Abstract
Background
Daratumumab was the first monoclonal CD38 antibody with single-agent activity approved for the treatment of multiple myeloma. Moreover, daratumumab demonstrated high response rates in relapsed immunoglobulin light-chain (AL) amyloidosis.
Patients and methods
In our single-center retrospective real-life case series, we analyzed the efficacy and safety of daratumumab as first-line treatment. Daratumumab was administered with low-dose dexamethasone alone or in combination with other multiple myeloma therapeutics
Results
Fourteen patients were eligible, including nine patients with cardiac stage IIIa or IIIb. Overall hematologic response rate was 100%, with 64.3% achieving complete response after a median of 16 cycles of treatment. Median time to hematologic response was 1.4 months. Organ response rates were 45.5% after a median of 4.0 months and 66.7% after a median of 10.0 months, for heart and kidney involvement, respectively. After a median follow-up of 20.5 months, two patients underwent successful autologous stem cell transplantation (ASCT), while another three patients were in preparation for ASCT. Three patients remained on daratumumab at the last follow-up. There were no unexpected toxicities and no grade III or IV adverse events, although more than half of our patients were in stage IIIa or IIIb.
Conclusion
Daratumumab proved to be highly effective in newly diagnosed AL amyloidosis with excellent hematologic and organ response rates, a remarkable safety profile, and good tolerability even in patients with advanced stage of disease.
Key words: amyloidosis, AL, light chain, immunotherapy, daratumumab
Highlights
-
•
Daratumumab yields high hematologic response rates in untreated AL amyloidosis.
-
•
Treatment is well tolerated even in advanced disease stages.
-
•
No unexpected toxicity was observed.
Introduction
Immunoglobulin light-chain (AL) amyloidosis is a paraprotein-driven systemic disease caused by the deposition of misfolded proteins in multiple organs.1 Accurate diagnosis is often delayed due to unspecific symptoms. If cardiac involvement is present, AL amyloidosis is associated with very poor outcomes even in young and otherwise healthy patients.2,3 Currently, there are no approved treatments available. However, several clinical trials using antimyeloma drugs are ongoing. Especially in advanced stages, such trials are difficult to conduct due to the high early mortality rate.4 The general treatment strategy is to reduce the production of the monoclonal protein, which over time leads to a clearance of pathologic monoclonal light chains from the involved organ tissue. In many cases, improvement of organ function can be achieved. Trials with agents that actively clear the deposited protein from the affected organs are ongoing, however, they are not yet ready to enter routine clinical practice. While primary autologous stem cell transplantation (ASCT) has been shown to be the most effective treatment option, this procedure is frequently not possible in heavily compromised patients with advanced stages of the disease. The use of regimens with proteasome inhibitors and/or immunomodulatory drugs is often limited by organ dysfunction and/or poor performance status of the patients.5 There is an unmet need for well-tolerated effective agents that can be used safely in AL amyloidosis while inducing a rapid reduction of free light chains (FLCs).
The anti-CD38 monoclonal antibody daratumumab, which was the second monoclonal antibody registered for multiple myeloma after elotuzumab, but the first one with single-agent activity, was associated with impressive hematologic responses in multiple myeloma patients6 and in heavily pretreated AL amyloidosis patients.7, 8, 9 Recently, the first results from the ANDROMEDA trial, evaluating the use of subcutaneous daratumumab in combination with bortezomib, cyclophosphamide, and dexamethasone in newly diagnosed AL amyloidosis with a median duration of follow-up of <12 months, have been presented as late-breaking abstract at EHA 2020.10 More mature data from the safety-run-in phase of the first 28 patients recruited were published in July 2020.11
Here, we present a retrospective real-life case series of 14 patients with AL amyloidosis that received daratumumab as a first-line agent.
Materials and methods
Diagnosis and staging
This analysis represents a retrospective real-life case series based on chart reviews of 14 patients with newly diagnosed AL amyloidosis treated with daratumumab as frontline therapy between April 2017 and November 2019.
