Abstract
Diphenyl phosphate (DPHP) has been increasingly detected in environmental samples, posing a potential hazard to humans and other organisms and arousing concern regarding its adverse effects. Biological degradation of DPHP is considered a promising and environmentally friendly method for its removal. In this study, the bagdpd gene was mined from the Bacillus altitudinis W3 genome and identified as a glycerophosphodiester phosphodiesterase by bioinformatics analysis. The enzyme was expressed and its biochemical properties were studied. When using bis(4-nitrophenyl) phosphate as substrate, enzyme activity was optimal at 55 °C and a pH of 8.5. The enzyme remained stable in the pH range of 8.0 − 10.0. The rBaGDPD enzyme degraded DPHP and the reaction product was identified as phenyl phosphate by LC–MS. This is the first report of a glycerophosphodiester phosphodiesterase exhibiting hydrolytic activity against DPHP. This study demonstrated that rBaGDPD could have the potential for bioremediation and industrial applications.
Keywords: Glycerophosphodiester phosphodiesterase, Bacillus altitudinis W3, Biochemical properties, Degradation, DPHP
Introduction
Glycerophosphodiester phosphodiesterases (GDPDs) (EC 3.1.4.46), which are widely expressed in prokaryotic and eukaryotic organisms, decompose glycerol phosphodiester into glycerol-3-phosphate (G3P) and the corresponding alcohol (Ohshima et al. 2008). In prokaryotes, GDPDs are involved in providing the essential G3P to cells by hydrolyzing glycerol phosphodiester (Cheng et al. 2011; Corda et al. 2014). The GDPDs in eukaryotes play a significant role in phospholipid metabolism, cytoskeleton modification, osmolyte regulation, and motor neuron differentiation (Corda et al. 2009; Yan et al. 2009; Okazaki et al. 2010). Periplasmic (GlpQ) and cytosolic (UgpQ) GDPDs have been found in E. coli, seven homologs of bacterial GDPDs (GDE1-GDE7) have been identified in mammals, and GpdQ has been discovered in Enterobacter aerogenes (Denloye et al. 2012; Daumann et al. 2014; Sockanathan and Park 2016). As yet, there have been relatively few studies on the characterization and application of this enzyme. In 2016, Wang et al. characterized the enzymatic properties of GDPD from Pyrococcus furiosus (pfGDPD) but no further research into its application has been conducted (Wang et al. 2016).
Diphenyl phosphate (DPHP) is one of the most common organophosphate esters (OPEs), usually used as a chemical additive and an industrial catalyst. It is also the main metabolite of many organophosphorus flame retardants, including triphenyl phosphate (TPHP) (Mitchell et al. 2019). Because DPHP is an additive chemical agent and does not bond with other chemicals, it can easily spread to the environment. Previous studies have detected DPHP in different environmental media all over the world, including in the urine of babies (Hoffman et al. 2015; Zhao et al. 2016) and pregnant women (Bjornsdotter et al. 2018). These studies suggest that humans and other organisms can be directly exposed to DPHP. Furthermore, DPHP has a long half-life in the environment and exhibits immunotoxicity and neurotoxicity toward organisms. The potential for exposure to DPHP and its possible toxicity have therefore received widespread attention. So far, research has suggested that OPEs in the environment can be removed by photocatalytic degradation (Antonopoulou et al. 2017), Fenton oxidation (Yasin et al. 2016), and biological degradation (Yamaguchi et al. 2016). Among these methods, the biological method is an effective and feasible approach to eliminate OPEs in the environment. Recently, researchers have found that the glycerophosphodiesterase (GpdQ) from Enterobacter aerogenes has the potential to hydrolyze OPEs (Daumann et al. 2014). Therefore, datamining of GDPDs that can degrade OPEs has become a research hotspot.
In this study, the phylogenetic relationships of the GDPD from Bacillus altitudinis W3 (BaGDPD) were constructed by bioinformatics analysis. The biochemical properties of the enzyme were further explored based on the expression and purification of recombinant BaGDPD (rBaGDPD). The enzyme activity was characterized using bis(4-nitrophenyl) phosphate (BpNPP), a substrate that is usually used to screen OPE-degrading enzymes with high activity. In addition, a liquid chromatography-mass spectrometer (LC–MS) was employed to identify the degradation products following enzymatic hydrolysis of DPHP.
