Abstract
Introduction
ModraDoc006 is a novel docetaxel tablet formulation that is co-administrated with the cytochrome P450 3A4 and P-glycoprotein inhibitor ritonavir (r): ModraDoc006/r.
Objectives
This study evaluated the effect of food consumed prior to administration of ModraDoc006/r on the pharmacokinetics of docetaxel and ritonavir.
Methods
Patients with advanced solid tumours were enrolled in this randomized crossover study to receive ModraDoc006/r in a fasted state in week 1 and after a standardized high-fat meal in week 2 and vice versa. Pharmacokinetic sampling was conducted until 48 h after both study drug administrations. Docetaxel and ritonavir plasma concentrations were determined using liquid chromatography with tandem mass spectrometry. Safety was evaluated with the Common Terminology Criteria for Adverse Events, version 4.03.
Results
In total, 16 patients completed the food-effect study. The geometric mean ratio (GMR) for the docetaxel area under the plasma concentration–time curve (AUC)0–48, AUC0–inf and maximum concentration (Cmax) were 1.11 (90% confidence interval [CI] 0.93–1.33), 1.19 (90% CI 1.00–1.41) and 1.07 (90% CI 0.81–1.42) in fed versus fasted conditions, respectively. For the ritonavir Cmax, the GMR was 0.79 (90% CI 0.69–0.90), whereas the AUC0–48 and AUC0–inf were bioequivalent. The most frequent treatment-related toxicities were grade ≤ 2 diarrhoea and fatigue. Hypokalaemia was the only observed treatment-related grade 3 toxicity.
Conclusions
The docetaxel and ritonavir exposure were not bioequivalent, as consumption of a high-fat meal prior to administration of ModraDoc006/r resulted in a slightly higher docetaxel exposure and lower ritonavir Cmax. Since docetaxel exposure is the only clinically relevant parameter in our patient population, the overall conclusion is that combined ModraDoc006 and ritonavir treatment may be slightly affected by concomitant intake of a high-fat meal. In view of the small effect, it is most likely that the intake of a light meal will not affect the systemic exposure to docetaxel.
ClinicalTrials.gov Identifier
NCT03147378, date of registration: May 10 2017.
Key Points
| This study evaluated the effect of a high-fat meal on the pharmacokinetics of ModraDoc006/r: a novel oral tablet formulation of docetaxel (ModraDoc006) co-administered with ritonavir (r). |
| Although intake of a high-fat meal before administration of ModraDoc006/r led to a slightly higher docetaxel exposure and lower peak concentration of ritonavir, the effect is considered small. |
| Patients are advised to take ModraDoc006/r at least 1 h before or at least 2 h after a high-fat meal. In view of the small effect, it is most likely that the intake of a light meal will not affect the systemic exposure to docetaxel. |
Introduction
Docetaxel retains a key role in the systemic treatment of patients with different types of solid tumours and is currently administered as a 3-weekly intravenous treatment [1–5]. Oral administration of docetaxel in a weekly schedule can have several advantages. Oral treatment avoids hypersensitivity reactions related to the excipients of intravenous taxane formulations, thereby avoiding the need for corticosteroid premedication [8]. In addition, oral administration of chemotherapy is more practical, is preferred by patients and can improve quality of life [9–13]. Furthermore, oral administration might improve the cost effectiveness of chemotherapy treatment [12, 14]. Finally, a weekly regimen might lead to better safety, as administration of docetaxel in a weekly instead of a three-weekly schedule was associated with less myelosuppression [6, 7].
However, the oral administration of docetaxel is hampered by its low oral bioavailability. This is the result of pharmaceutical and pharmacological factors: the poor water solubility of the drug and the extensive first-pass metabolism by cytochrome P450 (CYP) 3A4 and excretion by P-glycoprotein (P-gp) in the liver and gastro-intestinal tract [15, 16]. The bioavailability of oral docetaxel was improved in two ways with the novel ModraDOC006/r formulation. First, the first-pass effect was reduced by co-administration of ritonavir (r), an inhibitor of both CYP3A4 and P-gp [16, 17]. Second, water solubility was improved with the production of a tablet containing a spray-dried amorphous solid dispersion of docetaxel [18]. Treatment with ModraDoc006 in combination with ritonavir (ModraDoc006/r) was investigated in two phase I studies in patients with advanced solid tumours [19, 20]. A bi-daily schedule of weekly ModraDoc060/r was pursued for further clinical development [20].
All patients in these phase I studies were treated in fasted conditions. To potentially improve patient convenience, a food-interaction study was performed to investigate the effect of food on the pharmacokinetics of ModraDoc006/r [21–23]. Since ModraDoc006 was administered in fasted conditions in the previous phase I trials, the effect of food on the pharmacokinetics was never investigated. Studies with the other oral taxanes BMS-275183 and tesetaxel reported no pharmacokinetic interactions with food [24, 25]. It is reported that the pharmacokinetics of ritonavir can be affected by food, resulting in lower plasma exposure of ritonavir in a fed state, probably due to delayed gastric emptying [26–28]. However, other food-effect studies reported bioequivalent results or clinically irrelevant differences in ritonavir pharmacokinetics in fed and fasted states [29, 30]. Since ritonavir is used as a booster drug, any food interaction with ritonavir may also affect the exposure to docetaxel when ModraDoc006/r is administered with food. Therefore, this randomized crossover food-interaction study investigated the pharmacokinetics of docetaxel and ritonavir after administration of ModraDoc006/r, with and without prior intake of a high-fat meal, in patients with advanced solid tumours.
