Summary
Visible light photocatalysis has become a powerful tool in organic synthesis that uses photons as traceless, sustainable reagents. Most of the activities in the field focus on the development of new reactions via common photoredox cycles, but recently a number of exciting new concepts and strategies entered less charted territories. We survey approaches that enable the use of longer wavelengths and show that the wavelength and intensity of photons are import parameters that enable tuning of the reactivity of a photocatalyst to control or change the selectivity of chemical reactions. In addition, we discuss recent efforts to substitute strong reductants, such as elemental lithium and sodium, by light and technological advances in the field.
Subject areas: chemistry, catalysis, organic chemistry, green chemistry, chemical engineering
Graphical abstract
Chemistry; catalysis; organic chemistry; green chemistry; chemical engineering
Introduction
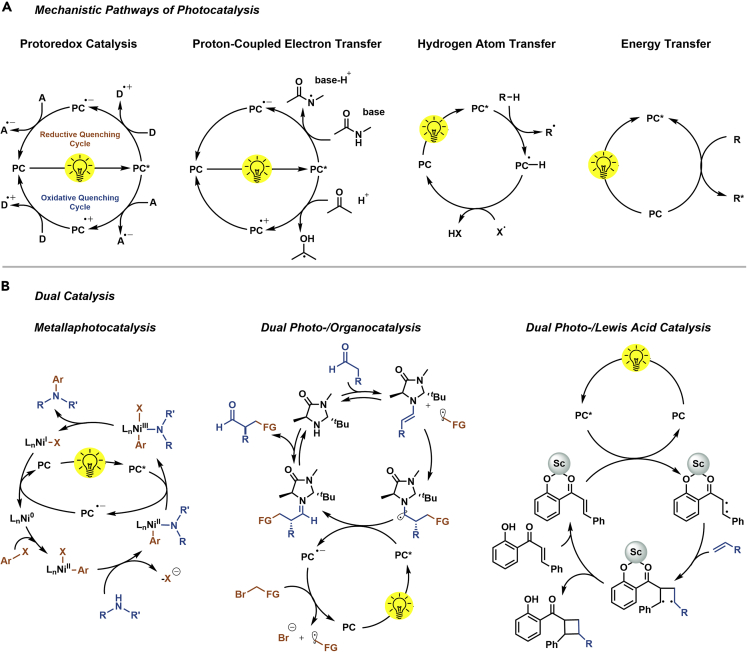
Using light to induce chemical reactions is attractive because photons are traceless reagents that provide energy to activate substrates, reagents, or catalytic intermediates under mild conditions. Traditionally, photochemical reactions were carried out using UV light to excite substrates or reagents (Hoffmann, 2008). The high energy of these light sources requires special equipment and often causes unselective reactions, which are difficult to predict and control. This has changed with the development of photocatalysts (PC) that can be activated with low-energy photons, paving the way for sustainable chemical synthesis that is driven by a non-hazardous and environment-friendly reagent: visible light (Crisenza and Melchiorre, 2020). Photocatalysts can initiate transformations via various mechanistic scenarios (Figure 1A) (Marzo et al., 2018). In particular, visible light photoredox catalysis has gained widespread recognition as a powerful tool in organic synthesis (Schultz and Yoon, 2014; Shaw et al., 2016). Upon irradiation, an excited catalyst (PC∗) accepts or donates a single electron, enabling oxidative or reductive quenching cycles depending on the substrates and reagents that are present in the reaction mixture. During an oxidative quenching cycle, the excited state catalyst reduces an electron acceptor (A), resulting in a strong oxidant (PC•+). This oxidized form of the catalyst can accept an electron from a suitable donor (D) to close the catalytic cycle. Depending on the reaction conditions, the inverse events can occur to complete a reductive quenching cycle. Redox events can be also accompanied by a concerted proton transfer (proton-coupled electron transfer [PCET]) (Gentry and Knowles, 2016). Photocatalytic hydrogen atom transfer (HAT), on the contrary, proceeds through homolytic cleavage of C–H bonds by the PC, or after single electron transfer (SET) events (Capaldo et al., 2020). Moreover, a PC can also transfer its excited state energy to a substrate or reagent that is not able to absorb light at the given wavelength, thereby inducing a chemical reaction (Strieth-Kalthoff et al., 2018).
Figure 1.
General mechanisms in photocatalytic synthesis
(A and B) Different modes of photocatalysis (A) and selected examples for dual catalysis (B).
Photocatalysis can be combined with “conventional” catalysis (dual catalysis) to enable reactions that are not possible using only one catalyst (Figure 1B) (Skubi et al., 2016). Merging photocatalysis with transition metal catalysis (metallaphotocatalysis) enables selective carbon-heteroatom and carbon-carbon cross-coupling reactions under mild conditions (Twilton et al., 2017). Key to the success is the modulation of the oxidation state of transition metal complexes by SET processes, radical additions, or photosensitization. This strategy is extensively studied using nickel complexes (Milligan et al., 2019; Twilton et al., 2017) and was further expanded to a range of other transition metals (Hopkinson et al., 2014; Skubi et al., 2016), including abundant first row metals such as cobalt (Kojima and Matsunaga, 2020), copper (Hossain et al., 2019), and iron (Neumeier et al., 2020; Ouyang et al., 2019). Combining photo- and organocatalysis involves SET of an excited PC and, for example, enamine intermediates to enable the α-functionalization of aldehydes with high enantioselectivity (Nicewicz and MacMillan, 2008). Catalytic amounts of Lewis acids form activated complexes with certain substrates, which can interact with a PC to trigger, for example, [2 + 2] photocycloadditions (Miller et al., 2017).
Photocatalysis in medicinal chemistry and the pharmaceutical industry
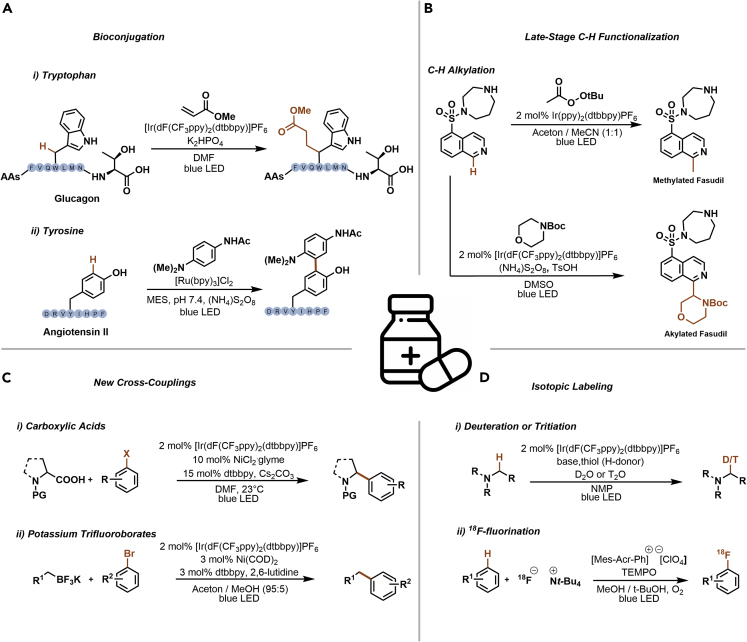
Although photocatalytic synthesis with visible light is a relatively young branch of organic chemistry, it quickly became an integral part of the synthetic chemists’ toolbox. The advantages associated with visible light photocatalysis resulted in various applications in medicinal chemistry, including drug discovery, bioconjugation, late-stage C–H functionalization, and isotopic labeling (Figure 2) (Douglas et al., 2016; Li et al., 2020).
Figure 2.
Representative examples of photocatalytic reactions of interest in medicinal chemistry
(A–D) Bioconjugations (A), late-stage C-H functionalization (B), Csp3-Csp2 cross-coupling reactions (C), isotopic labeling (D).
Chemoselective peptide modifications are achieved through the selective photocatalytic activation of tryptophan to induce Michael-type additions (Figure 2A) (Yu et al., 2018). The method was validated on the peptide hormone glucagon resulting in 16% isolated yield of the desired conjugate. The low selectivity was a result of bis-conjugation (tryptophan and C terminus), but remarkably, no conjugation on the His, Phe, Tyr, Arg, Met, Ser, Lys and Thr residues was observed.
Tyrosine residues were selectively modified using Ru(byp)3Cl2 (Sato and Nakamura, 2013). The authors could also show that the tyrosine modification can be carried out on a specific protein in cell lysates. Therefore, the ruthenium PC was equipped with a ligand that binds selectively to the target protein. This enables local SET reactions on the target protein, whereas the tyrosine amino acids of other proteins in the lysate stay untouched.