Materials and methods
Bacterial strains, media, and reagents
The gene bagdpd was screened from B. altitudinis W3 (GenBank No. CP011150.1), which was isolated by (Zhang et al. 2015). Then, the genome of B. altitudinis W3 was sequenced and annotated. The E. coli JM109 and E. coli BL21 strains were used as storage and protein expression hosts, respectively. The E. coli strains were cultured in Luria–Bertani broth media (LB medium) at 37 C (Yang et al. 2020). The plasmid pCold, genomic DNA extraction kit, plasmid miniprep kit, DNA gel extraction kit, and other molecular biology reagents were purchased from Takara Bio (Dalian, China). Ampicillin, ethylenediamine tetraacetic acid (EDTA), bis(4-nitrophenyl) phosphate (BpNPP), and p-Nitrophenol were purchased from Aladdin (Shanghai, China). All reagents used in this study were of analytical grade.
Bioinformatics analyses
B. altitudinis W3 was sequenced by Guan et al. (Guan et al. 2015). Based on genomic annotations, BaGDPD (GenBank No. MT512400) was screened from B. altitudinis W3. The primary structure and physicochemical properties of the protein were predicted using ExPASy ProtParam Server (Yang et al. 2020). The NCBI Conserved Domain Database (CDD) was used to analyze the amino acid sequence in the BaGDPD conserved domain (Aron et al. 2015). Clustal Omega was used for multiple sequence alignment and the alignment results were displayed using ESPript (Yang et al. 2020). A phylogenetic tree of BaGDPD and GDPDs from other species was built using MEGA 7.0 based on 1000 repeated pilot tests through the neighbor-joining method (Sudhir et al. 2016).
Cloning of GDPD gene
B. altitudinis W3 was cultured in the LB medium at 37 °C with agitation at 200 rpm overnight. Genomic DNA was extracted using the genomic DNA extraction kit. According to the gene sequence of bagdpd, primers (gdpd-F: 5′-GCCGGGTACCATGACGAAAATTTTTGCACATAGAGGCTT-3′, gdpd-R: 5′-GCCGGGATCCTTATTTCATTTCTTCTCTTATTTTGACGGCTCG-3′) with suitable restriction enzyme sites (Kpn1 and BamH1) were used to amplify the gene. The PCR amplification was performed according to the following method: preheating at 95 °C for 3 min, 25 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 15 s, extension at 72 °C for 1 min and a final extension at 72 °C for 10 min. Subsequently, the digested PCR fragment was ligated with the digested pCold II by T4 DNA ligase. The recombinant plasmid was then transformed into E. coli JM109, which was verified by DNA sequencing (Yixin Wuxi, China). The sequenced recombinant plasmid pCold II -gdpd was transformed into E. coli BL21 (DE3) for protein expression.
Expression and purification of the recombinant GDPD
The recombinant E. coli cells were cultured at 37 °C in LB medium containing ampicillin (100 mg mL−1). Under the same culture conditions, 1 ml of overnight culture broth was transferred to 50 ml of LB medium and incubated until the absorbance at 600 nm (OD600) reached 0.6. After cooling the medium at 15 °C for 30 min, protein expression was induced by the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG, 0.4 mM) and incubated at 15 °C for 24 h. The cells were harvested by centrifugation at 8000 rpm and 4 °C for 10 min. The pellet was resuspended in pH 7.0 phosphate lysis buffer and disrupted by ultrasonication in an ice bath. Unbroken cells and cell debris were removed by centrifugation (10,000 rpm, 4 °C, 15 min), leaving the crude enzyme in the supernatant.
The AKTA protein system (GE Healthcare) was used to purify the target protein. The crude enzyme was passed through a 0.22 μm filter and adsorbed onto a 5 mL Ni-HisTrap column. After washing the column with at least 50 mL of binding buffer (20 mM sodium phosphate buffer containing 500 mM NaCl and 5 mM imidazole, pH 7.4), the target enzyme was eluted with elution buffer (20 mM sodium phosphate buffer containing 500 mM NaCl and 500 mM imidazole, pH 7.4) using a linear gradient. Subsequently, the enzyme-containing fraction was desalted through a 5 mL Hitrap™ desalting column (Zhai et al. 2019).
