Abstract
Background
Intravenous drug administration is associated with potential complications, such as phlebitis. The physiochemical characteristics of the infusate play a very important role in some of these problems.
Aim
The aim of this study was to standardize the dilutions of intravenous drugs most commonly used in hospitalized adult patients and to characterize their pH, osmolarity and cytotoxic nature to better guide the selection of the most appropriate vascular access.
Methods
The project was conducted in three phases: (i) standardization of intravenous therapy, which was conducted using a modified double-round Delphi method; (ii) characterization of the dilutions agreed on in the previous phase by means of determining the osmolarity and pH of each of the agreed concentrations, and recording the vesicant nature based on the information in literature; and (iii) algorithm proposal for selecting the most appropriate vascular access, taking into account the information gathered in the previous phases.
Results
In total, 112 drugs were standardized and 307 different admixtures were assessed for pH, osmolarity and vesicant nature. Of these, 123 admixtures (40%), had osmolarity values >600 mOsm/L, pH < 4 or > 9, or were classified as vesicants. In these cases, selection of the most suitable route of infusion and vascular access device is crucial to minimize the risk of phlebitis-type complications.
Conclusions
Increasing safety of intravenous therapy should be a priority in the healthcare settings. Knowing the characteristics of drugs to assess the risk involved in their administration related to their physicochemical nature may be useful to guide decision making regarding the most appropriate vascular access and devices.
Key Points
| To our knowledge, this is the first nationwide approach towards intravenous therapy standardization in our country. The list of drugs and standard concentrations we present here are the result of a multidisciplinary team consensus in order to reduce variability and increase safety regarding intravenous drug management. |
| There is no information in literature related to pH or osmolarity in dilution of the most common drugs that are delivered through an intravenous line. This is the most extensive study addressing the osmolarities and pH of standard drug concentrations. |
| Current recommendations about vascular access selection include aspects such as length of therapy and patients’ requirements. This paper suggests adding different risk levels depending on pH and osmolarity of drugs to better guide the most appropriate vascular access for each patient. |
Introduction
The intravenous line is an essential device in medicine and is sometimes the only option for the delivery of medication and patient monitoring. It has been estimated that over 80% of hospitalized patients receive intravenous therapy [1, 2]. The most common reasons for intravenous therapy are to replace and maintain fluids and the electrolyte balance; to administer medications, blood or blood products; and to deliver nutrients and nutritional supplements [3]. Administration of intravenous therapy is performed through vascular access devices (VADs), either peripheral (including short peripheral catheters and midline catheters inserted into the upper arm) [4] or central (CVADs), including peripherally inserted central catheters, tunneled catheters, non-tunneled catheters and implanted ports [5].
The selection of a VAD depends on the clinical circumstances; a peripheral catheter is associated with fewer complications in venous access, and it is preferred if intravenous therapy is required for only a short period, provided the patient’s venous patrimony and medication needs are suitable for peripheral intravenous infusion [6]. CVADs, on the other hand, are the devices of choice for long-term therapies, for administering drugs that are potentially harmful to the vascular endothelium due to their physicochemical characteristics, or in cases of the inability or the failure of other forms of venous access [6, 7].
The use of VADs is associated with several complications, including phlebitis, infiltration and extravasation, nerve injuries, VAD occlusion, infection, air embolism and thrombosis [5]. While some of these complications, such as catheter-related bloodstream infections or venous air embolisms, are uncommon [8, 9], phlebitis has been reported to have an incidence of 31 per 100 catheters, and severe phlebitis occurs in 3.6% of patients [10]. The occurrence of these complications has an important impact on patients and society since they are associated with treatment delays, increased patient discomfort and dissatisfaction, and may result in suboptimal health care outcomes, including injury, permanent disability and death [4].
Factors associated with the occurrence of these complications are patient-related (advanced age, female sex, fragility, immunosuppression); use-related, which is closely related with staff training (suboptimal placement or inappropriate device management); and device-related (a large catheter diameter in relation to the vein size, a poorly secured device, the infusion set and the catheter composition). In addition to these factors, the physiochemical characteristics of the infusate play a very important role in phlebitis. Some infusates can harm tissues through direct venous damage (cytotoxic drugs), direct vasoconstriction, or by exposing cells to osmotic stress or a nonphysiologic pH [4, 11–13]. While oncology drug properties and their influence in VAD selection have been widely documented [14–18], there is limited data available regarding nononcologic drugs.
The objective of the work we present was to standardize the dilutions of nononcologic drugs that are most commonly used in hospitalized adult patients and to characterize these dilutions regarding their pH, osmolarity and cytotoxic nature to complement current knowledge in order to guide the selection of the most appropriate vascular access for each one.
Methods
A multidisciplinary team, the Expert Advisory Group (EAG), was created with 10 members from several scientific societies: one physician from the Spanish Society of Intensive, Critical and Coronary Care Medical Units (SEMICYUC); one physician from the Spanish Society for Preventive Medicine, Public Health and Hygiene (SEMPSPH); three nurses from the Spanish Society of Infusion and Vascular Access (SEINAV); and five pharmacists, four of them from the Spanish Society of Hospital Pharmacy (SEFH). One pharmacist experienced in intensive care drug management proposed a list of the drugs most commonly used in hospitalized and/or critically ill adults that are administered intravenously by continuous or intermittent infusion. Every member of the EAG reviewed the proposal and made suggestions according to their clinical experience to comprise the definitive list of drugs of the study. Drugs that required direct intravenous administration or those from a specific therapeutic area such as oncology, radiology, or pediatrics, were excluded.
The project was conducted in three phases: (i) standardization of intravenous therapy, (ii) characterization of the dilutions agreed on in the previous phase and (iii) algorithm proposal for selecting the most appropriate VAD, taking into account the information gathered in the previous phases.
Standardization of the Intravenous Therapy
After agreeing on the list of drugs, two pharmacists experienced in pharmacy practice risk management proposed one or more potential concentrations for each drug based on the recommendations available in the literature [19, 20], national or international intravenous therapy protocols [21–23] and their own experience. The drug concentrations suggested should encompass a broad range of clinical scenarios and fluid load requirements. All drug concentrations would be obtained by diluting the drug with sodium chloride 0.9% (NS) or dextrose 5% in water (D5W) (the two diluents most commonly used for intravenous mixtures in the hospital setting). Compatibility of the drug–diluent was checked against standard databases [19, 20, 24], and, in a case of incompatibility, only the compatible diluent was selected.
Parenteral dosage forms supplied as ready-to-use solutions were not intended for standardization consensus but were included in the final document with the characterization of their physicochemical properties.
The standardization process was carried out using a modified double-round Delphi method. The Delphi method is frequently applied to gather opinions from a group of experts in a structured way [25]. A group of 29 experts, including the EAG, with experience in intravenous therapy/vascular access, critical care and/or risk management, was assembled, comprising 6 physicians (4 from intensive care units, 1 anesthetist, and 1 from preventative medicine), 12 nurses, and 11 pharmacists. The list of drugs and concentrations was categorized depending on whether they should be administered by continuous or intermittent infusion. In the Delphi first round that was conducted between April 2019 and June 2019, each expert selected the concentrations that were considered appropriate for dealing with different clinical scenarios in daily practice; the experts could include comments regarding new drug concentrations to cover potential clinical situations not previously considered. Agreement on a specific drug concentration was reached when it was selected by at least 70% of the respondents. Drugs with a nonresponder rate ≥ 30% (meaning none of the suggested concentrations were selected, and no comments were made regarding possible alternatives) were directly excluded from the study. Concentrations selected by 40–69% of the respondents were analyzed in the second-round discussion. Concentrations selected by fewer than 40% of respondents were also excluded from the study.
Given that the process was not anonymous, all participants were informed via e-mail of the results of the first round. This allowed for a discussion, also via e-mail, regarding those concentrations that did not reach the threshold for agreement in the first round. The second round was carried out between June 2019 and July 2019 following the same approach.
Characterization of the Agreed Upon Infusion Solutions
The osmolarity and pH of each of the agreed concentrations were determined.
Osmotic pressure can be expressed as either osmolality or osmolarity. These concepts are usually misused by health professionals. Osmolality is defined as the number of milliosmoles of solute per kilogram of solvent and can be calculated experimentally using sodium chloride equivalents or determined with an osmometer [11, 26]. Osmolarity is the number of milliosmoles per liter of solution; it cannot be measured experimentally but instead can be calculated from osmolality using a conversion factor [11, 26]:
This method of expressing density seems to be an optimum combination of accuracy and practicality [27].
In clinical practice, osmolarity is preferred over osmolality since it expresses concentration as a function of volume [11, 26].
Osmolarity was experimentally measured using the Fiske Model 210 Micro Osmometer (John Morris Scientific Pty Ltd., Australia) that determines the osmolality of solutions using freezing point depression. The osmometer was calibrated with its specific calibration solution in the range of 0–2000 mOsm/kg H2O. The repeatability of the instrument was 0–400 mOsm/kg H2O: ± 2 mOsm/kg H2O (1 standard deviation [SD]); 400–2000 mOsm/kg H2O: ±0.5% (1 SD). The resolution was 1 mOsm/kg H2O.
Every drug concentration to be tested was prepared using the drugs available at the Pharmaceutical Technology Unit of the Hospital General Universitario Gregorio Marañón, Madrid, Spain. B. Braun Medical NS and D5W were used as diluents for every agreed concentration in order to study their influence on the physicochemical characteristics.
Density was measured with a Gay-Lussac pycnometer, with a capacity of 25 mL that was calibrated with bidistilled water at a temperature of 25 ºC, and used the following equation:
Osmolarity was then calculated with the above-mentioned equations and expressed as the mean (±SD) of three different measures.
pH was measured with a pH meter (Crison 2006, Hach Lange Spain, S.L.U., Spain) and expressed as the mean (±SD) of three different measures.
For each agreed dilution, aliquots of 50 mL were prepared. Then, 25 mL was used for the density determination by the pycnometer method, 60 µL divided into three aliquots of 20 µL was used to obtain three osmolality measures, and the remaining volume was used in the pH determinations.
Each drug was also characterized according to its vesicant nature based on the information provided in the corresponding summary of product characteristics and the published information [19, 20, 28, 29].
In cases where the osmolarity of the admixture was higher than 450 mOsm/L (see the Results and Discussion sections), and there was no other admixture at the same concentration with an osmolarity value <450 mOsm/L, the drug was diluted with 0.45% hypotonic saline solution (1/2S) to assess the potential changes in osmolarity and pH. Compatibility of the drug–diluent was checked against standard databases [19, 20, 24] and in case the admixture had not been tested, it was kept 8 hours under visual observation in order to assess precipitation or color changes.
If different brand names of the same drugs were available at the hospital at the time of this study, their osmolarity and pH were assessed in order to analyze the potential influence of different brands or excipients on their physicochemical properties.
Developing an Algorithm for Catheter Selection
The published literature regarding the influence of different factors on the selection of the type of vascular access and catheter was reviewed. Most national and international algorithms available consider the patient’s venous patrimony, duration of therapy and osmolarity of the drug to be infused. However, the role of pH, vesicant properties of nononcologic drugs and possible scenarios of different risk levels are not usually taken into account [30–35].
The EAG, led by the experienced nurses from the Spanish Society of Infusion and Vascular Access, agreed on three different risk levels (low, medium and high) regarding the osmolarity and pH of the infusate drugs in order to include all these items, together with the ones mentioned before, in an updated version of the vascular access selection algorithm.