Photocatalysis enabled the late-stage alkylation of biologically active heterocycles (Figure 2B). Methylation of Fasudil, an important rho-kinase inhibitor, was achieved via the generation of a methyl radical using t-butylperacetate and an iridium PC through a PCET mechanism (DiRocco et al., 2014). In a similar vein, Fasudil was functionalized with N-Boc-protected morpholine. In this case, the generation of an α-amino radical enabled the direct cross-dehydrogenative-coupling via a Minisci-type addition (Grainger et al., 2019). In both cases, high-throughput experimentation techniques were used to identify suitable catalytic cocktails.
Metallaphotocatalysis is at the forefront of light-mediated reactions and has a significant impact in small molecule synthesis (Figure 2C). In traditional, palladium-catalyzed transformations organometallic nucleophiles are coupled with aryl (pseudo)halides. These methods are effective for Csp2-Csp2 couplings, but Csp3-Csp2 couplings are challenging because of low rates of oxidative addition (OA) and reductive elimination (RE), as well as undesired side reactions via β-hydride elimination. In 2014, two independent publications showed that efficient Csp3-Csp2 cross-couplings can be achieved using dual photo/nickel catalysis via the photocatalytic generation of alkyl radicals from carboxylic acids (Zuo et al., 2014), or potassium trifluoroborates (Tellis et al., 2014). The radical adds to a Ni(II) complex that is formed via OA of an aryl halide with a Ni(0) catalyst. The resulting Ni(III) complex undergoes facile RE of the desired product, and a final photocatalytic SET event regenerates the Ni(0) catalyst. A range of similar strategies for other alkyl radical precursors (Milligan et al., 2019) and various carbon-heteroatom couplings (Zhu et al., 2020) were developed that all have the potential to have a high impact in the synthesis of active pharmaceutical ingredients (APIs).
Understanding the metabolic fate of a drug candidate is a key factor during its development. One common technique to gain better understanding of the biological behavior is to label its molecular framework with stable isotopes. Late-stage labeling of pharmaceutically active compounds with deuterium and tritium was recently realized via a photoredox-mediated HAT reaction (Figure 2D) (Loh et al., 2017). This method enables hydrogen-deuterium or hydrogen-tritium exchange reactions using isotopically labeled water (D2O or T2O) in a single step and was applied for several APIs. More recently, a direct arene C-H fluorination with 18F-containing salts was realized using an acridinium PC (Chen et al., 2019).
In addition, a series of new strategies and concepts for light-mediated methodologies were developed that have the potential to open up new horizons in medicinal chemistry and industrial applications. These protocols access hitherto undisclosed reactions, enable new ways to control selectivities, and overcome limitations of current approaches. Here, we discuss these developments using selected examples. We begin our survey with methods that enable the use of long wavelengths, which is key for efficient solar harvesting. Thereafter, we discus examples that show that the photons are not only a sustainable energy source to trigger reactions but also enable controlling selectivities in reactions by changing the photon energy/intensity. Next, we survey strategies to generate catalyst species that have oxidation/reduction potentials beyond those that are accessible by “standard” photoredox cycles and have the potential to serve as sustainable alternatives for reactions that, for example, require elemental sodium or lithium. Reaction technology is of utmost importance for reproducible and efficient photocatalytic reactions. In the last two sections, we discuss some recent technological achievements for photocatalytic transformations using batch and continuous flow strategies.
The wavelength matters
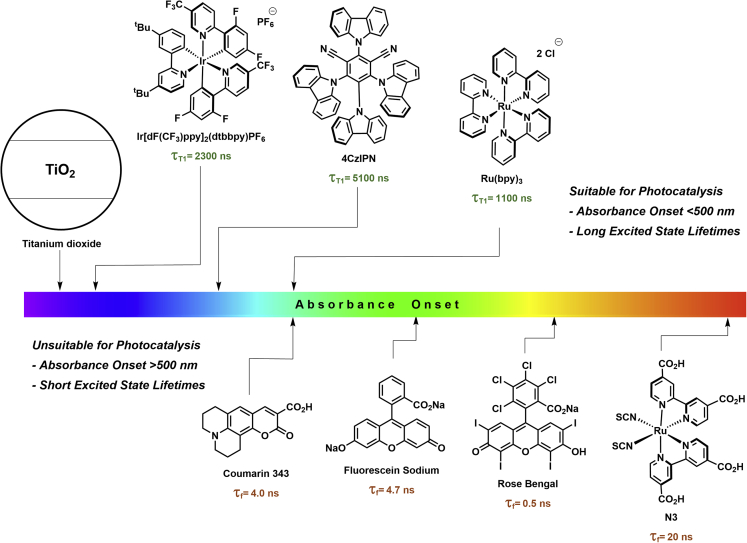
Most photocatalytic reactions, including the examples described earlier, rely on a small set of PCs, such as homogeneous iridium or ruthenium polypyridyl complexes (Arias-Rotondo and McCusker, 2016), a few organic dyes (Romero and Nicewicz, 2016), and some semiconducting materials (Gisbertz and Pieber, 2020) with suitable redox potentials or triplet energies, and long-lived excited states. One of the main drawbacks of these PCs is their limitation to highly energetic visible light (Figure 3) (Prier et al., 2013). Photocatalytic strategies that use the entire visible light spectrum would enable efficient solar harvesting and are key for performing sustainable photochemical reactions with sunlight instead of artificial light sources. Furthermore, photochemical systems that are able to use near-infrared (NIR) radiation allow the activation of PCs through barriers, such as skin and tissue, which bears high potential for biological and medical applications (Glaser et al., 2020). Many dyes absorb broadly across the visible light spectrum and their redox potentials and excited state energies are in theory suitable for photocatalysis, but these chromophores have low ISC rates, and therefore reach only short-lived singlet excited states that are not suitable for photocatalysis.
Figure 3.
Onset of absorption of selected dyes and semiconductors
The suitability for photocatalysis depends on excited state lifetimes.
Chromophores with short excited lifetimes have shown enormous potential in other research areas. A plethora of organic dyes are used as sensitizers in dye-sensitized solar cells (DSSCs) (Hagfeldt et al., 2010). In DSSCs, the dyes are adsorbed or bound to the surface of a semiconducting material, such as TiO2. Because of the resulting spatial proximity, even dyes with short excited state lifetimes efficiently inject electrons into the conduction band of the semiconductor. This results in charge-separated species that are sustained for several microseconds (Hagfeldt and Graetzel, 1995). Compared with molecular PCs with long-lived excited states, only a few reports of dye-sensitized semiconductors as PCs for organic synthesis were reported to date (Franchi and Amara, 2020).
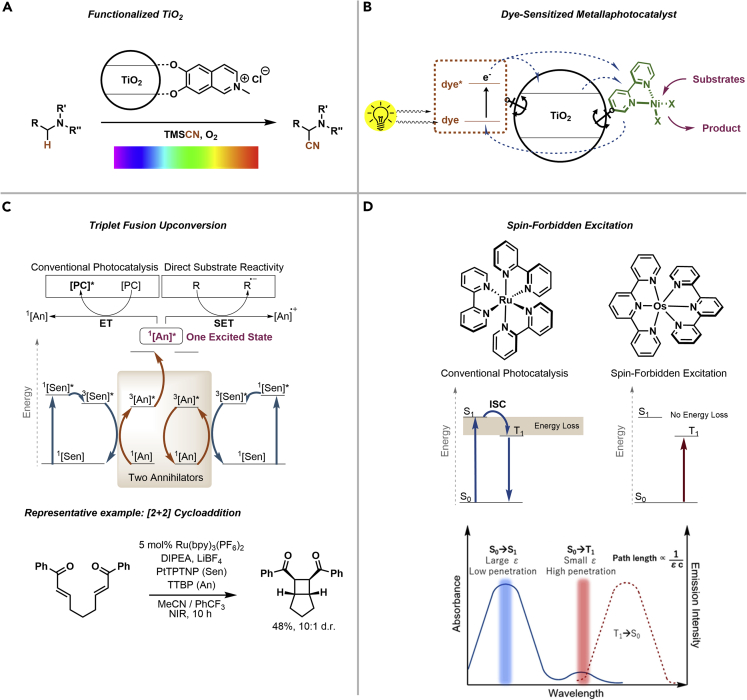
An intriguing example is the functionalization of TiO2 nanoparticles (NPs) with 6,7-dihydroxy-2-methylisoquinolium (DHMIQ), which results in a visible light PC that can catalyze the α-cyanation of tertiary amines (Figure 4A) (Nauth et al., 2018). DHMIQ is a combination of a chromophore and a catechol moiety that binds to the surface of the semiconductor. The redox active ligand was bound to spherical TiO2 NP, which were prepared via a hydrothermal synthesis of titanium(IV)butoxide with oleic acid via a post-synthetic ligand exchange. The resulting TiO2-DHMIQ hybrid absorbs across the entire visible light spectrum and in the NIR region. The catalytic activity was shown for the aerobic cyanation of several tertiary amines using trimethylsilyl cyanide in acetonitrile. A detailed study of all reaction conditions showed that traces of water lower the yield dramatically, presumably because of the formation of a hydration shell around the NPs that inhibits productive catalysis. The authors showed that the catalyst can be used with blue (462 nm), green (520 nm), yellow (592 nm), red (635 nm), and NIR (730 nm) irradiation for the title reaction.