The relative molecular mass of the purified enzyme was analyzed by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970), and the concentration of purified protein was determined using Bradford’s method (Bradford 1976).
Enzyme activity assay
The GDPD activity was determined by measuring the absorbance of the product p-nitrophenol at 410 nm using BpNPP as substrate according to the published method (Yip et al. 2011). The amount of enzyme required to release 1 nmol of p-nitrophenol per minute was defined as an enzyme activity unit.
Biochemical characterization of rBaGDPD
Using BpNPP as substrate, the effects of pH and temperature on rBaGDPD enzyme activity were investigated. The optimal pH was measured from 6.0 − 12.0 at 45 °C. The optimal temperature was determined at the optimal pH over the range of 25 − 75 °C. For pH stability, remaining enzyme activity was measured after incubating the enzyme in different pH buffers for 2 h at 4 °C. The following buffer systems (50 mM) were used: phosphate buffer for pH 6.0 − 7.0, Tris–HCl buffer for pH 8.0 − 9.0, glycineNaOH for pH 10.0 − 12.0. Thermostability was evaluated by incubating the enzyme for 20 min to 2 h at temperatures of 35 − 55 °C and measuring residual activity under the optimal conditions (pH 8.5 and 55 °C). Untreated enzyme activity was defined as 100%.
Using BpNPP as substrate, the effects of metal ions (Mg2+, Co2+, Mn2+, Ca2+, Fe3+, Cu2+, Ni2+, and Zn2+) on enzyme activity were measured at 55 °C and pH 8.5. Metal ion concentrations of 1.0– 100 mM were used in this study. The effect of 10 mM EDTA on enzyme activity was also studied. The effects of organic solvent (benzene, toluene, chloroform, n-Hexane, ethyl acetate, diethyl ether, dichloromethane) on enzyme activity were determined under the optimal conditions by incubating the enzyme in different organic solvents (50% concentration) at 4 °C for 1 h. Untreated enzyme activity was defined as a control.
Using different concentrations of BpNPP as substrate, the activity of rBaGDPD was determined at 55 °C and pH 8.5. The kinetic parameters of rBaGDPD were determined by nonlinear curve fitting. Measurements were conducted in triplicate for all tests.
Identification of degradation products
The DPHP (0.05 µg mL−1) was added to the purified rBaGDPD (diluted five times) and the mixture was shaken at 160 rpm at 40 °C for 24 h. After the reaction, the mixture was extracted three times with an equal volume of ethyl acetate. The combined ethyl acetate extracts were dried over anhydrous sodium sulfate and then concentrated using a vacuum rotary evaporator at 45 °C. Finally, the residue was dissolved in an equal volume of HPLC grade acetonitrile for analysis (Wei et al. 2018). The same procedure using inactivated enzyme was used as a control.
The samples were analyzed using a Waters Maldi SYNAPT Q-TOF mass spectrometer. The separation was performed on a C18 column (BEH C18, 2.1 × 150 mm, 1.7 μm). The mobile phases were composed of solution A (acetonitrile) and solution B (1% formic acid in water). Linear gradient elution was performed with 10 − 90% solution A for 10 min. The flow rate was 0.3 mL min−1, the injection volume was 5 μL and the detection wavelength was 260 nm. The MS analysis conditions were: cone voltage 30 V, capillary voltage 3.5 kV, source block temperature 100 °C and desolvation temperature 450 °C. All measurements were performed using positive electrospray and negative electrospray ionization (ESI).
Results and discussion
Bioinformatics analyses
Based on analyses of gene sequences and conserved domains, the putative GDPD gene bagdpd (GeneBank No: MT512400) was selected from B. altitudinis W3. The gene was 738 bp in length and encoded 245 amino acids. The theoretical molecular weight was 28 kDa. The results of phylogenetic analysis (Fig. 1a) indicated that BaGDPD had a very close relationship with GDPD from Bacillus safensis (GenBank No. AYJ89117.1).