Methods
Study Design and Treatment
The effect of food on the plasma exposure of docetaxel and ritonavir after administration of ModraDoc006/r was assessed in an open-label, two-period crossover design, as shown in Fig. 1. The maximum tolerated dose (MTD) for further clinical development was determined in a phase I dose-finding study in patients with advanced solid tumours as ModraDoc006 30 mg with ritonavir 100 mg in the morning and ModraDoc006 20 mg with ritonavir 100 mg in the afternoon [20]. In this food-effect study, a single dose of ModraDoc006 30 mg was administered simultaneously with ritonavir 100 mg (Norvir®). This corresponds to the highest single dose intended to be marketed in patients with advanced solid tumours, in accordance with the US FDA guidelines for food-effect studies [22].
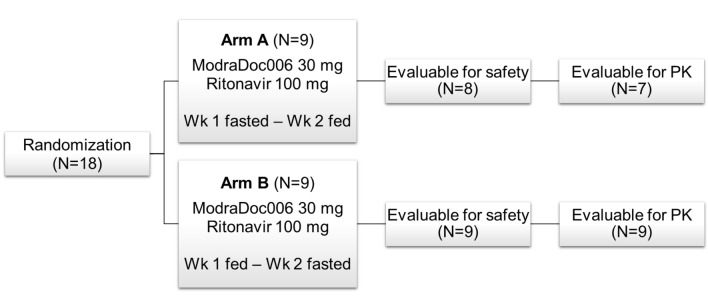
Fig. 1.
Study design and patients. In total, 18 patients were screened and randomized (1:1) to arm A or B. Each patient received ModraDoc006 30 mg in combination with ritonavir 100 mg with or without a high-fat meal in week 1 or 2, according to the randomization arm. In arm A, nine patients were randomized. One patient did not start with ModraDoc006/r because of symptomatic brain metastases diagnosed on the starting day and was excluded from safety and pharmacokinetic evaluation. A second patient in arm A received only one administration of ModraDoc006/r (in fasted condition) and discontinued in week 2 because of rapid clinical deterioration due to disease progression and development of a pneumosepsis; neither event was considered related to study medication. This patient was evaluable for safety but not for the pharmacokinetic food-interaction evaluation. In arm B, all nine randomized patients completed the food-effect study and were evaluable for safety and pharmacokinetic analysis. PK pharmacokinetics, Wk treatment week
Patient Eligibility
The study was conducted in adult patients with advanced solid tumours for whom no standard therapy of proven benefit existed and who might benefit from treatment with docetaxel. Patients with difficulties swallowing oral medication, bowel obstructions, motility disorders or previous surgery that could influence the absorption of drugs or intake of a high-fat meal were excluded, as were patients using comedication that could alter the pharmacokinetics of docetaxel and/or ritonavir, such as CYP3A4- or P-gp-modulating drugs. A World Health Organization (WHO) performance status (PS) of ≤ 1, a life expectancy of at least 3 months, adequate baseline bone marrow (haemoglobin ≥ 6.0 mmol/L, absolute neutrophil count ≥ 1.5 × 109/L, platelet count ≥ 100 × 109/L), renal function (creatinine ≤ 1.5 × upper limit of normal [ULN] or creatinine clearance ≥ 50 mL/min by Cockcroft–Gault formula) and hepatic function (bilirubin ≤ 1.5 × ULN, alanine aminotransferase and aspartate aminotransferase of ≤ 2.5 × ULN or ≤ 5 × ULN in case of hepatic metastases) were required for participation.
Study Procedures
Patients were randomized 1:1 into two treatment groups. Group A received ModraDoc006/r under fasting conditions the first week and under fed conditions during the second week of treatment. Group B received ModraDoc006/r under fed conditions the first week and under fasted conditions during the second week of treatment. Randomization was performed centrally at the Biometrics Department of the Netherlands Cancer Institute. Patients were considered evaluable for pharmacokinetics and safety when they complied with the fasting instructions, had eaten at least 75% of the high-fat meal within 30–45 min on the day of fed administration, completed the administrations of ModraDoc006/r (ModraDoc006 30 mg together with Norvir® 100 mg), did not vomit within 2 h after the intake and completed the pharmacokinetic blood sampling during the first 2 weeks of treatment.
The meal administered in the food-effect arm consisted of a high-fat meal in accordance with the FDA guidelines [22]. This meal, described in Table 1, was given 30 min prior to intake of ModraDoc006/r. In both treatment arms, an overnight fast of at least 10 h prior to treatment administration and a fasting period of 4 h post dose was applied.
Table 1.