Figure 4.
Strategies to access high wavelengths for photocatalytic synthesis
(A–D) Functionalized TiO2 with non-innocent ligands (A). Dye-sensitized metallaphotocatalysts (B). Triplet fusion upconversion (C). Spin-forbidden excitation of osmium complexes (D). Reproduced with permission from (Ravetz et al., 2020)
More recently, the immobilization of a chromophore and a nickel complex on the surface of TiO2 resulted in a single material that can be used for metallaphotocatalytic cross-couplings (Figure 4B) (Reischauer et al., 2020). These dye-sensitized metallaphotocatalysts (DSMPs) assemble in situ by adding a dye and a nickel complex, which both are equipped with a functional group that binds to the semiconducting material, and TiO2 to a solution of the substrates and a base. The operational simplicity in combination with the high modularity of the three-component catalyst enabled a straightforward screening of suitable dyes and ligands for various cross-couplings and different irradiation sources. The DSMP system was applied for C–O, C–S, C–N, and C–C couplings using blue (440 nm), green (525 nm), and red (666 nm) light. During a series of control experiments, the authors showed that productive catalysis was also achieved when the semiconductor TiO2 was replaced with insulating SiO2 and Al2O3, but no product was formed in the absence of a support. These results in combination with spectroscopic studies indicated that two mechanisms are responsible for catalytic activity. In case of TiO2, electrons are injected from the excited dye into the conduction band (CB) of TiO2 and transferred to the nickel complex (“through-particle”). If insulating materials are used, the excited dye molecules directly transfer energy or electrons to the nickel complex (“on particle”). The latter process is, however, significantly less efficient compared with the “through-particle” mechanism, resulting in very long reaction times.
A more general approach to use long wavelengths for photocatalytic synthesis is triplet fusion upconversion (Figure 4C) (Zhou et al., 2015). This process involves a sensitizer ([Sen]) that absorbs low-energy photons to reach a triplet excited state (3[Sen]∗), which transfers its energy to an annihilator ([An]) resulting in the triplet excited species 3[An]∗. Two triplet excited annihilators (3[An]∗) can undergo triplet fusion to generate a higher energy singlet exciton (1[An]∗) that decays via fluorescence by emitting a high-energy photon. The annihilator furanyldiketopyrrolopyrrole (FDPP) was combined with the sensitizer palladium(II) octabutoxyphthalocyanine (PdPc) to convert NIR photons into orange light, which in turn can excite the organic PCs eosin Y and rose bengal (Ravetz et al., 2019). This catalytic cocktail was used for hydrodehalogenations, oxidations and radical cyclizations using NIR light as energy source. Tetratertbutylperylene (TTBP) and platinum(II) tertraphenyltetranaphthoporphyrin (PtTBTNB) were used to convert NIR into blue light to activate a ruthenium bipyridyl complex that catalyzes a [2 + 2] cyclization. TTBP and PtTBTNB also triggered the polymerization of methyl methacrylate (MMA) via NIR irradiation in the absence of an additional PC. As NIR light penetrates through opaque media, the polymerization could be carried out by irradiating the reaction mixture through several materials, including pig skin. For similar reasons, this approach improved the scalability of the MMA polymerization significantly.
More recently, the use of NIR and deep red light was realized using Os(II) terpyridine complexes as PCs (Figure 4D) (Ravetz et al., 2020). These transition metal complexes undergo a spin-forbidden S0 → T1 transition upon irradiation with long wavelengths. Strategic ligand design resulted in a library of osmium complexes with different redox potentials. Several examples, including polymerizations, cycloadditions, radical methylations, smiles reactions, and metallaphotocatalytic transformations using NIR light showcased the broad applicability of this catalytic strategy. The extinction coefficients of Os(II) terpyridine complexes are lower when compared with common PCs, which improves the scalability as showcased for an efficient photocatalytic trifluoromethylation on a mole scale in batch using a vessel with a large cross-sectional area.
The energy and intensity of photons
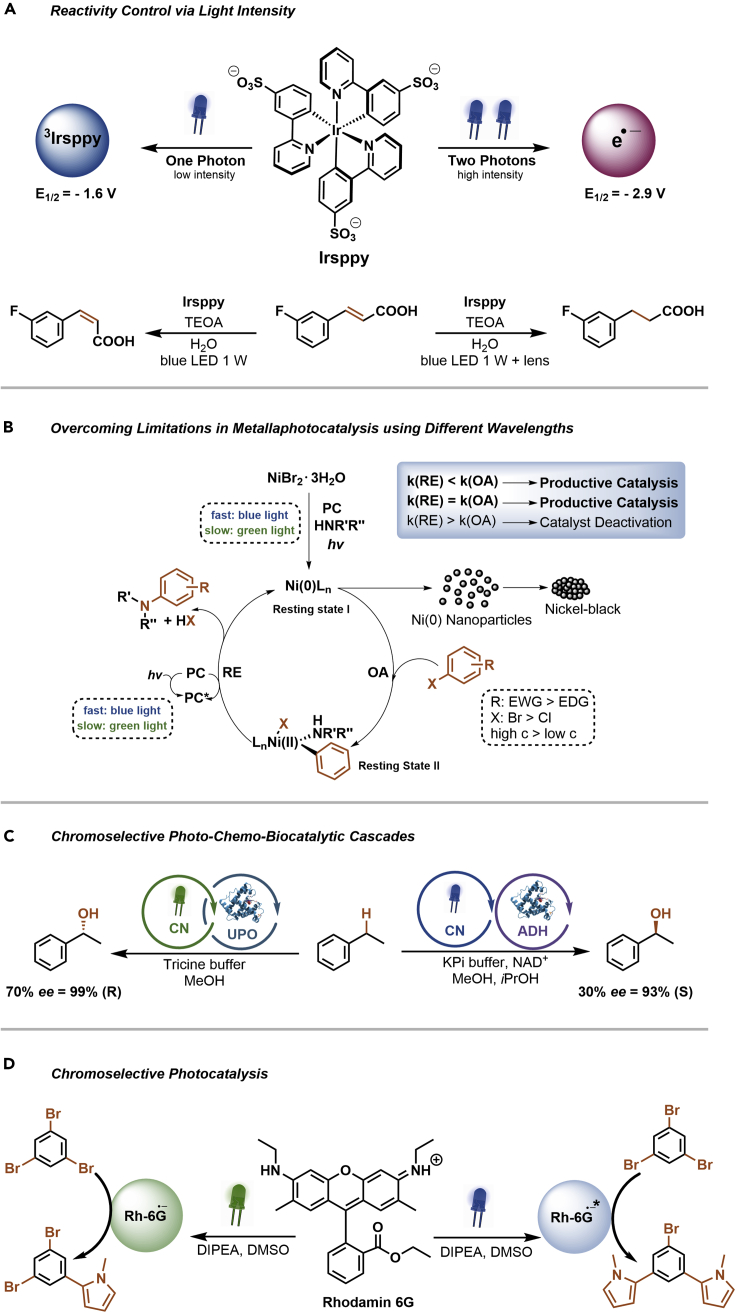
Light is more than only a traceless, sustainable reagent. The energy and intensity of photons are overlooked parameters that can be used to tune photocatalytic activities and influence the selectivities, or even reactivity of a PC (Figure 5) (Protti et al., 2019). The water-soluble iridium complex fac-tris[2-(5′-sulfonatophenyl)pyridine]iridate(III) pentahydrate (Irsppy) shows completely different reactivity depending on the intensity of blue light irradiation (Figure 5A) (Kerzig and Wenger, 2019). Low light intensity (one-photon excitation) results in 3Irsppy that has a triplet energy of 2.65 eV and redox potentials that are suitable for various photoredox reactions. At high light intensities (two-photon excitation), strongly reducing hydrated electrons (standard potential of −2.9 V versus NHE) can be generated in aqueous solutions. By using a 447-nm continuous wave laser, this can be used to achieve different selectivities and reactivities for several transformations. Upon irradiation with low photon intensities, 3Irsppy induces the E-Z isomerization of 3-fluorocinammic acid via an energy transfer mechanism. When a lens is placed between the light source and the reaction mixture to concentrate the light intensity, the hydrogenation of the alkene was observed, which is initiated via a single electron reduction by hydrated electrons. Similarly, this method allowed to obtain either the hydrogen atom abstraction (low intensity) or the dimerization product (high intensity) of 4-(chloromethyl)benzoic acid.
Figure 5.
Accessing different photocatalytic activities by controlling the energy and intensity of photons
(A–D) Reactivity control of an iridium photocatalyst through the light intensity (A). Overcoming limitations in metallaphotocatalysis using carbon nitride photocatalysis by changing the wavelength (B). Chromoselective photo-chemo-enzymatic cascade reactions with a carbon nitride photocatalyst (C). Chromoselective photocatalysis with Rhodamine 6G (D).