Fig. 1.
a Phylogenetic trees of BaGDPD and GDPDs from other sources. It was built using MEGA 7.0 based on 1000 repeated pilot tests through the neighbor-joining method. The sequence in the phylogenetic tree came from GenBank (accession number in parentheses). b Multiple sequence alignment of BaGDPD from Bacillus altitudinis W3 with those known GDPDs available in Protein Data Bank (PDB). Identical residues were marked with a red background, and highly conserved residues were shown in red font. The secondary structure of GDPD was shown above the comparison. The red triangles indicated the catalytic triad of two histidines. The blue triangles indicated the residues in the gap that bonded to the divalent cation. The yellow triangles indicated strictly conserved residues in GDPD. GDPD from Thermotoga maritima (PDB: 1O1Z);GDPD from Thermococcus kodakarensis KOD1(PDB: 4OEC); GDPD from Thermoanaerobacter tengcongensis (PDB: 2PZ0) were used for alignment
In CATH database, GDPD has been classified as a member of the phosphatidylinositol phosphodiesterases superfamily (Orengo et al. 2015). Members of the GDPD family have evolutionarily conserved sequence motifs containing active site residues and shared a catalytic domain with the phosphoinositide-specific phospholipase C family (Shi et al. 2008). In BaGDPD, highly conserved sequence motifs (HR(X)n EN(X)n EXD(X)n HD) containing active site residues were found according to multiple sequence alignments. In the BaGDPD, His7 and His49 are strictly conserved and play the roles of general base and acid in catalytic hydrolysis of phosphate diester bond (Shi et al. 2008). It was found that BaGDPD shared 44, 42.68, and 40.37% sequence identities with GDPD from Thermococcus kodakarensis (PDB: 4OEC), Thermoanaerobacter tengcongensis (PDB: 2PZ0), Thermomotoga maritima (PDB: 1O1Z), respectively. The phylogenetic relationship, high sequence identity, and the conserved sequence motif proved that the BaGDPD was a member of the GDPD superfamily (Fig. 1).
Gene cloning and recombinant protein expression
The B. altitudinis W3 whole genome was used as a template to amplify the bagdpd gene, which was ligated to the pCold II plasmid fused with His-tag. Soluble overexpression was successfully achieved in E. coli BL21. The SDS-PAGE analysis showed that the molecular weight of the purified protein was about 28 kDa (Fig. 2), showing a single band consistent with the theoretical value.
Fig. 2.

SDS-PAGE analysis of rBaGDPD. Lane M, protein markers (14–100 kDa); lane 1, E. coli BL21 (pCold II) after IPTG induction; lane 2, crude fraction of rBaGDPD; lane 3, rBaGDPD purified by nickel column affinity chromatography
Characterization of rBaGDPD
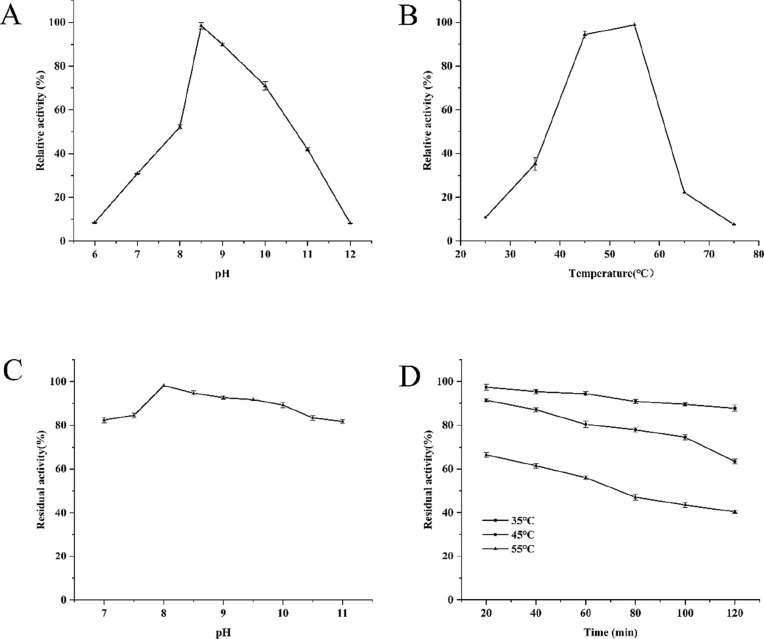
The effects of pH and temperature on enzyme activity and stability are shown in Fig. 3. The rBaGDPD showed activity toward BpNPP in the pH range of 6.0–12.0 and was most active at pH 8.5. The enzyme was highly tolerant to pH in the range of 7.0–11.0 (residual activity was more than 80%).