Results
Standardization of the Intravenous Therapy
Table 1 shows the results regarding the number of drugs and concentrations included and selected during the Delphi consensus. An initial list of 111 drugs was suggested by two pharmacists. Of these, 46 (41.4%) were for continuous administration and 71 (63.9%) for intermittent administration. Some drugs had concentrations for both continuous and intermittent infusions. In addition, 13 of these 111 drugs were provided as ready-to-use medications and were not subjected to the standardization discussion. Therefore, 98 drugs, a total of 205 concentrations, were included in the Delphi.
Table 1.
Double-round Delphi results
| Initial proposala | First round proposalb | Agreement after first roundb | Second round proposalb | Final agreementa | |
|---|---|---|---|---|---|
| Number of drugsc | 111 | 98 | 93 | 56 | 106 |
| Continuous infusion | 46 | 40 | 35 | 24 | 39 |
| Intermittent infusion | 71 | 62 | 62 | 32 | 71 |
| Number of concentrations | 221 | 205 | 82 | 81 | 183 |
| Continuous infusion | 109 | 102 | 24 | 41 (one new strength proposal) | 67 |
| Intermittent infusion | 112 | 103 | 58 | 40 (three new strengths proposals) | 116 |
Agreement for a definite concentration was achieved after the first round if it was selected by at least 70% of the panel members
Final agreement was achieved after the second round when concentrations with 40–69% votes were discussed
aReady-to-use drugs included (13 drugs; 16 different strengths)
bReady-to-use drugs excluded
cSome of the drugs are included in both continuous and intermittent infusions
After the first round, there was no agreement for any of the concentrations of five suggested drugs (alprostadil, lidocaine, octreotide, procainamide and tacrolimus), and therefore, these five drugs were directly excluded from the study. On the other hand, 82 specific concentrations out of 205 directly reached consensus as they were selected by at least 70% of the panel experts.