As described in the previous section, the broad absorption of PCs across the visible light spectrum improves solar harvesting, reduces the energy input using artificial light sources, and is beneficial for the scalability of light-mediated reactions. Moreover, the energy of photons can be used to control the activity of photochemical processes, thereby tuning the selectivity of a photocatalytic reaction. This was used to overcome substrate scope limitations and reproducibility issues in metallaphotocatalytic C–N cross-couplings of cyclic secondary amines with electron poor aryl halides (Figure 5B) (Gisbertz et al., 2020). This limitation is a result of catalyst deactivation via the formation of nickel black, which was attributed to the accumulation of low-valent nickel species due to the relatively slow OA in case of electron-rich aryl halides. To avoid this problem, the relative rate of OA has to be equal to or higher than the rate of RE. By using a heterogeneous PC (CN-OA-m) that absorbs weakly at longer wavelengths, the rate of RE was significantly reduced using green light, which was sufficient to avoid catalyst deactivation in certain cases. Blue light irradiation could be used when the rate of OA was increased using a high substrate concentration. These measures were, however, not successful in case of primary amines. For such substrates, the additive MTBD (7-methyl-1,5,7-triazabicyclo(4.4.0)dec-5-ene) was used to stabilize low-valent nickel intermediates, thereby decelerating the rate of nickel black formation. Similarly, decelerating a photocatalytic reaction at longer wavelengths was key to achieve high selectivities for the light-mediated benzyl ether deprotection with photoexcited 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (Cavedon et al., 2021).
More recently, it was shown that the oxidation potential of CN-OA-m differs depending on the irradiation wavelength (Figure 5C) (Schmermund et al., 2021). Irradiation with blue light leads to π-π∗ transitions that enable the oxidation of ethylbenzene to acetophenone, whereas no reaction was observed when n-π∗ transitions where induced using green light. This phenomenon was used for photo-chemo-enzymatic cascades that give either the (S)- or the (R)-enantiomer of chiral benzylic alcohols. Green light irradiation of a cocktail consisting of CN-OA-m, an unspecific peroxygenase from Agrocybe aegerita, and ethylbenzene in an aqueous buffer allows the selective formation of H2O2 that fuels the enantioselective biocatalytic hydroxylation of ethylbenzene to (R)-1-phenylethanol (99% enantiomeric excess [ee]). Blue light was used for the photocatalytic oxidation of the ethylbenzene to acetophenone, which in turn was reduced by an enantioselective alcohol dehydrogenase from Rhodococcus ruber to yield (S)-1-phenylethanol (93% ee).
Varying the irradiation wavelength also enables selective control between a 1- or 2-fold substitution of 1,3,5-tribromobenzene with N-methylpyrrole using Rhodamine 6G (Rh-6G) as PC (Figure 5D) (Ghosh and König, 2016). In the presence of N,N-diisopropylethylamine (DIPEA), the radical anion Rh-6G.- is formed upon irradiation with green light (530 nm). This species has a reduction potential of −1.0 V versus SCE, which is sufficient to activate aryl bromides with relatively low reduction potentials, resulting in a selective monosubstitution. When blue light (455 nm) was used as irradiation source the disubstituted products were obtained. This is possible because at short wavelengths, Rh-6G∙- is again excited, forming Rh-6G∙-∗ (reduction potential ca. −2.4 V versus SCE), which can activate aryl bromides with rather high reduction potentials.
Replacing elemental alkali metal reductants with light
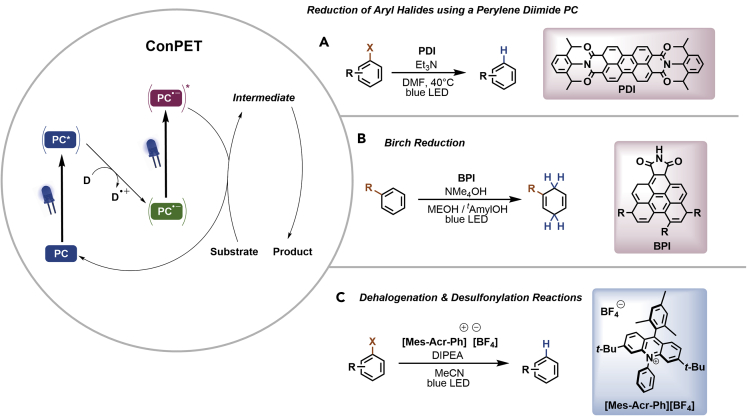
The wavelength-dependent approach described above that generates Rh-6G∙- or Rh-6G∙-∗, which have different redox potentials, is not limited to this specific xanthene dye. Similar approaches can be used to generate highly reductive species from other organic PCs (Figure 6) (Schmalzbauer et al., 2021). The common mechanistic feature of these strategies is that quenching of PC∗ with a sacrificial electron donor (SED) generates a relatively stable intermediate that is able to absorb another photon (consecutive photoinduced electron transfer [ConPET]). This results in excited states that are characterized by remarkably strong single electron reduction potentials that can be similar to elemental lithium (−3.28 V versus SCE) and sodium (−2.95 V versus SCE).
Figure 6.
Accessing strong photoreductants via consecutive photoinduced electron transfer (ConPET)
(A–C) Reduction of aryl halides using a perylene diimide PC (A). Photocatalytic Birch-type reductions using BPI (B). Dehalogenation using a mesityl acridinium salt (C).
The first reported example used the perylene diimide N,N-bis(2,6-diisopropylphenyl(perylene-3,4,9,10-bis(dicarboximide))) (PDI) as PC (Figure 6A) (Ghosh et al., 2014). Perylene diimides are fluorescent dyes that absorb broadly across the visible light spectrum and are used as pigments, colorants, electronic materials, and photoreceptors, with only a few applications in photocatalysis. Visible light excitation generates PDI∗, which is quenched by an SED yielding the moderately reducing PDI∙- (−0.43 V versus SCE). Absorption of a second photon generates PDI∙-∗ that reduces several aryl iodides, bromides, and chlorides in moderate to excellent yield. In the presence of radical trapping agents, such as substituted pyrroles, the corresponding C–C coupling products were obtained.
More recently, a benzo[ghi]perylene monoimide (BPI) was also shown to be able to undergo ConPET, forming the excited radical anion BPI∙-∗ (Figure 6B) (Cole et al., 2020). According to density functional theory calculations, this species is an extremely strong reductant (−2.43 to −4.28 V). In fact, the authors were able to show that BPI is able to reduce several arenes, including benzene (Ered < −3.42 V versus SCE) to the corresponding 1,4-dienes in the presence of NMe4OH and 405 nm irradiation via a Birch-type mechanism. Mechanistic investigations suggested that BPI∙-∗ releases a solvated electron that is responsible for substrate reduction rather than a direct SET between the excited radical anion and the substrate. By adapting the reaction conditions, BPI ConPET catalysis was also demonstrated to enable reductive deoxygenations, selective olefin reductions, reductive cylopropane ring-openings, and late stage dehalogenations.
Mesityl acridinium salts (Mes-Acr+) are among the most potent PCs for oxidative reactions (Romero and Nicewicz, 2016). Nicewicz and colleagues realized that the neutral aciridine radical (Mes-Acr∙), which is formed after quenching of ∗Mes-Acr+ with Hünig's base, is relatively stable under oxygen-free conditions (MacKenzie et al., 2020). Studies of the photophysical properties of this persistent radical revealed that absorption of a second photon (>350 nm) generates two new excited states, a lower-energy doublet, and a twisted intramolecular charge-transfer state that has strong reduction potentials (−2.91 and −3.36 V versus SCE, respectively). Based on this discovery, the authors developed protocols for the light-mediated reductive dehalogenation of aryl bromides and chlorides, and for the reductive detosylation of tosyl amines catalyzed by Mes-Acr-BF4 (Figure 6C). It is worth noting that ConPET pathways can be also realized using iridium complexes as PCs (Giedyk et al., 2020).
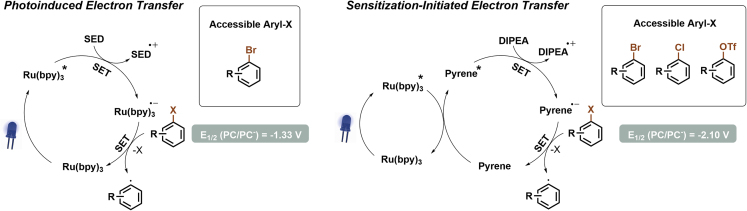
Polycyclic aromatic hydrocarbons (PAHs), such as pyrene and triphenylene, generate strongly reducing radical anions upon irradiation with UV light and quenching of the excited state by a single electron donor. König and colleagues hypothesized that these excited states could be also accessible using visible light via an energy transfer from a PC to a (PAH (Figure 7) (Ghosh et al., 2017). Key to the success was the selection of a PAH/PC couple that has similar triplet energies, shows fast EnT from the PC to the PAH, and fast SET between PAH∗ and the SED to generate the strongly reducing PAH radical anion. The authors identified that the combination of Ru(bpy)3Cl2, pyrene, and DIPEA as SED fulfills these requirements. Generation of the pyrene radical anion enabled the C–H arylation of aryl (hetero)aryl bromides, chlorides, or triflates, as well as light-mediated C–P couplings.
