Abstract
Immune checkpoint inhibitors (ICIs) are considered a new standard-of-care across many cancer indications. This review provides an update on ICIs approved by the Food and Drug Administration (FDA), with focus on monoclonal antibodies that target the programmed cell death 1 (PD-1) or its ligand, PD-1 ligand 1 (PD-L1), including information on their clinical indications and associated companion diagnostics. The information is further discussed with strategies for identifying predictive biomarkers to guide the clinical use of PD-1/PD-L1-targeted therapies.
KEY WORDS: cancer immunotherapy, immune checkpoint inhibitor, biomarker, companion diagnostic
INTRODUCTION
The development of cancer immunotherapies, harnessing the immune system to restore anti-tumor immunity, has transformed the treatment of certain cancers. The first immune checkpoint inhibitor (ICI), an antibody targeting the cytotoxic T lymphocyte antigen 4 (CTLA4), was approved by the Food and Drug Administration in 2011 (1, 2). Since then, six more ICIs have been approved by the FDA, exclusively targeting the T cell co-inhibitory programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) signaling pathway (3, 4), with clinical indications across 19 different cancer types and two tissue-agnostic conditions (Fig. 1). While there is great promise in ICIs, only a small population of patients achieve a durable response to monotherapy. As a result, predictive biomarkers are used to “identify individuals who are more likely than similar individuals without the biomarker to experience a favorable or unfavorable effect from exposure to a medical product or an environmental agent.” (5) These markers, which are measured using validated in vitro assays, can aid in the enrichment of a patient population for clinical trials and for stratification of biomarker-positive and -negative patients. PD-L1 status on immune cells or tumor cells was considered to be one of the first potential predictive biomarkers for response to ICI treatment (6). Three of these approved ICIs targeting the PD-1/PD-L1 pathway (Keytruda (pembrolizumab), Opdivo (nivolumab), and Tecentriq (atezolizumab)) require the measurement of PD-L1. Identifying the appropriate biomarkers for these products requires understanding their mechanisms of action (MOAs) and tumor pathophysiology in individual patients with specific tumor types. This review will provide an update on the regulatory approvals of anti-PD-1/PD-L1 therapeutics along with their companion and complementary diagnostic devices.
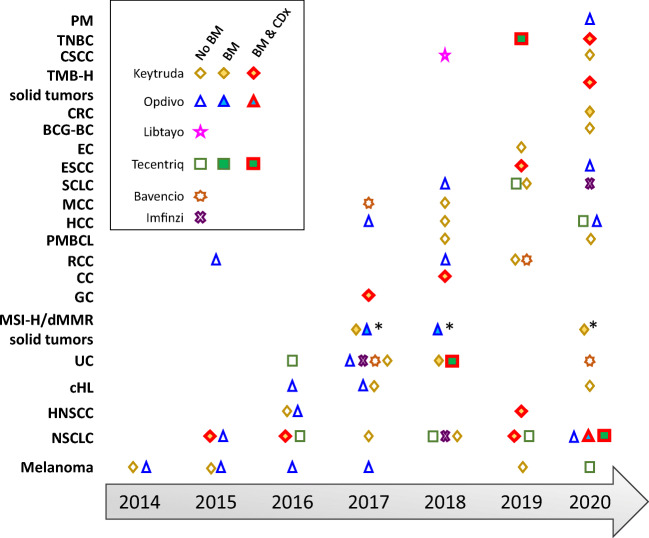
Fig. 1.
FDA approvals of PD-1/PD-L1 mAbs. As of December 2020, six anti-PD-1/PD-L1 mAbs have been approved with supplemental indications across 19 cancer types and two tissue-agnostic conditions. Shown are the approvals for each cancer indication, for Keytruda (pembrolizumab), Opdivo (nivolumab), Libtayo (cemiplimab), Tecentriq (atezolizumab), Bavencio (avelumab), and Imfinzi (durvalumab). Multiple approvals for a cancer indication within the same year are shown with only one symbol. The open symbols represent approvals without a biomarker (no BM). The full symbols represent approvals that incorporate a biomarker with an associated threshold for each indication (BM), which was measured using either a central laboratory test or complementary diagnostic that was not approved as a CDx for the drug. Symbols with a red outline represent approvals in which a companion diagnostic is indicated for biomarker measurement (BM + CDx). *: approval for MSI-H/dMMR colorectal cancer. PM, pleural mesothelioma; TNBC, triple-negative breast cancer; CSCC, cutaneous squamous cell carcinoma; TMB-H, tumor mutation burden high; CRC, colorectal cancer; BCG-BC, Bacillus Calmette-Guérin bladder cancer; EC, endometrial carcinoma; ESCC, esophageal squamous cell carcinoma; SCLC, small cell lung cancer; RCC, renal cell carcinoma; MCC, Merkel cell carcinoma; HCC, hepatocellular carcinoma; PMBCL, primary mediastinal large B cell lymphoma; CC, cervical cancer; GC, gastric cancer; MSI-H, microsatellite instability high; dMMR, mismatch repair-deficient; UC, urothelial carcinoma; cHL, classical Hodgkin’s lymphoma; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer. Information on approvals and supplemental approvals was gathered from Drugs@FDA
FDA-APPROVED ANTI-PD-1/PD-L1 THERAPIES
The standard of care for several cancer types currently includes treatment with monoclonal antibodies (mAbs) specific to PD-1 or PD-L1. PD-1 (CD279) is a co-inhibitory transmembrane protein that is expressed on antigen-stimulated T and B lymphocytes, natural killer (NK) cells, and myeloid suppressor dendritic cells (MDSCs). Following recognition of antigens or stimulation from cytokines, PD-1 is activated as a mechanism to modulate the intensity of the immune response (7). The engagement of PD-1 with its cognate ligands PD-L1 (B7-H1) or PD-L2 (B7-DC), which are widely expressed on tumor cells, results in the inhibition of T cell activation or proliferation and subsequently T cell exhaustion (3, 7, 8). While ICIs have demonstrated improved clinical efficacy, only a small proportion of patients respond to single-agent treatment. PD-L1 protein expression was the primary immuno-oncology biomarker, with the expression on immune cells and tumor cells being evaluated and quantified using immunohistochemistry (IHC) assays. The debate on whether PD-L1 expression levels are predictive of a response has been assessed through prospective or retrospective analysis, resulting in many ICI approvals with biomarker-independent treatment indications (1, 3). There remains a lack of universal predictive biomarker for patient selection for ICI treatment.
Anti-PD-1 mAbs
Three anti-PD-1 antibodies have been approved by the FDA: pembrolizumab (Keytruda), nivolumab (Opdivo), and cemiplimab (Libtayo).
Pembrolizumab (Keytruda)
Pembrolizumab, a humanized IgG4 antibody against PD-1, was initially approved by the FDA in September 2014 following results from the KEYNOTE-001 clinical trial (NCT01295827), studying patients with unresectable or metastatic melanoma and patients with non-small cell lung cancer (NSCLC). These cancer types were chosen as there were previously seen high levels of PD-L1 expression (9, 10). The approval was specified for the treatment of patients with unresectable or metastatic melanoma and disease progression after receiving ipilimumab and, in patients with BRAFV600 mutation, a BRAF inhibitor (11). Improvements were seen in overall response rate (ORR) and duration of response (12). This was later expanded to include treatment of patients with melanoma with involvement of lymph nodes following complete resection.
The incorporation of threshold inclusion criteria based on the expression level of PD-L1 protein was approved in 2015, for the treatment of patients with PD-L1-positive NSCLC as determined by an FDA-approved test along with the approval of the PD-L1 IHC 22C3 pharmDx (Dako). In the NSCLC cohort of the trial, patients were analyzed for their PD-L1 tumor proportion score (TPS), which is the percentage of tumor cells that express PD-L1 identified using IHC analysis (13, 14). Patients were separated into cohorts based on expression levels of < 1% TPS, 1–49% TPS, and ≥ 50% TPS, and considered positive if they had a TPS ≥ 1% (15). Patients with a TPS ≥ 1% had an increased ORR compared to those < 1%, with the highest benefit in the patients with ≥ 50% TPS (13, 14). The indication for metastatic NSCLC was expanded in 2016, to include patients with TPS ≥ 1% with disease progression on or after platinum-containing chemotherapy and metastatic NSCLC with high PD-L1 expression (TPS ≥ 50%) with no EGFR or ALK genomic tumor aberrations, and no prior systemic chemotherapy treatment. An improved overall survival rate was seen in patients with high PD-L1 expression (16, 17). Not all lung cancer indications require a PD-L1 protein measurement, including the first-line treatment of patients with squamous or non-squamous NSCLC as a single agent or in combination with carboplatin and either paclitaxel or nab-paclitaxel (18, 19) or patients with SCLC with disease progression on or after platinum-based chemotherapy, and at least one other prior line of therapy (20, 21). In these clinical studies, patients demonstrated benefit regardless of the level of PD-L1 expression.
