Abstract
Multimorbidity is an emerging public health priority. This study aims to assess the role of lifestyle and socioeconomic status in the prevalence of multimorbidity and chronic diseases by using two language groups that are part of the same genetic subgroup but differ by daily habits. We conducted a cross-sectional survey in 2016 with randomly selected population sample with 4173 responders (52.3%) aged 20–69 years in Western Finland. We included 3864 Finnish participants with Swedish (28.1%) or Finnish (71.9%) as a native language. We used a questionnaire to assess participants' chronic diseases and lifestyle. We determined multimorbidity as a disease count ≥ 2.
Finnish speakers were more likely to have a diagnosis of COPD, heart failure, diabetes, reflux disease, chronic kidney failure, and painful conditions than Swedish speakers. The prevalence of multimorbidity was higher for Finnish speakers in the age group of 60–69 years (41.0% vs. 32.0%, p = 0.018) than Swedish speakers. A higher proportion of Finnish speakers smoked, were obese, inactive, and had lower socioeconomic status compared to Swedish speakers. All these factors, in addition to age and female sex, were significant risk factors for multimorbidity. Prevalence of multimorbidity was different in two language groups living in the same area and was associated with differences in lifestyle factors such as smoking, physical inactivity and obesity.
Keywords: Multimorbidity, Risk factors, Health disparities, COPD, Obesity
1. Introduction
Multimorbidity, defined as patients living with two or more chronic health conditions, is of paramount public health concern to any aging population as the prevalence of multimorbidity increases with age (Barnett et al., 2012). The prevalence of multimorbidity seems to be increasing, at least in western countries (Buttorff et al., 2017, Lebenbaum et al., 2018).
To understand multimorbidity in a broader context, not only the inter-correlation between different diseases or their medication but also factors related to the daily life need to be considered (Violan et al., 2014a). Many prevalence studies on multimorbidity are register-based with limited information on participants’ lifestyle and BMI. One approach to overcome this problem is to compare multimorbidity between countries, in different nationalities or ethnic groups, known to have differing habits (Garin et al., 2016). However, this kind of analysis may be hampered by geographical factors, different political systems, healthcare organizations, and access to healthcare between ethnic groups.
Another way of solving this could be to study two genetically similar populations living in the same socio-cultural landscape. Western Finland is one of the most affluent areas in the country, which contributes to the health benefits in Western Finland when compared to the general population of Finland (Saarela and Finnäs, 2011). Finnish and Swedish speaking Finns are part of the same Finnish genetic subpopulation (Kerminen et al., 2017), living in the same geographical area sharing the same educational institutions and healthcare system but with differing daily habits.
Swedish speakers have less harmful drinking patterns (Paljarvi et al., 2009), lower rates of sickness allowance, less early retirement (Reini and Saarela, 2017, Saarela and Finnäs, 2002), and mortality (Saarela and Finnäs, 2011, Saarela and Finnäs, 2005). The mortality difference between language-groups is highest in deaths related to alcohol, suicide, and other external causes (Sipilä and Martikainen, 2010).
Compared to existing studies on socioeconomic inequalities (Ahmadi et al., 2016, Mathur et al., 2011), FinEsS Western Finland study population provides a different aspect as inequalities concern the majority in a population sharing several determinants of health but differing in lifestyle. Also, habits were asked from the study participants, instead of making assumptions based on how the different language groups differ on average. The aim was to assess prevalence of multimorbidity in Swedish and Finnish speaking people in Western Finland and to evaluate lifestyle factors associated with multimorbidity.
2. Methods
2.1. Study design and participants
In collaboration with Nordic EpiLung, the latest FinEsS' (Finland-Estonia-Sweden) survey for Western Finland was conducted in February 2016. Health questionnaires were sent to 8,000 randomly selected recipients aged 20–69 years in hospital districts of South Ostrobothnia and Vaasa. We identified recipients' personal information from the Finnish Population Register, and the sample reflected the population in the study area. The official native language of a recipient determined whether we used a Finnish or Swedish questionnaire. We sent two reminders to those not responding.
2.2. Compliance with ethical standards
Ethics Committee of the Department of Medicine of Helsinki University Central Hospital approved the study (approval number 200/13/03/00/15). Informed written consent was obtained from all individual participants included in the study. We followed the General Data Protection Regulation (EU) 2016/679. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
2.3. Questionnaire
The FinEsS questionnaire comprises questions on respiratory symptoms, respiratory diseases, diseases and morbidity in general, risk factors, occupation, and use of medication, and have previously been used in many studies in several countries (Honkamäki et al., 2019, Larsson et al., 2003, Pallasaho et al., 2011).
2.4. Variables
The assessment of multimorbidity was based on self-reported diseases or medical conditions and defined as disease count ≥ 2 in any individual responder (Valderas et al., 2009). The rationale for diseases included was based on three independent sources: (1) previous publication (Barnett et al., 2012), (2) the most common comorbidities found in adult asthma in the same area (Ilmarinen et al., 2016), and (3) comorbidities reported to be associated with asthma (Kankaanranta et al., 2016).
The question asked on diseases was 'Has a doctor diagnosed you with any one of the following diseases: asthma, chronic obstructive pulmonary disease (COPD), hypertension, coronary heart disease, atrial fibrillation or another cardiac arrhythmia, heart failure, stroke or transient ischemic attack, diabetes, depression, panic attack or anxiety, treated dyspepsia/reflux disease, chronic kidney failure, sleep apnea, osteoporosis, and painful condition requiring daily analgesic medication?' with tick boxes yes/no for each of the diseases.