Diagnosis was established through hematologic workup with serum and urine protein electrophoresis, immunofixation, and serum and urine FLC quantification to demonstrate an underlying monoclonal gammopathy. Bone marrow biopsy and immunohistochemical assessment of clonal plasma cell infiltration were performed in all patients. Congo red staining was used for the detection of amyloid deposits. Immunohistochemical confirmation of AL amyloidosis was obtained using specific antibodies to kappa and lambda light chain amyloid as well as to amyloid A and TTR amyloid (am-Y kit standard, amYmed, Germany). Biopsies of organs with suspected involvement were performed when silent biopsies were inconclusive.12
Cardiac workup consisting of transthoracic echocardiography with left ventricular strain analysis, cardiac magnetic resonance imaging including extracellular volume quantification using T1 mapping (modified Look-Locker inversion recovery sequence), and serum N-terminal pro–B-type natriuretic peptide (NT-pro-BNP) and troponin T testing was performed in all patients. Kidney biopsies and myocardial biopsies to determine renal or cardiac involvement were performed as necessary. All patients were staged according to the Mayo 2004 and 2012 criteria.2,13,14 FISH cytogenetics from the bone marrow were performed in all patients at time of diagnosis.
Treatment
Patients received daratumumab, which is not yet approved as treatment of AL amyloidosis, complying to the compassionate use guidelines of the Austrian Federal Office for Safety in Health Care (AGES).15
All patients were discussed in a special interdisciplinary tumor board for multiple myeloma and AL amyloidosis and evaluated for other treatment options and ongoing clinical trials. As there are no approved drugs for AL amyloidosis, treatment choice was based on renal and cardiac function as well as additional comorbidities and performance status. Furthermore, the use of daratumumab was evaluated and authorized by the board of medical directors of the General Hospital of Vienna (Allgemeines Krankenhaus Wien, AKH Wien). All patients provided their written informed consent agreeing to the treatment as part of an individual healing attempt.
Dosing was calculated at 16 mg/kg in 1000 ml sodium chloride 0.9%. The first two treatment cycles consisted one weekly infusion for 8 weeks; the interval was extended to every two weeks up to week 25, with 4-week dosing intervals thereafter. In selected cases, the dosing interval was increased to 8 weeks based on hematologic complete remission.
Initial infusion rate was set at 50 ml/h and increased by 50 ml/h until a maximum of 200 ml/h according to the summary of product characteristics. Usually, the first dose was administered in an inpatient setting to closely monitor the patients for side-effects, and the subsequent doses were administered in the outpatient clinic. Premedication with dexamethasone (20 mg administered intravenously), diphenhydramine (60 mg administered intravenously), and paracetamol (1000 mg administered perorally) was performed with each treatment. Additional antimyeloma agents were added if hematologic response was not deemed rapid or deep enough. The drug selection was based on the comorbidities of the individual patients.
Response assessment
Hematologic response as well as organ responses were assessed using the current consensus criteria for AL amyloidosis.16 In addition, for cardiac and renal response rates the newly proposed 2018 response criteria that stratify any organ response into complete response (CR), very good partial response (VGPR), and partial response (PR) were used.17 Cardiac response was based on the change from baseline troponin T and NT-pro-BNP levels. As this is a retrospective analysis, regular quantitative measurement of 24-h proteinuria was not available. We used the protein-to-creatinine ratio from spot urine samples as a surrogate for improvement of kidney function. The cutoff values were the same as proposed in the respective publications.16,17