In 2016, pembrolizumab was approved for the treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck (HNSCC). In 2019, an additional indication for HNSCC was approved, for patients whose tumors express PD-L1 for a combined positive score of more than 1 (CPS ≥ 1) as determined by an FDA-approved test (22). The CPS determines the amount of PD-L1-positive cells that are within the tumor, including the tumor cells, lymphocytes, and macrophages relative to the total viable cell counts. Patients with a positive PD-L1 expression (CPS > 1) derived benefit, and those patients who expressed a CPS > 20 were found to have the most benefit, with an increase in OS when treated with pembrolizumab with chemotherapy compared to cetuximab with chemotherapy (22). Approvals for indications for locally advanced or metastatic gastric or gastroesophageal junction carcinoma (23–25) and recurrent or metastatic cervical cancer (26, 27) both require the determination of a PD-L1 score of CPS ≥ 1 for treatment, while the approvals for locally advanced or metastatic urothelial carcinoma (28), locally advanced or metastatic squamous cell carcinoma of the esophagus (29), and locally recurrent unresectable or metastatic triple-negative breast cancer (TNBC) require the determination of a PD-L1 score of CPS ≥ 10 for treatment (30–32).
In 2017, a novel indication was approved, which included any solid tumor which had microsatellite instability (MSI-H) or dMMR status. This approval was the first time a cancer treatment was approved based on a common biomarker across cancer types, regardless of the cancer of origin (33). The data was collected from single-arm cohorts of clinical trials, for a pooled analysis, in which patient samples were analyzed retrospectively using a central laboratory-developed PCR test, and patients with an MSI-H had a significantly higher ORR and increased duration of response compared to patients with microsatellite-stable (MSS) tumors (25, 33). This was expanded to include patients with unresectable or metastatic, MSI-H or dMMR solid tumors or metastatic MSI-H, or dMMR colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan (34). Mutations in the mismatch repair genes (MLH1, MSH2, MSH6, and PMS2) can lead to MSI due to errors in the DNA microsatellites. Tumors with high levels of mismatch repair mutations are commonly associated with higher levels of neoantigen production (33), rendering the tumors susceptible to the ICI therapy.
In 2020, a new indication was added with a companion diagnostic, for the treatment of patients with tumor mutational burden high (TMB-H) cancer, as determined by an FDA-approved test (35, 36). TMB is defined by the number of somatic mutations per megabase (Mb) across an interrogated genomic sequence (35). Within the Keynote-158 (NCT02628067) clinical trial, retrospective analysis was performed on tumor samples and the TMB of ≥ 10 or ≥ 13 mutations (mut) per Mb was analyzed by the Foundation One CDx (36). Patients with TMB-H (≥ 10 mut/Mb) were found to have an ORR of 29% and patients with TMB ≥ 13 mut/Mb achieved an ORR of 37%. The higher mutational burden within a tumor is expected to correspond to a higher level of immunogenic neopeptides that would drive T cell-mediated anti-tumor immunity (35–37).
Several additional indications without biomarker requirements were approved over the past 5 years, including indications for the treatment of adult and pediatric patients with refractory classical Hodgkin’s lymphoma (38, 39), locally advanced or metastatic urothelial carcinoma for patients who are not eligible for cisplatin-containing chemotherapy or who have had disease progression during or following platinum-containing chemotherapy (40, 41), mediastinal large B cell lymphoma (42, 43), hepatocellular carcinoma (44), Merkel cell carcinoma (45, 46), patients with advanced renal cell carcinoma, recurrent or metastatic cutaneous squamous cell carcinoma (22, 47), and patients with Bacillus Calmette-Guerin unresponsive, high-risk, non-muscle invasive bladder cancer (48).
Nivolumab (Opdivo)
Nivolumab, an IgG4 mAb against PD-1, was approved following the pivotal trial CheckMate-037, on December 22, 2014, for the treatment of patients with unresectable of metastatic melanoma who have experienced disease progression following ipilimumab and, if BRAFV600 mutation-positive, a BRAF inhibitor. Between 2015 and 2020, new indications were approved for the treatment of patients with metastatic NSCLC with progression on or after platinum-based chemotherapy (49, 50), for treatment in combination with ipilimumab or as a single agent for unresectable or metastatic melanoma patients (51), for the treatment of patients with advanced renal cell carcinoma (52), classical Hodgkin’s lymphoma (53), recurrent or metastatic squamous cell carcinoma of the head and neck (HNSCC) (54), locally advanced or metastatic urothelial carcinoma, hepatocellular carcinoma (55), metastatic SCLC (56), metastatic or recurrent NSCLC (57), esophageal squamous cell carcinoma (ESCC) (58), and for patients with unresectable malignant pleural mesothelioma (59, 60).
In the CheckMate 017 phase 3 clinical trial studying squamous cell NSCLC, PD-L1 expression ( ≥ 1%, ≥ 5%, ≥ 10%) was used for retrospective analysis and stratification to determine efficacy, though expression levels were not found to be prognostic or predictive of benefit (49, 61). In the CheckMate 057 and CheckMate 063, similar retrospective stratification was performed, which demonstrated that PD-L1 expression was predictive of benefit to treatment with nivolumab (61, 62). PD-L1 positivity was determined as ≥ 5%, as previous studies did not distinguish a greater response when a threshold of 1% was used (50). Patients who had PD-L1-positive tumors had more objective response compared to patients with PD-L1-negative tumors, though this was not considered to be significant due to sample size.
The CheckMate 275 clinical trial, studying nivolumab as a first-line treatment of patients with metastatic or surgically unresectable urothelial carcinoma, determined PD-L1 expression at screening of patients using the Dako PD-L1 IHC 28-8 pharmDx kit, though this was not used as inclusion criteria (63). Patients experienced benefit from the treatment, irrespective of PD-L1 expression (63). Patient samples were further evaluated in the 2-year follow-up for novel biomarker discovery, in which retrospective analysis demonstrated that patients with higher TMB had improved ORR and OS, which was further improved when TMB was combined with PD-L1 status (64). TMB stratification was divided into three groups, with low < 85, medium 85–169, and high ≥ 170 missense somatic mutations per tumor (64). The TMB levels and PD-L1 expression were not correlated.
PD-L1 status was used to stratify the patients with resected stage IIIB–C or stage IV melanoma in the CheckMate 238 (51). Patients were randomized to receive either ipilimumab or nivolumab and stratified based on disease stage and PD-L1 status (≥ 5% of tumor cells compared to < 5 % or indeterminate staining). Recurrence-free survival was higher in patients with a higher PD-L1 expression, though all patients experienced greater benefit when treated with nivolumab compared to ipilimumab (51). In May 2020, treatment with nivolumab in combination with ipilimumab was approved for first-line treatment of patients with metastatic or recurrent NSCLC with no EGFR or ALK genomic tumor aberrations (65). Patients were enrolled in the CheckMate 9LA trial regardless of PD-L1 status, and randomized to receive nivolumab with ipilimumab and chemotherapy or chemotherapy alone, with cohorts stratified based on PD-L1 status (< 1% vs ≥ 1%) (57). Clinical benefit was seen across all groups that were treated with the ICI combination, regardless of biomarker status. Stratification by biomarker was also performed in the Attraction-3 clinical trial, in which patients with unresectable advanced, recurrent, or metastatic ESCC were enrolled regardless of PD-L1 status. PD-L1 expression was determined by the PD-L1 IHC 28-8 pharmDx assay at a central testing laboratory, and patients were randomized using PD-L1 ≥ 1% or < 1% or indeterminate staining. No clinical benefit was seen that was dependent on PD-L1 status, with all patients treated with nivolumab having significant improvement in OS (58). In May 2020, the inclusion of a biomarker was approved for nivolumab in the treatment of adult patients with metastatic or recurrent NSCLC whose tumors express PD-L1 (≥ 1%) as determined by an FDA-approved test (66). PD-L1 status was measured using the PD-L1 IHC 28-8 pharmDx assay and patients were randomized 1:1:1 to receive either nivolumab plus ipilimumab, nivolumab monotherapy, or chemotherapy. Patients with PD-L1-positive tumors treated with the combination treatment reported a longer overall survival rate and a longer median duration of response (66). The addition of the biomarker inclusion criteria was accompanied by the approval of the PD-L1 IHC 28-8 pharmDx assay as a companion diagnostic for the indication of Opdivo (67). Patient samples were also screened for tumor mutational burden for exploratory biomarker analysis, though no correlation was seen for TMB-high vs. TMB-low with overall survival benefit. TMB and PD-L1 status also did not report a correlation with benefit in this patient population (66).