Administered high-fat meal with caloric breakdown
| Ingredients | Amount (g) | kcal | Fat (g) | Protein (g) | Carbohydrate (g) |
|---|---|---|---|---|---|
| Two eggs | 120 | 184 | 10.6 | 12.5 | 1.1 |
| Sausage or 48+ cheese | 30 or 30 | 129 or 114 | 11.7 or 9.7 | 6.3 or 7.8 | 0.2 or 0 |
| Butter | 30 | 255 | 24 | 0.3 | 0 |
| Two slices of bread | 60 | 140 | 2.3 | 5.4 | 25.8 |
| Banana | 130 | 125 | 0.4 | 1.4 | 27 |
| Full-fat milk | 240 | 152 | 8 | 8 | 11 |
| Total with sausage | 985 | 57 | 33.9 | 65.1 | |
| Total with 48+ cheese | 970 | 55 | 35.4 | 64.9 |
g grams, kcal kilocalorie
Blood sampling for pharmacokinetic analysis was performed at 26 time points in week 1 and 2 up to 48 h after ModraDoc006/r administration. Venous blood was collected in 4 mL lithium heparin tubes. The samples were processed by centrifugation at 1500 g at 4 °C for 10 min. Plasma was stored within 1 h after sampling in Eppendorf tubes at − 20 °C. Docetaxel and ritonavir concentrations were determined by a validated bioanalytical liquid chromatography with tandem mass spectrometry assay [31]. Stable isotopically labelled docetaxel was used in the assay as internal standard, with a lower limit of quantification of 0.5 ng/mL for docetaxel and 2.0 ng/mL for ritonavir.
Safety measurements included weekly assessments of signs and symptoms, routine physical examination, vital signs, Eastern Cooperative Oncology Group/WHO PS, clinical haematology and chemistry laboratory tests and evaluation of concomitant medication. After completion of the food-interaction study, patients continued ModraDoc006/r treatment within a roll-over safety study. In this roll-over study, patients were treated with the previously established MTD of ModraDoc006/r [20]. For one patient discontinuing treatment with ModraDoc006/r, a safety follow-up 28 days after the last intake of ModraDoc006/r was conducted. All adverse events (AEs) were assessed according to the National Cancer Institute’s Common Terminology Criteria for AEs criteria (NCI-CTCAE v4.03). Toxicities that were assessed by the investigator as possibly, probably or definitely related to ModraDoc006 and/or ritonavir were considered as treatment-related AEs. Data on serious AEs were also collected.
Pharmacokinetic parameters were determined using validated scripts in the R software package (version 3.01) [32]. The mean, median, coefficient of variation and range of the following parameters of docetaxel and ritonavir were calculated: maximum plasma concentration (Cmax), time to Cmax (tmax), area under the plasma concentration versus time curve from zero to the last datapoint at 48 h (AUC0–48) and AUC from zero to infinity (AUC0–inf).
A total of 16 patients with evaluable pharmacokinetic data were planned for enrolment. The 90% confidence interval (CI) for the ratio of the population geometric means (GM) between fed and fasted treatments, based on log-transformed data, was calculated for AUC0–inf, AUC0–48 and Cmax. According to the FDA guidelines, no food effect should be concluded if both the lower and higher 90% CI values of the GM ratio are contained in the bioequivalence range of 0.80–1.25 for AUC0–inf, AUC0–48 and Cmax [22]. All analyses were performed in accordance with regulatory guidelines [22].
This study was compliant with current standards of International Conference for Harmonization—Good Clinical Practice and the WHO Declaration of Helsinki and with the Medical Research Involving Human Subjects Act. The study was approved by the Medical Ethical Committee of the Netherlands Cancer Institute. This study was registered at ClinicalTrials.gov, identifier number NCT03147378.
Results
Study Treatment and Patients
A total of 18 patients were randomized, 16 of whom completed the food-effect study, as shown in Fig. 1. In arm A, one patient did not start treatment with ModraDoc006/r and another patient received only 1 week of treatment, both because of disease-related complications. As shown in Table 2, of the 18 patients participating in the study, six were male and 12 were female. The median age was 58.5 years (range 45–76), and most patients (94.4%) were Caucasian. Ten patients were diagnosed with non-small-cell lung cancer, two with squamous cell carcinoma of the head and neck, two with ovarian cancer and four with another metastatic cancer type. The great majority of patients were pre-treated with chemotherapy (94.4%), immunotherapy (including targeted monoclonal antibodies, 72.2%) or radiotherapy (61.1%). Three patients had received one prior line of systemic anticancer therapy (including chemotherapy, immunotherapy and/or targeted therapy), five patients were pre-treated with two lines of systemic therapy and ten patients had received three or more lines of systemic therapy before enrolment in this study.
Table 2.