Results
A total of 14 patients were evaluated. The patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Characteristics | Total cohort (N = 14) | Dara/dex (N = 7) | Dara/dex + additional treatment (N = 7) |
|---|---|---|---|
| Sex male/female, n (%) | 9 (64.3)/5 (35.7) | 5 (71.4)/2 (28.6) | 4 (57.1)/3 (42.9) |
| Age, median (range) | 66.2 (47.8-80.9) | 71.8 (49.0-80.7) | 62.7 (47.8-80.3) |
| ECOG (0/1 versus ≥ 2), n (%) | 11 (78.6) versus 3 (21.4) | 4 (57.1) versus 3 (42.9) | 7 (100.0) versus 0 (0.0) |
| Involved free light chain, n (%) | |||
| Kappa | 2 (14.3) | 0 (0.0) | 2 (28.6) |
| Lambda | 12 (85.7) | 7 (100.0) | 5 (71.4) |
| Staging Mayo 2004, n (%) | |||
| I | 1 (7.1) | 1 (14.3) | 0 (0.0) |
| II | 4 (28.6) | 2 (28.6) | 2 (28.6) |
| IIIa | 7 (50.0) | 2 (28.6) | 5 (71.4) |
| IIIb | 2 (14.3) | 2 (28.6) | 0 (0.0) |
| Staging Mayo 2012, n (%) | |||
| I | 2 (14.3) | 1 (14.3) | 1 (14.3) |
| II | 2 (14.3) | 1 (14.3) | 1 (14.3) |
| III | 3 (21.4) | 1 (14.3) | 2 (28.6) |
| IV | 7 (50.0) | 4 (57.1) | 3 (42.9) |
| Plasma cells in bone marrow (%), median (range) | 15 (5-30) | 15 (5-20) | 20 (7-30) |
| Patients with ≥10% plasma cells, n (%) | 12 (85.7) | 6 (85.7) | 6 (85.7) |
| Number of organs involved, median (range) | 2 (1-4) | 2 (1-4) | 2 (1-4) |
| Number of organs involved ≥2, n (%) | 9 (64.3) | 4 (57.1) | 5 (71.4) |
| Number of organs involved ≥3, n (%) | 2 (14.3) | 1 (14.3) | 1 (14.3) |
| Organ involvement, n (%) | |||
| Heart | 11 (78.6) | 6 (85.7) | 5 (71.4) |
| Kidney | 9 (64.3) | 4 (57.1) | 5 (71.4) |
| Nervous system | 3 (21.4) | 1 (14.3) | 2 (28.6) |
| Gastrointestinal | 2 (14.3) | 1 (14.3) | 1 (14.3) |
| Other | 2 (14.3) | 1 (14.3) | 1 (14.3) |
| Lab parameters at diagnosis | |||
| Involved FLC level, median (range) | 277.5 (106-2960) | 297 (114-2960) | 258 (106-984) |
| dFLC (mg/dl), median (range) | 250.2 (21-2924.4) | 276.9 (100.6-2924.4) | 223.5 (21-954.6) |
| Patients with dFLC ≥180 mg/l, n (%) | 8 (57.1) | 4 (57.1) | 4 (57.1) |
| NT-pro-BNP (pg/ml), median (range)a | 3385 (387.3-177 668.0) | 4994 (387.3-17 768.0) | 3385 (1251-6245) |
| Troponin T (ng/ml), median (range)a | 73 (16-244) | 112 (16-244) | 73 (53-112) |
| Protein-to-creatinine ratio (mg/g), median (range)a | 3934.0 (123-9412) | 3613.5 (123-5393) | 5712 (701-9412) |
Dara/dex, daratumumab and dexamethasone; dFLC, difference in involved and uninvolved free light chains; NT-pro-BNP, N-terminal pro–B-type natriuretic peptide.
Only values for patients with the respective organ affected are given.
Median age was 66.2 years (range 47.8-80.9 years). Nine patients were categorized as stage III (seven with stage IIIa and two with stage IIIb) according to the 2004 Mayo staging system. If persistent proteinuria was present, a kidney biopsy was performed, which revealed AL amyloidosis in six out of eight biopsies. Endomyocardial biopsies were obtained in three patients in whom bone marrow biopsy did not allow the adequate subtyping of amyloidosis. Heart and kidney involvement were present in 11 (78.6%) and 9 (64.3%) patients, respectively. A median of two organs was affected (range 1-4), with nine patients showing an organ involvement of two or more. Interphase FISH cytogenetics revealed the translocation (t11;14) in six cases, and monosomy 8 and gain 1q in one case each. No anomalies could be detected in six patients due to low number of monoclonal plasma cells in the aspirate.