Indications for nivolumab treatment for patients with MSI-H or dMMR metastatic colorectal cancer as a single agent were approved in 2017 and in combination with ipilimumab in 2018 (68). MSI/dMMR status was determined either by PCR or IHC using a central testing laboratory assay. PD-L1 status was also determined using the Dako 28-8 pharmDx assay (≥ 1% or < 1%) (68, 69). Improved rate of disease control and ORR was reported for patients with dMMR/MSI-H when treated with nivolumab, regardless of PD-L1 status (68).
Cemiplimab-rwlc (Libtayo)
Cemiplimab is a human IgG4 anti-PD-1 mAb that was approved in 2018 for the treatment of patients with metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC who are not candidates for curative surgery or radiation (70). This cancer type was studied due to known high mutational burden. A response was seen in half of the patients in the pivotal phase II study with an acceptable safety profile (70, 71).
Anti-PD-L1 mAbs
Three anti-PD-L1 antibodies have been approved by the FDA: atezolizumab (Tecentriq), durvalumab (Imfinzi), and avelumab (Bavencio).
Atezolizumab (Tecentriq)
Atezolizumab, a humanized anti-PD-L1 mAb, was approved in 2016 for the treatment of patients with advanced or metastatic urothelial carcinoma. PD-L1 expression was evaluated on tumor specimens prospectively using the Ventana PD-L1 (SP142) Assay, using the threshold cutoff of more than 5% of the tumor area having PD-L1-positive tumor-infiltrating immune cells (IC) (72). This threshold only includes the PD-L1 positivity of the immune cells within the tumor microenvironment. PD-L1 expression was defined based on expression status of the immune cells and separated into cohorts of IC0 (< 1%), IC1 (≥ 1% but < 5%), and IC2/3 (≥ 5%). Response rate was seen to correlate with the increased expression of PD-L1 status on ICs (72). Genomic profiling was also conducted for exploratory biomarker analysis using the FoundationOne panel (72). Treatment with atezolizumab resulted in improved survival, with higher levels of PD-L1 expression on immune cells, though not with tumor cells, or higher TMB associated with higher response rate (73, 74). PD-L1 expression was then incorporated into FDA labeling in 2018 following the IMvigor210 clinical trial, to select patients who should receive Tecentriq treatment (74, 75). Tumor specimens were prospectively evaluated using the Ventana PD-L1 (SP142) assay and patients with high levels of PD-L1 expression had improved PR, CR, and ORR.
Clinical trials studying atezolizumab treatment in advanced cancers (NSCLC, melanoma, renal cell carcinoma, colorectal, gastric, and HSCC) led to approved indications due to increased response rates in patients treated with atezolizumab (76). Biomarker inclusion was studied in most of these trials but was not initially included in the FDA labeling. In NSCLC patients, there was a correlation between PD-L1 expression and response to treatment, in which patients were stratified based on PD-L1 status on tumor-infiltrating immune cells and tumor cells and randomized to receive either atezolizumab or docetaxel (76). Patients were classified as having high PD-L1 expression if more than 50% of their tumor cells or 10% of their immune cells expressed PD-L1 membranous staining. PD-L1 positivity correlated with improved OS, PFS, and ORR when treated with atezolizumab as a single agent (77–79). In May 2020, following the IMpower110 (NCT02409342) clinical trial, the inclusion criteria of high PD-L1 expression ≥ 50% of tumor cells or ≥ 10% of tumor-infiltrating immune cells as defined by an FDA-approved device were approved for the treatment of adult metastatic NSCLC with no EGFR or ALK genomic aberrations (78). The overall survival rate was 20.2 months for patients with PD-L1 high-expressing tumors treated with atezolizumab compared to 13.1 months for patients treated with chemotherapy, and patients with PD-L1-positive tumors (tumor proportion score > 1%) had an OS of 17.8 months in the atezolizumab-treated cohort compared to 14.1 months in the chemotherapy-treated (78). In March 2019, the FDA approved the new indication for treatment with atezolizumab in combination with nab-paclitaxel for the treatment of adult patients with unresectable locally advanced or metastatic TNBC whose tumors express PD-L1 (IC ≥ 1% of tumor area) as determined by an FDA-approved test. This was the first ICI approval for the treatment of patients with breast cancer, with significantly longer PFS compared to the placebo arm (80).
While PD-L1 status was shown to correlate with improved response rates in some clinical studies, the evaluation of PD-L1 expression is not always performed, depending on the study population and the primary endpoints evaluated. In patients with ES-SCLC, the IMpower133 clinical trial, studying the treatment of atezolizumab in combination with carboplatin and etoposide, the primary endpoints of PFS and OS were met, with improved survival for patients treated with atezolizumab without evaluation of PD-L1 status (81). The IMspire150 clinical trial stratified patients with BRAFV600 mutation-positive advanced or metastatic melanoma using lactate dehydrogenase concentration, demonstrating significantly increased PFS in patients treated with atezolizumab (82). The IMbrave150 clinical trial reported significant improvements in the OS and PFS in the atezolizumab-treated patients with hepatocellular carcinoma as a first-line treatment (83).
Durvalumab (Imfinzi)
Durvalumab is an IgG1κ anti-PD-L1 mAb that was first approved in 2017 for the treatment of locally advanced or metastatic urothelial carcinoma. PD-L1 expression was prospectively determined in patients with solid tumors using the Ventana PD-L1 (SP263) assay, in which expression levels were classified as PD-L1 high (if ICs involve > 1% of the tumor area, TC ≥ 25% or IC ≥ 25%; if ICs involve < 1% of the tumor area, TC ≥ 25% or IC = 100%) or PD-L1 low/negative (84, 85). In the urothelial carcinoma cohort, the PD-L1 high patients experienced an improved disease control rate but patients treated with durvalumab experienced response regardless of PD-L1 status. In 2018, an additional indication was approved for durvalumab, for the treatment of adult patients with ES-SCLC in combination with etoposide and either carboplatin or cisplatin, which reported significant improvement in OS compared to the control group (86).
Avelumab (Bavencio)
Avelumab is a fully human IgG1 anti-PD-L1 mAb that was approved under accelerated approval in 2017 for the treatment of patients with metastatic Merkel cell carcinoma (MCC), in which patients’ response to the therapy was not dependent on PD-L1 positivity. This was the first treatment for mMCC, with an ORR of 46.7% (87). Avelumab was then approved for the treatment of urothelial carcinoma patients and for the treatment of patients with advanced renal cell carcinoma (88). Though PD-L1 expression was evaluated and demonstrated an increase in ORR correlated with expression levels, the ORR was achieved in all expression cohorts. OS was not found to be correlated with PD-L1 expression; therefore, the protein expression was not considered predictive (88).