Participants were considered to be physically active if they were physically active at least three hours (≥180 min) per day, which was answered by the question 'How many hours in a day do you spend moving/physically active?'. We divided participants into current smokers, ex-smokers (when the participants stopped smoking more than 12 months before), and never smokers (neither a current smoker nor an ex-smoker). Body Mass Index (BMI) was based on self-reported height and weight and was categorized as follows: under and normal weight < 25 kg/m2, overweight 25.0–29.9 kg/m2, obesity grade I 30.0–34.9 kg/m2 and obesity grade II ≥ 35.0 kg/m2.
We asked each participant of their main occupation. Occupations were classified according to The International Standard Classification of Occupations 2008 (ISCO-08), providing a system for classifying professional skill levels in a four-level hierarchy. ISCO-08 skill level 1 is the primary level of education, and level 4 is higher education (Gaskin et al., 2014). Occupational exposure to vapors, gases, dust, or fumes (VGDF) was asked with a question. 'Are you now, or have you been heavily exposed to gases, dust, or fumes at work'?
2.5. Statistical analysis
Statistical analyses were performed by using IBM SPSS Statistics software version 26 (IBM SPSS, Armonk, NY, USA). Pearson chi-square –test was used for categorical variables. A p-value < 0.05 was considered significant.
Binary logistic regression models with multimorbidity as outcome were performed to calculate unadjusted Odds Ratios (OR) with 95% confidence intervals (CI) for age, sex, smoking status, BMI, physical activity, skill level, and native language. Multivariable binary logistic regression with multimorbidity as outcome was performed to calculate adjusted ORs with 95% CI including age, sex, smoking status, BMI, physical activity, skill level, and native language in the same model. Sensitivity analyses were performed with three different disease grouping models (see Appendix).
3. Results
3.1. Characteristics of the study participants
In total, 4173 participants of the 8000 invited responded, yielding a participation rate of 52.3%. Of the responders, 206 were excluded due to missing data on smoking, and 103 were excluded since their native language was not Finnish or Swedish. Altogether 3864 participants were included in the present study population (48.3%), of which 2780 (71.9%) were Finnish speaking, and 1084 (28.1%) were Swedish speaking. Fig. A1 shows a flow chart of the study.
The response rate was 51.4% (2932 out of 5704) for Finnish speakers and 60.0% (1132 out of 1886) for Swedish speakers. Non-responders were more often males and younger than responders (Table A1). The Finnish speaking group aged 20–39 years is most underrepresented. The age group 60–69 years has response rate of 73.8% (72.1% for Finnish speakers and 78.8% for Swedish speakers). For those over 40 years, the response rate was 61.7%.
Table 1 shows characteristics of the study participants. We observed a slight dominance of women over men in both language groups. The proportion of participants in the youngest age group was higher among Swedish speakers than Finnish speakers. The prevalence of obesity, active smoking and ex-smoking were higher in Finnish speakers than Swedish speakers. The level of physical activity was lower in Finnish speakers than Swedish speakers. Also, the ISCO-08 skill level was lower in Finnish speakers compared to Swedish speakers, whereas occupational exposure to VGDF was higher in Finnish speakers than Swedish speakers. Prevalence of family history of chronic bronchitis, COPD, or emphysema was higher in Finnish speakers than Swedish speakers.
Table 1.
Characteristics of the study participants.
| Finnish speakers n = 2780 |
Swedish speakers n = 1084 |
p-value | |
|---|---|---|---|
| Female | 1468 (52.8%) | 549 (50.6%) | 0.227 |
| Age groups 20–44 45–59 60–69 |
866 (32.3%) 895 (33.4%) 918 (34.3%) |
430 (41.3%) 293 (28.1%) 319 (30.6%) |
<0.001 |
| BMI < 25 BMI 25–29.99 BMI 30–34.99 BMI ≥ 35 |
1007 (37.0%) 1089 (40.0%) 447 (16.4%) 179 (6.6%) |
499 (47.1%) 392 (37.0%) 126 (11.9%) 43 (4.1%) |
<0.001 |
| Smoking status Ex-smoker Current smoker Non-smoker |
789 (28.4%) 605 (21.8%) 1386 (49.9%) |
277 (25.6%) 170 (15.7%) 637 (58.8%) |
<0.001 |
| ISCO-08 skill level 1 2 3 4 |
124 (5.3%) 1415 (60.1%) 489 (20.8%) 325 (13.8%) |
34 (3.8%) 511 (57.0%) 189 (21.1%) 162 (18.1%) |
0.008 |
| Occupational exposure to VGDF | 1105 (40.8%) | 290 (29.4%) | <0.001 |
| Family history of chronic bronchitis, COPD or emphysema | 342 (12.3%) | 71 (6.5%) | <0.001 |
| Duration of daily physical activity ≥ 3 h | 1309 (50.8%) | 676 (67.6%) | <0.001 |
Data is shown as n (%). Abbreviations: BMI (Body Mass Index), ISCO (International Standard Classification of Occupations), VGDF (Vapors, gases, dust and fumes), and COPD (Chronic obstructive pulmonary disease). Missing cases BMI 82 (2%), physical activity 287 (7%), and skill level 615 (16%) of total 3864.
3.2. Multimorbidity
Finnish speakers were more likely multi-morbid compared to Swedish speakers, with 26.0% prevalence of multimorbidity for Finnish speakers and 22.3% for Swedish speakers (p = 0.049). At the level of single diseases, Finnish speakers had more frequently a diagnosis of COPD (3.0% vs 1.3%, p = 0.002), heart failure (1.7% vs 0.5%, p = 0.002), diabetes (8.1% vs 5.1%, p = 0.001), treated dyspepsia/reflux disease (7.2% vs 4.9%, p = 0.008), chronic kidney failure (0.9% vs 0.2%, p = 0.016) and painful conditions (9.8% vs 5.1%, p < 0.001) compared to Swedish speakers (Table 2).
Table 2.