Patient characteristics
| Characteristics | Arm A (week 1 fasted, week 2 fed) (N = 9) | Arm B (week 1 fed, week 2 fasted) (N = 9) | Total (N = 18) |
|---|---|---|---|
| Sex | |||
| Male | 2 (22.2) | 4 (44.4) | 6 (33.3) |
| Female | 7 (77.8) | 5 (55.6) | 12 (66.7) |
| Age | |||
| Median (Q1–Q3) | 58 (54–65) | 59 (51–65) | 58.5 (53.3–65.0) |
| Minimum–maximum | 45–71 | 48–76 | 45–76 |
| Ethnicity | |||
| Caucasian | 9 (100) | 8 (88.9) | 17 (94.4) |
| Other | 0 (0) | 1 (11.1) | 1 (5.6) |
| WHO performance status | |||
|
0 1 |
3 (33.3) 6 (66.7) |
3 (33.5) 6 (66.7) |
6 (33.3) 12 (66.7) |
| Tumour type | |||
| Non-small-cell lung cancer | 5 (55.6) | 5 (55.6) | 10 (55.6) |
| SCCHN | 0 (0.0) | 2 (22.2) | 2 (11.1) |
| Ovarian cancer | 1 (11.1) | 1 (11.1) | 2 (11.1) |
| Othera | 3 (33.3) | 1 (11.1) | 4 (22.2) |
| Prior anticancer treatment | |||
| Chemotherapy | 9 (100) | 8 (88.9) | 17 (94.4) |
| Immunotherapy | 5 (55.6) | 8 (88.9) | 13 (72.2) |
| Radiotherapy | 5 (55.6) | 6 (66.7) | 11 (61.1) |
| Surgery | 3 (33.3) | 2 (22.2) | 5 (27.8) |
Data are presented as N (%) unless otherwise indicated
SCCHN squamous cell carcinoma of the head and neck, WHO World Health Organization
aAnal carcinoma (one patient), urothelial cell carcinoma (one patient), small-cell lung cancer (one patient), neuroendocrine carcinoma of the cervix (one patient)
Docetaxel Pharmacokinetics
The mean plasma concentration versus time curves and pharmacokinetic parameters of docetaxel are shown in Fig. 2 and Table 3, respectively. All 16 patients who completed the study were included in the pharmacokinetic analysis, except for the docetaxel AUC0–inf, as determination was not possible in two patients because of uncertainty in the regression line. As shown in Fig. 2, the mean plasma concentration–time curves of docetaxel show a similar shape in fasted and fed states. The mean AUC0–48, AUC0–inf and Cmax of docetaxel under fasted and fed conditions are reported in Table 3. In fed conditions, the mean docetaxel AUC0–inf was slightly higher (921.87 ± 804.62 vs. 731.47 ± 499.61 ng/mL × h) as was also observed for the Cmax (88.22 ± 75.40 vs. 69.55 ± 52.06 ng/mL).
Fig. 2.
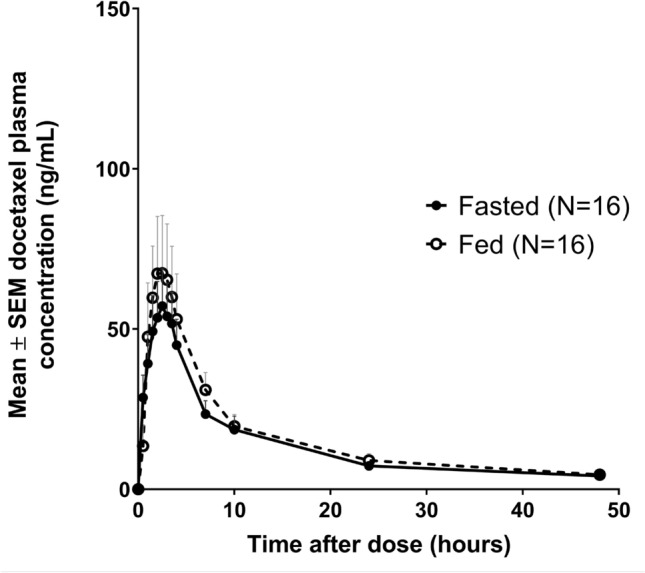
Mean plasma concentration versus time curves of docetaxel after administration of ModraDoc006/r in fasted and fed conditions. SEM standard error of the mean
Table 3.
Docetaxel pharmacokinetics
| Parameter | Fasted | Fed | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | GM | SD | N | Mean | GM | SD | |
| AUC0–48 (ng × h/mL) | 16 | 640.92 | 449.65 | 446.05 | 16 | 744.49 | 500.07 | 632.25 |
| AUC0–inf (ng × h/mL) | 14a | 731.47 | 509.74 | 499.61 | 14a | 921.87 | 604.65 | 804.62 |
| Cmax (ng × mL) | 16 | 69.55 | 51.26 | 52.06 | 16 | 88.22 | 54.78 | 75.40 |
| tmax (h) | 16 | 2.97 | 2.54 | 2.09 | 16 | 3.41 | 2.80 | 2.25 |
AUC0-48 area under the plasma concentration–time curve from 0 to the last time point at 48 h, AUC0–inf area under the plasma concentration–time curve from 0 to infinity, Cmax maximum concentration, GM geometric mean, SD standard deviation, tmax time at which Cmax was measured
aPatients with unreliable regression were removed for AUC0–nf. AUC0–inf was determined with extrapolation of the AUC to infinity using the terminal elimination constant (Ke)
As reported in Table 4, the GMs and their ratios for the fed versus fasted states were calculated according to the regulatory guidelines, where a GM ratio with a 90% CI 0.80–1.25 is considered bioequivalent [22]. The GM ratios for the docetaxel AUC0–48, AUC0–inf and Cmax were 1.11 (90% CI 0.93–1.33), 1.19 (90% CI 1.00–1.41) and 1.07 (90% CI 0.81–1.42) in fed versus fasted conditions, respectively. Since the upper limits of the 90% CI of the GM ratios are above the regulatory-defined 0.80–1.25 bioequivalence range for all docetaxel parameters, there might be a slightly higher docetaxel exposure in the fed state, so bioequivalence cannot be concluded.
Table 4.