FDA CLEARED DIAGNOSTICS FOR USE WITH ANTI-PD-1/PD-L1 THERAPEUTICS
The use of a companion or complementary diagnostic device for PD-L1 expression levels has been included in many clinical trials and FDA labeling across cancer types. While a companion diagnostic device is required for the therapeutic product’s safe and effective use, a complementary test is performed to provide information that is clinically meaningful and will aid in the decision regarding treatment (89). The first companion diagnostic for an ICI targeting the PD-1/PD-L1 signaling pathway was approved in 2015 through the Premarket Approval process, for use in identifying NSCLC patients for treatment with pembrolizumab (Fig. 2) (90). Since the initial approval, the PD-L1 IHC 22C3 pharmDx assay approval has been extended to patients with gastroesophageal and gastroesophageal junction cancer, cervical cancer, UC, HNSCC, and TNBC. As previously described, the device uses the TPS to identify NSCLC patients who are PD-L1-positive (TPS ≥ 1%) and CPS for the additional biomarker-dependent indications, either CPS ≥ 1 or CPS ≥ 10. TPS identifies the percentage of PD-L1-positive tumor cells relative to the viable tumor cells within the sample, whereas CPS identifies the PD-L1-positive cells, including tumor cells, lymphocytes, and macrophages (91, 92).
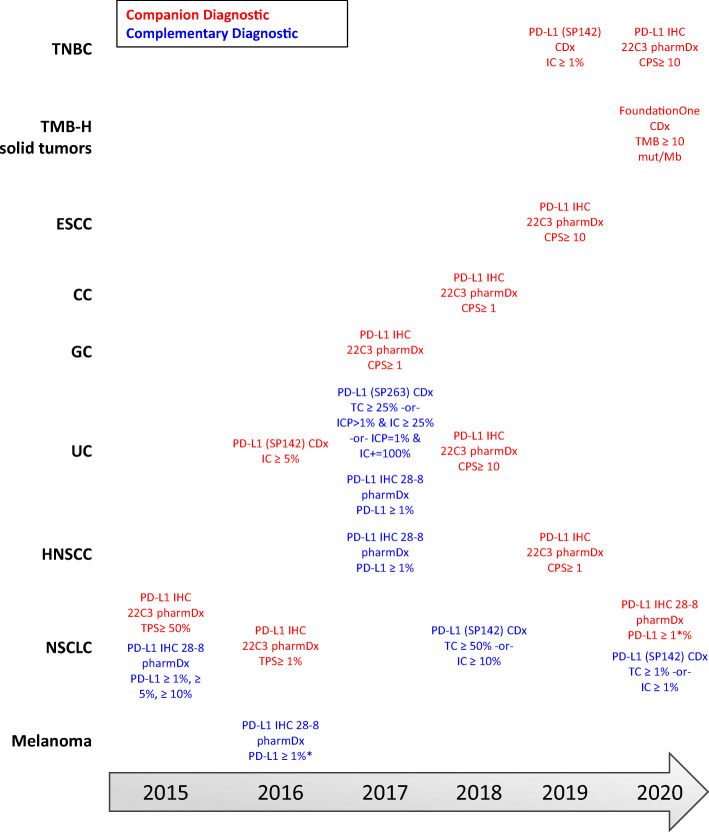
Fig. 2.
FDA approvals of companion and complementary diagnostic assays. As of December 2020, there are five companion diagnostics that have been approved to identify patients across seven tissue types and one tissue-agnostic condition who may benefit from treatment with an anti-PD-1/PD-L1 mAb. Shown are the approvals for each companion (indicated in red text) and complementary (indicated in blue text) device with the associated threshold per cancer type. Tumor proportion score (TPS) measured the membranous staining of tumors cells and is reported as a percentage of the total viable tumor cells. Combined proportion score (CPS) measures the membranous staining of tumor cells, lymphocytes, and macrophages and is reported as a percentage of the total viable tumor cells and multiplied by 100. PD-L1 percentage (as measured by the 28-8 pharmDx) reports the number of tumor cells with complete circumferential or partial linear plasma membrane staining of PDL1 out of 100 viable tumor cells. IC and TC measure the proportion of tumor area occupied by PD-L1 expressing tumor-infiltrating immune cells (IC) or the percentage of PD-L1-positive tumor cells (TC) and is reported as a percentage of the tumor area. The percentage of immune cells present (ICP) is reported as the percentage of tumor area occupied by any tumor-associated immune cells. *The melanoma indication was withdrawn from the PD-L1 IHC 28-8 pharmDx label on 07 March 2019. TNBC, triple-negative breast cancer; TMB-H, tumor mutation burden high; ESCC, esophageal squamous cell carcinoma; CC, cervical cancer; GC, gastric cancer; UC, urothelial carcinoma; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer. Information was collected from the “List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools) (https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools)
The Ventana PD-L1 (SP142) assay was specifically developed for use with atezolizumab and was tested in the pivotal studies that led to the therapeutics approval and the approval of the assay as a complementary diagnostic (93). This device uses IHC to determine partial or circumferential membrane or associated cytoplasmic staining of IC or TC. The approval was updated to include the assay as a companion diagnostic for the identification of patients with urothelial carcinoma (≥ 5% IC cutoff) (94), TNBC (≥ 1% IC cutoff) (95), and NSCLC (≥ 50% TC cutoff or ≥ 10% IC) with the approved therapeutic product labeling. The Ventana PD-L1 (SP263) assay was developed for clinical trial enrollment of patients intended for treatment with durvalumab, to determine the percentage of tumor cells and tumor-associated immune cells with any membrane staining of PD-L1 and is used as a complementary diagnostic.
The approval of the PD-L1 IHC 28-8 pharmDx as a companion diagnostic intended for use in the detection of PD-L1 protein to identify NSCLC patients for treatment with nivolumab in combination with ipilimumab was granted in May 2020 (67). The assay was originally developed for use with clinical trials with nivolumab and was approved as a complementary diagnostic device for second-line treatment of NSCLC, as patients treated with nivolumab demonstrated increased response rate regardless of PD-L1 status (96). PD-L1 expression as a predictive biomarker was evaluated using retrospective analysis, in which there was a statistically significant difference in OS of PD-L1-positive patients treated with nivolumab in combination with ipilimumab compared to docetaxel treatment. The device may also be used to determine the PD-L1 protein expression in patients with SCCHN and UC, but as a complementary diagnostic device.
Comparability across assays and their diagnostic use has been discussed, as approvals indicate specific devices for the different therapeutics with varying biomarker thresholds (96). The consistency in identifying PD-L1-positive patients and the concordance across devices has been studied in the Blueprint PD-L1 IHC Assay Comparison Project, in which concordance was seen across the 28-8, 22C3, and SP263 devices, though not for the SP142 assay (97, 98). As PD-L1 positivity is still being evaluated as a predictive biomarker in clinical trials, in which patients with negative or non-evaluable tumor samples have also demonstrated a response, additional biomarkers are being assessed to determine their correlation with response rates and to better identify those patients who will respond.
In the Keynote-158 clinical trial, patients with solid tumors were enrolled and a tumor sample was taken for biomarker analysis. These samples were assessed by the FoundationOne CDx assay to determine the tissue tumor mutation burden (tTMB), with a threshold cutoff of more than 10 mutations per megabase as determined by whole exome sequencing (36, 99). The association between the efficacy of pembrolizumab, as determined by CR or PR and high tTMB resulted in the approval of the assay as a companion diagnostic to determine treatment with pembrolizumab in patients with TMB-high solid tumors. The approval of the FoundationOne CDx as a companion diagnostic also indicates there is a universal threshold for TMB across tumor types in determining treatment with pembrolizumab (99).
EXPANDED BIOMARKER DISCOVERY AND POTENTIAL COMBINATION THERAPIES
To better identify the population who will most benefit from ICI treatment and those who may be susceptible to immune-related adverse events, strategies are being implemented to expand the use of ICIs and develop novel biomarkers using proteomic, genomic, and transcriptomic analysis. This involves a deeper understanding of evolving resistance mechanisms, primary resistance, and the factors that impact ICI efficacy. As described above, the use of PD-L1 as a predictive biomarker to identify those patients who are most likely to benefit from ICI treatment remains difficult due to different assays used for each therapeutic, difference in threshold cutoffs across indications, tumor heterogeneity within and across patient populations, the diversity of patients’ treatment history, and the dynamic status of the tumor microenvironment. In some cases, a single-parameter biomarker (e.g., PD-L1) may not be sufficient to accurately stratify patients for ICI therapy (100).