Prevalence of chronic diseases for Finnish and Swedish speaking responders.
| Finnish speakers | Swedish speakers | p-value | |
|---|---|---|---|
| Asthma | 319 (11.5%) | 125 (11.5%) | 0.955 |
| COPD | 83 (3.0%) | 14 (1.3%) | 0.002 |
| Hypertension | 634 (22.8%) | 253 (23.3%) | 0.733 |
| Coronary heart disease | 84 (3.0%) | 29 (2.7%) | 0.597 |
| Atrial fibrillation and other cardiac arrhythmias | 215 (7.7%) | 70 (6.5%) | 0.193 |
| Heart failure | 47 (1.7%) | 5 (0.5%) | 0.002 |
| Stroke and transient ischemic attack | 61 (2.2%) | 29 (2.7%) | 0.406 |
| Diabetes | 224 (8.1%) | 55 (5.1%) | 0.001 |
| Depression | 278 (10%) | 101 (9.3%) | 0.548 |
| Panic attack or anxiety | 160 (5.8%) | 69 (6.4%) | 0.495 |
| Treated dyspepsia, reflux disease | 201 (7.2%) | 53 (4.9%) | 0.008 |
| Chronic kidney failure | 25 (0.9%) | 2 (0.2%) | 0.016 |
| Sleep apnea | 139 (5.0%) | 50 (4.6%) | 0.678 |
| Osteoporosis | 75 (2.7%) | 24 (2.2%) | 0.429 |
| Painful condition | 272 (9.8%) | 55 (5.1%) | <0.001 |
Data is shown as n (%).
3.3. Multimorbidity in different age groups
There was no significant difference in multimorbidity between Finnish and Swedish speakers in age groups 20–39 years (8.9% vs. 12.1%, p = 0.202) or 40–59 years (22.6% vs. 20.4%, p = 0.427). However, in the age group 60–69 years, Finnish speakers were more often multimorbid (41.0% vs. 32.0%, p = 0.018) (Fig. A2).
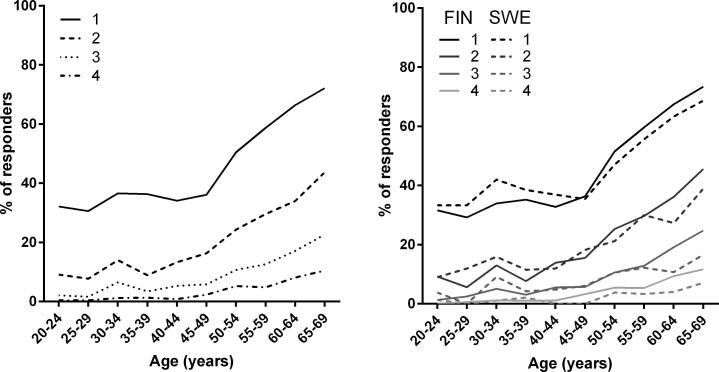
In the whole study population, the percentage of persons suffering from at least 1, 2, 3, or 4 diseases, as well as the morbidity count, increased with age (Fig. 1a). The percentage of respondents with at least 1, 2, 3, or 4 diseases seemed to be higher in Finnish speakers than Swedish speakers in older age groups (Fig. 1b).
Fig. 1.
Prevalence of participants with at least 1, 2, 3 or 4 diseases according to age in the whole study sample (A) and separately in Finnish and Swedish speakers (B).
3.4. Risk factors for multimorbidity
Significant independent risk factors for multimorbidity in both unadjusted and adjusted analyses were age, current and ex-smoking and overweight and obesity (Table 3). Swedish language was a risk-reducing factor for multimorbidity only in unadjusted model. Lower skill levels 1 and 2 were risk factors for multimorbidity only in unadjusted model. Physical inactivity was significant risk factor in adjusted model. Age over 60 (OR = 5.91) and obesity grade II (OR = 5.62) were the most significant risk factors for multimorbidity.
Table 3.
Factors associated with multimorbidity (morbidity count ≥ 2) in univariate and multivariate logistic regression analyses.
| Crude OR (95% CI) |
*Adjusted OR (95% CI) |
|
|---|---|---|
| Age groups (20–39 yrs. ref group) | ||
| 40–59 yrs. | 2.55 (2.01–3.24) | 2.35 (1.74–3.16) |
| 60–69 yrs. | 5.85 (4.65–7.37) | 5.91 (4.40–7.93) |
| Female | 1.03 (0.89–1.19) | 1.32 (1.09–1.60) |
| Swedish-speaking | 0.82 (0.69–0.96) | 1.13 (0.92–1.40) |
| Smoking status (never smoker ref group) | ||
| Current smoker | 1.55 (1.28–1.88) | 1.85 (1.43–2.38) |
| Ex-smoker | 2.09 (1.77–2.48) | 1.82 (1.47–2.25) |
| BMI (<25 ref group) | ||
| Overweight (25–29.9) | 1.90 (1.58–2.29) | 1.53 (1.23–1.91) |
| Obesity grade I (30–34.99) | 3.55 (2.85–4.42) | 2.73 (2.09–3.56) |
| Obesity grade II (≥35-) | 7.20 (5.33–9.73) | 5.62 (3.88–8.15) |
| Duration of daily physical activity < 3 h | 1.15 (0.99–1.35) | 1.23 (1.01–1.49) |
| ISCO-08 Skill level (4 ref group) | ||
| 1 | 1.94 (1.29–2.90) | 1.58 (1.00–2.51) |
| 2 | 1.59 (1.24–2.04) | 1.24 (0.93–1.65) |
| 3 | 0.94 (0.75–1.36) | 0.98 (0.71–1.36) |
Adjusted for age, sex, native language, smoking status, BMI, physical activity, and skill level. Bold indicates p < 0.05. Abbreviations: BMI (Body Mass Index) and ISCO (International Standard Classification of Occupations).