Fig. 3.
The effect of pH and temperature on the activity and stability of BaGDPD. a The highest activity at pH 8.5 (4254.18 ± 64.24 µg−1) was defined as 100%. b The effect of temperature on enzyme activity; the highest activity at 55 °C (4362.0 ± 70.93 µg−1) was defined as 100%. c pH stability; the enzyme activity of untreated enzyme (4450.71 ± 52.3 µg−1) was defined as 100%. d Thermostability; the enzyme activity of untreated enzyme (4273.36 ± 50.7 µg−1) was defined as 100%
The optimal temperature of the enzyme was 55 °C and it maintained high activity in the range of 40–60 °C. When the temperature was below 25 °C or above 75 °C, the enzyme activity was very low. Thermostability studies showed that the enzyme maintained more than 62% of its activity after incubation at 45 °C for 2 h, so could be considered as a thermostable enzyme. Enzyme activity decreased faster when the temperature was increased to 55 °C.
According to (Wang et al. 2016), pfGDPD had the same optimal temperature and pH as rBaGDPD. However, rBaGDPD hydrolyzed BpNPP over a wider pH range, indicating that it could have greater potential for degradation of environmental OPEs.
As shown in Table 1, Mg2+, Co2+, Mn2+, Ca2+, Cu2+, and Ni2+ all stimulated enzyme activity to a certain extent. Enzyme activity could not be detected when Fe3+ and Zn2+ were added. The greatest activation of enzyme activity occurred when 50 mM Ni2+ was added. No activity was detected after addition of EDTA, indicating that the enzyme was a metalloenzyme. The effects of various organic solvents on enzyme activity were also evaluated. The results (Table 1) showed that various organic solvents had a certain inhibitory effect on enzyme activity.
Table 1.
The effects of different additives on enzymatic activity
| Ions | Concentration (mM) |
Enzymatic activity (µg−1) | Organic solvents | Concentration (%) | Enzymatic activity (µg−1) |
|---|---|---|---|---|---|
| None | – | N.D. | Control | – | 4622 ± 42 |
| EDTA | 10 | N.D. | Diethyl ether | 50 | 3542 ± 47 |
| Ni2+ | 1 | 2957 ± 12 | Chloroform | 50 | 2141 ± 67 |
| 5 | 2964 ± 8 | Dichloromethane | 50 | 2887 ± 37 | |
| 10 | 3023 ± 22 | n-Hexane | 50 | 2516 ± 50 | |
| 50 | 6041 ± 22 | Ethyl acetate | 50 | 4150 ± 29 | |
| 100 | 2665 ± 23 | Toluene | 50 | 2647 ± 67 | |
| Co2+ | 1 | 2884 ± 43 | Benzene | 50 | 2479 ± 92 |
| 5 | 2944 ± 23 | ||||
| 10 | 3155 ± 41 | ||||
| 50 | 4835 ± 65 | ||||
| 100 | 2630 ± 34 | ||||
| Mn2+ | 1 | 4525 ± 87 | |||
| 5 | 3944 ± 5 | ||||
| 10 | 3187 ± 94 | ||||
| 50 | 2428 ± 29 | ||||
| 100 | 1187 ± 95 | ||||
| Ca2+ | 1 | 736 ± 34 | |||
| 5 | 972 ± 20 | ||||
| 10 | 1370 ± 30 | ||||
| 50 | 2375 ± 34 | ||||
| 100 | 2733 ± 114 | ||||
| Mg2+ | 1 | 557 ± 59 | |||
| 5 | 1845 ± 33 | ||||
| 10 | 2095 ± 47 | ||||
| 50 | 2368 ± 32 | ||||
| 100 | 1600 ± 24 | ||||
| Cu2+ | 50 | 305 ± 28 | |||
| Fe3+ | 10 | N.D. | |||
| Zn2+ | 10 | N.D. |
N.D. represented no enzyme activity was detected
GDPDs are α/β sandwich structural folds belonging to the metal ion-dependent phosphatase family (Natasa et al. 2006). Calcium or other metal ions (Zn2+, Mg2+, Fe2+, etc.) have been found in the active sites of prokaryotes GDPDs with confirmed structures (Shi et al. 2008). Usually, these enzymes have special metal ion characteristics related to their physiological functions (Wang et al. 2016). It is therefore very important to investigate the natural metal ion preference of the enzyme to understand its physiological function and catalytic mechanism. In this study, Ni2+ and Co2+ significantly activated rBaGDPD, while no activity was detected in the presence of Fe3+ and Zn2+, which differed from other reported GDPD enzymes (Wang et al. 2016). The particular metal ion preference further indicated the novelty of this enzyme.