Figure 7.
Photoinduced electron transfer (PET) versus sensitization-initiated electron transfer (SenI-ET)
Better, faster, scalable: technological aspects and developments
The impact of visible light photocatalysis in academia and industry cannot be understated, and the continuous advancements and developments of new concepts, catalysts, and strategies will likely increase the impact of such reactions for industrial processes in the future. Nevertheless, photocatalysis faces some problems that require the attention of practitioners in industrial and university settings.
First, the reported photocatalytic protocols can be difficult to reproduce, which renders the adaption of developed methods by other laboratories difficult. “Conventional” reactions require heating or cooling, which is in most cases easy to duplicate in other research laboratories. The reproducibility of photocatalytic transformations strongly depends on the experimental setup. Light-mediated reactions are usually carried out using light-emitting diode (LED) lamps that often have different specifications, including emission spectra and photon outputs, which can have a dramatic influence on a photochemical transformation. The standardization of photochemical reactors using dedicated, commercial equipment might be an ideal solution (Le et al., 2017), but is unlikely to happen due to low prices of self-made setups. In an excellent comment, researchers from GSK, Pfizer, Merck, and AbbVie discussed problems related to photochemical setups and called for more accurate descriptions of light sources and reactor arrangements when reporting experimental procedures (Bonfield et al., 2020).
Second, photocatalytic transformations rely on efficient irradiation of the reaction mixture. Solvents, starting materials, products, photosensitizers, and PCs, at the point of incident light, can all act as filters reducing the light intensity available for the rest of the reaction mixture. This attenuation effect of photon transport (Beer-Lambert law) becomes particularly problematic on larger scales. The Beer-Lambert law states the correlation between the absorption (A) and the molar extinction coefficient (ε) of the molecule(s), their concentration (c), and the optical path length of the light (l) (Equation 1).
| (Equation 1) |
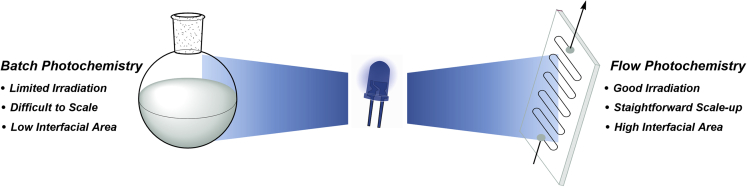
This trade-off has serious implications, especially for scaling a photoreaction in batch. Continuous flow (micro)reactors are the technology of choice to overcome this bottleneck (Plutschack et al., 2017) (Cambié et al., 2016). The narrow channel dimensions of flow reactors provide opportunities to ensure a uniform irradiation of the entire reaction mixture. Consequently, photochemical reactions can be substantially accelerated and scaled to higher quantities compared with batch reactors (Figure 8). Flow chemistry is also the technology of choice for transformations involving multiple phases. The high surface-area-to-volume ratios are a consequence of the small reactor size, leading to efficient mass transfer between two (or even three) phases. In case of gaseous reagents, flow reactors further offer the opportunity to control the stoichiometry of gasses with mass-flow controllers and are easily pressurized, which increases the solubility of gasses in the reaction mixture.
Figure 8.
Photochemistry in batch and flow reactors
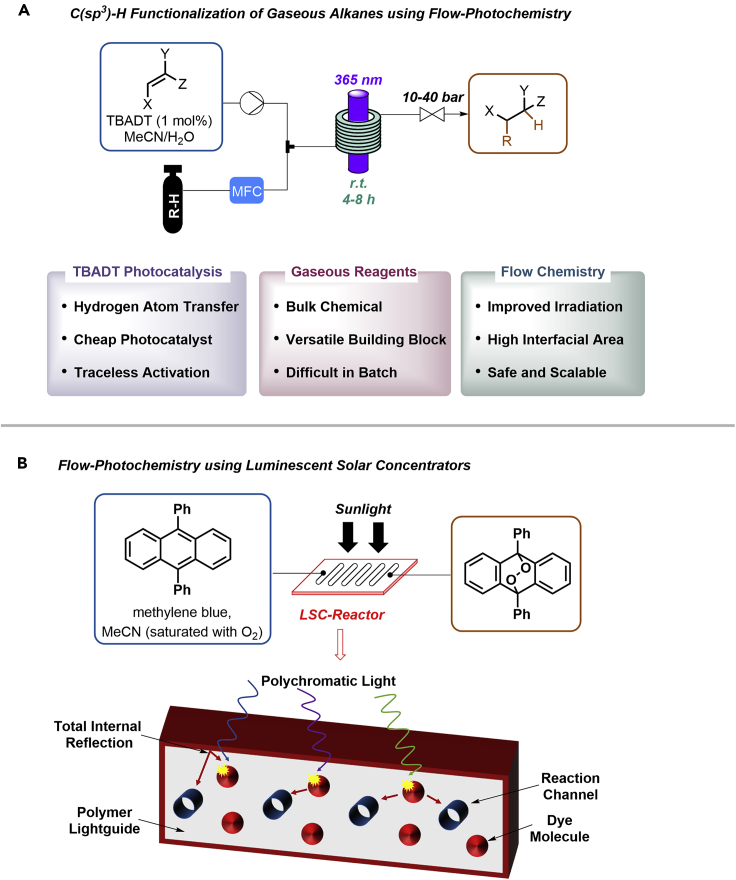
All these advantages were recently combined in a photocatalytic gas/liquid process that enables the direct functionalization of light hydrocarbons via HAT (Figure 9A) (Laudadio et al., 2020). A solution of the HAT catalyst tetrabutylammoniumdecatungstate (TBADT) and an olefin starting material in a suitable solvent was mixed with gaseous methane, ethane, propane, or isobutene and fed into a 365-nm flow photoreactor at back pressures of 10–45 bar. Upon irradiation, the excited PC abstracts a hydrogen atom from the respective alkane. The resulting nucleophilic C-centered radical undergoes a conjugate addition onto the olefin. A subsequent hydrogen back-donation results in the desired product and restores TBADT. This transformation is difficult to access in batch reactors as efficient irradiation and high pressures are crucial for product formation.
Figure 9.
Flow photocatalysis
(A and B) C(sp3)-H functionalization of light hydrocarbons using photocatalysis in flow (A). Luminescent solar concentrator for energy-efficient flow chemistry using sunlight (B).
Similar to the use of gaseous feedstock chemicals such as methane, the use of sunlight as energy source can greatly benefit from continuous flow chemistry. One approach to use sunlight efficiently combines continuous flow technology with luminescent solar concentrators (LSCs, Figure 9B) (Cambié et al., 2017). LSCs are made by dispersing a luminophore in a waveguide, such as polymeric materials or glass. Light penetrates the surface of the waveguide and is absorbed by the luminophore. Re-emitted photons are guided and concentrated by total internal reflection toward the edge of the device. The adaption of this principle to continuous flow synthesis was realized using a chip-based reactor made out of PDMS that was doped with the fluorescent dye Lumogen F red 305. This dye absorbs visible light from ∼400–600 nm and re-emits light at ∼600–700 nm. The emitted light perfectly overlaps with the absorption spectrum of methylene blue, a common triplet photosensitizer. The authors studied the singlet oxygen cycloaddition to 9,10-diphenylanthracene using sunlight during a cloudy day and showed that this reactor is significantly more efficient than non-doped reactors.
Flow chemistry is, however, not the ultimate solution to all problems in (photo)chemical synthesis and has still several limitations. One of the biggest bottlenecks of flow chemistry is the handling of solid materials, such as heterogeneous PCs, which have advantages over homogeneous PCs (Gisbertz and Pieber, 2020). Packed bed reactors with heterogeneous catalysts embedded between filter units are unsuitable for opaque PCs, because photons will be exclusively absorbed at the outer region, whereas the inner particles are shielded. Efficient irradiation can be ensured by pumping a suspension through a coil reactor, but the solid catalyst will settle, leading to a heterogeneous distribution, irreproducible results, or clogging.