3.5. Multimorbidity prevalence in association to smoking status, physical activity, and BMI
Since current and ex-smoking, overweight, obesity and physical inactivity were significant risk factors for multimorbidity, we calculated the prevalence of multimorbidity in association with smoking status, physical activity, and BMI.
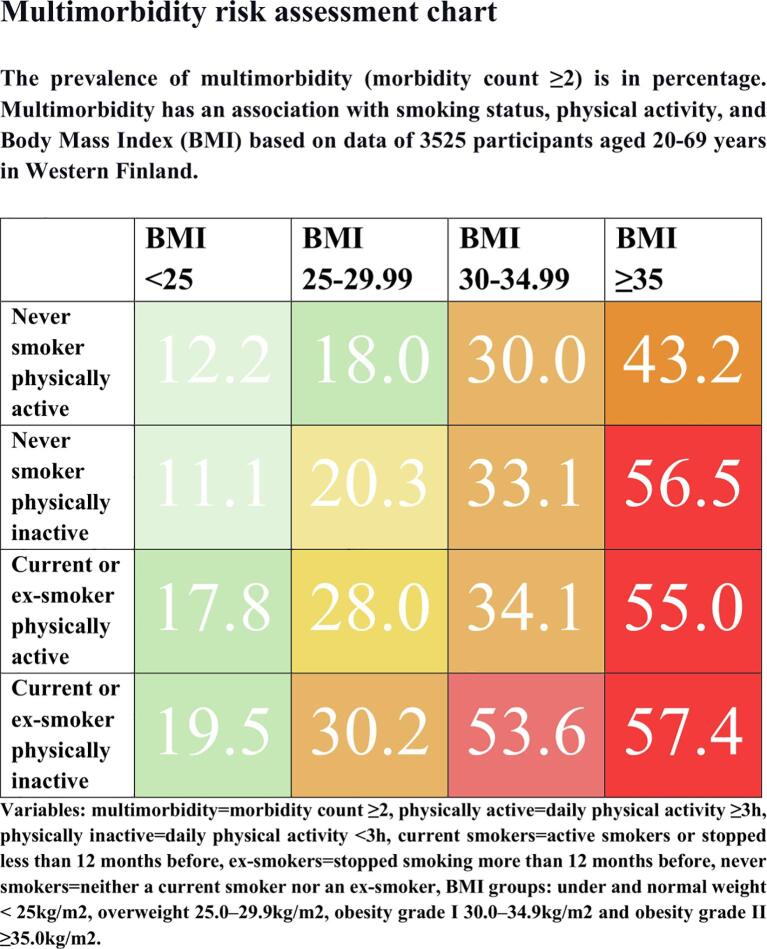
Ex-smokers had the highest multimorbidity prevalence in all BMI groups (Table A2). Table A3 shows that among obese participants, the prevalence of multimorbidity was higher in the physically inactive group compared to the physically active group. We had all data on the four variables for 3525 out of 3864 participants. We divided participants into four groups: never smokers with daily physical activity ≥ 3 h (n = 968), never smokers with daily physical activity < 3 h (n = 916), current or ex-smokers with daily physical activity ≥ 3 h (n = 1017), and current or ex-smokers with daily physical activity < 3 h (n = 676) and calculated multimorbidity prevalence in BMI groups: under and normal weight < 25 kg/m2, overweight 25.0–29.9 kg/m2, obesity grade I 30.0–34.9 kg/m2 and obesity grade II ≥ 35.0 kg/m2. The results are shown in Table A4. In the group with BMI < 25 kg/m2, never smokers and physically active responders, the prevalence of multimorbidity was 12.2% and in the group with BMI ≥ 35.0 kg/m2, current or ex-smokers and physically inactive participants the prevalence of multimorbidity was 57.4%. Results modified for patient education are shown in the multimorbidity risk assessment chart in Fig. 2.
Fig. 2.
Multimorbidity risk assessment chart.
3.6. Sensitivity analyses
We constructed disease groups to take into account the possible interrelationships between diseases. The three models composed are described in Appendix. The difference in multimorbidity prevalence between language groups remained after accounting one disease group as one disease (Table A5). In multivariable binary logistic regression analysis, results remained similar, except physical inactivity lost significance (Table A6).
4. Discussion
In this study, we found that multimorbidity was more prevalent in Finnish speakers compared to Swedish speakers in Western Finland. Finnish speakers had a higher BMI, they smoked more often, had a lower social status based on occupation, and were physically less active when compared to Swedish speakers. These lifestyle-related and socioeconomic aspects were associated with multimorbidity and might explain the difference between the two language groups. Finnish speakers had significantly more often 6 out of 16 self-reported diagnoses, and they had more often a diagnosis of COPD, heart failure, diabetes, treated dyspepsia/reflux disease, chronic kidney failure, and painful condition.
The disease count ≥ 2 is a simple and widely accepted concept to define multimorbidity, but it has limitations; it does not consider disease severity or inter-relationships. For example, hypertension, and diabetes interrelate to cardiovascular disease along with lifestyle-associated factors (Straus et al., 2002, Yusuf et al., 2004). Risk factors can be associated, or there may be true causality, both options lead to disease clusters. Therefore, causal models of multimorbidity should be interpreted with high caution (Valderas et al., 2009). Considering the possible disease interrelations, we carried out three sensitivity analyses with disease grouping based on disease co-occurrence in our cohort and the difference between langue groups remained.
The prevalence of multimorbidity in this study was 26% for Finnish speakers and 22% for Swedish speakers, similar to the prevalence in the UK (23%) (Barnett et al., 2012), but lower than overall pooled multimorbidity prevalence (33%) in a recent literature review (Nguyen et al., 2019). In Sweden, 38% were multimorbid in the age group 60–74 years (Marengoni et al., 2016), compared to 41% vs. 32% in our Finnish and Swedish speaking Finns in the age group 60–69 years.