The kinetic parameters of rBaGDPD in the hydrolysis of BpNPP are shown in Table 2. The Km value was 3.56 ± 0.20 mM and Kcat/Km is 0.40 s−1 mM−1. Compared with the glycerophosphodiesterase from Enterobacter aerogenes (Mirams et al. 2008), rBaGDPD had a lower Km value and, therefore, a higher affinity for BpNPP.
Table 2.
The kinetic parameters of rBaGDPD for BpNPP
| Enzyme | Km (mM) | Kcat (s−1) | Kcat/Km (s−1mM−1) |
|---|---|---|---|
| rBaGDPD | 3.56 ± 0.20 | 1.42 ± 0.06 | 0.40 |
Identification of degradation products
The products from the degradation of DPHP by rBaGDPD were identified by LC–MS. Deprotonated molecular ions were obtained by negative electrospray ionization to obtain the DPHP mass spectrum, which contained a fragment peak with m/z value of 248.99 (Fig. 4a). After the reaction for 24 h, HPLC analysis detected a new peak in addition to DPHP. The mass spectrum of the new peak under negative electrospray ionization is shown in Fig. 4b. The fragment ion of the new peak was located at m/z 172.98, corresponding to the loss of one − C6H4 from DPHP. Therefore, the degradation product was identified as phenyl phosphate (PHP).
Fig. 4.
LC–MS analysis of DPHP degradation products after rBaGDPD treatment. a MS analysis result of DPHP treated with inactivated rBaGDPD. b MS analysis result of DPHP after rBaGDPD treatment
In previous research, Yamaguchi et al. found that Phaeobacte sp., Ruegeria sp., and Thalassospira sp. in marine bacteria contained phosphotriesterase activity and could be used to degrade OPEs (Yamaguchi et al. 2016). In subsequent research, Wei et al. found that Brevibacillus brevis could be used to biodegrade TPHP (Wei et al. 2018). In this study, we used rBaGDPD to hydrolyze DPHP and identified the reaction product by LC–MS as PHP. The present report is the first example of DPHP degradation by GDPDs. Further research is still in progress to demonstrate the potential of rBaGDPD to degrade organic phosphates for bioremediation.
Conclusion
In this study, a GDPD enzyme from B. altitudinis W3 was cloned and heterologously-expressed in E. coli strain BL21(DE3). Furthermore, the interesting discovery that rBaGDPD was able to hydrolyze DPHP into PHP suggested that it could be applied to eliminate DPHP in the environment. Further research will focus on evaluating and improving the application of this enzyme in bioremediation. It is hopeful that rBaGDPD can be applied for the degradation of other toxic organophosphates to bioremediate the environment.
Acknowledgments
This work was financially supported by the Collaborative Innovation Involving Production, Teaching and Research Funds of Jiangsu Province (BY2014023-28). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Author’s contributions
RR: conducting experiment, data analysis, manuscript writing. LZ: writing-review and editing. QT: writing-review and editing. DM: writing-review and editing. YC: supervision, fund acquisition. ZG: supervision, fund acquisition. XL: writing-review and editing, supervision, funding acquisition. All authors read and approved the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interests.