In the second round, 77 different concentrations that had been selected by 40–69% of respondents, together with four additional new ones suggested by several panel members, were the subject of discussion. Finally, the whole panel agreed that all of them should be included as they were necessary to represent different feasible scenarios in clinical practice.
After Delphi consensus, 106 drugs were included with 183 different concentrations (including ready-to-use drugs), 67 (36.6%) for continuous infusion and 116 (63.4%) for intermittent infusion.
Characterization of the Agreed-on Infusion Solutions
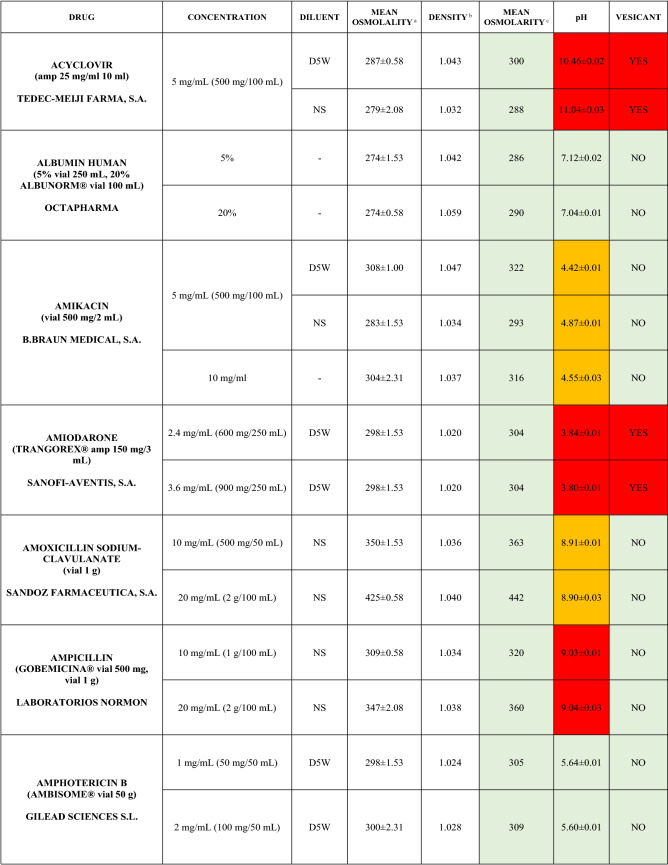
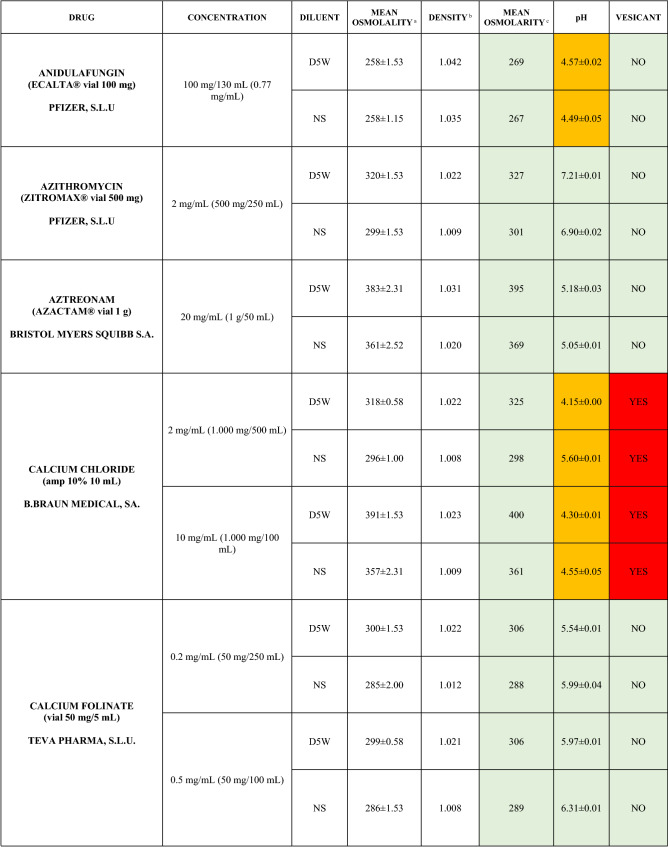
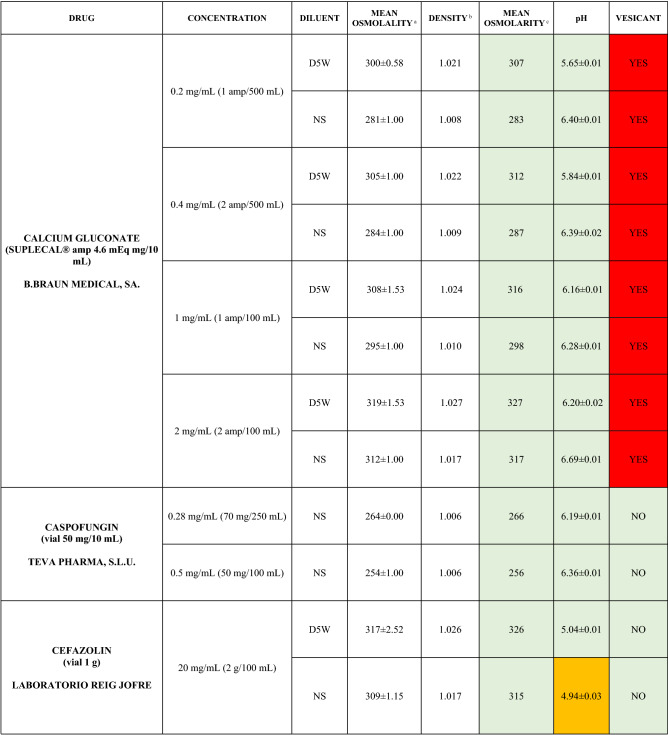
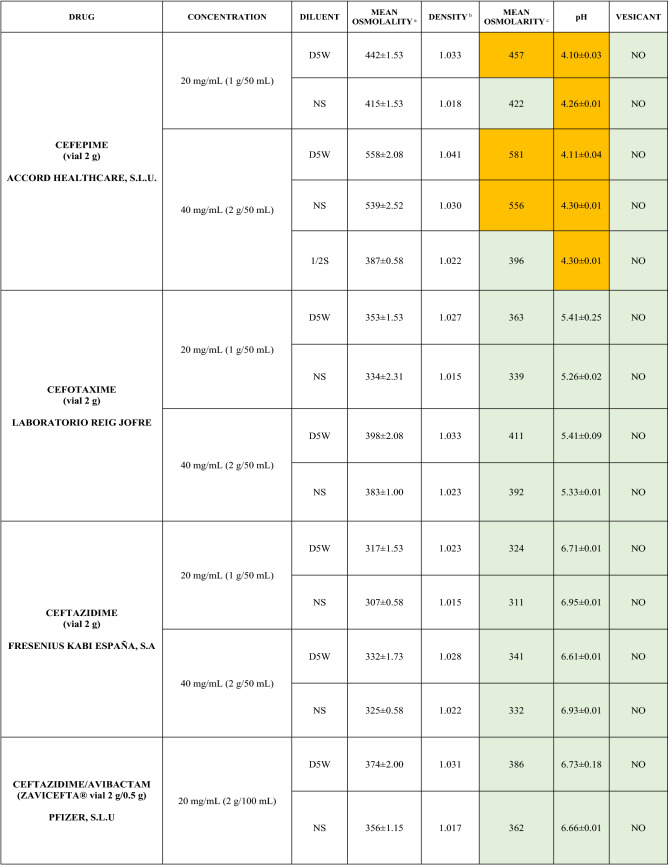
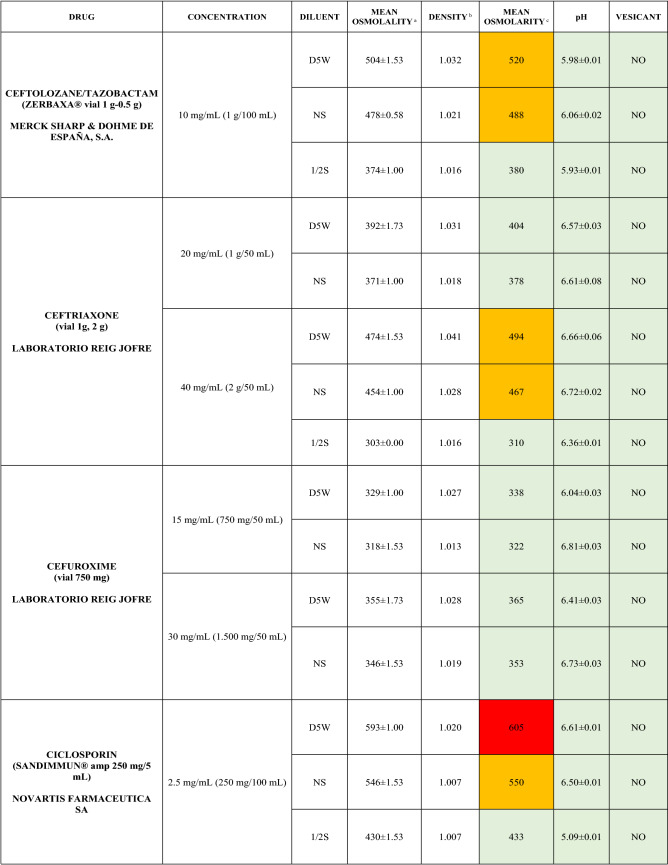