In a previous study, multimorbidity occurred in deprived areas 10–15 years earlier than in the most affluent areas (Mercer and Watt, 2007). In our study, low socioeconomic status had a relationship with multimorbidity consistent with current evidence (Donovan et al., 1996, Marmot, 2005, Salisbury et al., 2011, van den Akker et al., 1998, Violan et al., 2014, Walker, 2007). According to our results, Finnish speakers with lower socioeconomic status might have poorer health and health behaviors. Earlier studies showed that better psychosocial living conditions in childhood and working conditions in adulthood among Swedish speakers facilitates advanced individual's sense of coherence (Volanen et al., 2006). Sense of coherence is an individual’s capacity to manage stress and sustain healthy habits and is related to health in general (Eriksson and Lindström, 2005) which may contribute to the difference seen in multimorbidity between Swedish and Finnish speakers. Social participation is considered to be related to good self-rated health and social capita among Swedish speaking Finns (Hyyppä and Mäki, 2001, Hyyppä and Mäki, 2003).
Few previous studies have shown a higher prevalence of multimorbidity among minority ethnic populations in East London and Iran (Ahmadi et al., 2016, Mathur et al., 2011). However, we describe these health disparities in the majority population and with fewer confounding factors, like ethnicity, access to education or healthcare, than in previous studies.
We found that multimorbidity is positively associated with obesity. Previously in a longitudinal study in Canada, the most considerable increase in multimorbidity was found among seniors living with obesity (Lebenbaum et al., 2018). Multimorbidity was highly associated with increasing BMI and OR 2.73 for obesity grade I was higher than previously reported OR 1.65–2.20 (Agborsangaya et al., 2012, Booth et al., 2014). In our study, obesity grade II (OR 5.62) and age of 60–69 years (OR 5.91) were equal risk factors for multimorbidity. Therefore, obesity is the most influential treatable risk factor for multimorbidity.
Our study shows that physical inactivity defined as daily activity/movement less than three hours a day is a risk factor for multimorbidity. The association between physical inactivity was in line with existing evidence (Ahmadi et al., 2016, Keats et al., 2017, Wikström et al., 2015), although the definition of physical inactivity varies between studies. In the Iranian cohort (Ahmadi et al., 2016), physical activity was defined only based on occupational activity. The Finnish FINRISK study (Wikström et al., 2015) used a complex Physical Activity Questionnaire combined with a smaller cohort with activity measurements. A previous study used a cut-point of four hours daily to evaluate the association between physical inactivity and lung function decline in adult-onset asthma (Loponen et al., 2018). Paradoxically, Finnish speaking schoolchildren aged 14–15 showed more leisure-time exercise despite higher amount of alcohol consumption, smoking, and physician-diagnosed diseases than Swedish speakers in Western Finland (Saarela and Finnäs, 2004). How regular exercise and physical activity relate to other health behaviors needs further studies.
COPD prevalence has been shown to be higher in minorities and among people with low socioeconomic status due to differences in health behaviors, mainly smoking, and differences in occupational exposure to inhalant toxins. Low socioeconomic status is also associated with worsened COPD health outcomes (Axelsson et al., 2018, Dransfield and Bailey, 2006, Holt et al., 2011, Pleasants et al., 2016, Tran et al., 2011). In our study, the Finnish speaking majority had lower socioeconomic status and, hence, more smoking and occupational exposure to VGDF. Finnish speakers had two times as frequently diagnosis of COPD compared to Swedish speakers. A family history of COPD was more common among Finnish speakers than Swedish speakers, presumably due to family's habits and occupation.
We identified ex-smokers as having a higher probability for multimorbidity than current smokers, similar to a study in the UK (Booth et al., 2014). In contrast to our study, an Australian study showed that current smokers had a higher probability for multimorbidity; in sub-analyses in the age group >60 years, however, ex-smoking was a higher risk for multimorbidity than current smoking (Taylor et al., 2010). By the age of 60 years, diseases associated with smoking are possible, and hence people stop smoking. In a cohort of adult-onset asthma, multimorbidity increased dose-dependently with smoked pack-years (Tommola et al., 2019), and in that study, the risk for multimorbidity was rather associated with pack-years than with current smoking status. We do not have information on smoked pack-years for the responders, and we do not know if ex-smokers had more pack-years than current smokers. Also, other possible explanations exist. Firstly, healthcare integrates interventions to stop smoking into care, and there is contact with healthcare at the time of diagnosis. Secondly, smoking cessation is associated with weight gain, and paradoxically might worsen glycemic control and increase the risk for diabetes, even though smoking is a risk factor for diabetes (Bush et al., 2016). Regardless of smoking cessation being associated with short-term risk of type 2 diabetes, there is still a benefit on cardiovascular and all-cause mortality (Hu et al., 2018).
Overall, studies on multimorbidity combining information on obesity, smoking status, and physical activity are rare, and therefore findings of our study provide further evidence on the association between obesity and multimorbidity. The prevalence of multimorbidity was 12% in physically active, never smoking participants with normal weight and 57% in physically inactive, smoking participants with BMI ≥ 35.0 kg/m2. Our results demonstrate the phenomenon in a motivating way, and the multimorbidity risk assessment chart might be important for patient education within smoking cessation, diabetes, and weight reduction.
The major strengths of this study are; random sample, a large sample size, and established structured questionnaire. This study was based on self-report data, which could be considered as a limitation of this study. Another limitation is the lack of knowledge on the disease severity. However, the FinEsS questionnaire has been validated in several previous Nordic studies, and as a large-scale questionnaire, it has several benefits compared to register-based data. The self-reported data is valuable as it combines information like smoking, exercise, and BMI not readily available in register-based data. As another limitation of the study, nutritional factors and alcohol consumption were not included in the questionnaire. However, we can assume Swedish speakers have a healthier diet and more moderate alcohol consumption based on two earlier studies (De Oliveira Figueiredo et al., 2019, Paljarvi et al., 2009).