Strategies being explored for the development of novel biomarkers also include further understanding of the tumor microenvironment. The cancer immunity cycle is initiated when the accumulation of genetic mutations within cancer cell results in the production of neoantigens, which are able to bind to major histocompatibility complex (MHC) molecules on the cancer cell plasma membrane (101). As cancer cells die during tumor growth, neoantigens are released and captured by dendritic cells (DCs) or antigen-presenting cells (APCs), which migrate to the lymphoid organs. The DCs present the antigens to T cells to prime and activate the T cells using co-stimulatory (CD28, CD80, CD86) and co-inhibitory molecules (PD-L1, CTLA4), to regulate the tumor-specific T cells and encourage the T cells to become effector cells (101). The T cells then target the foreign antigen/tumor cells through binding of T cell receptor (TCR) to the antigen-bound MHCs on the cancer cells, leading to cell lysis and further antigen release (101). Identification of these neoantigens or how these proteins are involved in the cancer immunity cycle may help identify novel predictive biomarkers.
Exploring how biomarkers interact may also aid in the design of combination strategies, to maximize their benefit (99). Various clinical trials are studying the sequential treatment of ICIs either prior to or following chemotherapies, to determine if this treatment can turn “cold” non-immunogenic tumor to a “hot” tumor, which would respond to ICI treatment (NCT00527735, NCT02499367). The goal of these combinations is to modulate the immune suppressive microenvironment and initiate tumor cell death, recruiting effector T cells to the tumor and increasing the efficacy of the ICIs. Using retrospective analysis, potential biomarkers that may correlate with increased response rate to ICIs include the neutrophil to lymphocyte ratio (NLR) (102) or an absolute eosinophil count (103). Due to the dynamic nature of PD-1/PD-L1, it has been challenging to detect the changing PD-1/PD-L1 expression using solid tumor tissue biopsy during tumor progress or treatment. As a result, several trials are also incorporating liquid biopsies to monitor the soluble PD-1/PD-L1 in the peripheral blood (104, 105).
Combination therapies are in the limelight for PD-1/PD-L1 clinical trials (106). Ongoing clinical trials are testing combination and sequential therapies, such as additional immuno-oncology treatments that target parallel signaling pathways, chemotherapies known to increase antigen release, or radiotherapies (106, 107). PD-L1 expression is known to change following chemotherapy, radiation therapy, and several targeted therapies (108, 109). Understanding how these therapies may work together may require a continuous monitoring of biomarkers to aid in treatment decisions, to evaluate whether a first-line treatment switches a tumor from TMB-low to TMB-high or modulates PD-L1 expression above a threshold cutoff. Determining the optimal sequence, dosing, and timing of combination therapies to modify the tumor microenvironment while evading acquired resistance may further expand the use of ICIs. Examination of the changing tumor microenvironment during these combinations, with understanding the contribution of genomic or proteomic biomarkers to response rates, will hopefully improve patient response and expand the potential patient population who will benefit from these therapies.
CONCLUSION
With the rapid growth of PD-1/PD-L1 blockade in clinical use, an effective biomarker to identify patients who are likely or unlikely to benefit from these therapies becomes increasingly necessary. The currently available biomarkers (PD-L1 expression, TMB-H, dMMR/MSI-H) are helpful to the selection of patients for PD-1/PD-L1 therapy in several tumor types. However, due to the intra- and inter-tumoral heterogeneity, there are still many challenges for their expanded use across different products and tumor types. PD-L1 thresholds that determine a biomarker-positive patient are inconsistent across different assays, differing in thresholds both within and across tumor types. Biomarker positivity is dependent on the assay, which varies in measuring PD-L1 expression on tumor cells alone or in conjunction with tumor-infiltrating immune cells and is specific to each immuno-oncology product. Harmonization of the different diagnostic assays and their scoring metrics is critical to provide patients with consistent information regarding the selection of optimal treatment strategies. The tissue-agnostic signature such as TMB-H and dMMR/MSI-H holds promise to guide the prescription of PD-1/PD-L1 therapy, but their predictive value is limited by the lack of pre-defined criteria for each product in a specific tumor. Given the complexity of the immune system, a single-parameter biomarker (e.g., PD-L1) may not be sufficient to accurately predict therapeutic benefit in individual patients. Composite biomarkers of multiple variables may be able to better predict patient outcomes. Regardless, prospective randomized trials are required to establish the roles of predictive biomarkers in specific clinical settings.
Acknowledgements
The authors would like to thank Dr. Elaine Chang (Office of New Drugs, CDER, FDA) for helpful discussions and sharing information, and Drs. Shen Luo, Ancy Nalli, and Serge Beaucage (Office of Biotechnology Products, CDER, FDA) for their critical review on the manuscript.
Funding
This work was funded by the U.S. Food and Drug Administration. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations
Conflict of Interest
The authors declare no conflict of interest.
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Julianne D. Twomey, Email: Julianne.Twomey@fda.hhs.gov
Baolin Zhang, Email: Baolin.zhang@fda.hhs.gov.
References
- 1.Liu X, Guo C-Y, Tou F-F, Wen X-M, Kuang Y-K, Zhu Q, Hu H. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: a systematic review and meta-analysis. International Journal of Cancer. 2020;147(1):116–127. doi: 10.1002/ijc.32744. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. Journal for ImmunoTherapy of Cancer. 2019;7(1):278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaver JA, Tzou A, Blumenthal GM, McKee AE, Kim G, Pazdur R, et al. An FDA perspective on the regulatory implications of complex signatures to predict response to targeted therapies. Clin Cancer Res. 2017;23(6):1368–1372. doi: 10.1158/1078-0432.CCR-16-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [updated 11/13/2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK338448/. [PubMed]
- 6.Patel SP, Kurzrock R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Molecular Cancer Therapeutics. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 7.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111):111ra20. [DOI] [PMC free article] [PubMed]
- 9.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 11.Kang SP, Gergich K, Lubiniecki GM, de Alwis DP, Chen C, Tice MAB, Rubin EH. Pembrolizumab KEYNOTE-001: an adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann Oncol. 2017;28(6):1388–1398. doi: 10.1093/annonc/mdx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raedler LA. Keytruda (pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Health Drug Benefits. 2015;8(Spec Feature):96-100. [PMC free article] [PubMed]
- 13.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 14.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 15.Kang SP, Gergich K, Lubiniecki GM, de Alwis DP, Chen C, Tice MAB, Rubin EH. Pembrolizumab KEYNOTE-001: an adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann Oncol. 2017;28(6):1388–1398. doi: 10.1093/annonc/mdx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu YL, Zhang L, Fan Y, Zhou J, Zhang L, Zhou Q, et al. Randomized clinical trial of pembrolizumab versus chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China study. Int J Cancer. 2020. [DOI] [PMC free article] [PubMed]