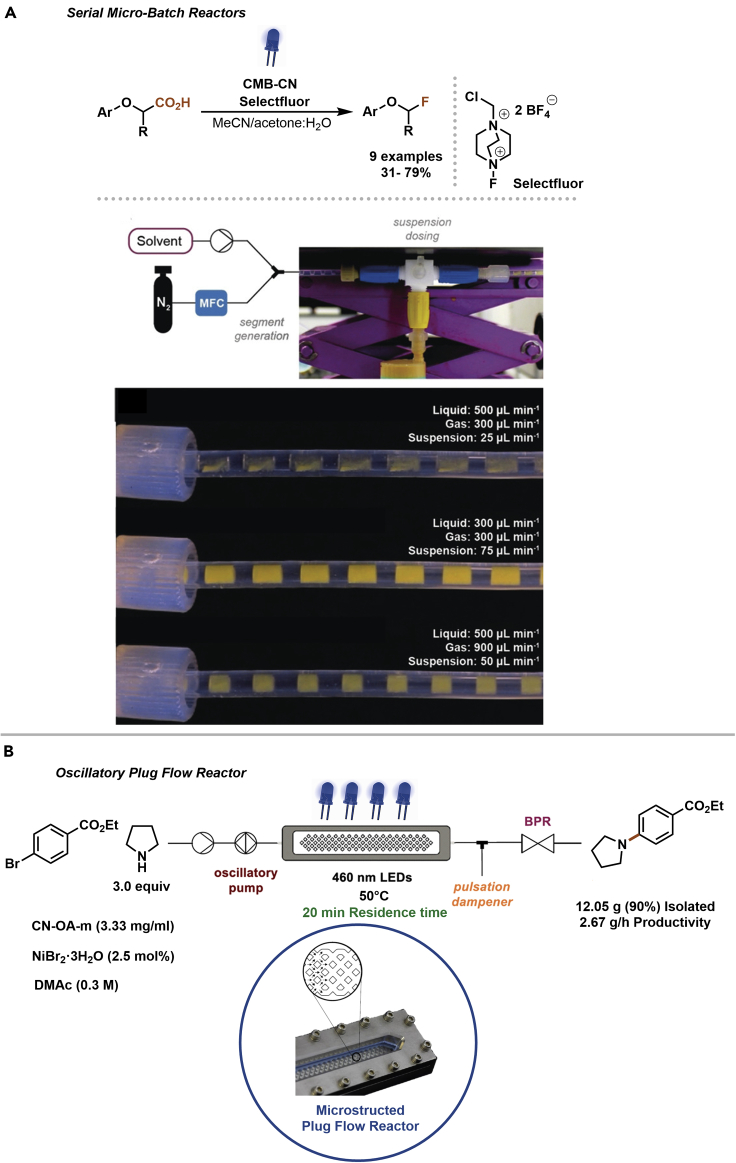
This problem was recently tackled on laboratory scale by a system that generates serial micro-batch reactors (SMBRs, Figure 10A) (Pieber et al., 2018). SMBRs are a series of small solid-liquid compartments, which contain all ingredients for the chemical transformation, and are separated by an inert gas spacer within a coil reactor tubing. This was realized by dosing a heterogeneous carbon nitride PC to a stable gas-liquid segmented flow. The resulting triphasic mixture can be conveniently pumped through an irradiated coil reactor that is submerged in a thermostatic bath to perform photocatalytic reactions. The natural Taylor flow mixes the slug to continuously re-suspend the material, ensuring efficient irradiation and reproducible processing. In this system, the reaction time can be adapted by changing the gas and/or liquid flow rate or the reactor volume, whereas the catalyst stoichiometry can be varied by changing the rate of suspension dosing. The system was evaluated and optimized using the photocatalytic decarboxylative fluorination of phenoxyacetic acids and phenylacetic acid derivatives using Selectfluor and a carbon nitride PC made from cyanuric acid, melamine, and barbituric acid (CMB-CN).
Figure 10.
Heterogeneous photocatalyst in flow
(A and B) Decarboxylative fluorination of phenoxyacetic acids in flow using serial micro-batch reactors (A). Reproduced with permission from (Pieber et al., 2018). Dual nickel/carbon nitride amination using an oscillatory plug flow reactor (B). Reproduced with permission from (Rosso et al., 2020).
More recently, the combination of an oscillatory pump and a microstructured plug flow photoreactor was shown to be also capable of processing heterogeneous PCs (Figure 10B) (Rosso et al., 2020). Careful tuning of the pulsation frequency and amplitude was crucial for controlling the residence time distribution. The nickel/carbon nitride-catalyzed C–N cross-coupling described earlier (Figure 5B) was significantly intensified and was achieved in reaction times as low as 20 min. To demonstrate the scalability, a 4.5-h experiment provided a model compound on a 12 g scale (2.67 g/h). Additionally, an intermediate of tetracaine, a local anesthetic, was synthesized on a gram scale.
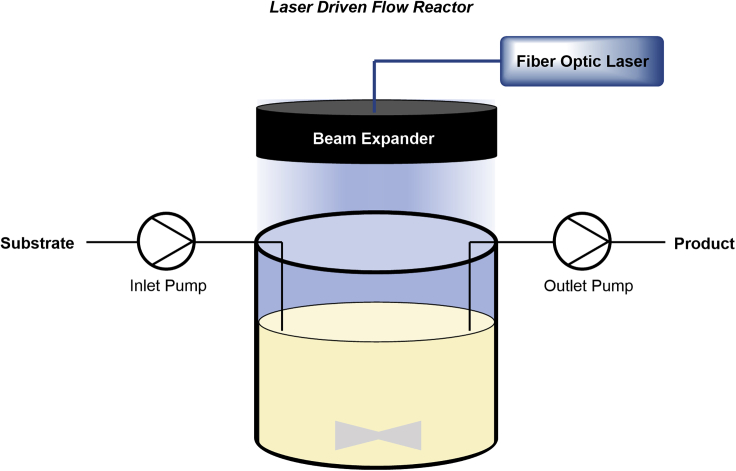
A metallaphotocatalytic C-N cross-coupling was also used as benchmark reaction for a continuous stirred tank reactor (CSTR) equipped with a continuous wave laser that achieves high productivities (Figure 11) (Harper et al., 2019). Continuous wave lasers have several advantages over standard LEDs, including the ability to measure the output power, the coherence of light, the ability to shape the beam, and a significantly higher intensity. The researchers coupled a 405-nm laser with an adjustable beam expander and studied the reaction kinetics of a model C–N coupling using a homogeneous iridium complex as PC in combination with a nickel salt. During these studies, the authors realized that the optimal reaction performance is correlated to the concentration of the PC, the solution depth, and the power density and can be entirely determined by the Beer-Lambert law. Coupling the laser setup with a CSTR and applying the optimized conditions allowed them to perform a continuous experiment over 32 h under steady-state conditions to produce 1.85 kg of the desired coupling product.
Figure 11.
Continuous stirred tank reactor (CSTR) for large-scale laser-driven photocatalysis
Photon-free photocatalysis
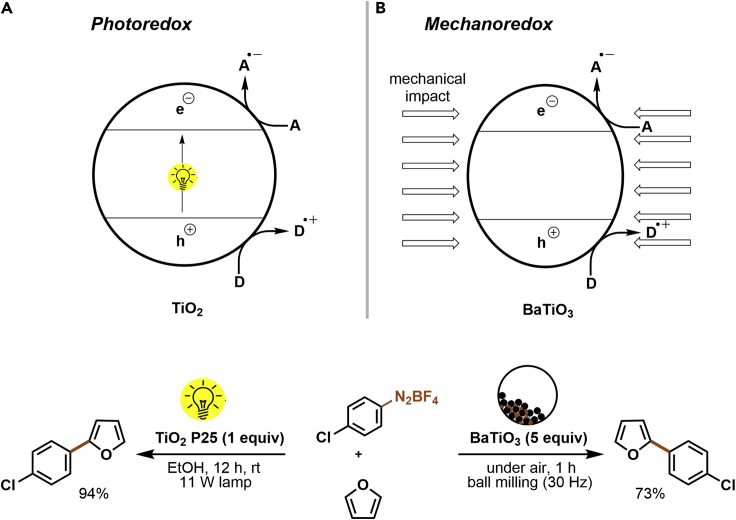
The “unique” feature of many homogeneous PCs is their ability to trigger redox events upon excitation through oxidative or reductive quenching cycles (Figure 1). Heterogeneous semiconductors, such as TiO2 or carbon nitrides, are essentially operating by similar mechanisms. When a semiconductor absorbs photons with sufficiently high energy, electrons are excited from the valence band (VB) to the CB, generating simultaneously an oxidizing and a reducing species on a single particle. The generated electron holes can oxidize electron donors, whereas the electrons in the VB are able to reduce electron acceptors via SET (Figure 12A).
Figure 12.
Photon-free photocatalysis
Comparison of photoredox catalysis (A) and mechanoredox catalysis (B).