Docetaxel food-effect calculations
| Parameter, ratio fed/fasted | N | GM ratio | CV | Minimum | Maximum | 90% CI | Assessment |
|---|---|---|---|---|---|---|---|
| AUC0–48 (ng × h/mL) | 16 | 1.1121 | 0.4169 | 0.5686 | 1.9183 | 0.9332–1.3254 | Not bioequivalent |
| AUC0–inf (ng × h/mL) | 14 | 1.1862 | 0.3700 | 0.6143 | 2.0390 | 1.0012–1.4054 | Not bioequivalent |
| Cmax (ng × mL) | 16 | 1.0686 | 0.7169 | 0.3941 | 4.5434 | 0.8058–1.4171 | Not bioequivalent |
AUC0–48 area under the plasma concentration–time curve from 0 to the last time point at 48 h, AUC0–inf area under the plasma concentration–time curve from 0 to infinity, CI confidence interval, Cmax maximum concentration, CV coefficient of variation, GM geometric mean
Ritonavir Pharmacokinetics
Figure 3 shows the mean plasma concentration versus time curves for ritonavir. The pharmacokinetic parameters are listed in Table 5. With the exception of the Cmax, the mean curves have a similar pattern in fasted and fed states. The mean Cmax of ritonavir was slightly but not significantly lower in fed conditions (709.84 ± 486.41 vs. 820.81 ± 436.45 ng/mL). The mean tmax of ritonavir was 4.0 ± 2.0 in fasted conditions compared with 6.3 ± 5.3 in fed conditions.
Fig. 3.
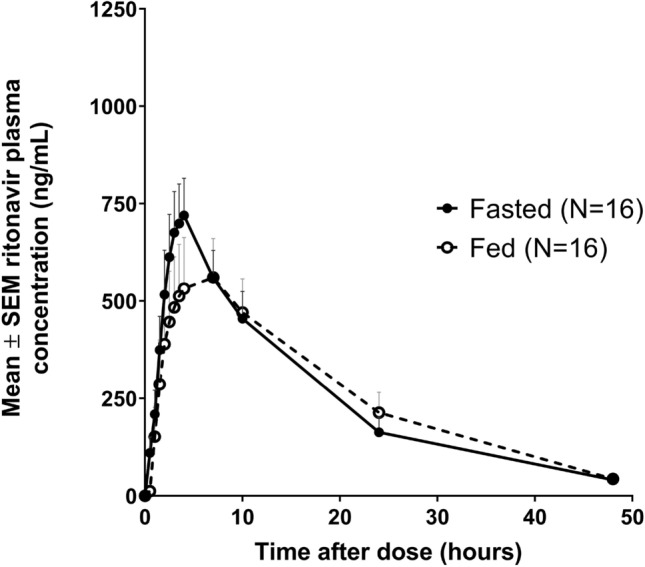
Mean plasma concentration versus time curves of ritonavir after administration of ModraDoc006/r in fasted and fed conditions. SEM standard error of the mean
Table 5.
Ritonavir pharmacokinetics
| Parameter | Fasted | Fed | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | GM | SD | N | Mean | GM | SD | |
| AUC0–t (ng × h/mL) | 16 | 11,198.69 | 8686.94 | 7721.01 | 16 | 11,471.61 | 7919.02 | 9520.49 |
| AUC0–inf (ng × h/mL) | 14a | 11,514.69 | 9081.88 | 7372.01 | 13a | 12,627.06 | 8321.47 | 11,177.55 |
| Cmax (ng × mL) | 16 | 820.81 | 682.79 | 436.45 | 16 | 709.84 | 539.88 | 486.41 |
| tmax (h) | 16 | 4.0 | 3.62 | 2.02 | 16 | 6.34 | 5.08 | 5.25 |
AUC0–48 area under the plasma concentration–time curve from 0 to the last time point at 48 h, AUC0–inf area under the plasma concentration–time curve from 0 to infinity, Cmax maximum concentration, GM geometric mean, SD standard deviation, tmax time at which Cmax was measured
aPatients with unreliable regression were removed for AUC0-inf. AUC0-inf was determined with extrapolation of the AUC to infinity using the terminal elimination constant (Ke)
The GM ratios for ritonavir in fed versus fasted states are reported in Table 6. The GM ratios for the ritonavir AUC0–48, AUC0–inf and Cmax were 0.91 (90% CI 0.81–1.03), 0.94 (90% CI 0.81–1.08) and 0.79 (90% CI 0.69–0.90) in fed versus fasted conditions, respectively. The lower limit of the 90% CI of the GM ratio of the Cmax was below the 0.80–1.25 bioequivalence range. Intake of a high-fat meal before administration of ModraDoc006/r led to lower peak concentrations of ritonavir in the fed state, with an approximately 21% decrease in the Cmax. The GM ratios and 90% CIs of the ritonavir AUC0–48 and AUC0–inf were contained within the 0.80–1.25 bioequivalence range for fed versus fasted conditions. Therefore, according to the regulatory guidelines, although a food interaction was observed for the ritonavir Cmax, the total systemic exposure was considered bioequivalent in fed and fasted states [22].
Table 6.
Ritonavir food-effect calculations
| Parameter, ratio fed/fasted | N | GM ratio | CV | Minimum | Maximum | 90% CI | Assessment |
|---|---|---|---|---|---|---|---|
| AUC0–t (ng × h/mL) | 16 | 0.9116 | 0.2829 | 0.6045 | 1.5379 | 0.8072–1.0295 | Bioequivalent |
| AUC0–inf (ng × h/mL) | 13 | 0.9352 | 0.3035 | 0.6073 | 1.5679 | 0.8076–1.0830 | Bioequivalent |
| Cmax (ng × mL) | 16 | 0.7907 | 0.3141 | 0.5398 | 1.5251 | 0.6912–0.9045 | Not bioequivalent |
AUC0–48 area under the plasma concentration–time curve from 0 to the last time point at 48 h, AUC0–inf area under the plasma concentration–time curve from 0 to infinity, CI confidence interval, Cmax maximum concentration, CV coefficient of variation, GM geometric mean
Safety
All related AEs are listed in Table 7, according to the worst occurring treatment-related AE per patient in fasted and fed states. Although the docetaxel exposure was slightly higher, this did not lead to a higher incidence of AEs in the fed state. The most frequently occurring AEs were grade 1 and 2 fatigue and diarrhoea, each experienced by a total of four patients in the fasted state, whereas two patients experienced nausea and vomiting in the fed state.