Disease count did not include information on malignancies and HIV, thus the Charlson Comorbidity Index or the ACG System measures could not be calculated. Finland is a low HIV prevalence country. Disease counts perform as well as sophisticated measures in predicting the outcome (Huntley et al., 2012). Due to the lack of standardization of diseases included in multimorbidity studies, comparison between studies is challenging (Garin et al., 2016, Marengoni et al., 2011).
The response rate was moderate in the present study. In our study, non-responders were younger and more often males compared to responders, consistent with previous findings (Rönmark et al., 2009). The participation rate was higher for Swedish speakers, similar to the FINRISK Study 2012 (Tolonen et al., 2018). However, to minimize bias, we used age groups in statistical analysis. We conclude that this study might have included some non-responder bias that mainly affects younger and males; still, as the main difference in multimorbidity was found in older age groups and their response rate was higher, we consider the main results to be reliable.
Taken together, we have shown that smoking, overweight, obesity, and physical inactivity were associated with multimorbidity. To reduce the disease-burden the general population as well as the specific target groups need more information about these findings. Therefore, we provided a multimorbidity risk assessment chart for patient education. This study gives population-based insight that it might be possible to reduce multimorbidity and thus, the general disease burden at older age with smoking cessation, weight reduction, and increased physical activity although other determinants of health may also play a role.
4.1. Conclusion
In this study we found that Finnish speakers were more multimorbid than Swedish speakers, and COPD and diabetes were among diseases more common in Finnish speakers. Lifestyle-associated risk factors for multimorbidity smoking, overweight, obesity, and physical inactivity should be targeted in health interventions.
5. Ethics approval
The study was approved by the Ethics Committee of the Department of Medicine of Helsinki University Central Hospital (approval number 200/13/03/00/15).
6. Data statement section
All data generated or analyzed during this study are included in this published article (and its Supplementary Information File). According to ethical permission and data-protection laws of Finland, single person data cannot be made available.
Funding
This work was supported by Tampere Tuberculosis Foundation (Tampere, Finland), The Finnish Anti-Tuberculosis Association Foundation (Helsinki, Finland), The Research Foundation of The Pulmonary Diseases (Helsinki, Finland), The Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (Tampere, Finland) and The Medical Research Fund of Seinäjoki Central Hospital (Seinäjoki, Finland). We also acknowledge Nord Forsk for possibilities of collaborations between Finland, Sweden, and Norway. None of the sponsors had any involvement in the planning, execution, drafting, or write-up of this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful for Dr. Paula Pallasaho for participating in translating and modifying the original questions in Finnish language form, and Mr. Antti Sepponen, technician, and Mrs. Aino Sepponen, RN for their input with Western Finland FinEsS sample.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2021.101338.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agborsangaya C.B., Lau D., Lahtinen M., Cooke T., Johnson J.A. Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health. 2012;12(1) doi: 10.1186/1471-2458-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi B., Alimohammadian M., Yaseri M., Majidi A., Boreiri M., Islami F., Poustchi H., Derakhshan M.H., Feizesani A., Pourshams A., Abnet C.C., Brennan P., Dawsey S.M., Kamangar F., Boffetta P., Sadjadi A., Malekzadeh R. Multimorbidity: Epidemiology and risk factors in the golestan cohort study, Iran a cross-sectional analysis. Med. (United States) 2016;95(7):e2756. doi: 10.1097/MD.0000000000002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson S., Mulinari S., Wemrell M., Leckie G., Perez R., Merlo J. SSM - Population Health Chronic Obstructive Pulmonary Disease in Sweden: an intersectional multilevel analysis of individual heterogeneity and discriminatory accuracy. SSM - Popul. Heal. 2018;4:334–346. doi: 10.1016/j.ssmph.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett K., Mercer S.W., Norbury M., Watt G., Wyke S., Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Booth H.P., Prevost A.T., Gulliford M.C. Impact of body mass index on prevalence of multimorbidity in primary care: Cohort study. Fam. Pract. 2014;31(1):38–43. doi: 10.1093/fampra/cmt061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush T., Lovejoy J.C., Deprey M., Carpenter K.M. The effect of tobacco cessation on weight gain, obesity, and diabetes risk. Obesity. 2016;24(9):1834–1841. doi: 10.1002/oby.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttorff, C., Ruder, T., Bauman, M., 2017. Multiple Chronic Conditions in the United States, Multiple Chronic Conditions in the United States. https://doi.org/10.7249/tl221.