References
- Antonopoulou M, Giannakas A, Bairamis F, Papadaki M, Konstantinou I. Degradation of organophosphorus flame retardant tris (1-chloro-2-propyl) phosphate (TCPP) by visible light N, S-codoped TiO 2 photocatalysts. Chem Eng J. 2017;318:231–239. doi: 10.1016/j.cej.2016.06.124. [DOI] [Google Scholar]
- Aron MB, Derbyshire MK, Gonzales NR, Shennan L, Farideh C, Geer LY, Geer RC, Jane H, Marc G, Hurwitz DI. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;D1:D222. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter MK, Romera-Garcia E, Borrull J, de Boer J, Rubio S, Ballesteros-Gomez A. Presence of diphenyl phosphate and aryl-phosphate flame retardants in indoor dust from different microenvironments in Spain and the Netherlands and estimation of human exposure. Environ Int. 2018;112:59–67. doi: 10.1016/j.envint.2017.11.028. [DOI] [PubMed] [Google Scholar]
- Bradford M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of potein-dye binding. Anal Chem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng YX, Zhou WB, El Sheery NI, Peters C, Li MY, Wang XM, Huang JR. Characterization of the arabidopsis glycerophosphodiester phosphodiesterase (gdpd) family reveals a role of the plastid-localized atgdpd1 in maintaining cellular phosphate homeostasis under phosphate starvation. Plant J. 2011;66(5):781–795. doi: 10.1111/j.1365-313X.2011.04538.x. [DOI] [PubMed] [Google Scholar]
- Corda D, Kudo T, Zizza P, Iurisci C, Kawai E, Kato N, Yanaka N, Mariggio S. The developmentally regulated osteoblast phosphodiesterase GDE3 is glycerophosphoinositol-specific and modulates cell growth. J Biol Chem. 2009;284(37):24848–24856. doi: 10.1074/jbc.M109.035444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda D, Mosca MG, Ohshima N, Grauso L, Yanaka N, Mariggio S. The emerging physiological roles of the glycerophosphodiesterase family. Febs J. 2014;281(4):998–1016. doi: 10.1111/febs.12699. [DOI] [PubMed] [Google Scholar]
- Daumann LJ, Larrabee JA, Ollis D, Schenk G, Gahan LR. Immobilization of the enzyme GpdQ on magnetite nanoparticles for organophosphate pesticide bioremediation. J Inorg Biochem. 2014;131:1–7. doi: 10.1016/j.jinorgbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Denloye T, Dalal S, Klemba M. Characterization of a glycerophosphodiesterase with an unusual tripartite distribution and an important role in the asexual blood stages of Plasmodium falciparum. Mol Biochem Parasitol. 2012;186(1):29–37. doi: 10.1016/j.molbiopara.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Guan ZB, Cai YJ, Zhang YZ, Zhao H, Liao XR. Complete genome sequence of Bacillus pumilus W3: A strain exhibiting high laccase activity. J Biotechnol. 2015;207:8–9. doi: 10.1016/j.jbiotec.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: Hand wipes and house dust. Environ Health Perspect. 2015;123(2):160–165. doi: 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mirams RE, Smith SJ, Hadler KS, Ollis DL, Schenk G, Gahan LR. Cadmium(II) complexes of the glycerophosphodiester-degrading enzyme GpdQ and a biomimetic N O ligand. J Biol Inorg Chem. 2008;13(7):1065–1072. doi: 10.1007/s00775-008-0392-5. [DOI] [PubMed] [Google Scholar]
- Mitchell CA, Reddam A, Dasgupta S, Zhang S, Stapleton HM, Volz DC. Diphenyl phosphate-induced toxicity during embryonic development. Environ Sci Technol. 2019;53(7):3908–3916. doi: 10.1021/acs.est.8b07238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natasa M, Smith SJ, Neves A, Guddat LW, Gahan LR, Schenk G. The catalytic mechanisms of binuclear metallohydrolases. Chem Rev. 2006;106:3338–3363. doi: 10.1021/cr050318f. [DOI] [PubMed] [Google Scholar]
- Ohshima N, Yamashita S, Takahashi N, Kuroishi C, Shiro Y, Takio K. Escherichia coli cytosolic glycerophosphodiester phosphodiesterase (ugpq) requires mg2+, co2+, or mn2+ for its enzyme activity. J Bacteriol. 2008;190(4):1219–1223. doi: 10.1128/JB.01223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y, Ohshima N, Yoshizawa I, Kamei Y, Mariggio S, Okamoto K, Maeda M, Nogusa Y, Fujioka Y, Izumi T, Ogawa Y, Shiro Y, Wada M, Kato N, Corda D, Yanaka N. A novel glycerophosphodiester phosphodiesterase, GDE5, controls skeletal muscle development via a non-enzymatic mechanism. J Biol Chem. 2010;285(36):27652–27663. doi: 10.1074/jbc.M110.106708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orengo CA, Bray JE, Buchan DWA, Harrison A, Lee D, Pearl FMG, Sillitoe I, Todd AE, Thornton JM. The CATH protein family database: a resource for structural and functional annotation of genomes. Proteomics. 2015;2(1):11–21. doi: 10.1002/1615-9861(200201)2:1<11::AID-PROT11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Shi L, Liu JF, An XM, Liang DC. Crystal structure of glycerophosphodiester phosphodiesterase (gdpd) from thermoanaerobacter tengcongensis, a metal ion-dependent enzyme: Insight into the catalytic mechanism. Proteins. 2008;72(1):280–288. doi: 10.1002/prot.21921. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Park S (2016) Modulators of glycerophosphodiester phosphodiesterase proteins.