Table 7.
Treatment-related adverse events for all patients in fasted and fed conditions
| AEa | Fasted (N = 17) | Fed (N = 16) | ||||
|---|---|---|---|---|---|---|
| CTCAE grade | CTCAE grade | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Fatigue | 1 (6%) | 3 (18%) | – | – | – | – |
| Diarrhoea | 3 (18%) | 1 (6%) | – | – | – | – |
| Nausea | 1 (6%) | – | – | 2 (13%) | – | – |
| Vomiting | – | – | – | 2 (13%) | – | – |
| Anorexia | 1 (6%) | – | – | – | – | – |
| Rectal haemorrhage | – | – | – | 1 (6%) | – | – |
| Stoma site haemorrhage | – | – | – | 1 (6%) | – | – |
| Hypokalaemia | – | – | 1 (6%) | – | – | – |
| Hypomagnesaemia | 1 (6%) | – | – | – | – | – |
AE adverse event, CTCAE Common Terminology Criteria for AEs
aFor each AE, the total number of patients is reported. In case of repeating similar AEs reported by one patient in fed or in fasted state, these AEs are counted once, and only the worst grade is reported
Hypokalaemia was the only grade 3 treatment-related AE observed, occurring in a patient who received intensive intravenous fluid replacement because of pneumosepsis that was not considered related to the study treatment. Because this patient also experienced diarrhoea, as a result of either the antibiotics or the ModraDoc006/r treatment, the hypokalaemia was evaluated as possibly related to the study treatment. This patient only received ModraDoc006/r in the fasted state and discontinued before the start of the second week of treatment in the fed state because of these disease-related complications. No serious AEs considered related to ModraDoc006/r were observed in the study.
Discussion
The docetaxel exposure after intake of ModraDoc006/r in fed and fasted states cannot be considered bioequivalent, as the GM ratios with their corresponding 90% CIs were not completely contained within the regulatory-defined 0.80–1.25 equivalence range for the AUC0–48, AUC0–inf and Cmax. Therefore, the docetaxel exposure was slightly higher in the fed state. For ritonavir, the lower range of the 90% CI of the GM ratio for the Cmax was below 0.80, indicating that the intake of a high-fat meal resulted in a lower peak concentration of ritonavir. However, the total exposure of ritonavir seemed to be unaffected by food, as the 90% CIs of the AUC0–48 and AUC0–inf were within the bioequivalence range.
Since ritonavir is used as a booster drug for docetaxel, only the docetaxel exposure is considered clinically relevant for our patients. As observed in this study, the lower Cmax of the booster drug did not lead to a lower systemic uptake of ModraDoc006. In fact, the docetaxel exposure was slightly higher after intake of ModraDoc006/r in the fed state. The effect of this 19% higher docetaxel AUC0–inf in the fed state (based on the GMs) was considered small. Moreover, minor differences were expected based on the observed intra-patient variability in docetaxel exposure in the other phase I studies performed with ModraDoc006/r, where patients were treated with ModraDoc006/r in fasted conditions [19, 20]. Therefore, although the GM ratios for the docetaxel AUC0–48, AUC0–inf and Cmax and the ritonavir Cmax were not bioequivalent in this food-interaction study, treatment with ModraDoc006/r will most likely not be affected by the concomitant intake of a light meal. This might further enhance the convenience of the oral chemotherapy treatment.
ModraDoc006/r treatment is currently being developed in the clinic as a bi-daily weekly schedule, dosed as ModraDoc006 30 mg with ritonavir 100 mg in the morning and ModraDoc006 20 mg with ritonavir 100 mg in the evening [20]. In line with the currently available regulatory guidelines, food interaction was investigated for the highest single dose that is used in the bi-daily schedule (i.e., ModraDoc006 30 mg in combination with ritonavir 100 mg) [22]. Although the Cmax of ritonavir was lower in the fed state, the docetaxel exposure was higher in the fed state. Therefore, the lower ritonavir Cmax did not diminish the boosting effect needed for the systemic uptake of ModraDoc006, so the effect of food interaction is expected to be small with twice-daily administration.
ModraDoc006/r was well tolerated in both the fed and the fasted state. The observed AEs in this food-effect study were generally mild. However, the safety results should be interpreted with caution because of the short duration of treatment and the low administered dose of ModraDoc006/r. Diarrhoea, fatigue, nausea and vomiting were the most frequently reported AEs, in line with the already known safety profile of ModraDoc006/r [19, 20].