- De Oliveira Figueiredo R.A., Viljakainen J., Viljakainen H., Roos E., Rounge T.B., Weiderpass E. Identifying eating habits in Finnish children: A cross-sectional study. BMC Public Health. 2019;19(1):312. doi: 10.1186/s12889-019-6603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eachus J., Williams M., Chan P., Smith G.D., Grainge M., Donovan J., Frankel S. Deprivation and cause specific morbidity: evidence from the Somerset and Avon survey of health. BMJ. 1996;312(7026):287–292. doi: 10.1136/bmj.312.7026.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield M.T., Bailey W.C. COPD: racial disparities in susceptibility, treatment, and outcomes. Clin. Chest Med. 2006;27(3):463–471. doi: 10.1016/j.ccm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Eriksson M., Lindström B. Validity of Antonovsky’s sense of coherence scale: a systematic review. J. Epidemiol. Community Health. 2005;60:376–381. doi: 10.1136/jech.2003.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin N., Koyanagi A., Chatterji S., Tyrovolas S., Olaya B., Leonardi M., Lara E., Koskinen S., Tobiasz-Adamczyk B., Ayuso-Mateos J.L., Haro J.M. Global Multimorbidity Patterns: A Cross-Sectional, Population-Based, Multi-Country Study. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2016;71(2):205–214. doi: 10.1093/gerona/glv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin D.J., Thorpe R.J., McGinty E.E., Bower K., Rohde C., Young J.H., LaVeist T.A., Dubay L. Disparities in diabetes: the nexus of race, poverty, and place. Am. J. Public Health. 2014;104(11):2147–2155. doi: 10.2105/AJPH.2013.301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J.B., Zhang X., Presley-Cantrell L., Croft J.B. Geographic disparities in chronic obstructive pulmonary disease (COPD) hospitalization among Medicare beneficiaries in the United States. Int. J. COPD. 2011;6:321–328. doi: 10.2147/COPD.S19945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkamäki J., Hisinger-Mölkänen H., Ilmarinen P., Piirilä P., Tuomisto L.E., Andersén H., Huhtala H., Sovijärvi A., Backman H., Lundbäck B., Rönmark E., Lehtimäki L., Kankaanranta H. Age- and gender-specific incidence of new asthma diagnosis from childhood to late adulthood. Respir. Med. 2019;154:56–62. doi: 10.1016/j.rmed.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zong G., Liu G., Wang M., Rosner B., Pan A.n., Willett W.C., Manson J.E., Hu F.B., Sun Q.i. Smoking cessation, weight change, type 2 diabetes, and mortality. N. Engl. J. Med. 2018;379(7):623–632. doi: 10.1056/NEJMoa1803626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley A.L., Johnson R., Purdy S., Valderas J.M., Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann. Fam. Med. 2012;10(2):134–141. doi: 10.1370/afm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyyppä M.T., Mäki J. Individual-level relationships between social capital and self-rated health in a bilingual community. Prev. Med. (Baltim) 2001;32(2):148–155. doi: 10.1006/pmed.2000.0782. [DOI] [PubMed] [Google Scholar]
- Hyyppä M.T., Mäki J. Social participation and health in a community rich in stock of social capital. Health Educ. Res. 2003;18(6):770–779. doi: 10.1093/her/cyf044. [DOI] [PubMed] [Google Scholar]
- Ilmarinen P., Tuomisto L.E., Niemelä O., Danielsson J., Haanpää J., Kankaanranta T., Kankaanranta H. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur. Respir. J. 2016;48(4):1052–1062. doi: 10.1183/13993003.02198-2015. [DOI] [PubMed] [Google Scholar]
- Kankaanranta H., Kauppi P., Tuomisto L.E., Ilmarinen P. Emerging comorbidities in adult asthma: risks, clinical associations, and mechanisms. Mediators Inflamm. 2016;2016:1–23. doi: 10.1155/2016/3690628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats M.R., Cui Y., DeClercq V., Dummer T.J.B., Forbes C., Grandy S.A., Hicks J., Sweeney E., Yu Z.M., Parker L. Multimorbidity in Atlantic Canada and association with low levels of physical activity. Prev. Med. (Baltim) 2017;105:326–331. doi: 10.1016/j.ypmed.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Kerminen S., Havulinna A.S., Hellenthal G., Martin A.R., Sarin A.-P., Perola M., Palotie A., Salomaa V., Daly M.J., Ripatti S., Pirinen M. Fine-Scale Genetic Structure in Finland. G3 (Bethesda) 2017;7(10):3459–3468. doi: 10.1534/g3.117.300217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M.L., Loit H-M., Meren M., Põlluste J., Magnusson A., Larsson K., Lundbäck B. Passive smoking and respiratory symptoms in the FinEsS study. Eur. Respir. J. 2003;21(4):672–676. doi: 10.1183/09031936.03.00033702. [DOI] [PubMed] [Google Scholar]
- Lebenbaum M., Zaric G.S., Thind A., Sarma S. Trends in obesity and multimorbidity in Canada. Prev. Med. (Baltim) 2018;116:173–179. doi: 10.1016/j.ypmed.2018.08.025. [DOI] [PubMed] [Google Scholar]
- Loponen J., Ilmarinen P., Tuomisto L.E., Niemelä O., Tommola M., Nieminen P., Lehtimäki L., Kankaanranta H. Daily physical activity and lung function decline in adult-onset asthma: a 12-year follow-up study. Eur. Clin. Respir. J. 2018;5(1):1533753. doi: 10.1080/20018525.2018.1533753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A., Angleman S., Meinow B., Santoni G., Mangialasche F., Rizzuto D., Fastbom J., Melis R., Parker M., Johnell K., Fratiglioni L. Coexisting chronic conditions in the older population: variation by health indicators. Eur. J. Intern. Med. 2016;31:29–34. doi: 10.1016/j.ejim.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Marengoni A., Angleman S., Melis R., Mangialasche F., Karp A., Garmen A., Meinow B., Fratiglioni L. Aging with multimorbidity: a systematic review of the literature. Ageing Res. Rev. 2011;10(4):430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- Mathur R., Hull S.A., Badrick E., Robson J. Cardiovascular multimorbidity: the effect of ethnicity on prevalence and risk factor management. Br. J. Gen. Pract. 2011;61(586):e262–e270. doi: 10.3399/bjgp11X572454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer S.W., Watt G.C.M. The inverse care law: clinical primary care encounters in deprived and affluent areas of Scotland. Ann. Fam. Med. 2007;5(6):503–510. doi: 10.1370/afm.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H., Manolova G., Daskalopoulou C., Vitoratou S., Prince M., Prina A.M. Prevalence of multimorbidity in community settings: a systematic review and meta-analysis of observational studies. J. Comorbidity. 2019;9 doi: 10.1177/2235042X19870934. 2235042X1987093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paljarvi T., Suominen S., Koskenvuo M., Winter T., Kauhanen J. The differences in drinking patterns between Finnish-speaking majority and Swedish-speaking minority in Finland. Eur. J. Public Health. 2009;19(3):278–284. doi: 10.1093/eurpub/ckp007. [DOI] [PubMed] [Google Scholar]