Treatment modalities
Median follow-up was 20.5 months (2.2-33.4) during which a median of 16 (3-21) cycles of daratumumab were administered. Seven patients received treatment with daratumumab and dexamethasone (dara/dex) only, while seven patients were treated, sometimes sequentially, with additional antimyeloma drugs (all seven patients received proteasome inhibitors, four received also immunomodulatory drugs, and one patient received additional chemotherapy). Of these seven patients, five received more than one additional agent. The compounds used are listed in Table 2.
Table 2.
Treatment modalities
| Agent | Dara/dex + additional agents (N = 7), n (%) |
|---|---|
| Proteasome inhibitora | 7 (100.0) |
| Bortezomib | 4 (57.2) |
| Carfilzomib | 3 (42.9) |
| Ixazomib | 3 (42.9) |
| Immunomodulatory drugs | 4 (57.1) |
| Lenalidomide | 1 (25.0) |
| Pomalidomide | 3 (75.0) |
| Chemotherapy | 1 (14.3) |
| Bendamustine | 1 (100.0) |
| Cyclophosphamide | 1 (100.0) |
Dara/dex, daratumumab and dexamethasone.
Several patients received more than one proteasome inhibitor.
Treatment intensification with additional antimyeloma drugs was initiated because of nonsufficient hematologic responses, meaning hematologic response was not rapid or deep enough (i.e. less than PR). The first combination partner was added after a median of four cycles (i.e. after 12 daratumumab infusions, range 3-8 cycles) of dara/dex. Subsequent changes of combination partners were either due to insufficient response, rising paraprotein levels, or toxicity.
At the time of last follow-up, three patients, all of them in CR, were still on treatment after 16, 20, and 21 cycles. Their treatment interval, however, was increased to 8 weeks after their clinical and hematological evaluation suggested sustained stabilization of disease. Three patients were lost to follow-up: one patient had to discontinue treatment due to pre-existing psychiatric comorbidities aggravated by the use of corticosteroids; one patient moved to a different part of Austria and had ASCT at a local hematological center. In one patient, the reason for loss of follow-up could not be determined. Seven patients discontinued treatment: one patient had to halt treatment following infectious complications, while two patients were taken off treatment when reaching CR after 15 and 21 cycles of daratumumab. One patient who achieved VGPR after 21 cycles, partly in combination with bortezomib was taken off treatment before a planned shoulder surgery.
Two patients received ASCT during follow-up. One patient with Mayo 2004 stage IIIb with severe isolated heart involvement, who achieved rapid hematologic VGPR without organ response, first underwent heart transplantation and subsequent successful ASCT after improvement of cardiac function. Unfortunately, he passed away 41 days after ASCT due to pulmonary hemorrhage and subsequent hemorrhagic shock during sepsis after having been discharged from hospital following ASCT. Another patient with Mayo 2004 stage II and isolated kidney involvement, who achieved hematologic CR and had an organ response (VGPR according to 2018 proposed criteria) underwent successful ASCT with MEL140 mg/m2 after 10 cycles and remains in CR as of last follow-up.
At the time of the last follow-up visit, three more patients were in preparation for ASCT: one patient with stage I with hematologic VGPR and no organ response; and two with stage IIIa who both achieved hematologic CR with organ response. All patients were initially not eligible for upfront ASCT, as their organ function and performance status did not permit ASCT.
Successful stem cell harvest was performed in four patients using subcutaneous granulocyte-colony-stimulating factor only. No compromise of the mobilization necessitating the use of plerixafor was observed during daratumumab treatment. One patient with severe cardiac impairment required two sessions for the harvest, which were performed in an intensive care unit setting as hemodynamic instability was anticipated during the procedure. However, no complications were observed.
It is important to note that only one death was observed, although the outcome of the three patients that were lost to follow-up remains undetermined.
There were no differences in hematologic, serologic, or molecular features between patients responding to treatment with daratumumab alone and those requiring further antimyeloma treatment, with the exception of the frequency of translocation (t11;14) which was present in two patients in the daratumumab monotherapy arm versus four patients in the combination arm. This corroborates previous data that have shown poor prognosis in (t11;14)-positive AL amyloidosis.18
Infusion-related reactions (IRRs) were observed in 28.6% of patients during the first infusion, with only one patient experiencing an IRR grade 3 which necessitated a complete infusion stop. No IRRs were observed during the following administrations. As for toxicities, no unexpected side-effects occurred. The most common issue was upper respiratory tract infections due to low gamma-immunoglobulin concentrations sometimes requiring intravenous immunoglobulins substitution.