Ito and colleagues showed that electron-hole pairs can be also generated mechanochemically by generating an “excited-state” barium titanate via ball-milling (Figure 12B) (Kubota et al., 2019). Upon agitation, the piezoelectric material becomes temporarily highly polarized and generates an electrochemical potential that is suitable for the activation of redox-active aryl diazonium salts, which was previously reported using, for example, TiO2 photocatalysis (Zoller et al., 2015). The authors could show that ball-milling of a mixture of aryldiazonium salts, BaTiO3, and heterocycles, such as furan, thiophene, or protected pyrrole, results in C–C coupling products. Moreover, the borylation of aryldiazonium salts with bis(pinacolato)diboron was achieved via the same approach. The methodology was proved to be scalable during a gram-scale synthesis. Recycling experiments further showed that BaTiO3 can be reused three times before the catalytic activity decreases. This approach is an interesting alternative to photocatalysis that can be carried out in the absence of solvents and light, which overcomes some of the problems related to photochemistry. The method is also reported to be very robust as reactions were even induced by “wrapping all ingredients in weighing paper and striking it with a hammer” instead of dedicated ball-milling equipment.
Conclusion and outlook
In summary, it can be stated without any doubt that photocatalysis has already significantly expanded the organic chemists’ toolbox and provides sustainable opportunities for synthesis in academia and industry. The steadily increasing amounts of hitherto undisclosed reactions that can be realized using visible light, such as replacing elemental alkali metal reductants, will certainly have a significant impact in industry and academia. The recent efforts to use the entire visible light spectrum for efficient photocatalysis might be a small step toward a chemical industry that was proposed more than 100 years ago, where “forests of glass tubes will extend over the plains and glass buildings will rise everywhere; inside of these will take place the photochemical processes that hitherto have been the guarded secret of the plants, but that will have been mastered by human industry which will know how to make them bear even more abundant fruit than nature” (Ciamician, 1912). It is, however, more realistic that artificial light sources will be the energy source of choice for most photocatalytic reactions in academia and industry, due to the better control over reaction conditions. Additionally, several recent examples indicate that the photon intensity and energy can be used as tunable reaction parameters that enable selectivity and reactivity control. Although this has been often overlooked in the past, we believe that the future will see more examples showcasing “chromoselective photocatalysis.” Photochemistry tremendously benefits from technological developments including laser technology and flow chemistry, and interdisciplinary research programs between chemists and chemical engineers will be of utmost importance for the implementation of photocatalytic transformations in industrial setting.
Acknowledgments
We gratefully acknowledge the Max Planck Society for generous financial support. We thank the German Chemical Industry Fund (Fonds der Chemischen Industrie, FCI) for funding through a Liebig Fellowship. B.P. thanks the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy—EXC 2008–390540038—UniSysCat for financial support.
Author contributions
S.R. and B.P. conceived the structure of the review and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- Arias-Rotondo D.M., McCusker J.K. The photophysics of photoredox catalysis: a roadmap for catalyst design. Chem. Soc. Rev. 2016;45:5803–5820. doi: 10.1039/c6cs00526h. [DOI] [PubMed] [Google Scholar]
- Bonfield H.E., Knauber T., Lévesque F., Moschetta E.G., Susanne F., Edwards L.J. Photons as a 21st century reagent. Nat. Commun. 2020;11:804. doi: 10.1038/s41467-019-13988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambié D., Bottecchia C., Straathof N.J.W., Hessel V., Noël T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 2016;116:10276–10341. doi: 10.1021/acs.chemrev.5b00707. [DOI] [PubMed] [Google Scholar]
- Cambié D., Zhao F., Hessel V., Debije M.G., Noël T. A leaf-inspired luminescent solar concentrator for energy-efficient continuous-flow photochemistry. Angew. Chem. Int. Ed. 2017;56:1050–1054. doi: 10.1002/anie.201611101. [DOI] [PubMed] [Google Scholar]
- Capaldo L., Quadri L.L., Ravelli D. Photocatalytic hydrogen atom transfer: the philosopher's stone for late-stage functionalization? Green. Chem. 2020;22:3376–3396. [Google Scholar]
- Cavedon C., Sletten E.T., Madani A., Niemeyer O., Seeberger P.H., Pieber B. Visible-light-mediated oxidative debenzylation enables the use of benzyl ethers as temporary protecting groups. Org. Lett. 2021;23:514–518. doi: 10.1021/acs.orglett.0c04026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Huang Z., Tay N.E.S., Giglio B., Wang M., Wang H., Wu Z., Nicewicz D.A., Li Z. Direct arene C–H fluorination with 18F- via organic photoredox catalysis. Science. 2019;364:1170–1174. doi: 10.1126/science.aav7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciamician G. The photochemistry of the future. Science. 1912;36:385–394. doi: 10.1126/science.36.926.385. [DOI] [PubMed] [Google Scholar]
- Cole J.P., Chen D.-F., Kudisch M., Pearson R.M., Lim C.-H., Miyake G.M. Organocatalyzed Birch reduction driven by visible light. J. Am. Chem. Soc. 2020;142:13573–13581. doi: 10.1021/jacs.0c05899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisenza G.E.M., Melchiorre P. Chemistry glows green with photoredox catalysis. Nat. Commun. 2020;11:803. doi: 10.1038/s41467-019-13887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRocco D.A., Dykstra K., Krska S., Vachal P., Conway D.V., Tudge M. Late-stage functionalization of biologically active heterocycles through photoredox catalysis. Angew. Chem. Int. Ed. 2014;53:4802–4806. doi: 10.1002/anie.201402023. [DOI] [PubMed] [Google Scholar]
- Douglas J.J., Sevrin M.J., Stephenson C.R.J. Visible light photocatalysis: applications and new disconnections in the synthesis of pharmaceutical agents. Org. Process. Res. Dev. 2016;20:1134–1147. [Google Scholar]
- Franchi D., Amara Z. Applications of sensitized semiconductors as heterogeneous visible-light photocatalysts in organic synthesis. ACS Sustain. Chem. Eng. 2020;8:15405–15429. [Google Scholar]
- Gentry E.C., Knowles R.R. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 2016;49:1546–1556. doi: 10.1021/acs.accounts.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh I., Ghosh T., Bardagi J.I., König B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science. 2014;346:725–728. doi: 10.1126/science.1258232. [DOI] [PubMed] [Google Scholar]
- Ghosh I., König B. Chromoselective photocatalysis: controlled bond activation through light-color regulation of redox potentials. Angew. Chem. Int. Ed. 2016;55:7676–7679. doi: 10.1002/anie.201602349. [DOI] [PubMed] [Google Scholar]
- Ghosh I., Shaikh R.S., König B. Sensitization-initiated electron transfer for photoredox catalysis. Angew. Chem. Int. Ed. 2017;56:8544–8549. doi: 10.1002/anie.201703004. [DOI] [PubMed] [Google Scholar]
- Giedyk M., Narobe R., Weiß S., Touraud D., Kunz W., König B. Photocatalytic activation of alkyl chlorides by assembly-promoted single electron transfer in microheterogeneous solutions. Nat. Catal. 2020;3:40–47. [Google Scholar]
- Gisbertz S., Pieber B. Heterogeneous photocatalysis in organic synthesis. ChemPhotoChem. 2020;4:1–21. [Google Scholar]
- Gisbertz S., Reischauer S., Pieber B. Overcoming limitations in dual photoredox/nickel-catalysed C–N cross-couplings due to catalyst deactivation. Nat. Catal. 2020;3:611–620. [Google Scholar]
- Glaser F., Kerzig C., Wenger O.S. Multi-photon excitation in photoredox catalysis: concepts, applications, methods. Angew. Chem. Int. Ed. 2020;59:10266–10284. doi: 10.1002/anie.201915762. [DOI] [PubMed] [Google Scholar]
- Grainger R., Heightman T.D., Ley Steven V., Lima F., Johnson C.N. Enabling synthesis in fragment-based drug discovery by reactivity mapping: photoredox-mediated cross-dehydrogenative heteroarylation of cyclic amines. Chem. Sci. 2019;10:2264–2271. doi: 10.1039/c8sc04789h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagfeldt A., Boschloo G., Sun L., Kloo L., Pettersson H. Dye-sensitized solar cells. Chem. Rev. 2010;110:6595–6663. doi: 10.1021/cr900356p. [DOI] [PubMed] [Google Scholar]
- Hagfeldt A., Graetzel M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995;95:49–68. [Google Scholar]
- Harper K.C., Moschetta E.G., Bordawekar S.V., Wittenberger S.J. A laser driven flow chemistry platform for scaling photochemical reactions with visible light. ACS Cent. Sci. 2019;5:109–115. doi: 10.1021/acscentsci.8b00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008;108:1052–1103. doi: 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]