- Pallasaho P., Juusela M., Lindqvist A., Sovijärvi A., Lundbäck B., Rönmark E. Allergic rhinoconjunctivitis doubles the risk for incident asthma - results from a population study in Helsinki, Finland. Respir. Med. 2011;105(10):1449–1456. doi: 10.1016/j.rmed.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Pleasants R.A., Riley I.L., Mannino D.M. Defining and targeting health disparities in chronic obstructive pulmonary disease. Int. J. COPD. 2016;11:2475–2496. doi: 10.2147/COPD.S79077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reini K., Saarela J. Differences in sickness allowance receipt between Swedish speakers and Finnish Speakers in Finland. Finnish Yearb. Popul. Res. 2017;52:43–58. doi: 10.23979/fypr.66598. [DOI] [Google Scholar]
- Rönmark E.P., Ekerljung L., Lötvall J., Torén K., Rönmark E., Lundbäck B. Large scale questionnaire survey on respiratory health in Sweden: Effects of late- and non-response. Respir. Med. 2009;103(1807) doi: 10.1016/j.rmed.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Saarela J., Finnäs F. Family origin and mortality: prospective Finnish cohort study. BMC Public Health. 2011;11:385. doi: 10.1186/1471-2458-11-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela J., Finnäs F. The health of Swedishspeaking and Finnishspeaking schoolchildren in Finland. Child Care Health Dev. 2004;30(1):51–58. doi: 10.1111/j.1365-2214.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- Saarela J., Finnäs F. Mortality inequality in two native population groups. Popul. Stud. (NY) 2005;59(3):313–320. doi: 10.1080/00324720500249372. [DOI] [PubMed] [Google Scholar]
- Saarela J., Finnäs F. Language-group differences in very early retirement in Finland. Demogr. Res. 2002;7:49–66. doi: 10.4054/DemRes.2002.7.3. [DOI] [Google Scholar]
- Salisbury C., Johnson L., Purdy S., Valderas J.M., Montgomery A.A. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br. J. Gen. Pract. 2011;61(582):e12–e21. doi: 10.3399/bjgp11X548929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipilä P., Martikainen P. Language-group mortality differentials in Finland: the effects of local language composition. Heal. Place. 2010;16(3):446–451. doi: 10.1016/j.healthplace:2009.11.014. [DOI] [PubMed] [Google Scholar]
- Straus S.E., Majumdar S.R., McAlister F.A. New evidence for stroke prevention: scientific review. J. Am. Med. Assoc. 2002;288:1388–1395. doi: 10.1001/jama.288.11.1388. [DOI] [PubMed] [Google Scholar]
- Taylor A.W., Price K., Gill T.K., Adams R., Pilkington R., Carrangis N., Shi Z., Wilson D. Multimorbidity - not just an older person’s issue. Results from an Australian biomedical study. BMC Public Health. 2010;10:718. doi: 10.1186/1471-2458-10-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolonen H., Koponen P., Borodulin K., Männistö S., Peltonen M., Vartiainen E. Language as a determinant of participation rates in Finnish health examination surveys. Scand. J. Public Health. 2018;46:240–243. doi: 10.1177/1403494817725243. [DOI] [PubMed] [Google Scholar]
- Tommola M., Ilmarinen P., Tuomisto L.E., Lehtimäki L., Niemelä O., Nieminen P., Kankaanranta H. Cumulative effect of smoking on disease burden and multimorbidity in adult-onset asthma. Eur. Respir. J. 2019;54(3):1801580. doi: 10.1183/13993003.01580-201810.1183/13993003.01580-2018.Shareable1. [DOI] [PubMed] [Google Scholar]
- Tran H.N., Siu S., Iribarren C., Udaltsova N., Klatsky A.L. Ethnicity and risk of hospitalization for Asthma and chronic obstructive pulmonary disease. Ann. Epidemiol. 2011;21(8):615–622. doi: 10.1016/j.annepidem.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Valderas J.M., Starfield B., Sibbald B., Salisbury C., Roland M. Defining comorbidity: Implications for understanding health and health services. Ann. Fam. Med. 2009;7(4):357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Akker M., Buntinx F., Metsemakers J.F.M., Roos S., Knottnerus J.A. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J. Clin. Epidemiol. 1998;51(5):367–375. doi: 10.1016/S0895-4356(97)00306-5. [DOI] [PubMed] [Google Scholar]
- Violan C., Foguet-Boreu Q., Flores-Mateo G., Salisbury C., Blom J., Freitag M., Glynn L., Muth C., Valderas J.M., Scuteri A. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7):e102149. doi: 10.1371/journal.pone.0102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volanen S.-M., Suominen S., Lahelma E., Koskenvuo M., Silventoinen K. Sense of coherence and its determinants: a comparative study of the Finnish-speaking majority and the Swedish-speaking minority in Finland. Scand. J. Public Health. 2006;34(5):515–525. doi: 10.1080/14034940600585812. [DOI] [PubMed] [Google Scholar]
- Walker A.E. Multiple chronic diseases and quality of life: patterns emerging from a large national sample, Australia. Chronic Illn. 2007;3(3):202–218. doi: 10.1177/1742395307081504. [DOI] [PubMed] [Google Scholar]
- Wikström K., Lindström J., Harald K., Peltonen M., Laatikainen T. Clinical and lifestyle-related risk factors for incident multimorbidity: 10-year follow-up of Finnish population-based cohorts 1982–2012. Eur. J. Intern. Med. 2015;26(3):211–216. doi: 10.1016/j.ejim.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Yusuf S., Hawken S., Ôunpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.