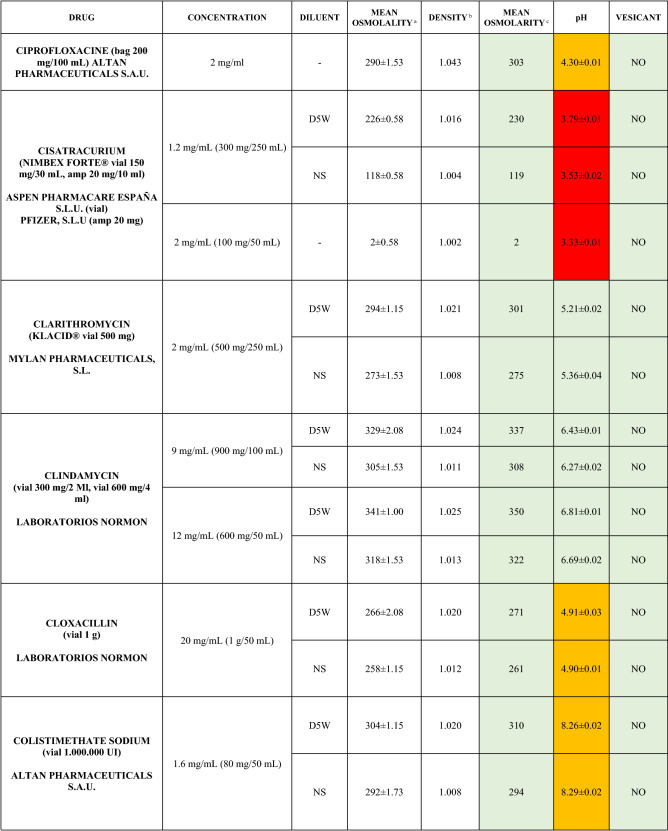
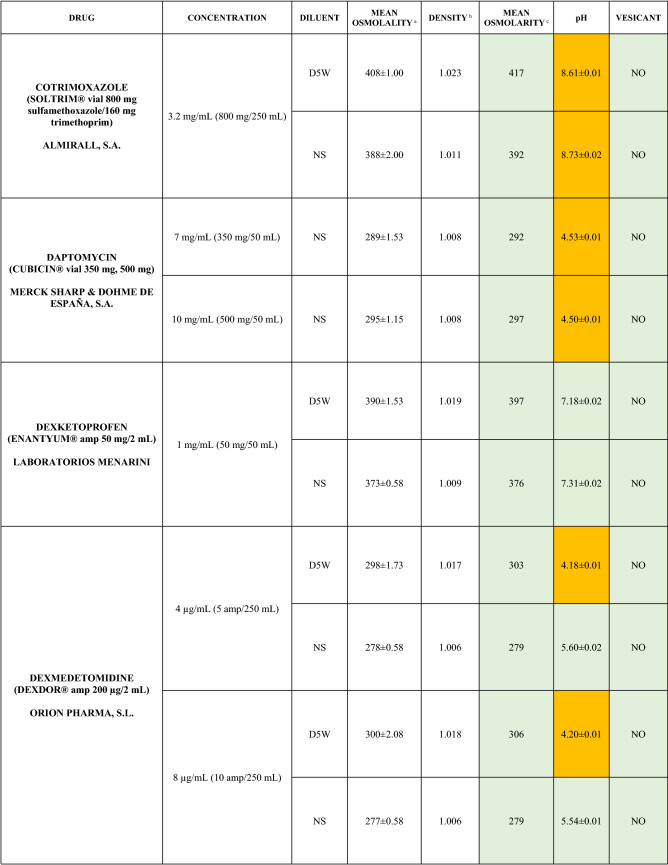
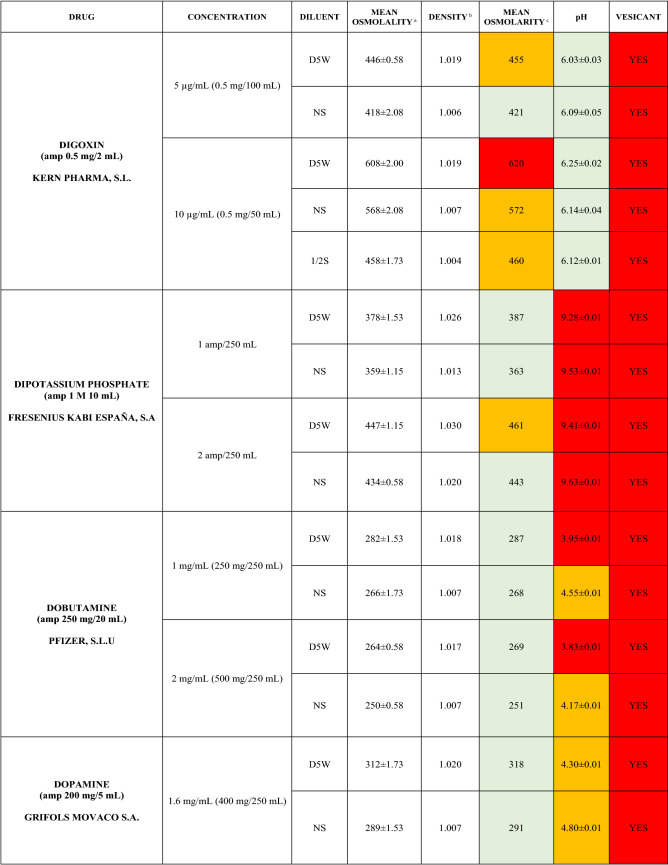
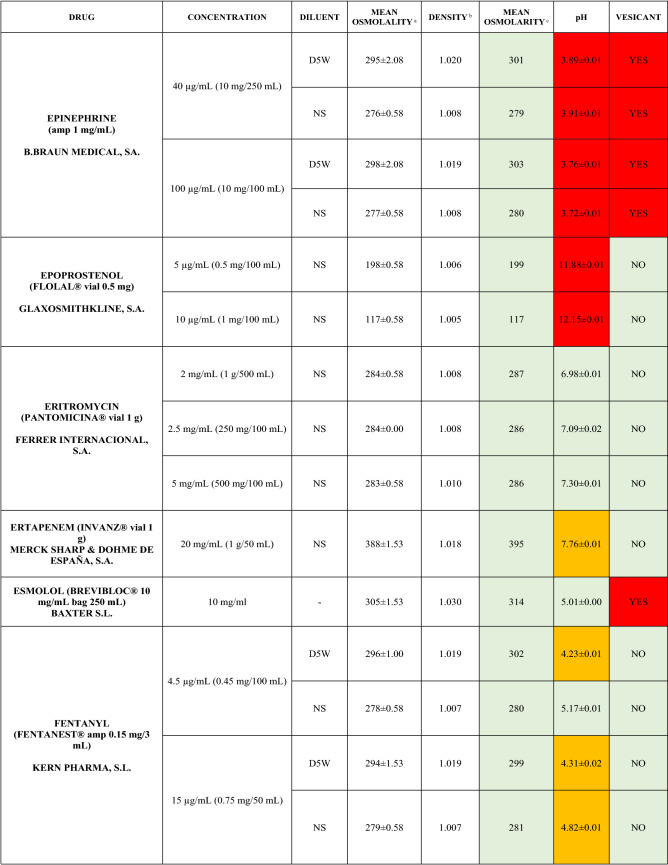
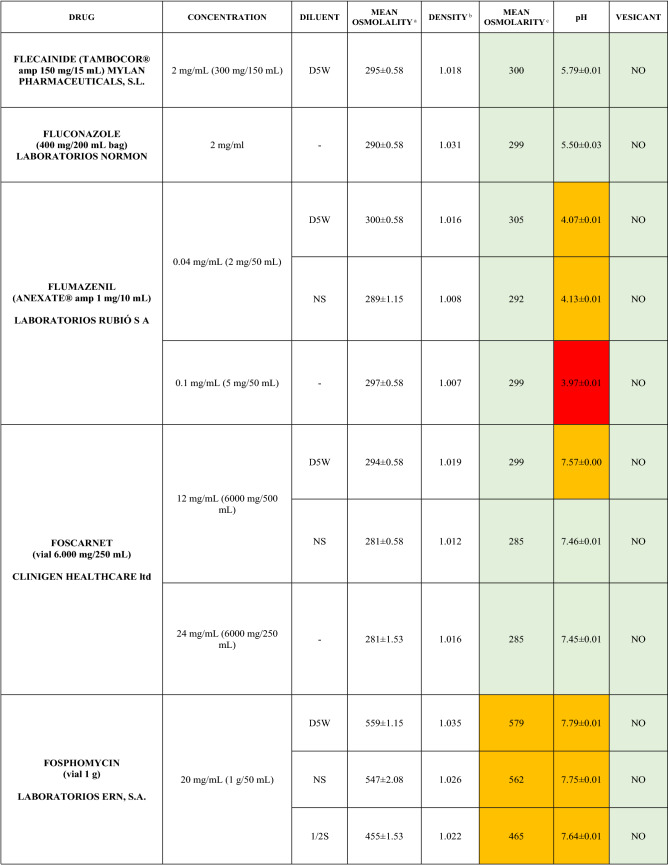
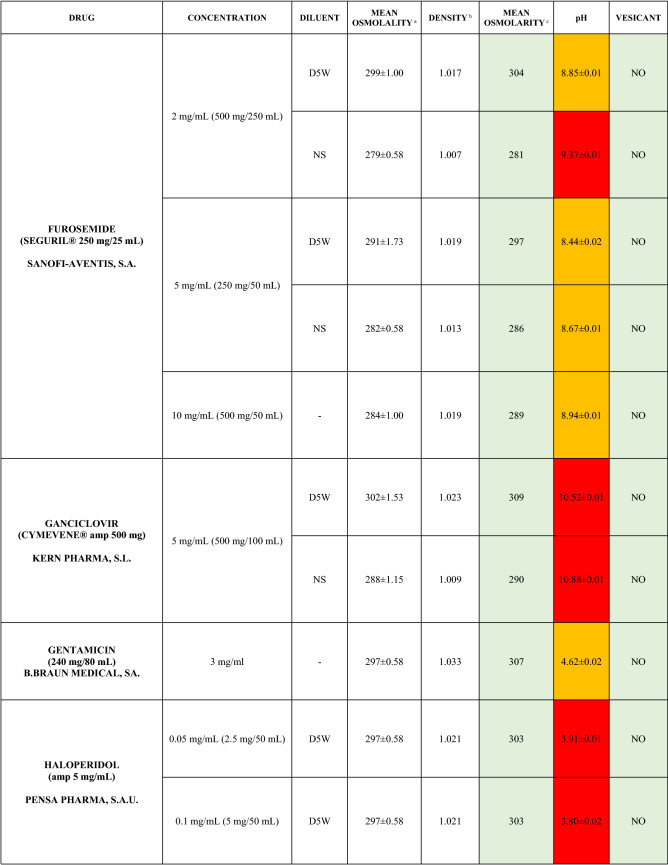
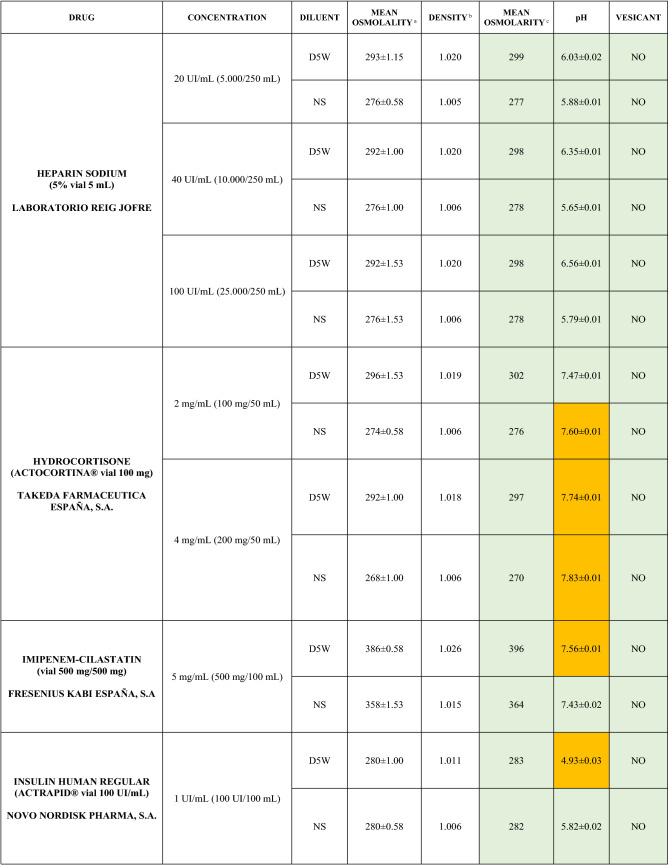
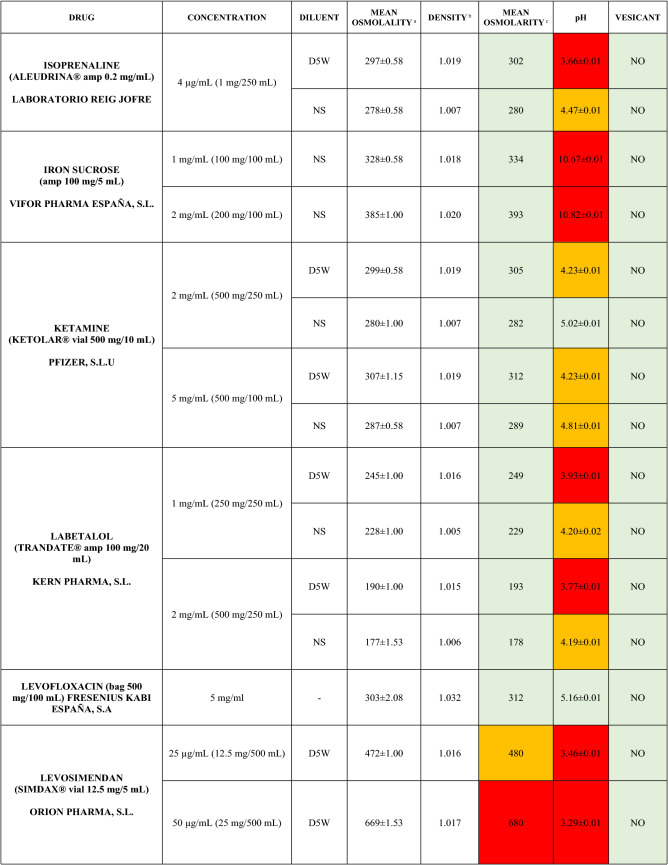
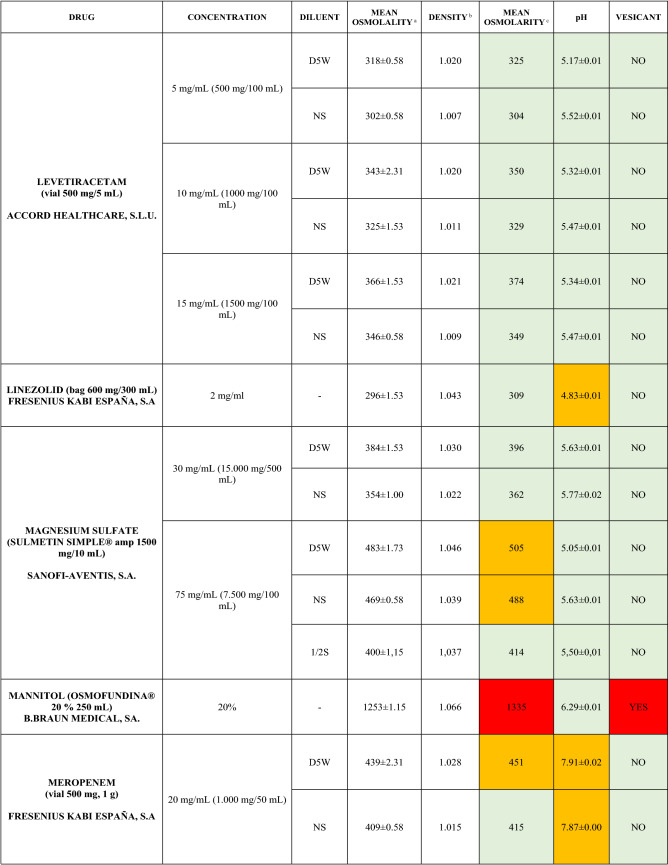
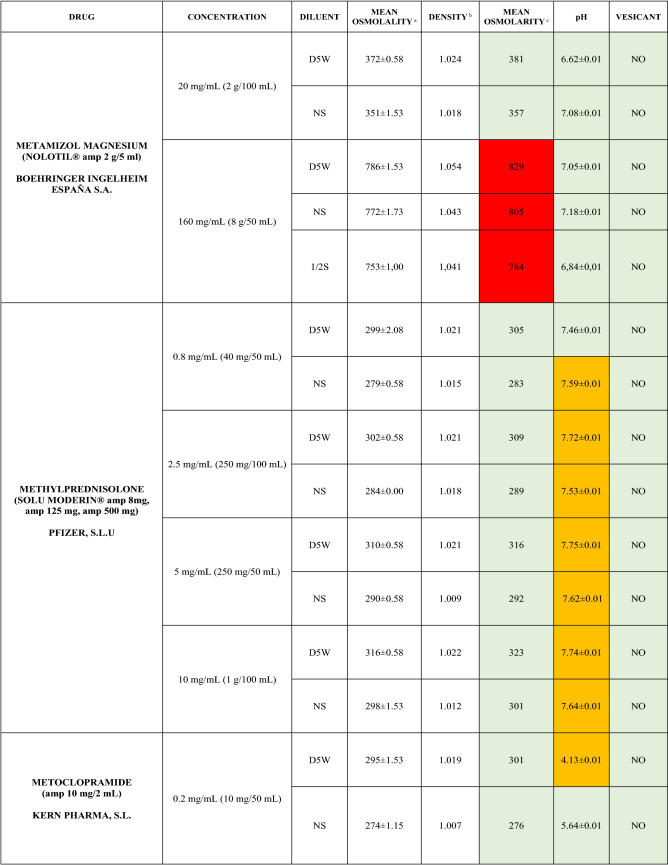
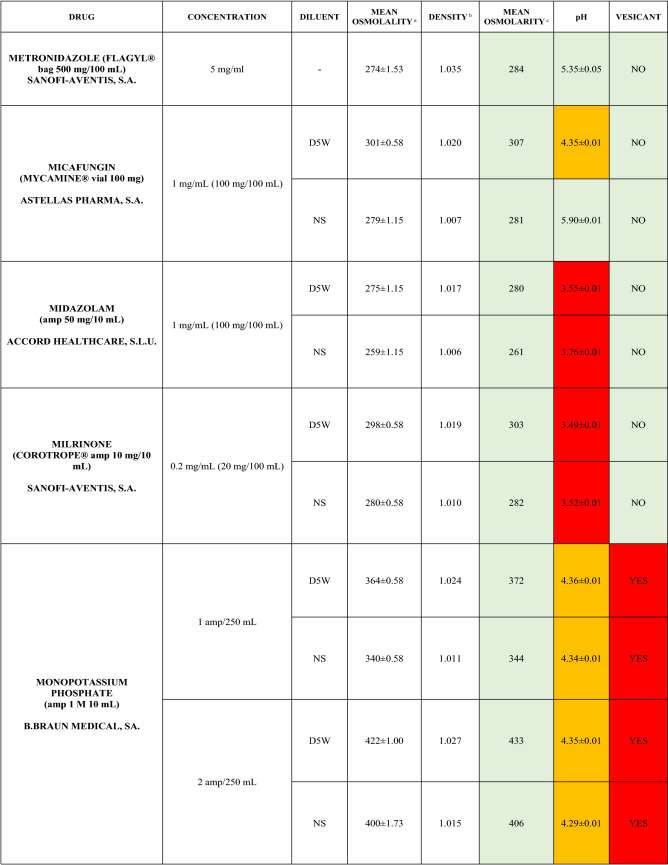
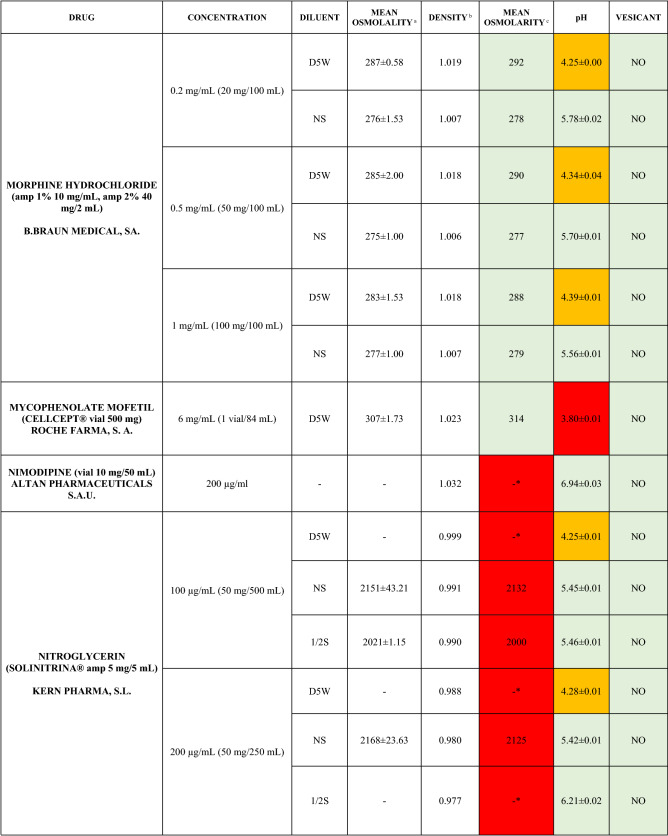
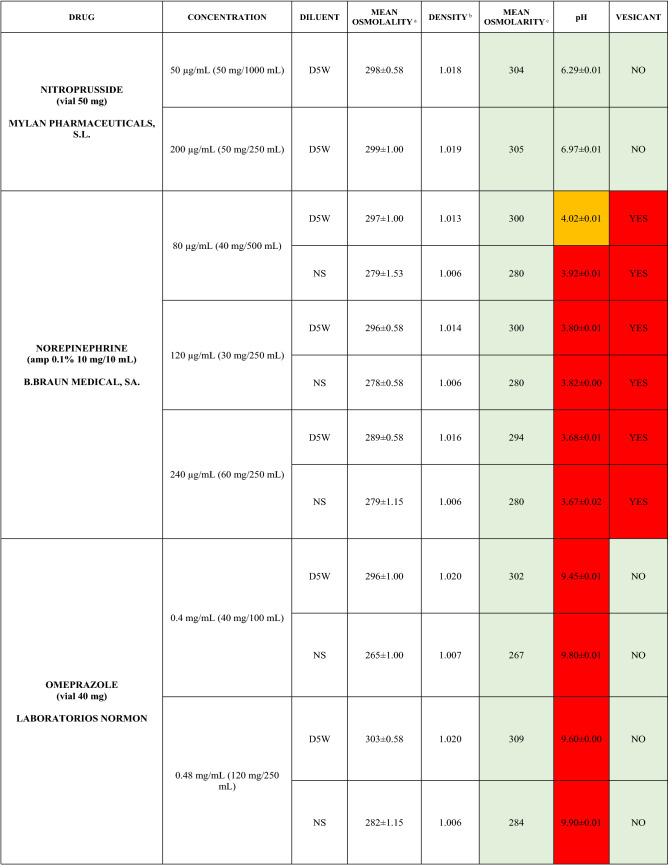
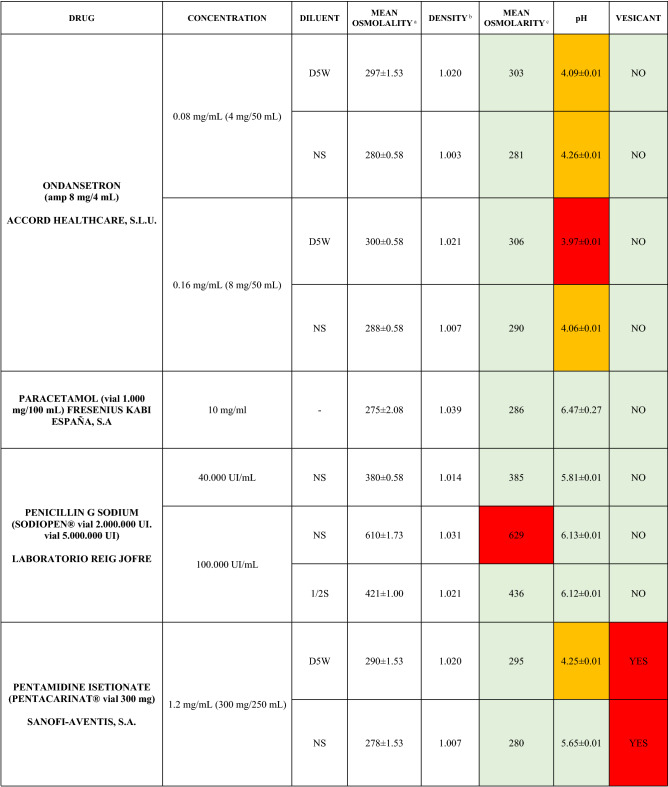
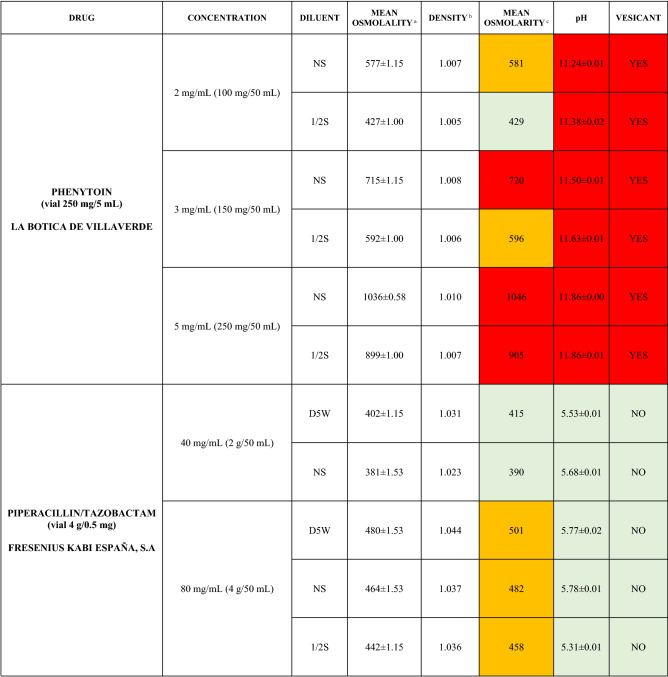
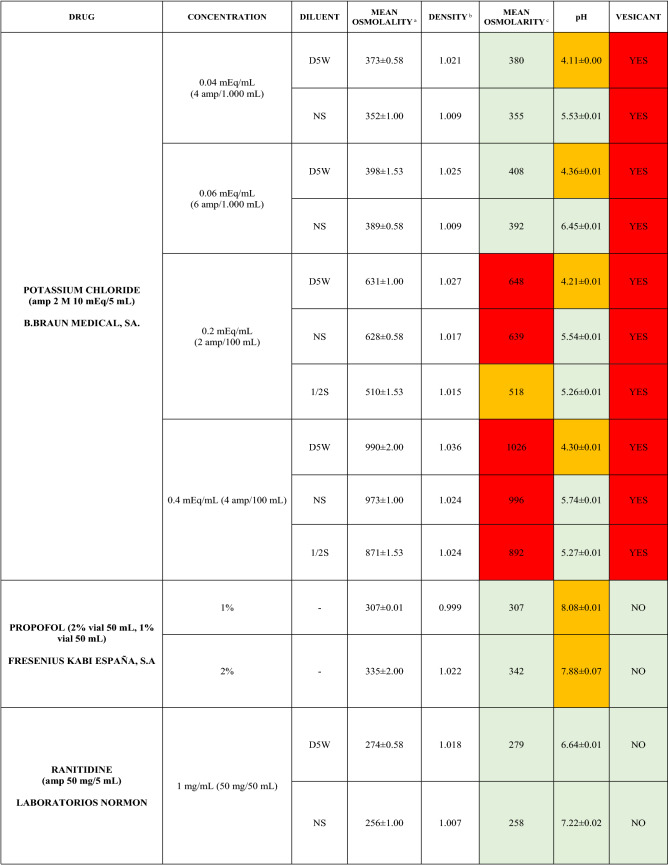
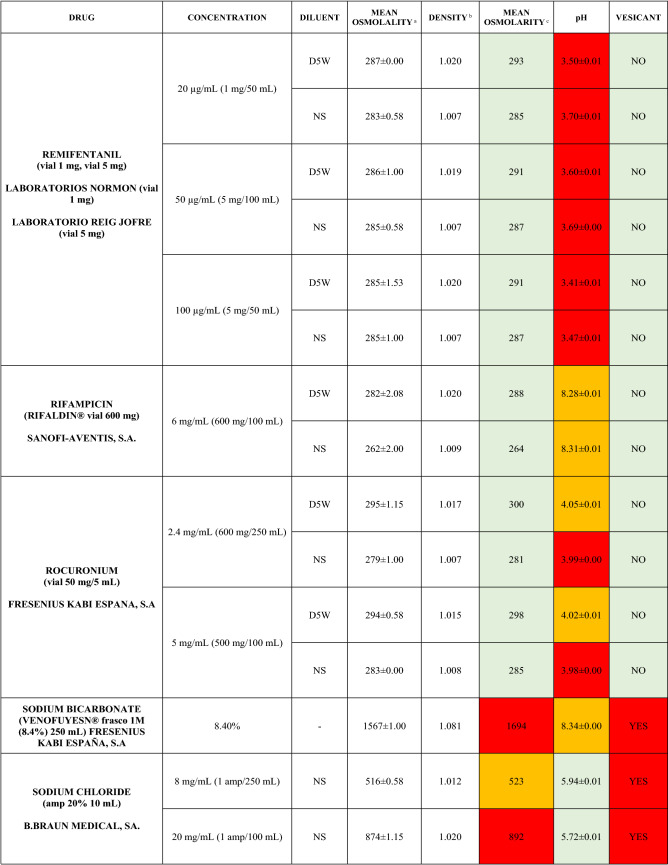
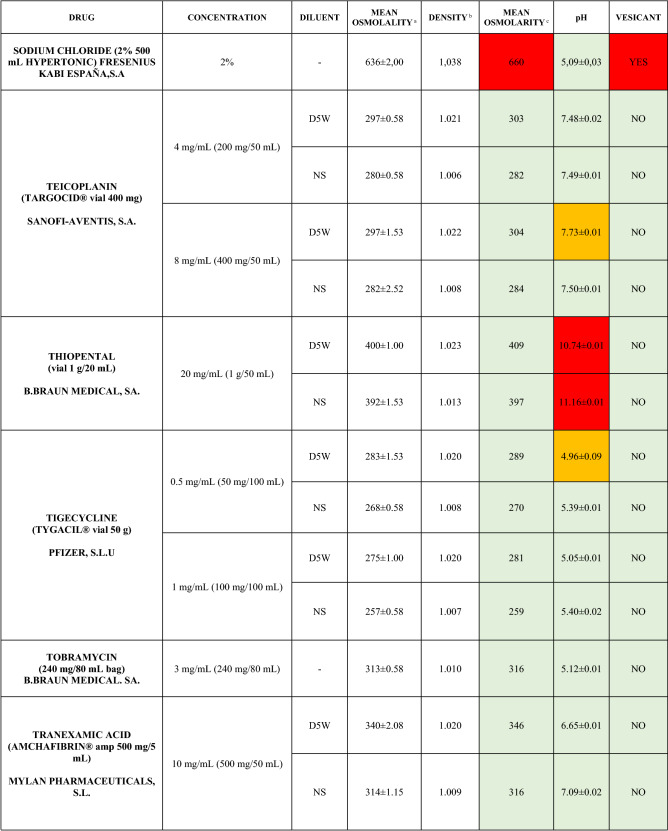
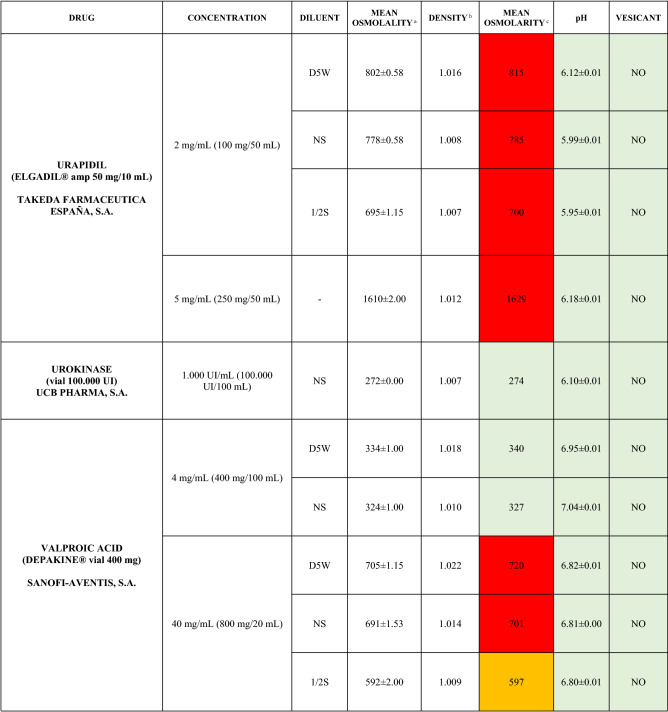
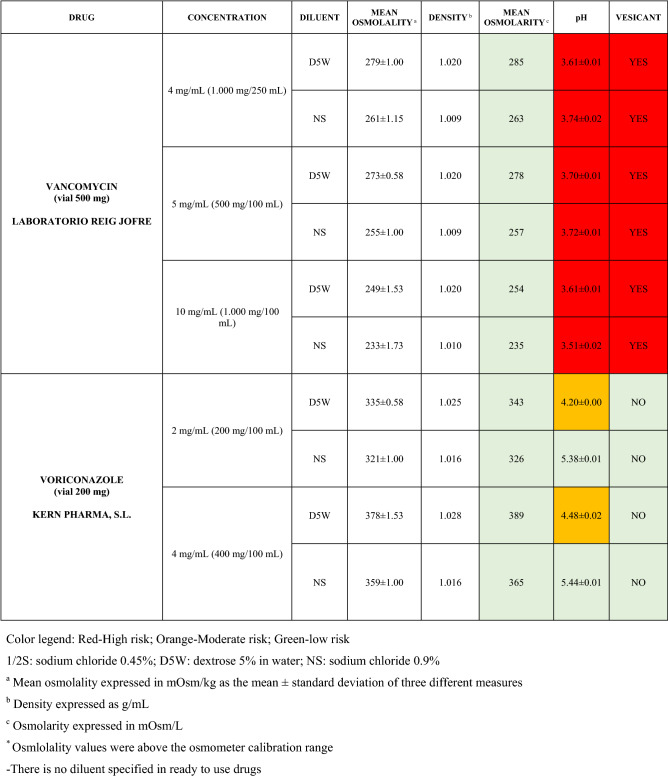
The characteristics of the 106 drugs, corresponding to 183 different concentrations and 307 different admixtures, are shown in Table 2.