- Hopkinson M.N., Sahoo B., Li J.-L., Glorius F. Dual catalysis sees the light: combining photoredox with organo-, acid, and transition-metal catalysis. Chem. Eur. J. 2014;20:3874–3886. doi: 10.1002/chem.201304823. [DOI] [PubMed] [Google Scholar]
- Hossain A., Bhattacharyya A., Reiser O. Copper’s rapid ascent in visible-light photoredox catalysis. Science. 2019;364:eaav9713. doi: 10.1126/science.aav9713. [DOI] [PubMed] [Google Scholar]
- Kerzig C., Wenger O.S. Reactivity control of a photocatalytic system by changing the light intensity. Chem. Sci. 2019;10:11023–11029. doi: 10.1039/c9sc04584h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Matsunaga S. The merger of photoredox and cobalt catalysis. Trends Chem. 2020;2:410–426. [Google Scholar]
- Kubota K., Pang Y., Miura A., Ito H. Redox reactions of small organic molecules using ball milling and piezoelectric materials. Science. 2019;366:1500–1504. doi: 10.1126/science.aay8224. [DOI] [PubMed] [Google Scholar]
- Laudadio G., Deng Y., van der Wal K., Ravelli D., Nuño M., Fagnoni M., Guthrie D., Sun Y., Noël T. C(sp3)–H functionalizations of light hydrocarbons using decatungstate photocatalysis in flow. Science. 2020;369:92–96. doi: 10.1126/science.abb4688. [DOI] [PubMed] [Google Scholar]
- Le C.C., Wismer M.K., Shi Z.-C., Zhang R., Conway D.V., Li G., Vachal P., Davies I.W., MacMillan D.W.C. A general small-scale reactor to enable standardization and acceleration of photocatalytic reactions. ACS. Cent. Sci. 2017;3:647–653. doi: 10.1021/acscentsci.7b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Terrett J.A., Zbieg J.R. Visible-light photocatalysis as an enabling technology for drug discovery: a paradigm shift for chemical reactivity. ACS Med. Chem. Lett. 2020;11:2120–2130. doi: 10.1021/acsmedchemlett.0c00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y.Y., Nagao K., Hoover A.J., Hesk D., Rivera N.R., Colletti S.L., Davies I.W., MacMillan D.W.C. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science. 2017;358:1182–1187. doi: 10.1126/science.aap9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie I.A., Wang L., Onuska N.P.R., Williams O.F., Begam K., Moran A.M., Dunietz B.D., Nicewicz D.A. Discovery and characterization of an acridine radical photoreductant. Nature. 2020;580:76–80. doi: 10.1038/s41586-020-2131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo L., Pagire S.K., Reiser O., König B. Visible-light photocatalysis: does it make a difference in organic synthesis? Angew. Chem. Int. Ed. 2018;57:10034–10072. doi: 10.1002/anie.201709766. [DOI] [PubMed] [Google Scholar]
- Miller Z.D., Lee B.J., Yoon T.P. Enantioselective crossed photocycloadditions of styrenic olefins by Lewis acid catalyzed triplet sensitization. Angew. Chem. Int. Ed. 2017;56:11891–11895. doi: 10.1002/anie.201706975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J.A., Phelan J.P., Badir S.O., Molander G.A. Alkyl carbon–carbon bond formation by nickel/photoredox cross-coupling. Angew. Chem. Int. Ed. 2019;58:6152–6163. doi: 10.1002/anie.201809431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauth A.M., Schechtel E., Dören R., Tremel W., Opatz T. TiO2 nanoparticles functionalized with non-innocent ligands allow oxidative photocyanation of amines with visible/near-infrared photons. J. Am. Chem. Soc. 2018;140:14169–14177. doi: 10.1021/jacs.8b07539. [DOI] [PubMed] [Google Scholar]
- Neumeier M., Chakraborty U., Schaarschmidt D., de la Pena O'Shea V., Perez-Ruiz R., Jacobi von Wangelin A. Combined photoredox and iron catalysis for the cyclotrimerization of alkynes. Angew. Chem. Int. Ed. 2020;59:13473–13478. doi: 10.1002/anie.202000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicewicz D.A., MacMillan D.W.C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science. 2008;322:77–80. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X.-H., Li Y., Song R.-J., Hu M., Luo S., Li J.-H. Intermolecular dialkylation of alkenes with two distinct C(sp3)—H bonds enabled by synergistic photoredox catalysis and iron catalysis. Sci. Adv. 2019;5:eaav9839. doi: 10.1126/sciadv.aav9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieber B., Shalom M., Antonietti M., Seeberger P.H., Gilmore K. Continuous heterogeneous photocatalysis in serial micro-batch reactors. Angew. Chem. Int. Ed. 2018;57:9976–9979. doi: 10.1002/anie.201712568. [DOI] [PubMed] [Google Scholar]
- Plutschack M.B., Pieber B., Gilmore K., Seeberger P.H. The hitchhiker’s guide to flow chemistry. Chem. Rev. 2017;117:11796–11893. doi: 10.1021/acs.chemrev.7b00183. [DOI] [PubMed] [Google Scholar]
- Prier C.K., Rankic D.A., MacMillan D.W.C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti S., Ravelli D., Fagnoni M. Wavelength dependence and wavelength selectivity in photochemical reactions. Photochem. Photobio. Sci. 2019;18:2094–2101. doi: 10.1039/c8pp00512e. [DOI] [PubMed] [Google Scholar]
- Ravetz B.D., Pun A.B., Churchill E.M., Congreve D.N., Rovis T., Campos L.M. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature. 2019;565:343–346. doi: 10.1038/s41586-018-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetz B.D., Tay N.E.S., Joe C.L., Sezen-Edmonds M., Schmidt M.A., Tan Y., Janey J.M., Eastgate M.D., Rovis T. Development of a platform for near-infrared photoredox catalysis. ACS Cent. Sci. 2020;6:2053–2059. doi: 10.1021/acscentsci.0c00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischauer S., Strauss V., Pieber B. A modular, self-assembling metallaphotocatalyst for cross couplings using the full visible-light spectrum. ACS. Catal. 2020;10:13269–13274. [Google Scholar]
- Romero N.A., Nicewicz D.A. Organic photoredox catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Rosso C., Gisbertz S., Williams J.D., Gemoets H.P.L., Debrouwer W., Pieber B., Kappe C.O. An oscillatory plug flow photoreactor facilitates semi-heterogeneous dual nickel/carbon nitride photocatalytic C–N couplings. React. Chem. Eng. 2020;5:597–604. [Google Scholar]
- Sato S., Nakamura H. Ligand-directed selective protein modification based on local single-electron-transfer catalysis. Angew. Chem. Int. Ed. 2013;52:8681–8684. doi: 10.1002/anie.201303831. [DOI] [PubMed] [Google Scholar]
- Schmalzbauer M., Marcon M., König B. Excited state anions in organic transformations. Angew. Chem. Int. Ed. 2021 doi: 10.1002/anie.202009288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmermund L., Reischauer S., Bierbaumer S., Winkler C.K., Diaz-Rodriguez A., Edwards L.J., Kara S., Mielke T., Cartwright J., Grogan G. Chromoselective photocatalysis enables stereocomplementary biocatalytic pathways. Angew. Chem. Int. Ed. 2021 doi: 10.1002/anie.202100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D.M., Yoon T.P. Solar synthesis: prospects in visible light photocatalysis. Science. 2014;343:1239176. doi: 10.1126/science.1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M.H., Twilton J., MacMillan D.W.C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubi K.L., Blum T.R., Yoon T.P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 2016;116:10035–10074. doi: 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieth-Kalthoff F., James M.J., Teders M., Pitzer L., Glorius F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 2018;47:7190–7202. doi: 10.1039/c8cs00054a. [DOI] [PubMed] [Google Scholar]
- Tellis J.C., Primer D.N., Molander G.A. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science. 2014;345:433–436. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twilton J., Le C., Zhang P., Shaw M.H., Evans R.W., MacMillan D.W.C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017;1:0052. [Google Scholar]
- Yu Y., Zhang L.-K., Buevich A.V., Li G., Tang H., Vachal P., Colletti S.L., Shi Z.-C. Chemoselective peptide modification via photocatalytic tryptophan β-position conjugation. J. Am. Chem. Soc. 2018;140:6797–6800. doi: 10.1021/jacs.8b03973. [DOI] [PubMed] [Google Scholar]
- Zhou J., Liu Q., Feng W., Sun Y., Li F. Upconversion luminescent materials: advances and applications. Chem. Rev. 2015;115:395–465. doi: 10.1021/cr400478f. [DOI] [PubMed] [Google Scholar]
- Zhu C., Yue H., Jia J., Rueping M. Recent advances in nickel-catalyzed C-heteroatom cross-coupling reactions under mild conditions via facilitated reductive elimination. Angew. Chem. Int. Ed. 2020 doi: 10.1002/anie.202013852. [DOI] [PubMed] [Google Scholar]
- Zoller J., Fabry D.C., Rueping M. Unexpected dual role of titanium dioxide in the visible light heterogeneous catalyzed C–H arylation of heteroarenes. ACS Catal. 2015;5:3900–3904. [Google Scholar]
- Zuo Z., Ahneman D.T., Chu L., Terrett J.A., Doyle A.G., MacMillan D.W.C. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science. 2014;345:437–440. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]