- Sudhir K, Glen S, Koichiro T. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Lai L, Liu Y, Yang B, Wang Y. Expression and characterization of a novel glycerophosphodiester phosphodiesterase from pyrococcus furiosus DSM 3638 that possesses lysophospholipase D activity. Int J Mol Sci. 2016;17(6):831. doi: 10.3390/ijms17060831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Yin H, Peng H, Lu G, Dang Z. Bioremediation of triphenyl phosphate by Brevibacillus brevis: Degradation characteristics and role of cytochrome P450 monooxygenase. Sci Total Environ. 2018;627:1389–1395. doi: 10.1016/j.scitotenv.2018.02.028. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Arisaka H, Seki M, Adachi M, Kimura K, Tomaru Y. Phosphotriesterase activity in marine bacteria of the genera Phaeobacter, Ruegeria, and Thalassospira. Int Biodeter Biodegradation. 2016;115:186–191. doi: 10.1016/j.ibiod.2016.08.019. [DOI] [Google Scholar]
- Yan Y, Sabharwal P, Rao M, Sockanathan S. The antioxidant enzyme prdx1 controls neuronal differentiation by thiol-redox-dependent activation of GDE2. Cell. 2009;138(6):1209–1221. doi: 10.1016/j.cell.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhai L, Huang L, Meng D, Li J, Hao Z, Guan Z, Cai Y, Liao X. Mining of alkaline proteases from Bacillus altitudinis W3 for desensitization of milk proteins: their heterologous expression, purification, and characterization. Int J Biol Macromol. 2020;153:1220–1230. doi: 10.1016/j.ijbiomac.2019.10.252. [DOI] [PubMed] [Google Scholar]
- Yasin S, Behary N, Giraud S, Perwuelz A (2016) In situ degradation of organophosphorus flame retardant on cellulosic fabric using advanced oxidation process: a study on degradation and characterization. Polym. Degrad. Stabil. 126(Apr):1–8
- Yip SH-C, Foo J-L, Gerhard S, Gahan LR, Carr PD, Ollis DL. Directed evolution combined with rational design increases activity of GpdQ toward a non-physiological substrate and alters the oligomeric structure of the enzyme. Protein Eng Des Sel. 2011;24(12):861–872. doi: 10.1093/protein/gzr048. [DOI] [PubMed] [Google Scholar]
- Zhai L, Yang S, Lai Y, Meng D, Tian Q, Guan Z, Cai Y, Liao X. Mining of aminotransferase gene ota3 from Bacillus pumilus W3 via genome analysis, gene cloning and expressing for compound bioamination. Gene. 2019;686:21–28. doi: 10.1016/j.gene.2018.10.082. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li X, Xi R, Guan Z, Cai Y, Liao X. Characterization of an acid-stable catalase KatB isolated from Bacillus altitudinis SYBC hb4. Ann Microbio. 2015;66(1):131–141. doi: 10.1007/s13213-015-1089-y. [DOI] [Google Scholar]
- Zhao F, Wan Y, Zhao H, Hu W, Mu D, Webster TF, Hu J. Levels of blood organophosphorus flame retardants and association with changes in human sphingolipid homeostasis. Environ Sci Technol. 2016;50(16):8896. doi: 10.1021/acs.est.6b02474. [DOI] [PubMed] [Google Scholar]