Table 2.
Agreed standard concentrations and physicochemical characterization
Most admixtures (281 [91.5%], corresponding to 101 drugs) had an osmolarity <600 mOsm/L. On the other hand, 26 admixtures, corresponding to 15 drugs, had an osmolarity > 600 mOsm/L.
Regarding the pH, 142 admixtures [46.25%], corresponding to 60 drugs, had a pH between 5 and 7.5. However, 68 admixtures [20.15%] corresponding to 27 drugs had an extreme pH < 4 (18 drugs) or > 9 (9 drugs).
Admixtures prepared with D5W had an osmolarity slightly higher than those prepared with NS. The pH was more similar among admixtures prepared with D5W than among those prepared with NS.
Nineteen drugs were categorized as vesicants, irrespective of their concentration, but only eight had extreme pH values (three drugs had at least one admixture with pH values > 9; five drugs had at least one admixture with pH values < 4)
Based on the literature [11, 32, 36, 37] and the experience of this panel of experts, drugs were categorized into different levels of tissue damage risk:
‘high risk’ drugs: osmolarity > 600 mOsm/L, pH <4 or >9, or a vesicant;
‘moderate risk’ drugs: osmolarity 450–600 mOsm/L, or pH 4–5 or 7.5–9 and not a vesicant;
‘low risk’ drugs: osmolarity < 450 mOsm/L, pH 5–7.5 and not a vesicant.
Overall, 123 (40.0%) of the admixtures involving 45 (40%) of the drugs were categorized as ‘high risk’. In contrast, 99 (32.2%) admixtures involving 47 (44.3%) drugs were categorized as ‘low risk’.
To assess the influence of the diluents, Table 2 also shows the change in osmolarity and pH for the same concentrations of drugs that only had osmolarity values > 450 mOsm/L when diluted in sodium chloride 0.45% to yield the same concentration. Some of these drugs are usually delivered through a peripheral line but were classified as moderate or high risk according to their osmolarity and pH values. None of the drugs diluted in sodium chloride 0.45% seemed to be incompatible with this diluent according to our study.
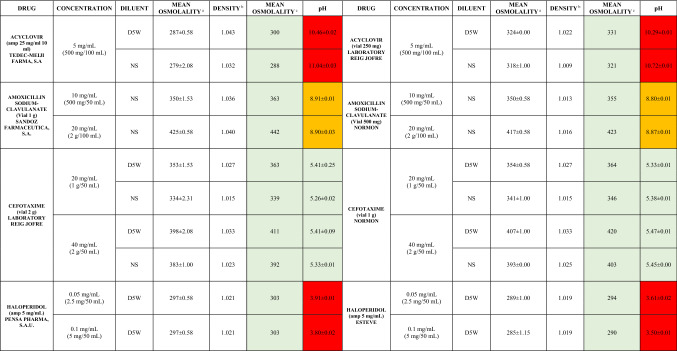
Table 3 shows the comparative osmolarity and pH measurements for the same drugs with different brand names. This approach could only be carried out for acyclovir, amoxicillin/clavulanate, cefotaxime and haloperidol, as these were the only drugs with different brand names for different doses available at the center. All of the paired brands received the same categorization of risk.
Table 3.
Different brand-name drugs comparison
D5W dextrose 5% in water, NS sodium chloride 0.9%
aMean osmolality expressed in mOsm/kg as the mean ± standard deviation of three different measures
bDensity expressed as g/mL
cOsmolarity expressed in mOsm/L
Color legend: Red high risk, Orange moderate risk, Green low risk
Developing an Algorithm for the Catheter Selection
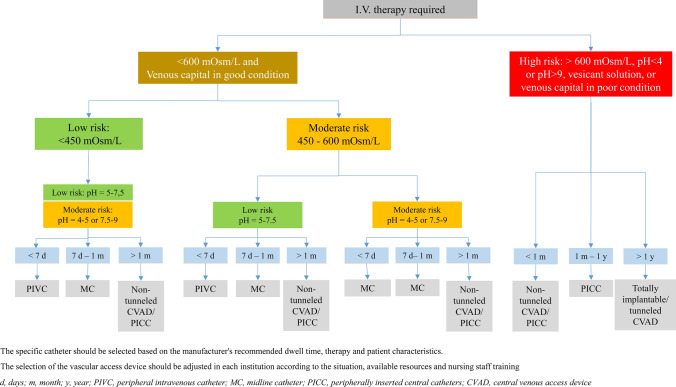
Based on the drugs’ risk classification, the quality of the patients’ venous access, and the duration of the therapy, the group of experts included some specific recommendations regarding osmolarity and pH risk in the general management of VADs and agreed on an updated proposed algorithm for the selection of the venous access, which is presented in Fig. 1.
Fig. 1.
Algorithm for vascular access device selection. The specific catheter should be selected based on the manufacturer's recommended dwell time, therapy and patient characteristics. The selection of the vascular access device should be adjusted in each institution according to the situation, available resources and nursing staff training. CVAD central venous access device, d days, I.V. intravenous, m month, MC midline catheter, PICC peripherally inserted central catheters, PIVC peripheral intravenous catheter, y year
According to this and on a general basis, three different types of catheters can be used:
CVADs are the preferred choice for long-term therapies, patients with difficult venous access, vesicant drugs and infusions with high osmolarity values (> 600 mOsm/L) or extreme pH values (< 4 or > 9). Peripherally inserted central catheters (PICCs), tunneled, non-tunneled and implanted ports could be selected depending on other factors regarding therapy, catheter indications and patient characteristics, that were not the subject of study in this paper.
Short peripheral catheters are the preferred choice for short-term therapies, provided the osmolarity and pH of the infusate are at low risk for at least one of these features and the patient’s venous patrimony is in good condition.
Midline catheters play a role for intermediate length therapies and could also be a suitable choice for drugs with an osmolarity and pH of moderate risk that are intended to be delivered in short course treatments.
Discussion
Standardizing Intravenous Therapy
Intravenous therapy can be administered in a wide range of different settings. Although it is delivered to the vast majority of hospitalized patients, it is well known that intravenous administration is potentially associated with relevant complications [5, 38]. It has been reported that in certain settings, more than half of the adverse drug events are associated with intravenous medications [39, 40], and almost 60% of them occur during the administration phase, mainly due to the use of incorrect intravenous concentrations [40]. Therefore, it is widely recognized that there is a need for standards that may serve as a guide for safe practice to ensure the best patient outcomes [5, 41–43]. Standardization of infusion therapy may reduce variability in clinical practice and minimize the opportunity for errors [44]. Although there is an increasing interest in this strategy among national and international institutions, there is still room for improvement. The Institute for Safe Medication Practices (ISMP) recommends standardization of high-risk intravenous drugs in order to increase safety in this area [45]. In response to the release of this guideline, The American Society of Health-System Pharmacists (ASHP) has become the first professional association to promote a nationwide initiative, known as ‘Standardize 4 Safety’, aimed at achieving the same objective [46].
There are several local groups that have addressed this subject. However, nationwide leadership is needed in order to accomplish this goal. Our study is consistent with this identified need and has been based on the same methodology followed by other international institutions [47].
The drugs and concentrations that finally reached a consensus were those most frequently used in hospitalized adult patients, seemed to cover all possible clinical conditions and are consistent with other concentrations suggested and published in the literature [21, 48]. However, if drug standardization protocols are compared among institutions, differences might be noticed due to variations in procedures, preferences and the availability of different brand drug names that may influence the choice of one drug strength over another.
Limited experience with the five drugs excluded from the study led to a higher variability in possible concentrations that could not reach the consensus threshold and therefore could not be taken into account in the characterization process.
Characterizing the Physicochemical Properties of Standard Therapy
Characterization of the physicochemical properties of standard therapies could provide very useful information that could guide the selection of the most appropriate vascular access for the patient. Despite an increasing body of evidence regarding the management of intravenous therapy, most of the recommendations on this topic are based on a low level of evidence, and the precise role of drugs according to their physicochemical characteristics remain uncertain.
Unfortunately, human tolerance of pH and osmolarity has not been well studied, but general recommendations exist in this regard for minimization or prevention of vascular damage due to extremes of pH or osmolarity [5, 11].
The administration of intravenous solutions that are not isotonic, especially hypertonic solutions, may induce osmotic changes that, in turn, may lead to several adverse events, including erythrocyte destruction, phlebitis and even necrosis at the injection site [26, 49]. Reducing the osmolarity may reduce the risk of thrombophlebitis [50]. Taking osmolarity into account is, therefore, important when preparing medication for intravenous infusion [26]. Although osmolality can be measured without major difficulties, in clinical practice osmolarity is preferred as a measure of the osmotic properties of the solution since it expresses the concentration as a function of volume [11, 26]. For dilute solutions, the difference between osmolarity and osmolality is insignificant, and this is the most common scenario in intravenous therapy.
The labels and literature for products for which osmotic strength is important should state the osmolality, and in many cases, the osmolarity. However, real-world evidence shows these data are often missing in the summary of product characteristics.
The physicochemical properties of the admixtures presented in this work had to be determined experimentally due to the lack of published data, both in the literature and in the labels of the drugs. The data presented in this study have been obtained from pharmaceutical commercial presentations available at the center at the time of the study, which are stated in Table 2.
To date, as far as we know, the work we are presenting is the most extensive study addressing the osmolarities and pH of standard drug concentrations.
In our analysis of the 307 admixtures of 106 drugs, we found that osmolality and osmolarity are almost interchangeable since the density of the solutions was close to 1.0 g/mL (Table 2).
Although the type of infusion fluid may affect the osmolality and pH [51], overall, we found that these parameters did not differ much between admixtures prepared with D5W or NS. Osmolarity was slightly higher in D5W and pH was slightly more acidic in D5W. Differences from theoretical data may be justified due to the non-ideal behavior of solutions that may not completely dissociate and may have interionic attractions or solvations [27]. Therefore, the selection of the preferred infusion fluid should not be based only on these characteristics.