Conclusion
Administration of a high-fat meal prior to administration of ModraDoc006/r resulted in a slightly higher docetaxel exposure and a lower Cmax of ritonavir. The 90% CI of the GM ratios of the docetaxel AUC0–48, AUC0–inf and docetaxel and ritonavir Cmax were just outside the regulatory-defined 0.80–1.25 bioequivalence range, indicative of a food interaction. Since docetaxel exposure is the only clinically relevant parameter in our patient population, the overall conclusion is that ModraDoc006 and ritonavir treatment may be slightly affected by concomitant intake of a high-fat meal. Therefore, patients are advised to take ModraDoc006/r at least 1 h before or at least 2 h after a high-fat meal. In view of the small effect, it is most likely that intake of a light meal will not affect the systemic exposure to docetaxel.
Acknowledgements
The authors thank all patients and their families for their participation in the trial.
Declarations
Funding
The study was funded and ModraDoc006 tablets provided by Modra Pharmaceuticals BV. Modra Pharmaceuticals is a commercial pharmaceutical company that was founded as a spin-off of the Netherlands Cancer Institute. ModraDoc006/r is currently in clinical development as the lead product of Modra Pharmaceuticals.
Conflict of interest
J.H. Beijnen is a (part-time) employee, patent holder and stockholder of Modra Pharmaceuticals BV. Marianne Keessen is a full-time employee of Modra Pharmaceuticals BV. Marit A.C. Vermunt, Vincent A. de Weger, Julie M. Janssen, Marta I. Lopez-Yurda, Bas Thijssen, Hilde Rosing, and Alwin D.R. Huitema Serena Marchetti have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study was approved by the Medical Ethical Committee of the Netherlands Cancer Institute. The study was performed in compliance with current standards of International Conference for Harmonization—Good Clinical Practice and the WHO Declaration of Helsinki and in accordance with the Medical Research Involving Human Subjects Act.
Consent to participate
All patients received written information and an explanation of the study and signed written informed consent before participation in the trial. The informed consent procedure was conducted compliant with current standards of International Conference for Harmonization—Good Clinical Practice.
Consent for publication
Consent for publication was not applicable since all collected data were anonymised before storage, analysis and publication.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
MV, VW, MK, JB and SM contributed to the protocol development and initiation of the trial. MV, VW and SM contributed to the conduct of the clinical trial. MV and VW contributed to the data collection. VW, JJ, BT, HR and AH contributed to the pharmacokinetic analysis. ML contributed to the statistical analysis. The first draft of the manuscript was written by MV. All authors commented on previous versions of and read and approved the final manuscript.
Footnotes
Marit A. C. Vermunt and Vincent A. de Weger equal contributions.
References
- 1.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Fossella FV, Devore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non–small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18(12):2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 3.Ghersi D, Wilson ML, Chan MM, Simes J, Donoghue E, Wilcken N. Taxane-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2015;6:CD003366. doi: 10.1002/14651858.CD003366.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernier J, Vrieling C. Docetaxel in the management of patients with head and neck squamous cell carcinoma. Expert Rev Anticancer Ther. 2008;8(7):1023–1032. doi: 10.1586/14737140.8.7.1023. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E. The treatment of advanced gastric cancer: new findings on the activity of the taxanes. Oncologist. 2004;9(2):9–15. doi: 10.1634/theoncologist.9-suppl_2-9. [DOI] [PubMed] [Google Scholar]
- 6.Kuroi K, Bando H, Saji S, Toi M. Weekly schedule of docetaxel in breast cancer: evaluation of response and toxicity. Breast Cancer. 2003;10(1):10–14. doi: 10.1007/BF02967619. [DOI] [PubMed] [Google Scholar]
- 7.Joerger M. Treatment regimens of classical and newer taxanes. Cancer Chemother Pharmacol. 2015;77(2):221–233. doi: 10.1007/s00280-015-2893-6. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz JR. Dexamethasone premedication for prophylaxis of taxane toxicities: can the doses be reduced when paclitaxel or docetaxel are given weekly? J Oncol Pharm Pract. 2011;18(2):250–256. doi: 10.1177/1078155211409473. [DOI] [PubMed] [Google Scholar]
- 9.Payne SA. A study of quality of life in cancer patients receiving palliative chemotherapy. Soc Sci Med. 1992;35(12):1505–1509. doi: 10.1016/0277-9536(92)90053-S. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–115. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 11.Borner M, Scheithauer W, Twelves C, Maroun J, Wilke H. Answering patients’ needs: oral alternatives to intravenous therapy. Oncologist. 2001;6(4):12–16. doi: 10.1634/theoncologist.6-suppl_4-12. [DOI] [PubMed] [Google Scholar]