- 17.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G, Adamchuk G, Ahn MJ, Alexandru A, Altundag O, Alyasova A, Andrusenko O, Aoe K, Araujo A, Aren O, Arrieta Rodriguez O, Ativitavas T, Avendano O, Barata F, Barrios CH, Beato C, Bergstrom P, Betticher D, Bolotina L, Bondarenko I, Botha M, Buddu S, Caglevic C, Cardona A, Castro G, Jr, Castro H, Cay Senler F, Cerny CAS, Cesas A, Chan GC, Chang J, Chen G, Chen X, Cheng S, Cheng Y, Cherciu N, Chiu CH, Cho BC, Cicenas S, Ciurescu D, Cohen G, Costa MA, Danchaivijitr P, de Angelis F, de Azevedo SJ, Dediu M, Deliverski T, de Marchi PRM, de The Bustamante Valles F, Ding Z, Doganov B, Dreosti L, Duarte R, Edusma-Dy R, Emelyanov S, Erman M, Fan Y, Fein L, Feng J, Fenton D, Fernandes G, Ferreira C, Franke FA, Freitas H, Fujisaka Y, Galindo H, Galvez C, Ganea D, Gil N, Girotto G, Goker E, Goksel T, Gomez Aubin G, Gomez Wolff L, Griph H, Gumus M, Hall J, Hart G, Havel L, He J, He Y, Hernandez Hernandez C, Hespanhol V, Hirashima T, Ho CMJ, Horiike A, Hosomi Y, Hotta K, Hou M, How SH, Hsia TC, Hu Y, Ichiki M, Imamura F, Ivashchuk O, Iwamoto Y, Jaal J, Jassem J, Jordaan C, Juergens RA, Kaen D, Kalinka-Warzocha E, Karaseva N, Karaszewska B, Kazarnowicz A, Kasahara K, Katakami N, Kato T, Kawaguchi T, Kim JH, Kishi K, Kolek V, Koleva M, Kolman P, Koubkova L, Kowalyszyn R, Kowalski D, Koynov K, Ksienski D, Kubota K, Kudaba I, Kurata T, Kuusk G, Kuzina L, Laczo I, Ladrera GEI, Laktionov K, Landers G, Lazarev S, Lerzo G, Lesniewski Kmak K, Li W, Liam CK, Lifirenko I, Lipatov O, Liu X, Liu Z, Lo SH, Lopes V, Lopez K, Lu S, Martinengo G, Mas L, Matrosova M, Micheva R, Milanova Z, Miron L, Mok T, Molina M, Murakami S, Nakahara Y, Nguyen TQ, Nishimura T, Ochsenbein A, Ohira T, Ohman R, Ong CK, Ostoros G, Ouyang X, Ovchinnikova E, Ozyilkan O, Petruzelka L, Pham XD, Picon P, Piko B, Poltoratsky A, Ponomarova O, Popelkova P, Purkalne G, Qin S, Ramlau R, Rappaport B, Rey F, Richardet E, Roubec J, Ruff P, Rusyn A, Saka H, Salas J, Sandoval M, Santos L, Sawa T, Seetalarom K, Seker M, Seki N, Seolwane F, Shepherd L, Shevnya S, Shimada AK, Shparyk Y, Sinielnikov I, Sirbu D, Smaletz O, Soares JPH, Sookprasert A, Speranza G, Srimuninnimit V, Sriuranpong V, Stara Z, Su WC, Sugawara S, Szpak W, Takahashi K, Takigawa N, Tanaka H, Tan Chun Bing J, Tang Q, Taranov P, Tejada H, Tho LM, Torii Y, Trukhyn D, Turdean M, Turna H, Ursol G, Vanasek J, Varela M, Vallejo M, Vera L, Victorino AP, Vlasek T, Vynnychenko I, Wang B, Wang J, Wang K, Wu Y, Yamada K, Yang CH, Yokoyama T, Yokoyama T, Yoshioka H, Yumuk F, Zambrano A, Zarba JJ, Zarubenkov O, Zemaitis M, Zhang L, Zhang L, Zhang X, Zhao J, Zhou C, Zhou J, Zhou Q, Zippelius A. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 18.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 19.Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell SF, Cheng SYS, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Garon EB, Novello S, Rubio-Viqueira B, Boyer M, Kurata T, Gray JE, Yang J, Bas T, Pietanza MC, Garassino MC. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 20.Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M, Yang JCH, Mazieres J, Orlandi FJ, Luft A, Gümüş M, Kato T, Kalemkerian GP, Luo Y, Ebiana V, Pietanza MC, Kim HR, on behalf of the KEYNOTE-604 Investigators Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito G, Palumbo G, Carillio G, Manzo A, Montanino A, Sforza V, et al. Immunotherapy in small cell lung cancer. Cancers (Basel). 2020;12(9). [DOI] [PMC free article] [PubMed]
- 22.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, Borg C, Doi T, Yoon HH, Savage MJ, Wang J, Dalal RP, Shah S, Wainberg ZA, Chung HC. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22(4):828–837. doi: 10.1007/s10120-018-00909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fashoyin-Aje L, Donoghue M, Chen H, He K, Veeraraghavan J, Goldberg KB, Keegan P, McKee AE, Pazdur R. FDA Approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24(1):103–109. doi: 10.1634/theoncologist.2018-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schellens JHM, Marabelle A, Zeigenfuss S, Ding J, Pruitt SK, Chung HC. Pembrolizumab for previously treated advanced cervical squamous cell cancer: preliminary results from the phase 2 KEYNOTE-158 study. Journal of Clinical Oncology. 2017;35(15_suppl):5514-.
- 27.U.S. Food and Drug Administration. FDA approves pembrolizumab for advanced cervical cancer with disease progression during or after chemotherapy 2018 [updated 06/13/2020. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-cervical-cancer-disease-progression-during-or-after-chemotherapy.
- 28.Vuky J, Balar AV, Castellano D, O’Donnell PH, Grivas P, Bellmunt J, Powles T, Bajorin D, Hahn NM, Savage MJ, Fang X, Godwin JL, Frenkl TL, Homet Moreno B, de Wit R, Plimack ER. Long-term outcomes in KEYNOTE-052: phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol. 2020;38(23):2658–2666. doi: 10.1200/JCO.19.01213. [DOI] [PubMed] [Google Scholar]
- 29.Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT, Lockhart AC, Arkenau HT, el-Hajbi F, Gupta M, Pfeiffer P, Liu Q, Lunceford J, Kang SP, Bhagia P, Kato K. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5(4):546–550. doi: 10.1001/jamaoncol.2018.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid P, Salgado R, Park YH, Muñoz-Couselo E, Kim SB, Sohn J, Im SA, Foukakis T, Kuemmel S, Dent R, Yin L, Wang A, Tryfonidis K, Karantza V, Cortés J, Loi S. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569–581. doi: 10.1016/j.annonc.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 32.Simmons CE, Brezden-Masley C, McCarthy J, McLeod D, Joy AA. Positive progress: current and evolving role of immune checkpoint inhibitors in metastatic triple-negative breast cancer. Ther Adv Med Oncol. 2020;12:1758835920909091. doi: 10.1177/1758835920909091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clinical Cancer Research. 2019;25(13):3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 34.PD-1 inhibitor bests chemo for colorectal cancer. Cancer Discov. 2020;10(7):Of2. [DOI] [PubMed]
- 35.Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A. Sinicrope FA. Cancer Discov: Tumor mutational burden as a predictive biomarker in solid tumors; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH, Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 37.Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O’Neil BH, Mehnert JM, Lopez J, Doi T, van Brummelen EMJ, Cristescu R, Yang P, Emancipator K, Stein K, Ayers M, Joe AK, Lunceford JK. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 38.Pembrolizumab improves progression-free survival in relapsed/refractory hodgkin lymphoma. Oncologist. 2020;25 Suppl 1(Suppl 1):S18-s9. [DOI] [PMC free article] [PubMed]