Hematologic response
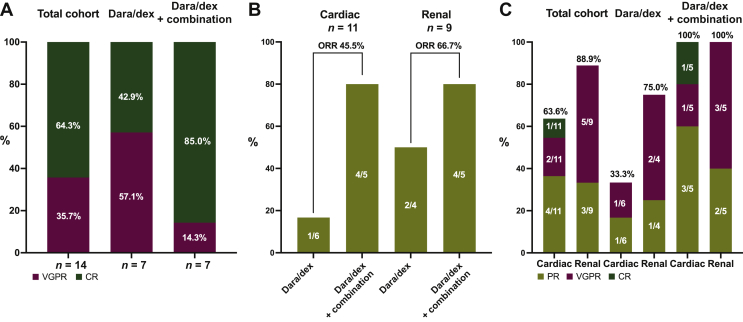
The overall hematologic response rate (ORR) was 100% (best hematologic response CR 64.3%, VGPR 35.7%; Figure 1A).
Figure 1.
Hematologic (A) and organ response rates according to the 2012 (B) and proposed 2018 (C) criteria. CR, complete response; Dara/dex, daratumumab and dexamethasone; ORR, overall response rate; PR, partial response; VGPR, very good partial response.
The depth of response remained unchanged at time of last follow-up. A nadir of the difference in involved and uninvolved FLCs (dFLCs) of <10 mg/l was observed in eight patients (57.2%). In cases where CR was not reached, this was due to the paraprotein still detectable in immunofixation in the serum or urine, while all patients had a normalized FLC ratio and a dFLC of <40. PR was the first response in 50.0% of patients, while 50.0% patients had VGPR as their first response. Four patients who required combination therapy did not reach at least PR before the combination partner was added. Usually, onset of hematologic response was rapid with a median time to first response of 1.4 months (range 0.2-8.3), while median time to best hematologic response was 11.2 months (range 0.5-21.0). The median best change in dFLC was 99.3% (range 79.2%-100.0%), with a median nadir of 3.7 mg/l after a median time of 11.2 months showing deepening response over time.
Organ response
Of the 11 patients with heart involvement, 1 patient could not be evaluated due to a baseline NT-pro-BNP level of <650 ng/l. Five patients (45.5%) showed an organ response as classified by the 2012 organ response criteria (Figure 1B), while one patient had an organ progression. No response was seen in four patients. After stratifying the organ response rates according to the proposed 2018 response criteria the ORR was 63.6%. Median time to first response according to the 2012 response criteria was 4.0 months (range 2.9-10.4 months), which was less than when the 2018 proposed criteria were applied (9.2 months, range 2.9-20.5 months). This striking difference in the median time to first response was due to a patient fulfilling the 2018 criteria for PR after 20.5 months. Because the 2018 proposed criteria allow for better quantification and description of the response depth, an increase in the VGPR rate to 18.2% and in the CR rate to 9.1% could be observed (Figure 1C). The median best change from baseline NT-pro-BNP was 70.2% (48.2-88.5) after a median of 17.6 months.
As mentioned before, regular quantitative measurement of 24-h proteinuria was not available, which is why protein-to-creatinine ratio in the spot urine was used instead. Of the nine patients with renal involvement, the organ response rate according to the 2012 criteria was 66.7% (Figure 1B), while 88.9% were classified as renal responders according to the 2018 proposed criteria. One organ progression was observed regardless of the set of criteria used. As in heart involvement, a deepening of response rates according to the 2018 proposed criteria could be observed, with five patients who were initially classified as PR having achieved VGPR (Figure 1C). Best change from baseline was 74.7% (range 49.7-96.9) with a median nadir of 1170.5 mg/g (100.0-4395.0) after a median time of 17.7 months (16.7-21.8).