Among drugs with different concentrations, drug dilution did not seem to modify pH in a significant manner.
Most admixtures had an osmolarity < 600 mOsm/L, but when osmolarity was > 450 mOsm/L, changing the NS diluent to hypotonic sodium chloride proved to be a valuable strategy for some drugs in order to reduce their osmolarity and subsequent potential risks. This finding is consistent with other authors’ work demonstrating that for drugs with high osmolarities, 0.45% sodium chloride or sterile water may be used as the diluent for injection [26] (Table 2). None of the drugs diluted in sodium chloride 0.45% seemed to be incompatible with this diluent. However, this study was not aimed to assess drug–diluent compatibility, so in case of lack of information in literature these findings should be interpreted with caution.
The osmolarity that peripheral veins are able to tolerate depends not only on the osmolarity value but also on the infusion rate [52]. Therefore, modifying the infusion rate and diluting the drug further might be effective strategies aimed at reducing the risk of phlebitis associated with infusion solutions [53].
Regarding pH, 68 admixtures (22.2%) corresponding to 27 drugs had a pH < 4 or > 9, which is usually, but not always, associated with the vesicant nature of the drug. Vesicant drugs can also be in the physiological range of pH and osmolarity and still induce tissue damage via alternative mechanisms of toxicity [54]. Very acidic or basic drugs can damage the vein’s delicate inner layer, so proper dilutions and the correct VAD selection are of critical importance.
These results show almost half of the drugs most commonly used in hospitalized adult patients may be delivered at a concentration that might put patients’ venous patrimony at risk, so this is a key point to take into account when selecting the right venous access and the most appropriate VAD in order to minimize potential harm.
To assess the potential influence of different brand-name drugs on osmolarity and pH values, a comparative analysis among five different drugs for which different brand names were available at the center was carried out. Although this subanalysis represents a tiny percentage of all of the drugs included, it seems that changes in brand-name drugs do not alter the risk level assigned to each drug as osmolarity and pH slightly vary.
Although they are not expected to identify important differences between different commercial brands, as shown in Table 3, we should be cautious when interpreting this information.
Selecting VADs
There is literature proposing decision algorithms for the selection of vascular access, mainly taking into account the duration of the therapy, the conditions of the patient's venous patrimony, the osmolarity and the vesicant nature of the solutions to be infused. In this sense, there is unanimity in recommending central catheters for patients with poor vascular access, long treatments and/or hyperosmolar drugs; however, the osmolarity threshold above which a drug is not considered optimal for peripheral infusion, as well as the role of the pH of the intravenous mixtures, are not well defined [33–35].
The current ‘Infusion Therapy Standard of Practice’ considers an osmolarity of 900 mOsm/L as a threshold for selecting central venous access but makes no recommendation on pH [5]. A previous version of this manual recommended an osmolarity threshold value for central venous access of 600 mOsm/L and a pH range of 5–9 [33]. The available literature varies regarding recommendations on the osmolarity limit for solutions suitable for peripheral infusions, and some authors suggest a threshold of approximately 600 mOsm/L [11, 32, 36].
This group of experts, consistent with the recommendations of other authors [55–57], considered a threshold of 450–600 mOsm/L more appropriate for avoiding irritant solutions for peripheral administration. Due to variability in recommendations, it seems reasonable to define different risk levels. Therefore, drugs with an osmolarity value < 450 mOsm/L would be of low risk, moderate risk if osmolarity was 450–600 mOsm/L and high risk if osmolarity was > 600 mOsm/L [11, 36, 55–57].
As far as pH is concerned, experimental studies have suggested that if the pH is not lower than 6.5, peripheral veins are able to tolerate the infusion without phlebitis [58]. The plasma pH is between 7.35 and 7.45; however, because of the plasma's buffering power, it seems reasonable to state that drugs with a pH between 5 and 7.5 can be suitable for peripheral administration.
Although pH is not considered a restrictive factor on its own for the peripheral administration of intravenous medications, some authors believe its influence could be relevant, especially when the pH is <4 or >9 [59, 60]. Taking all this into account, the authors believe it seems reasonable to define three risk levels regarding pH: high risk (pH < 4 or > 9), low risk (pH 5–7.5) and moderate risk for those intermediate situations when pH is 4–5 or 7.5–9 in order to improve the rational and safe use of VADs [11, 32, 36].
The drug nature should also be assessed. It has been proven that vesicant drugs may damage tissues even though their osmolarity and pH values are within a physiological range. Though oncology drugs are well characterized [61], there is no accepted standard for classifying a noncytotoxic solution or medication as a vesicant, and therefore clinicians should rely on the information provided in the summary of product characteristics, case reports and the published literature. The Nurse Infusion Society published a review of vesicant non-cytotoxic drugs with higher evidence in the literature [62] that allowed the authors in this project to identify vesicants drugs in Table 2.
In view of this evidence and controversy, agreeing on different risk levels and including them in classical decision support algorithms might be a useful approach [37].
According to the expert panel and consistent with current evidence, CVADs should be used when delivering drugs with an osmolarity >600 mOsm/L, extreme pH drugs, vesicant drugs, long treatments or patients with a poor vascular access condition [33–35].
Peripheral catheters should be used for short therapies, patients with a good vascular access condition and drugs with osmolarity and/or pH of low-moderate risk. Midline catheters are peripheral catheters inserted into the upper arm via the basilic, cephalic, or brachial vein [5]. They play their part in treatments involving peripherally appropriate solutions that will likely exceed 6 days and for patients requiring infusions of up to 14 days [63, 64]. What this paper adds is that these catheters might be an alternative to short peripheral catheters and a good choice for drugs of a moderate osmolarity and pH risk that are intended to be delivered through a peripheral line for short course treatments.
The suggested algorithm does not differ from the current recommendations regarding peripheral or central access when including risk levels of pH and osmolarity of infusates. However, having a deeper knowledge of the physicochemical properties of therapies can help ensure a safe and suitable decision is made according to the therapy, type of patient and available resources.
This algorithm is a general approach that applies in ideal situations with low complexity patients, availability of resources and trained personnel. If these recommendations cannot be followed due to emergency scenarios, lack of resources or failure to canalize a central venous access safely, some effective strategies that might minimize the potential harm of a vesicant drug or one with extreme osmolarity or pH include assessing the dilution, in order to change the diluent or increase the dilution, and slowing the rate of infusion [5, 59].
Conclusions
Ensuring the safety of intravenous drug administration should be a priority in all health care organizations. It should be noted that when categorizing admixtures based on the pH, osmolarity and vesicant nature of the infusates, it was found that 40% of the admixtures involving one-third of the drugs were categorized as ‘high risk’. This highlights the importance of properly characterizing intravenous solutions and medications in order to guarantee patient safety and preserve their venous patrimony. Having tools that allow health professionals to know the characteristics of the drugs to be administered and to assess the risk involved in their administration in relation to other possible patient-related factors can be useful to guide decision making regarding the most suitable type of vascular access and device of choice in each particular case.
Acknowledgments
The authors thank all healthcare professionals that participated in this study for their involvement in the project. Thanks to Becton Dickinson, S.A. for supporting the study. The corresponding author would like to acknowledge the work of Fernando Rico-Villademoros for his help in writing and editing this manuscript.
Declarations
Funding
This study was funded by Sociedad Española de Farmacia Hospitalaria.
Conflicts of interest/Competing interests
Author Irene Heras-Hidalgo has received a research grant from Becton Dickinson S.A.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
Authors’ contributions
Conceptualization: SMR, AHA, MSS. Formal analysis and investigation: SMR, IHH, MSPL, MDCRP, MBSM, MACP; VVR, NCR, ELC, MDCMO, EBL, CDS, PLS, MLGC, RAM, JFMP, MVR, EDC, IAG, IPA, MHG, FGV, PRS, EGP, LJFS, SFC, CLP, LGS, MJA. Writing: SMR. Review and editing: IHH, AHA, MSS, MDCRP, MBSM, MACP, MDCMO, MCMD. Funding acquisition: AHA.
Ethics approval
Because data related to patient management was not the subject of this study, the Hospital Ethics Committee exempted the project from approval.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
The original online version of this article was revised. The units of potassium chloride in Table 2 should are incorrect. Instead of 0,04 mEq/L, 0,06 mEq/L and 0,2 mEq/L, it should read as 0,04 mEq/mL, 0,06 mEq/mL and 0,2 mEq/mL. Norepinephrine: It says 80 µg/mL (40 mg/50 mL) and it should read as 80 µg/mL (40 mg/500 mL)
Change history
6/7/2021
A Correction to this paper has been published: 10.1007/s40268-021-00347-2
References
- 1.Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs. 2012;35:84–91. doi: 10.1097/NAN.0b013e31824237ce. [DOI] [PubMed] [Google Scholar]
- 2.Zingg W, Pittet D. Peripheral venous catheters: an under-evaluated problem. Int J Antimicrob Agents. 2009;34(Suppl 4):S38–42. doi: 10.1016/S0924-8579(09)70565-5. [DOI] [PubMed] [Google Scholar]
- 3.Doyle GR, McCutcheon A. Clinical procedures for safer patient care. Victoria: BCcampus; 2018. [PubMed] [Google Scholar]
- 4.Mattox EA. Complications of peripheral venous access devices: prevention, detection, and recovery strategies. Crit Care Nurse. 2017;37:e1–14. doi: 10.4037/ccn2017657. [DOI] [PubMed] [Google Scholar]
- 5.Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy. Standard of practice. J Infus Nurs. 2016;39:S1–159. [Google Scholar]
- 6.Frank RL. Peripheral venous access in adults. UpToDate. 2020.
- 7.Jamshidi R. Central venous catheters: indications, techniques, and complications. Semin Pediatr Surg. 2019;28:26–32. doi: 10.1053/j.sempedsurg.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Gordy S, Rowell S. Vascular air embolism. Int J Crit Illn Inj Sci. 2013;3:73–76. doi: 10.4103/2229-5151.109428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado-Capel M, Capdevila-Morell JA, Sauca-Subias G, Ballester-Joya L, Vidal-Diez E, Yebenes-Reyes JC. Incidence of catheter-related bloodstream infection in a general hospital using two different detection methods. Enferm Infecc Microbiol Clin. 2012;30:613–617. doi: 10.1016/j.eimc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Lv L, Zhang J. The incidence and risk of infusion phlebitis with peripheral intravenous catheters: a meta-analysis. J Vasc Access. 2020;21:342–349. doi: 10.1177/1129729819877323. [DOI] [PubMed] [Google Scholar]
- 11.Stranz M, Kastango ES. A Review of pH and osmolarity. Int J Pharm Compd. 2002;6:216–220. [PubMed] [Google Scholar]
- 12.Reynolds PM, MacLaren R, Mueller SW, Fish DN, Kiser TH. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617–632. doi: 10.1002/phar.1396. [DOI] [PubMed] [Google Scholar]
- 13.Loubani OM, Green RS. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. J Crit Care. 2015;30(653):e9–17. doi: 10.1016/j.jcrc.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Jackson-Rose J, Del Monte J, Groman A, Dial LS, Atwell L, Graham J, O'Neil Semler R, O'Sullivan M, Truini-Pittman L, Cunningham TA, Roman-Fischetti L, Costantinou E, Rimkus C, Banavage AJ, Dietz B, Colussi CJ, Catania K, Wasko M, Schreffler KA, West C, Siefert ML, Rice RD. Chemotherapy extravasation: establishing a national benchmark for incidence among cancer centers. Clin J Oncol Nurs. 2017;21:438–445. doi: 10.1188/17.CJON.438-445. [DOI] [PubMed] [Google Scholar]
- 15.Schulmeister L. Extravasation management: clinical update. Semin Oncol Nurs. 2011;27:82–90. doi: 10.1016/j.soncn.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Sanborn RE, Sauer DA. Cutaneous reactions to chemotherapy: commonly seen, less described, little understood. Dermatol Clin. 2008;26(103–19):ix. doi: 10.1016/j.det.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Viale PH. Chemotherapy and cutaneous toxicities: implications for oncology nurses. Semin Oncol Nurs. 2006;22:144–151. doi: 10.1016/j.soncn.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Gallieni M, Pittiruti M, Biffi R. Vascular access in oncology patients. CA Cancer J Clin. 2008;58:323–346. doi: 10.3322/CA.2008.0015. [DOI] [PubMed] [Google Scholar]
- 19.Trissel LA. Handbook on injectable drugs. Bethesda: American Society of Health-System Pharmacists; 2009. [Google Scholar]
- 20.IBM micromedex. 2020. http://www.micromedexsolutions.com/. Accessed Sept 2020.