- 39.Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, Tomita A, von Tresckow B, Shipp MA, Lin J, Kim E, Nahar A, Balakumaran A, Moskowitz CH. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144–1153. doi: 10.1182/blood.2019000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 41.Suzman DL, Agrawal S, Ning YM, Maher VE, Fernandes LL, Karuri S, Tang S, Sridhara R, Schroeder J, Goldberg KB, Ibrahim A, McKee AE, Pazdur R, Beaver JA. FDA approval summary: atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist. 2019;24(4):563–569. doi: 10.1634/theoncologist.2018-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armand P, Rodig S, Melnichenko V, Thieblemont C, Bouabdallah K, Tumyan G, Özcan M, Portino S, Fogliatto L, Caballero MD, Walewski J, Gulbas Z, Ribrag V, Christian B, Perini GF, Salles G, Svoboda J, Zain J, Patel S, Chen PH, Ligon AH, Ouyang J, Neuberg D, Redd R, Chatterjee A, Balakumaran A, Orlowski R, Shipp M, Zinzani PL. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37(34):3291–3299. doi: 10.1200/JCO.19.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zinzani PL, Ribrag V, Moskowitz CH, Michot JM, Kuruvilla J, Balakumaran A, Zhang Y, Chlosta S, Shipp MA, Armand P. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130(3):267–270. doi: 10.1182/blood-2016-12-758383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M, Alistar A, Asselah J, Blanc JF, Borbath I, Cannon T, Chung K, Cohn A, Cosgrove DP, Damjanov N, Gupta M, Karino Y, Karwal M, Kaubisch A, Kelley R, van Laethem JL, Larson T, Lee J, Li D, Manhas A, Manji GA, Numata K, Parsons B, Paulson AS, Pinto C, Ramirez R, Ratnam S, Rizell M, Rosmorduc O, Sada Y, Sasaki Y, Stal PI, Strasser S, Trojan J, Vaccaro G, van Vlierberghe H, Weiss A, Weiss KH, Yamashita T. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 45.Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, Friedlander PA, Daud A, Kluger HM, Reddy SA, Boulmay BC, Riker AI, Burgess MA, Hanks BA, Olencki T, Margolin K, Lundgren LM, Soni A, Ramchurren N, Church C, Park SY, Shinohara MM, Salim B, Taube JM, Bird SR, Ibrahim N, Fling SP, Homet Moreno B, Sharon E, Cheever MA, Topalian SL. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol. 2019;37(9):693–702. doi: 10.1200/JCO.18.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradford D, Demko S, Jin S, Mishra-Kalyani P, Beckles AR, Goldberg KB, Lemery S, Ward A, Keegan P, Pazdur R. FDA accelerated approval of pembrolizumab for recurrent locally advanced or metastatic Merkel cell carcinoma. Oncologist. 2020;25(7):e1077–e1e82. doi: 10.1634/theoncologist.2020-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 48.Kamat AM, Shore N, Hahn N, Alanee S, Nishiyama H, Shariat S, Nam K, Kapadia E, Frenkl T, Steinberg G. KEYNOTE-676: phase III study of BCG and pembrolizumab for persistent/recurrent high-risk NMIBC. Future Oncol. 2020;16(10):507–516. doi: 10.2217/fon-2019-0817. [DOI] [PubMed] [Google Scholar]
- 49.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. The New England journal of medicine. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. The Lancet Oncology. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–1477. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 52.Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6). [DOI] [PMC free article] [PubMed]
- 53.Ramchandren R, Domingo-Domènech E, Rueda A, Trněný M, Feldman TA, Lee HJ, Provencio M, Sillaber C, Cohen JB, Savage KJ, Willenbacher W, Ligon AH, Ouyang J, Redd R, Rodig SJ, Shipp MA, Sacchi M, Sumbul A, Armand P, Ansell SM. Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: safety and efficacy in the phase II CheckMate 205 study. J Clin Oncol. 2019;37(23):1997–2007. doi: 10.1200/JCO.19.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borel C, Jung AC, Burgy M. Immunotherapy breakthroughs in the treatment of recurrent or metastatic head and neck squamous cell carcinoma. Cancers (Basel). 2020;12(9). [DOI] [PMC free article] [PubMed]
- 55.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11). [DOI] [PMC free article] [PubMed]
- 56.Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, Spigel DR, Moreno V, Chau I, Hann CL, Eder JP, Steele NL, Pieters A, Fairchild J, Antonia SJ. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14(2):237–244. doi: 10.1016/j.jtho.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reck M, Ciuleanu T-E, Dols MC, Schenker M, Zurawski B, Menezes J, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. Journal of Clinical Oncology. 2020;38(15_suppl):9501-.
- 58.Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 59.Wright K. FDA approves nivolumab plus ipilimumab for previously untreated unresectable malignant pleural mesothelioma. Oncology (Williston Park). 2020;34(11):502–503. doi: 10.46883/ONC.2020.3411.0502. [DOI] [PubMed] [Google Scholar]
- 60.Mankor JM, Disselhorst MJ, Poncin M, Baas P, Aerts J, Vroman H. Efficacy of nivolumab and ipilimumab in patients with malignant pleural mesothelioma is related to a subtype of effector memory cytotoxic T cells: translational evidence from two clinical trials. EBioMedicine. 2020;62:103040. doi: 10.1016/j.ebiom.2020.103040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iafolla MAJ, Juergens RA. Update on programmed death-1 and programmed death-ligand 1 inhibition in the treatment of advanced or metastatic non-small cell lung cancer. Frontiers in oncology. 2017;7:67-. [DOI] [PMC free article] [PubMed]
- 62.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. The New England journal of medicine. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, Arranz JÁ, Pal S, Ohyama C, Saci A, Qu X, Lambert A, Krishnan S, Azrilevich A, Galsky MD. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 64.Galsky MD, Saci A, Szabo PM, Han GC, Grossfeld G, Collette S, Siefker-Radtke A, Necchi A, Sharma P. Nivolumab in patients with advanced platinum-resistant urothelial carcinoma: efficacy, safety, and biomarker analyses with extended follow-up from CheckMate 275. Clin Cancer Res. 2020;26(19):5120–5128. doi: 10.1158/1078-0432.CCR-19-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nasser NJ, Gorenberg M, Agbarya A. First line immunotherapy for non-small cell lung cancer. Pharmaceuticals (Basel). 2020;13(11). [DOI] [PMC free article] [PubMed]
- 66.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O’Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. New England Journal of Medicine. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 67.U.S. Food and Drug Administration. Approval Order P150025-S013. 2020.
- 68.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 70.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 71.Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer. 2020;8(1). [DOI] [PMC free article] [PubMed]
- 72.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. The Lancet. 2017;389(10064):67–76. [DOI] [PMC free article] [PubMed]
- 74.Gartrell BA, He T, Sharma J, Sonpavde G. Update of systemic immunotherapy for advanced urothelial carcinoma. Urol Oncol. 2017;35(12):678–686. doi: 10.1016/j.urolonc.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 75.Bernard-Tessier A, Bonnet C, Lavaud P, Gizzi M, Loriot Y, Massard C. Atezolizumab (Tecentriq(®)): activity, indication and modality of use in advanced or metastatic urinary bladder carcinoma. Bull Cancer. 2018;105(2):140–145. doi: 10.1016/j.bulcan.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 76.Santabarbara G, Maione P, Rossi A, Palazzolo G, Gridelli C. Novel immunotherapy in the treatment of advanced non-small cell lung cancer. Expert Rev Clin Pharmacol. 2016;9(12):1571–1581. doi: 10.1080/17512433.2016.1236681. [DOI] [PubMed] [Google Scholar]
- 77.Peters S, Gettinger S, Johnson ML, Jänne PA, Garassino MC, Christoph D, Toh CK, Rizvi NA, Chaft JE, Carcereny Costa E, Patel JD, Chow LQM, Koczywas M, Ho C, Früh M, van den Heuvel M, Rothenstein J, Reck M, Paz-Ares L, Shepherd FA, Kurata T, Li Z, Qiu J, Kowanetz M, Mocci S, Shankar G, Sandler A, Felip E. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1–selected advanced non–small-cell lung cancer (BIRCH) Journal of Clinical Oncology. 2017;35(24):2781–2789. doi: 10.1200/JCO.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, Özgüroğlu M, Zou W, Sandler A, Enquist I, Komatsubara K, Deng Y, Kuriki H, Wen X, McCleland M, Mocci S, Jassem J, Spigel DR. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 79.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 80.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New England Journal of Medicine. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 81.Armstrong SA, Liu SV. Dashing decades of defeat: long anticipated advances in the first-line treatment of extensive-stage small cell lung cancer. Curr Oncol Rep 2020;22(2):20. [DOI] [PubMed]