In the three patients with involvement of the nervous system (all presenting with sensomotoric polyneuropathy) stabilization or, in one case, slight improvement with the support of gabapentin was observed. Two patients presented with diarrhea due to gastrointestinal amyloidosis with both experiencing slight improvement of their symptoms and subsequent weight gain.
Discussion
Daratumumab, a monoclonal anti-CD38 antibody, has shown convincing results in patients with multiple myeloma.19 Early data have been published by three groups on pretreated AL amyloidosis patients receiving daratumumab-based therapy at recurrence of disease.7, 8, 9 All publications report highly promising results with rapid hematologic response; however, these pertain to pretreated patients. The study results showed that response is convincing, with either CR or VGPR in most patients. These results were corroborated by data from a phase II clinical trial.20 The first retrospective case series with 25 relapsed patients with a median of three prior lines of therapy showed an overall ORR of 76% (CR 36%, VGPR 24%, PR 16%).8 A subsequent report of a similar cohort by Khouri et al.9 observed an ORR of 86% (CR 33%, VGPR 53%).
In our cohort of untreated patients, the ORR was 100% with a CR rate of 64.3%. Onset of first hematologic response was rapid at about 1.4 months, which is in accordance with median time to response in pretreated AL amyloidosis of about 1 month.8 Response deepened over time and dFLC nadir was reached after a median 11.2 months. Despite the excellent overall response to treatment, there was a subgroup of patients who required treatment with additional antimyeloma drugs to achieve sufficient hematologic response (or in the case of four patients any hematologic response at all). The first drugs of physicians' choice were bortezomib or pomalidomide. Both drugs are used in the treatment of AL amyloidosis, given their safety in renal impaired patients and effectiveness to reduce production of the paraprotein.12
In several patients, the combination partners had to be changed due to either insufficient response or toxicity. In one patient with stage IIIa disease, a total of five additional combination partners had to be added to the dara/dex backbone during course of the treatment. During the whole treatment period of 20 months, the NT-pro-BNP decreased from 5745 at baseline to 2355 pg/ml. At last follow-up, the respective patient was in preparation for an ASCT.
In our cohort, two patients with stage II and IIIb underwent ASCT after improvement of their cardiac condition. Another three patients, two with stage IIIa, were in preparation for ASCT at last follow-up. This highlights the importance of a highly individualized approach in the treatment of AL amyloidosis to achieve the best possible hematologic response and improvement of organ function. Thus, we have the chance to improve overall survival rates and quality of life, as well as to reduce the risk of transplant-related mortality once ASCT is performed. We favor this strategy over a fixed duration or fixed drug combination of induction therapy.
Recently, the first results of the ANDROMEDA trial, which investigates the triplet of cyclophosphamide, bortezomib, and dexamethasone (CyBorD) with or without daratumumab in previously untreated AL amyloidosis, were presented at the 2020 EHA meeting.10 It provides data on 388 patients treated with 6 cycles of CyBorD and subcutaneous daratumumab followed by daratumumab maintenance for a maximum of 24 cycles versus patients treated with 6 cycles of CyBorD alone. In the late-breaking abstract session at EHA 2020 the data on the first interim analysis evaluated after a median follow-up duration of <12 months were reported. Patient characteristics were similar to our cohort. After a median of 9.6 months in the daratumumab arm and 5.3 months in the control arm, the hematologic ORR was 92% and 77%, respectively. The ≥VGPR rate was 79% versus 49%. In our real-life cohort with a median follow-up of 20.5 months the hematologic ORR was 100% in both groups. More importantly, even the dara/dex group's response rate was superior to the ANDROMEDA's CyBorD arm (CR 42.9% versus 18%), which underlines the possible role of dara/dex monotherapy in newly diagnosed AL amyloidosis. This may be of particular relevance in patients with stage IIIb cardiac amyloidosis, who were excluded from the ANDROMEDA trial, but represent a subgroup of patients that often do not tolerate intensified treatment with CyBorD. This is highlighted by the proportion of patients with ECOG status ≥2 in the dara/dex group (42.9%). It is tempting to speculate whether our patients would have met the inclusion criteria or managed to successfully receive CyBorD, as this regimen does carry the risk of increased treatment-related toxicities.