- 21.Nottingham University Hospitals. Pharmacy drug guidelines folder. 2017. https://studylib.net/doc/25267078/critical-care-pharmacy-drug-guidelines. Accessed Sept 2020.
- 22.Phillips MS. Standardizing i.v. infusion concentrations: National survey results. Am J Health Syst Pharm. 2011;68:2176–2182. doi: 10.2146/ajhp110001. [DOI] [PubMed] [Google Scholar]
- 23.Castells Lao G, Rodriguez Reyes M, Roura Turet J, Prat Dot M, Soy Muner D, Lopez CC. Compatibility of drugs administered as Y-site infusion in intensive care units: a systematic review. Med Intensiva. 2020;44:80–87. doi: 10.1016/j.medin.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Stabilis®. Stabilité et compatibilité des médicaments. 2020. https://www.stabilis.org/. Accessed Sept 2020.
- 25.Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67:401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Wermeling DP, Rapp RP, DeLuca PP, Piecoro JJ., Jr Osmolality of small-volume intravenous admixtures. Am J Hosp Pharm. 1985;42:1739–1744. [PubMed] [Google Scholar]
- 27.Deardorff DL. Osmotic strength, osmolality, and osmolarity. Am J Hosp Pharm. 1980;37:504–509. [PubMed] [Google Scholar]
- 28.Agencia Española de Medicamentos y Productos Sanitarios. Centro de información online de medicamentos de la AEMPS - CIMA. 2020. https://cima.aemps.es/cima/publico/home.html. Accessed Sept 2020.
- 29.The Infusion Nurses Society (INS). Noncytotoxic vesicant medications and solutions. 2020. https://www.learningcenter.ins1.org/products/noncytotoxic-vesicant-medications-and-solutions. Accessed Sept 2020.
- 30.Sou V, McManus C, Mifflin N, Frost SA, Ale J, Alexandrou E. A clinical pathway for the management of difficult venous access. BMC Nurs. 2017;16:64. doi: 10.1186/s12912-017-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Registered Nurses' Association of Ontario. Care and maintenance to reduce vascular access complications. 2005. https://rnao.ca/bpg/guidelines/care-and-maintenance-reduce-vascular-access-complications. Accessed Sept 2020.
- 32.Sociedad Española de Medicina Preventiva, Salud Pública e Higiene. Proyecto piloto multicéntrico estrategia multifactorial "flebitis zero" - resumen. 2020. https://www.sempsph.com/es/noticias/calidad-seguridad-y-gestion/proyecto-piloto-multicentrico-estrategia-multifactorial-qflebitis-zeroq-resumen.html. Accessed Sept 2020.
- 33.Infusion Nurses Society. Infusion nursing standards of practice. 2020. incativ.es/documentos/guias/INS_Standards_of_Practice_2011[1].pdf. Accessed Sept 2020.
- 34.Alexander M. Infusion nursing. An evidence-based approach. St Louis: Saunders; 2010. [Google Scholar]
- 35.Infusion Nurses Society. Policies and procedures for infusion therapy. Norwood, MA: Infusion Nurses Society (INS); 2016.
- 36.Suárez Mier B, Carmen Martínez Ortega C. Prevención de complicaciones relacionadas con accesos vasculares de inserción periférica. Programa Flebitis Zero. Madrid, Spain: Agencia Española de Medicamentos y Productos Sanitarios (AEMPS); 2019.
- 37.Carballo M, Llinas M, Feijoo M. Flebitis en catéteres periféricos. Incidencia y factores de riesgo. ROL Enferm. 2004;27:585–592. [PubMed] [Google Scholar]
- 38.Lyons I, Furniss D, Blandford A, Chumbley G, Iacovides I, Wei L, Cox A, Mayer A, Vos J, Galal-Edeen GH, Schnock KO, Dykes PC, Bates DW, Franklin BD. Errors and discrepancies in the administration of intravenous infusions: a mixed methods multihospital observational study. BMJ Qual Saf. 2018;27:892–901. doi: 10.1136/bmjqs-2017-007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, Goldmann DA. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 40.Ross LM, Wallace J, Paton JY. Medication errors in a paediatric teaching hospital in the UK: five years operational experience. Arch Dis Child. 2000;83:492–497. doi: 10.1136/adc.83.6.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nickel B. Peripheral intravenous access: applying infusion therapy standards of practice to improve patient safety. Crit Care Nurse. 2019;39:61–71. doi: 10.4037/ccn2019790. [DOI] [PubMed] [Google Scholar]
- 42.Keeling P, Scales K, Keeling S, Borthwick M. Towards IV drug standardization in critical care. Br J Nurs. 2010;19:S30–S33. doi: 10.12968/bjon.2010.19.Sup9.79313. [DOI] [PubMed] [Google Scholar]
- 43.Bullock J, Jordan D, Gawlinski A, Henneman EA. Standardizing IV infusion medication concentrations to reduce variability in medication errors. Crit Care Nurs Clin North Am. 2006;18:515–521. doi: 10.1016/j.ccell.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Manrique-Rodríguez S, Fernández-Llamazares CM. Standardization for safety: a feasible challenge. Farm Hosp. 2020;44:79–80. doi: 10.7399/fh.11448. [DOI] [PubMed] [Google Scholar]
- 45.Instituto Para el Uso Seguro de los Medicamentos. Instituto Para el Uso Seguro de los Medicamentos. Delegación española del Institute for Safe Medication Practices (ISMP). 2020. http://www.ismp-espana.org/. Accessed Sept 2020.
- 46.American Society of Hospital Pharmacists. Standardize 4 safety. 2016. https://www.ashp.org/-/media/assets/pharmacy-practice/s4s/docs/s4s-iv-adult-continuous-infusion-guiding-principles.ashx. Accessed Sept 2020.
- 47.Sutherland A, Christiansen N, Wignell A, Harris D. Fixed concentration infusions. A national consensus for paediatric and neonatal care in the United Kingdom. Manchester, United Kingdom; 2017.
- 48.Walroth TA, Dossett HA, Doolin M, McMichael D, Reddan JG, Degnan D, Fuller J. Standardizing concentrations of adult drug infusions in Indiana. Am J Health Syst Pharm. 2017;74:491–497. doi: 10.2146/ajhp151018. [DOI] [PubMed] [Google Scholar]
- 49.Santeiro ML, Sagraves R, Allen LV., Jr Osmolality of small-volume i.v. admixtures for pediatric patients. Am J Hosp Pharm. 1990;47:1359–1364. [PubMed] [Google Scholar]
- 50.Madan M, Alexander DJ, Mellor E, Cooke J, McMahon MJ. A randomised study of the effects of osmolality and heparin with hydrocortisone on thrombophlebitis in peripheral intravenous nutrition. Clin Nutr. 1991;10:309–314. doi: 10.1016/0261-5614(91)90059-l. [DOI] [PubMed] [Google Scholar]
- 51.Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer's solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88:999–1003. doi: 10.1097/00000539-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Kuwahara T, Asanami S, Kubo S. Experimental infusion phlebitis: tolerance osmolality of peripheral venous endothelial cells. Nutrition. 1998;14:496–501. doi: 10.1016/s0899-9007(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 53.Kuwahara T, Asanami S, Tamura T, Kubo S. Dilution is effective in reducing infusion phlebitis in peripheral parenteral nutrition: an experimental study in rabbits. Nutrition. 1998;14:186–190. doi: 10.1016/s0899-9007(97)00440-1. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez-Garcia C, Mata-Peon E, Avanzas-Fernandez S. Related factors with extravasation of non-cytostatic agents in peripheral vein catheters. Enferm Clin. 2017;27:71–78. doi: 10.1016/j.enfcli.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Wojnar DG, Beaman ML. Peripherally inserted central catheter: compliance with evidence-based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36:291–296. doi: 10.1097/NAN.0b013e318297c1a8. [DOI] [PubMed] [Google Scholar]
- 56.Hallam C, Weston V, Denton A, Hill S, Bodenham A, Dunn H, Jackson T. Development of the UK Vessel Health and Preservation (VHP) framework: a multi-organisational collaborative. J Infect Prev. 2016;17:65–72. doi: 10.1177/1757177415624752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pittiruti M, Hamilton H, Biffi R, MacFie J, Pertkiewicz M, ESPEN ESPEN guidelines on parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications) Clin Nutr. 2009;28:365–377. doi: 10.1016/j.clnu.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Kuwahara T, Asanami S, Kawauchi Y, Kubo S. Experimental infusion phlebitis: tolerance pH of peripheral vein. J Toxicol Sci. 1999;24:113–121. doi: 10.2131/jts.24.113. [DOI] [PubMed] [Google Scholar]
- 59.Gorski LA, Hagle ME, Bierman S. Intermittently delivered IV medication and pH: reevaluating the evidence. J Infus Nurs. 2015;38:27–46. doi: 10.1097/NAN.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 60.Kokotis K. Preventing chemical phlebitis. Nursing. 1998;28:41–46. doi: 10.1097/00152193-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 61.Magallon-Pedrera I, Perez-Altozano J, Virizuela Echaburu JA, Beato-Zambrano C, Borrega-Garcia P, de la Torre-Montero JC. ECO-SEOM-SEEO safety recommendations guideline for cancer patients receiving intravenous therapy. Clin Transl Oncol. 2020 doi: 10.1007/s12094-020-02347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorski LA, Stranz M, Cook LS, Joseph JM, Kokotis K, Sabatino-Holmes P, Van Gosen L, Infusion Nurses Society Vesicant Task Force Development of an evidence-based list of noncytotoxic vesicant medications and solutions. J Infus Nurs. 2017;40:26–40. doi: 10.1097/NAN.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 63.Adams DZ, Little A, Vinsant C, Khandelwal S. The midline catheter: a clinical review. J Emerg Med. 2016;51:252–258. doi: 10.1016/j.jemermed.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 64.Moureau N, Chopra V. Indications for peripheral, midline and central catheters: summary of the MAGIC recommendations. Br J Nurs. 2016;25:S15–24. doi: 10.12968/bjon.2016.25.8.S15. [DOI] [PubMed] [Google Scholar]