging. 2011;4:592–601. doi: 10.1016/j.jcmg.2011.01.020. [DOI] [PubMed] [Google Scholar]