In our analysis, after a median follow-up of 20.5 months substantial organ response rates could be observed, which are also mirrored by the ANDROMEDA study. It is important to note that the currently established organ response criteria do not allow for grading of response depth and only define response as a ≥50% reduction of NT-pro-BNP or 24-h proteinuria from baseline.16 The response criteria proposed by Muchtar et al.,17 however, differentiate between PR, VGPR, and CR, which also translates into a better survival. In our cohort this increased the overall organ response rate. Another advantage of this set of criteria could be a better selection of patients for additional treatment, as they allow for early detection of organ response, or rather the absence of it, because the threshold for PR is lower at ≥30% in NT-pro-BNP or 24-h proteinuria. In clinical practice, response assessment can prove challenging, in particular in the setting of combination therapies. Pomalidomide for instance routinely increases the NT-pro-BNP levels and may induce or aggravate dyspnea.21 This, however, should not be misinterpreted as progression of the disease itself and should not lead to treatment cessation.
In our experience, careful and frequent (i.e. twice a month) hematologic assessment should be performed in these patients to adequately capture their response to treatment. Furthermore, thorough patient education on such transient worsening is paramount to ensure adherence to treatment.
Regarding the relationship between serological and clinical response, it was previously shown by Palladini et al.22 that, even in patients with a clear decrease in FLCs and NT-pro-BNP, left ventricular hypertrophy improves minimally and slowly. This was reflected by our patients' follow-up, where a rapid decrease in dFLC was found, while organ response was seen after a longer period. It will remain an important endpoint in future studies to elucidate the period of time between serum FLC decrease, clinical improvement, and measurable organ response. The 2018 response criteria may help to improve response assessment.
In 2016, an open-label phase II study on daratumumab found that IRRs occurred at a high frequency.23 Low-grade (grade 1 or 2) IRRs were also reported in 60% of AL amyloidosis patients treated by Kaufman et al.8 As a consequence, Popat et al.24 suggested slower infusion rates and recently reported decreasing frequencies of IRR with continued daratumumab use. In our report, IRRs during first infusion were observed in 28.6% of patients, although they were usually not severe and infusion could be continued in three out of four patients after administration of additional doses of steroids or antihistamines. One patient experienced severe dyspnea and fluid retention, which led to discontinuation of the first dose and administration of antihistamines, steroids, and diuretics. However, it is important to note that patients with cardiac amyloidosis are prone to cardiac decompensation following fluid overload. This issue could be ameliorated by a subcutaneous formulation of daratumumab, which is used in the ANDROMEDA trial.
A limitation of this retrospective case series is the small number of patients in a single-center study. However, despite existing data on daratumumab treatment in other diseases and as a second-line agent, our results reveal important information concerning the efficacy of daratumumab as a novel first-line treatment strategy in AL amyloidosis patients.