- 82.Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, Pereira RP, Eigentler T, Rutkowski P, Demidov L, Manikhas GM, Yan Y, Huang KC, Uyei A, McNally V, McArthur GA, Ascierto PA. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 83.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 84.Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zajac M, Ye J, Mukhopadhyay P, Jin X, Ben Y, Antal J, Gupta AK, Rebelatto MC, Williams JA, Walker J. Optimal PD-L1-high cutoff for association with overall survival in patients with urothelial cancer treated with durvalumab monotherapy. PLoS One. 2020;15(4):e0231936. doi: 10.1371/journal.pone.0231936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW, Batagelj E, Casarini I, Pastor AV, Sena SN, Zarba JJ, Burghuber O, Hartl S, Hochmair MJ, Lamprecht B, Studnicka M, Alberto Schlittler L, Augusto Martinelli de Oliveira F, Calabrich A, Colagiovanni Girotto G, Dos Reis P, Fausto Nino Gorini C, Rafael Martins de Marchi P, Serodio da Rocha Baldotto C, Sette C, Zukin M, Conev NV, Dudov A, Ilieva R, Koynov K, Krasteva R, Tonev I, Valev S, Venkova V, Bi M, Chen C, Chen Y, Chen Z, Fang J, Feng J, Han Z, Hu J, Hu Y, Li W, Liang Z, Lin Z, Ma R, Ma S, Nan K, Shu Y, Wang K, Wang M, Wu G, Yang N, Yang Z, Zhang H, Zhang W, Zhao J, Zhao Y, Zhou C, Zhou J, Zhou X, Havel L, Kolek V, Koubkova L, Roubec J, Skrickova J, Zemanova M, Chouaid C, Hilgers W, Lena H, Moro-Sibilot D, Robinet G, Souquet PJ, Alt J, Bischoff H, Grohe C, Laack E, Lang S, Panse J, Reinmuth N, Schulz C, Bogos K, Csánky E, Fülöp A, Horváth Z, Kósa J, Laczó I, Losonczy G, Pajkos G, Pápai Z, Pápai Székely Z, Sárosi V, Somfay A, Somogyiné Ezer É, Telekes A, Bar J, Gottfried M, Heching NI, Zer Kuch A, Bartolucci R, Bettini AC, Delmonte A, Garassino MC, Minelli M, Roila F, Verderame F, Atagi S, Azuma K, Goto H, Goto K, Hara Y, Hayashi H, Hida T, Hotta K, Kanazawa K, Kanda S, Kim YH, Kuyama S, Maeda T, Morise M, Nakahara Y, Nishio M, Nogami N, Okamoto I, Saito H, Shinoda M, Umemura S, Yoshida T, Claessens N, Cornelissen R, Heniks L, Hiltermann J, Smit E, Staal van den Brekel A, Kazarnowicz A, Kowalski D, Mańdziuk S, Mróz R, Wojtukiewicz M, Ciuleanu T, Ganea D, Ungureanu A, Dvorkin M, Luft A, Moiseenko V, Poltoratskiy A, Sakaeva D, Smolin A, Statsenko G, Vasilyev A, Vladimirova L, Anasina I, Chovanec J, Demo P, Godal R, Kasan P, Stresko M, Urda M, Cho EK, Ji JH, Kim JH, Kim SW, Lee GW, Lee JS, Lee KH, Lee KH, Lee YG, Amelia Insa Molla M, Domine Gomez M, Ignacio Delgado Mingorance J, Isla Casado D, Lopez Brea M, Majem Tarruella M, Morán Bueno T, Navarro Mendivil A, Paz-Ares Rodríguez L, Ponce Aix S, Rosario Garcia Campelo M, Chang GC, Chen YH, Chiu CH, Hsia TC, Lee KY, Li CT, Wang CC, Wei YF, Wu SY, Alacacıoğlu A, Çiçin I, Demirkazik A, Erman M, Göksel T, Özgüroğlu M, Adamchuk H, Bondarenko I, Kolesnik O, Kryzhanivska A, Ostapenko Y, Shevnia S, Shparyk Y, Trukhin D, Ursol G, Voitko N, Voitko O, Vynnychenko I, Babu S, Chen Y, Chiang A, Chua W, Dakhil S, Dowlati A, Goldman JW, Haque B, Jamil R, Knoble J, Lakhanpal S, Mi K, Nikolinakos P, Powell S, Ross H, Schaefer E, Schneider J, Spahr J, Spigel D, Stilwill J, Sumey C, Williamson M. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 87.Walker JW, Lebbé C, Grignani G, Nathan P, Dirix L, Fenig E, Ascierto PA, Sandhu S, Munhoz R, Benincasa E, Flaskett S, Reed J, Engelsberg A, Hariharan S, Kasturi V. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: experience from a global expanded access program. Journal for immunotherapy of cancer. 2020;8(1):e000313. doi: 10.1136/jitc-2019-000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Apolo AB, Ellerton JA, Infante JR, Agrawal M, Gordon MS, Aljumaily R, et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed]
- 89.U.S. Food and Drug Administration. In vitro companion diagnostic devices: guidance for industry and Food and Drug Administration staff. In: Center for Devices and Radiological Health, editor. 2014.
- 90.Jørgensen JT. Companion diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Expert Rev Mol Diagn. 2016;16(2):131–133. doi: 10.1586/14737159.2016.1117389. [DOI] [PubMed] [Google Scholar]
- 91.Ilie M, Khambata-Ford S, Copie-Bergman C, Huang L, Juco J, Hofman V, Hofman P. Use of the 22C3 anti–PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLOS ONE. 2017;12(8):e0183023. doi: 10.1371/journal.pone.0183023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park Y, Koh J, Na HY, Kwak Y, Lee K-W, Ahn S-H, Park DJ, Kim HH, Lee HS. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res Treat. 2020;52(3):661–670. doi: 10.4143/crt.2019.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vennapusa B, Baker B, Kowanetz M, Boone J, Menzl I, Bruey jm, et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Applied Immunohistochemistry & Molecular Morphology. 2018;27:1. [DOI] [PMC free article] [PubMed]
- 94.Kim HS, Jang WS, Ham WS, Jung SI, Lee DH, Ku JH, Ha HK, Ku JY, Choi SY, Chang IH, Choi T, Song W, Jeon SS, Jeong BC, Kim SH, Seo HK. Programmed cell death-ligand 1 expression status in urothelial carcinoma according to clinical and pathological factors: a multi-institutional retrospective study. Front Oncol. 2020;10:568809. doi: 10.3389/fonc.2020.568809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, Vennapusa B, Chang CW, Tran D, Nakamura R, Sumiyoshi T, et al. Prevalence study of PD-L1 SP142 assay in metastatic triple-negative breast cancer. Appl Immunohistochem Mol Morphol. 2020;Publish Ahead of Print. [DOI] [PMC free article] [PubMed]
- 96.Büttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F, Penault-Llorca F, Viale G, Wotherspoon AC, Kerr KM, Tsao MS. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35(34):3867–3876. doi: 10.1200/JCO.2017.74.7642. [DOI] [PubMed] [Google Scholar]
- 97.Velcheti V, Patwardhan PD, Liu FX, Chen X, Cao X, Burke T. Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PLoS One. 2018;13(11):e0206370. doi: 10.1371/journal.pone.0206370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, Mistry A, Kalamegham R, Averbuch S, Novotny J, Rubin E, Emancipator K, McCaffery I, Williams JA, Walker J, Longshore J, Tsao MS, Kerr KM. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 99.Strickler JH, Hanks BA, Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masucci GV, Cesano A, Eggermont A, Fox BA, Wang E, Marincola FM, Ciliberto G, Dobbin K, Puzanov I, Taube J, Wargo J, Butterfield LH, Villabona L, Thurin M, Postow MA, Sondel PM, Demaria S, Agarwala S, Ascierto PA. The need for a network to establish and validate predictive biomarkers in cancer immunotherapy. Journal of Translational Medicine. 2017;15(1):223. doi: 10.1186/s12967-017-1325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 102.Bumma N, Jeyakumar G, Kim S, Galasso C, Thakur MK, Gadgeel SM, et al. Neutrophil lymphocyte ratio (NLR) as a predictive biomarker for PD-1/PD-L1 directed therapy in metastatic non-small cell lung cancer (NSCLC). Journal of Clinical Oncology. 2017;35(15_suppl):e20633-e.
- 103.Simon SCS, Hu X, Panten J, Grees M, Renders S, Thomas D, et al. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology. 2020;9(1):1727116-. [DOI] [PMC free article] [PubMed]
- 104.Hofman P, Heeke S, Alix-Panabières C, Pantel K. Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol. 2019;30(9):1448–1459. doi: 10.1093/annonc/mdz196. [DOI] [PubMed] [Google Scholar]
- 105.Gunasekaran M, Russo A, Cardona AF, de Miguel PD, Lapidus R, Cooper B, et al. Exosomal PD-L1 expression as non-invasive biomarker for immune checkpoint inhibitors in non-small cell lung cancer. The Journal of Immunology. 2020;204(1 Supplement):90.10–0.
- 106.Upadhaya S, Neftelino ST, Hodge JP, Oliva C, Campbell JR, Yu JX. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov. 2020. [DOI] [PubMed]
- 107.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, et al. Anti–CTLA-4 therapy broadens the melanoma-reactive CD8<sup>+</sup> T cell response. Science Translational Medicine. 2014;6(254):254ra128-254ra128. [DOI] [PubMed]
- 108.Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Research. 2020;30(6):507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]