- 12.Banna GL, Collovà E, Gebbia V, Lipari H, Giuffrida P, Cavallaro S, et al. Anticancer oral therapy: emerging related issues. Cancer Treat Rev. 2010;36(8):595–605. doi: 10.1016/j.ctrv.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Jensen LH, Osterlind K, Rytter C. Randomized cross-over study of patient preference for oral or intravenous vinorelbine in combination with carboplatin in the treatment of advanced NSCLC. Lung Cancer. 2008;62(1):85–91. doi: 10.1016/j.lungcan.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Le Lay K, Myon E, Hill S, Riou-Franca L, Scott D, Sidhu M, et al. Comparative cost-minimisation of oral and intravenous chemotherapy for first-line treatment of non-small cell lung cancer in the UK NHS system. Eur J Health Econ. 2007;8(2):145–151. doi: 10.1007/s10198-006-0034-1. [DOI] [PubMed] [Google Scholar]
- 15.Jibodh RA, Lagas JS, Nuijen B, Beijnen JH, Schellens JH. Taxanes: old drugs, new oral formulations. Eur J Pharmacol. 2013;717(1–3):40–46. doi: 10.1016/j.ejphar.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Bardelmeijer HA, Ouwehand M, Buckle T, Huisman MT, Schellens JHM, Beijnen JH, et al. Low systemic exposure of oral docetaxel in mice resulting from extensive first-pass metabolism is boosted by ritonavir. Cancer Res. 2002;62(21):6158–6164. [PubMed] [Google Scholar]
- 17.Oostendorp RL, Huitema A, Rosing H, Jansen RS, Ter Heine R, Keessen M, et al. Coadministration of ritonavir strongly enhances the apparent oral bioavailability of docetaxel in patients with solid tumors. Clin Cancer Res. 2009;15(12):4228–4233. doi: 10.1158/1078-0432.CCR-08-2944. [DOI] [PubMed] [Google Scholar]
- 18.Sawicki E, Beijnen JH, Schellens JH, Nuijen B. Pharmaceutical development of an oral tablet formulation containing a spray dried amorphous solid dispersion of docetaxel or paclitaxel. Int J Pharm. 2016;511(2):765–773. doi: 10.1016/j.ijpharm.2016.07.068. [DOI] [PubMed] [Google Scholar]
- 19.De Weger VA, Stuurman FE, Koolen SLW, Moes JJ, Hendrikx JJMA, Sawicki E, et al. A phase I dose escalation study of once-weekly oral administration of docetaxel as ModraDoc001 capsule or ModraDoc006 tablet in combination with ritonavir. Clin Cancer Res. 2019;25(18):5466–5474. doi: 10.1158/1078-0432.CCR-17-2299. [DOI] [PubMed] [Google Scholar]
- 20.De Weger VA, Stuurman FE, Hendrikx JJMA, Moes JJ, Sawicki E, Huitema ADR, et al. A dose-escalation study of bi-daily once weekly oral docetaxel either as ModraDoc001 or ModraDoc006 combined with ritonavir. Eur J Cancer. 2017;86:217–225. doi: 10.1016/j.ejca.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Mathijssen RH, Sparreboom A, Verweij J. Determining the optimal dose in the development of anticancer agents. Nat Rev Clin Oncol. 2014;11(5):272–281. doi: 10.1038/nrclinonc.2014.40. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry. Food-effect Bioavailability and Fed Bioequivalence Studies. 2002; https://www.fda.gov/media/70945/download. Accessed 15 Jan 2017
- 23.Farha M, Masson E, Tomkinson H, Mugundu G. Food effect study design with oral drugs: lessons learned from recently approved drugs in oncology. J Clin Pharmacol. 2019;59(4):463–471. doi: 10.1002/jcph.1351. [DOI] [PubMed] [Google Scholar]
- 24.Bröker LE, Valdivieso M, Pilat MJ, DeLuca P, Zhou X, Parker S, et al. Effect of food on the pharmacokinetic behavior of the potent oral taxane BMS-275183. Clin Cancer Res. 2008;14(13):4186–4191. doi: 10.1158/1078-0432.CCR-07-4594. [DOI] [PubMed] [Google Scholar]
- 25.Danesi H, Dudek A, Spindler E, Alcorn H. Clinical evaluation of food effects on pharmacokinetics of the novel oral taxane, tesetaxel. J Clin Oncol. 2011;29(15_suppl):e13059. doi: 10.1200/jco.2011.29.15_suppl.e13059. [DOI] [Google Scholar]
- 26.Ng J, Klein CE, Chui YL, Awni WM, Morris JB, Podsadecki TK, et al. The effect of food on ritonavir bioavailability following administration of ritonavir 100 mg film-coated tablet in healthy adult subjects. J Int AIDS Soc. 2008;11(1):247. doi: 10.1186/1758-2652-11-S1-P247. [DOI] [Google Scholar]
- 27.Hsu A, Granneman GR, Bertz RJ. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35(4):275–291. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 28.Kakuda T, Sekar V, Lavreys L, De Paepe E, Stevens T, Vanstockem M, et al. Pharmacokinetics of darunavir after administration of an oral suspension with low dose ritonavir and with or without food. Clin Pharmacol Drug Dev. 2014;3(5):346–352. doi: 10.1002/cpdd.88. [DOI] [PubMed] [Google Scholar]
- 29.Ibarra M, Fagiolino P, Vázquez M, Ruiz M, Vega M, Bellocq B, et al. Impact of food administration on lopinavir-ritonavir bioequivalence studies. Eur J Pharm Sci. 2012;46(5):516–521. doi: 10.1016/j.ejps.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Sekar V, Kestens D, Spinosa-Guzman S, De Pauw M, De Paepe E, Vangeneugden T, et al. The effect of different meal types on the pharmacokinetics of darunavir (TMC114)/ritonavir in HIV-negative healthy volunteers. J Clin Pharmacol. 2007;47(4):479–484. doi: 10.1177/0091270006298603. [DOI] [PubMed] [Google Scholar]
- 31.Hendrikx JJMA, Hillebrand MJX, Thijssen B, Rosing H, Schinkel AH, Schellens JHM, et al. A sensitive combined assay for the quantification of paclitaxel, docetaxel and ritonavir in human plasma using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(28):2984–2990. doi: 10.1016/j.jchromb.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 32.R development Core Team. R: a language and environment for statistical computing. 2009; https://www.r.poject.org/. Accessed 15 Jan 2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.