Here, we report on daratumumab as an efficient first-line treatment strategy in AL amyloidosis in a retrospective case series of 14 patients in a real-life setting. All patients rapidly achieved hematological response that deepened over time. A substantial proportion also showed cardiac and renal response. The treatment was generally well tolerated with no unexpected side-effects from neither daratumumab itself nor from any of the combination partners that were added over the course of the treatment. This was also true for frail patients with cardiac involvement stage IIIa and IIIb.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Dispenzieri A., Merlini G. Immunoglobulin light chain systemic amyloidosis. Cancer Treat Res. 2016;169:273–318. doi: 10.1007/978-3-319-40320-5_15. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S., Dispenzieri A., Lacy M.Q. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kristen A.V., Brokbals E., Aus dem Siepen F. Cardiac amyloid load: a prognostic and predictive biomarker in patients with light-chain amyloidosis. J Am Coll Cardiol. 2016;68(1):13–24. doi: 10.1016/j.jacc.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 4.Phillips E.H., Nash S., Adedayo T. Pitfalls in conducting prospective trials in stage III cardiac amyloidosis - experience from the REVEAL study. Amyloid. 2017;24(4):242–244. doi: 10.1080/13506129.2017.1385453. [DOI] [PubMed] [Google Scholar]
- 5.Jelinek T., Kufova Z., Hajek R. Immunomodulatory drugs in AL amyloidosis. Crit Rev Oncol Hematol. 2016;99:249–260. doi: 10.1016/j.critrevonc.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Lokhorst H.M., Plesner T., Laubach J.P. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 7.Sher T., Fenton B., Akhtar A. First report of safety and efficacy of daratumumab in 2 cases of advanced immunoglobulin light chain amyloidosis. Blood. 2016;128(15):1987–1989. doi: 10.1182/blood-2016-06-722496. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman G.P., Schrier S.L., Lafayette R.A. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130(7):900–902. doi: 10.1182/blood-2017-01-763599. [DOI] [PubMed] [Google Scholar]
- 9.Khouri J., Kin A., Thapa B. Daratumumab proves safe and highly effective in AL amyloidosis. Br J Haematol. 2019;185(2):342–344. doi: 10.1111/bjh.15455. [DOI] [PubMed] [Google Scholar]
- 10.Kastritis E, Palladini G, Minnema MC, et al. Subcutaneous daratumumab + cyclophosphamide, bortezomib, and dexamethasone (CyBorD) in patients with newly diagnosed light chain (AL) amyloidosis: primary results from the phase 3 ANDROMEDA study. EHA Late breaking abstract LB2604 2020. https://library.ehaweb.org/eha/2020/eha25th/303396/efstathios.kastritis.subcutaneous.daratumumab.2B.cycl%20ophosphamide.bortezomib.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D3%2Ace_i.
- 11.Palladini G., Kastritis E., Maurer M.S. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136(1):71–80. doi: 10.1182/blood.2019004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gertz M.A. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol. 2020;95:848–860. doi: 10.1002/ajh.25819. [DOI] [PubMed] [Google Scholar]
- 13.Dispenzieri A., Gertz M.A., Kyle R.A. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Wechalekar A.D., Schonland S.O., Kastritis E. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420–3427. doi: 10.1182/blood-2012-12-473066. [DOI] [PubMed] [Google Scholar]
- 15.https://www.basg.gv.at/en/companies/medicinal-products/compassionate-use
- 16.Comenzo R.L., Reece D., Palladini G. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26(11):2317–2325. doi: 10.1038/leu.2012.100. [DOI] [PubMed] [Google Scholar]
- 17.Muchtar E., Dispenzieri A., Leung N. Depth of organ response in AL amyloidosis is associated with improved survival: grading the organ response criteria. Leukemia. 2018;32(10):2240–2249. doi: 10.1038/s41375-018-0060-x. [DOI] [PubMed] [Google Scholar]
- 18.Bryce A.H., Ketterling R.P., Gertz M.A. Translocation t(11;14) and survival of patients with light chain (AL) amyloidosis. Haematologica. 2009;94(3):380–386. doi: 10.3324/haematol.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateos M.V., Dimopoulos M.A., Cavo M. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–528. doi: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 20.Sanchorawala V., Sarosiek S., Schulman A. Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: results of a phase II study. Blood. 2020;135:1541–1547. doi: 10.1182/blood.2019004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchorawala V., Shelton A.C., Lo S. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood. 2016;128(8):1059–1062. doi: 10.1182/blood-2016-04-710822. [DOI] [PubMed] [Google Scholar]
- 22.Palladini G., Lavatelli F., Russo P. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107(10):3854–3858. doi: 10.1182/blood-2005-11-4385. [DOI] [PubMed] [Google Scholar]
- 23.Lonial S., Weiss B.M., Usmani S.Z. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560. doi: 10.1016/S0140-6736(15)01120-4. [DOI] [PubMed] [Google Scholar]
- 24.Popat R., Dowling E., Achhala S. Real world data of the impact of first cycle daratumumab on multiple myeloma and AL amyloidosis services. Br J Haematol. 2018;182(6):936–939. doi: 10.1111/bjh.14897. [DOI] [PubMed] [Google Scholar]